HIGHLIGHTS
-
•
A total of 80% of patients admitted for COVID-19 at a safety-net hospital were Black or Hispanic.
-
•
The prevalence of long COVID was 34% in this cohort after a median of 255 days.
-
•
The severity of acute COVID-19 was associated with the risk of long COVID.
Keywords: Long COVID, post-COVID-19 syndrome, post-acute sequelae of COVID-19, health equity
Abstract
Introduction
Little is known about the burden of long COVID among Black and Hispanic patients in the U.S. We surveyed adult patients hospitalized for COVID-19 at John H. Roger, Jr. Hospital of Cook County, a safety-net hospital predominantly serving Black and Hispanic patients in Chicago, for persistent symptoms after hospitalization to assess prevalence and identify risk factors.
Methods
Cross-sectional data were obtained over 6 months after discharge from patients hospitalized at John H. Roger, Jr. Hospital of Cook County who tested positive for SARS-CoV-2 between October 1, 2020 and January 12, 2021. Multivariable logistic regression was used to analyze the associations between patient characteristics and symptom persistence.
Results
Of 145 patients surveyed at a median follow-up period of 255 days (IQR=238–302), 80% were Black or Hispanic, and 50 (34%) reported at least 1 symptom. In multivariable logistic regression, the risk of long COVID was associated with the severity of acute COVID-19 illness, consistent with findings from population-based cohort studies.
Conclusions
Long COVID prevalence remains high 7 months to a year after an initial illness in a majority Black and Hispanic hospitalized cohort. There is a long-term and ongoing need to assess and address the burden of long COVID, especially among minority communities disproportionately affected by acute COVID-19.
INTRODUCTION
Post-acute or long-haul coronavirus disease 2019 (COVID-19) entails new or ongoing symptoms that persist 4–12 weeks after a confirmed or probable diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1,2 Estimates of long COVID prevalence in the general U.S. population range from 10% to 30% of all cases of acute infection, with higher rates among older, female, hospitalized, and unvaccinated patients.3, 4, 5 Although it is recognized that acute COVID-19 disproportionately impacted racial/ethnic minorities in the U.S., the prevalence and characteristics of long COVID in socially vulnerable minority communities remain poorly understood.6,7
Public safety-net hospitals in the U.S. are at the frontlines of addressing the racial/ethnic and social inequities exposed by the pandemic.8 We carried out a cross-sectional survey with adult patients hospitalized for COVID-19 at John H. Stroger, Jr. Hospital of Cook County, which is the primary inpatient facility of Cook County Health, the only public safety-net health system for metropolitan Chicago. The vast majority of patients seen in our health system are uninsured or insured through Medicaid, the means-tested state and federal insurance program for people with low income. Previous research conducted at our institution during the first wave of the pandemic showed that the vast majority of patients hospitalized for acute COVID-19 were Hispanic or non-Hispanic Black and that the average social vulnerability index of the neighborhoods from which patients came was in the highest quartile.9
This study was carried out as a pilot to assess the prevalence and characteristics of long COVID among patients hospitalized for COVID-19 at John H. Roger, Jr. Hospital as well as to analyze the potential sociodemographic and clinical risk factors associated with long COVID. It also piloted the use of telephone screening for long-term follow-up for patients hospitalized with COVID-19 at our institution, constituting a first and necessary step for mitigating inequities for long COVID care in a resource-constrained healthcare setting.
METHODS
Starting on June 28, 2021, we conducted a cross-sectional survey of patients aged ≥18 years admitted to John H. Roger, Jr. Hospital diagnosed with COVID-19 infection on the basis of a positive reverse transcriptase polymerase chain reaction test between October 1, 2020 and January 12, 2021, shortly before the Alpha variant became the predominant variant in circulation.10 The exclusion criteria applied were patients who died or were discharged to hospice, patients who were asymptomatic at the time of hospitalization and incidentally tested positive for COVID-19, and patients who were part of Cermak Health Services of Cook County (a correctional health system).
We recruited patients by telephone with the aid of an interpreter when the patient's primary language was not English. Telephone numbers were obtained from the electronic health record of John H. Roger, Jr. Hospital. We adapted the Newcastle post-COVID syndrome follow-up survey designed to screen for symptoms 10–12 weeks after the acute phase of the illness.11 The survey included 1 yes/no question regarding whether the patient feels fully recovered from their COVID-19 illness and 12 yes/no questions that screened for specific symptoms of dyspnea, fatigue, cough, palpitations, myalgia, and 7 other symptoms described with post–COVID-19 syndrome. Patients who needed further care were referred to their primary care provider or to the Cook County Health General Medicine Clinic for further evaluation.
We compared clinical and demographic characteristics, including age; sex; race/ethnicity; comorbidities; smoking status; severity of acute illness; and treatments received between all eligible patients, patients who provided consent to be surveyed, and patients who screened positive for long COVID. All patient characteristics were extracted from medical records. Categorical variables were created for age (18–45, 46–55, 56–65, ≥65 years), sex (male or female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian, and other/unknown), and smoking status (never, former, current). A dichotomous variable for BMI was created around the cut off of 30 kg/m2. Patients’ comorbidities were stratified using the Charlson comorbidity score on the basis of the presence of ICD-10 codes for 20 chronic conditions (categorized as scores from 0 to 3 or more).12 Patients’ acute illnesses were stratified on admission as mild, moderate, or severe/critical, as per the NIH definition.5 Mild disease was described as the presence of symptoms suggestive of acute COVID-19 infection, with a positive COVID-19 reverse transcriptase polymerase chain reaction test. Moderate illness represented the presence of radiographic evidence of pneumonia and an SpO2 (saturation of peripheral oxygen) of 94%. Severe illness corresponded to the presence of radiographic findings suggestive of pneumonia with an SpO2 <94%. Critical illness included patients admitted to the intensive care unit (ICU).13
Univariate chi-square analysis was used to test the significance of associations between individual characteristics and recovery status. Multivariable logistic regression model was then used to test the associations of disease severity at the time of acute illness presentation with post-acute COVID-19 recovery status, adjusted for relevant clinical and demographic characteristics. Measures of illness severity we used included the NIH classification of illness severity at the time of presentation, admission to the medical ICU, and being discharged with oxygen. Only the NIH classification was included in the final regression model in consideration of its superior external validity and to avoid issues with multicollinearity. Statistical significance was defined by p<0.05 for all analyses. All statistical analyses were conducted using Stata, Version 14.2. The study protocol was reviewed and approved by the Cook County Health IRB (21-045-Stroger).
RESULTS
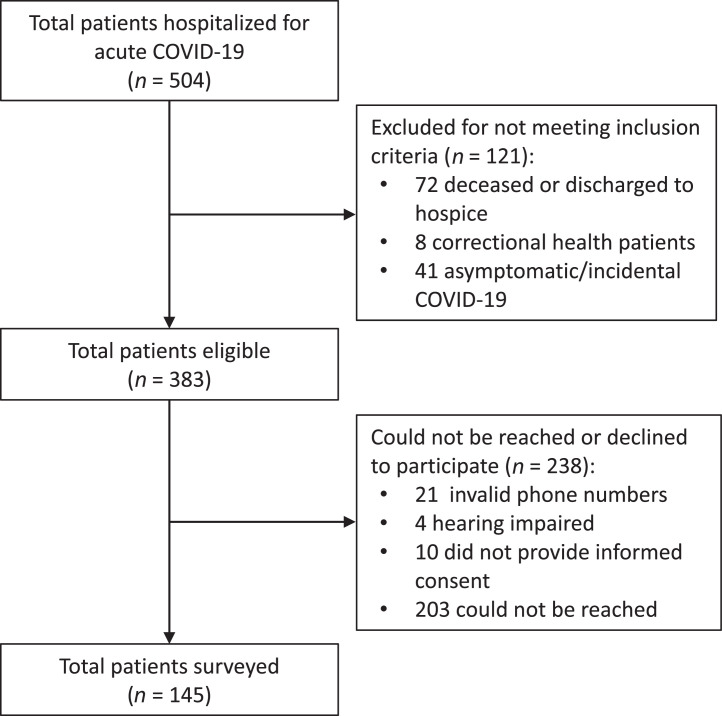
A total of 504 patients were hospitalized with COVID-19 at John H. Roger, Jr. Hospital between October 1, 2020 and January 12, 2021. Of these patients, 72 died or were discharged to hospice, 8 were patients of Cermak Health Services, and 41 had no documented symptoms of COVID-19 and incidentally tested positive at the time of hospitalization, leaving 383 patients eligible to participate in the study. Of these, 32 had an invalid phone number, 4 were hearing impaired and so could not provide consent by phone, and 202 were unable to be reached for unspecified reasons. Ultimately, 155 respondents were reached by phone, of whom 145 consented to be surveyed, resulting in an effective response rate of 38% (145/383) (Figure 1). The median time of assessment was 255 days (IQR=238–302) after the date of discharge.
Figure 1.
Patients hospitalized, eligible, and surveyed.
The average age of patients surveyed was 53.8 years, with an SD of 14.1 years (Table 1). A majority were male (64%), and 80% were Hispanic (48%) or non-Hispanic Black (32%). Most patients surveyed (66%) had a BMI of 30 kg/m2 or greater. Most were never smokers (65%). Among survey respondents, 8.3% were admitted to the ICU, and 21% were discharged on oxygen. All patients admitted to the ICU and 93% of patients discharged on oxygen had a moderate or severe illness presentation.
Table 1.
Demographic and Clinical Characteristics of Eligible Discharged, Surveyed, and Symptomatic Patients
| Characteristics | All eligible patients N=383, n (%) |
Patients consented n=145, n (%) |
Patients not recovered n=50, n (%) |
|---|---|---|---|
| Male sex | 236 (62) | 93 (64) | 30 (60) |
| Age, years | |||
| 0–45 | 100 (26) | 39 (27) | 13 (26) |
| 46–55 | 99 (26) | 34 (23) | 17 (34) |
| 55–65 | 96 (23) | 39 (27) | 12 (24) |
| ≥65 | 88 (23) | 33 (23) | 8 (16) |
| Race/ethnicity | |||
| Non-Hispanic White | 37 (9.7) | 17 (12) | 7 (14) |
| Non-Hispanic Black | 98 (26) | 47 (32) | 12 (24) |
| Hispanic | 225 (59) | 70 (48) | 27 (54) |
| Asian | 17 (4.4) | 6 (4.1) | 2 (4) |
| Other/unknown | 6 (1.6) | 5 (3.5) | 2 (4) |
| Admission severity | |||
| Mild | 114 (30) | 54 (37) | 13 (26) |
| Moderate | 136 (36) | 50 (34) | 21 (42) |
| Severe or critical | 133 (35) | 41 (28) | 15 (30) |
| Charlson comorbidity score | |||
| 0 | 158 (41) | 63 (43) | 23 (46) |
| 1 | 126 (33) | 41 (28) | 18 (36) |
| 2 | 57 (15) | 25 (17) | 5 (10) |
| ≥3 | 42 (11) | 16 (11) | 4 (8) |
| BMI | |||
| <30 kg/m2 | 173 (45) | 49 (34) | 10 (20) |
| ≥30 kg/m2 | 210 (55) | 96 (66) | 40 (80) |
| Smoking status | |||
| None | 253 (66) | 94 (65) | 30 (60) |
| Former | 93 (24) | 38 (26) | 15 (30) |
| Current | 36 (9.4) | 13 (9.0) | 5 (10) |
| Admitted to ICU | 44 (11) | 12 (8.3) | 8 (16) |
| Discharged with oxygen | 83 (22) | 30 (21) | 16 (32) |
Note: All values are n (%).
ICU, intensive care unit.
Fifty (34%) patients reported lingering symptoms after their hospitalization for acute COVID-19. In univariate chi-square analysis, BMI≥30 kg/m2, ICU admission, and being discharged on oxygen were significantly associated with a higher risk of reporting at least 1 long COVID symptom (p<0.05).
In multivariable logistic regression modeling, the severity of acute illness was associated with an increased risk of long COVID after adjusting for age, sex, race/ethnicity, BMI status, smoking status, and Charlson comorbidity score (Table 2). Around a threefold increase in the risk of long COVID was associated with moderate (adjusted OR=3.1, 95% CI=1.2, 8.3) or severe/critical (adjusted OR=2.7, 95% CI=1.0, 7.6) illness compared with that associated with mild illness at presentation, although the association with severe/critical presentation did not reach statistical significance (p=0.052).
Table 2.
Multivariable Logistic Regression for Patient Characteristics Associated With Long COVID
| Characteristics | AORa (95% CI) |
|---|---|
| Sex | |
| Female | ref |
| Male | 1.3 (0.9, 2.0) |
| Age, years | |
| <45 | ref |
| 46–55 | 1.3 (0.4, 3.6) |
| 55–65 | 0.6 (0.2, 1.9) |
| >65 | 0.4 (0.1, 1.5) |
| Smoking | |
| None | ref |
| Former | 2.1 (0.8, 5.5) |
| Current | 2.8 (0.6, 12.1) |
| Charlson comorbidity score | |
| 0 | ref |
| 1 | 1.4 (0.5, 3.6) |
| 2 | 0.6 (0.2, 2.0) |
| ≥3 | 1.0 (0.2, 4.1) |
| BMI | |
| <30 kg/m2 | ref |
| ≥30 kg/m2 | 2.5 (1.0, 6.3) |
| Admission severity | |
| Mild | ref |
| Moderate | 3.1 (1.2, 8.3) |
| Severe/critical | 2.7 (1.0, 7.6) |
Logistic regression adjusted for all variables listed in Table 2.
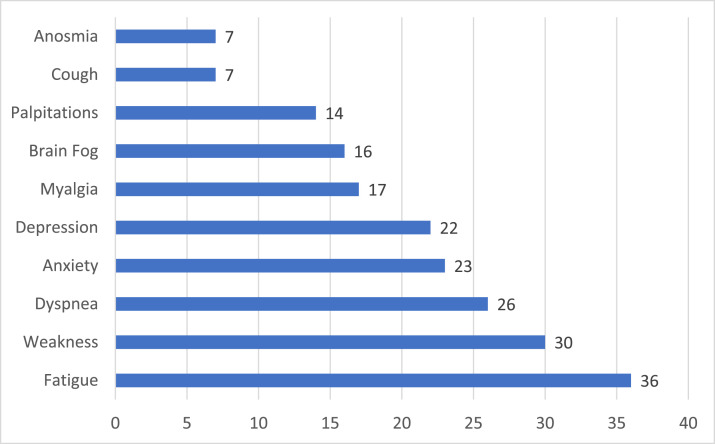
The most common long COVID symptoms reported were fatigue, weakness, and dyspnea, which were reported by over 50% of patients surveyed (Figure 2). Increased symptoms of anxiety and depression were reported by 46% and 44% of patients, respectively. Problems with memory and concentration suggestive of brain fog, a typical and serious long-term sequela of COVID-19, were reported by 32% of patients surveyed.
Figure 2.
Frequency of patient self-reported long COVID-19 symptoms (N=50).
DISCUSSION
In patients hospitalized at a public safety-net hospital in Chicago, we estimated the prevalence of at least 1 symptom of long COVID after discharge to be 34% even after >7 months. Our estimate is consistent with the U.S. Centers for Disease Control and Prevention's current estimate of long COVID prevalence 6 months after hospitalization (30%).1 A large cohort study of both hospitalized and nonhospitalized patients that included SARS-CoV-2–negative controls showed a similar incidence rate of neuropsychiatric sequelae (33.62%) 6 months after initial infection.4 Another large cohort study of patients in the U.S. Veteran's Administration database estimated a lower rate of long COVID prevalence at 15.8% among hospitalized patients beyond 12 weeks of initial illness.14 By contrast, our prevalence estimate is considerably lower than those of other studies on the basis of hospitalized patients, which have presented long COVID prevalence estimates of around 50%–80%, depending on the outcome assessed.3,15 Nevertheless, our finding that the severity of acute illness is associated with future risk of long COVID is consistent with these and those of other studies.16 The foremost symptoms of fatigue and dyspnea reported by our survey respondents are also consistent with those of previous studies.17,18 It bears emphasizing that long COVID encompasses multiple phenotypes and outcomes, and prevalence estimates can differ widely depending on the outcomes tracked and the methods used.
Compared with the racial/ethnic composition of Chicago residents, of whom 29.2% and 28.6% were reported to be Black and Hispanic, respectively, in the most recent U.S. census estimates,19 the patients we surveyed were more likely to be Black or Hispanic. Although we did not replicate previous findings that minority race/ethnic status might be associated with a higher risk of developing long-term post-COVID symptoms,16,20 this would not contradict findings of higher burdens of long COVID in Black and Hispanic communities in the U.S. Indeed, given higher rates of acute COVID-19 infections and deaths among predominantly Black and Hispanic neighborhoods and lower rates of vaccination among Black Chicagoans, it is likely that long COVID would also be more prevalent.
Limitations
This study has several limitations. The sample size was small and potentially underpowered to reveal the significance of certain associations, such as between BMI and long COVID in multivariable regression modeling. Our focus on patients hospitalized at a safety-net hospital limits how much we can generalize our findings to the larger burden of community-based COVID-19 infections. Lack of a negative control group also limits the interpretability of our prevalence estimates.
The response rate to our survey was only 38%, which is low by some measures, albeit within the range of published health research.21 Given the precipitous decline in response rates to telephone surveys in recent years, our response rate is comparable with and exceeds those of some national surveys.22 Although a low survey response rate could introduce nonresponder bias, there is evidence that it correlates weakly with how well the survey sample represents the target population.23 Reliance on self-reported symptoms may also limit the reliability of our findings. Despite its limitations, telephone survey remains an indispensable method of outreach to patients.
CONCLUSIONS
Long COVID poses a significant burden of morbidity for patients hospitalized with COVID-19. There is moreover an urgent need to assess and address the burden of COVID-19–related chronic disease and disability among socially vulnerable Black and Hispanic persons most adversely affected by the pandemic in the U.S. and have historically faced barriers to healthcare access. Through a telephonic survey, we were able to reach 40% (155/383) and survey 38% of patients previously hospitalized for COVID-19, some of whom have had no other post-hospitalization contact with our health system. Although the burden of long COVID from patients who were never hospitalized is likely greater, the severity of initial disease is predictive of the likelihood of developing long COVID, as shown in our study and many previous studies. This suggests that screening of patients hospitalized with COVID-19 even 7–12 months after discharge could prove to be a high-yield way for safety-net institutions to connect patients at risk of developing long COVID to clinical services. This pilot study has thus informed the broadening of ongoing assessment for long COVID in our health system over successive waves of the pandemic, both through telephonic surveys and in-person clinical assessments for a subset of patients endorsing long COVID symptoms. In addition to clarifying the risks and burdens of long COVID and characterizing its predominant phenotypes in our patients, the allocation of dedicated follow-up services in outpatient primary and specialty care settings constitutes a step toward addressing the long shadow of the pandemic's inequitable impact.
CRediT authorship contribution statement
Miao Jenny Hua: Conceptualization, Formal analysis, Funding acquisition, Investigation, Software, Visualization, Writing – original draft. Sriram Gonakoti: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing. Ruhi Shariff: Conceptualization, Investigation, Project administration, Supervision, Methodology. Carlos Corpuz: Investigation, Visualization. R. Alfonso Hernandez Acosta: Data curation, Investigation, Writing – review & editing. Hillary Chang: Investigation, Writing – review & editing. Iriagbonse Asemota: Investigation, Writing – review & editing. Elizabeth Gobbi: Investigation. Katayoun Rezai: Conceptualization, Funding acquisition, Investigation, Supervision, Methodology.
ACKNOWLEDGMENTS
The authors would like to thank Hisham Laswi, Ancy Jacob, and Gregory Holden for their help with conducting telephonic surveys.
This work was supported by the American College of Preventive Medicine and the American Medical Association grant (CDC OT18-1802).
This research was presented as a poster entitled “Prevalence and Characteristics of Post-Acute Sequelae of COVID-19 Among Urban Safety-Net Hospital Patients” at the Preventive Medicine 2022 conference.
MJH discloses being the recipient of funding from the American College of Preventive Medicine and the American Medical Association under the grant, “Improving Capacity of Physician-led Practices to Prevent, Mitigate and Treat COVID-19” (CDC OT18-1802) for implementing clinical services around long COVID at Cook County Health. She has no financial disclosures. No other disclosures were reported.
REFERENCES
- 1.Long COVID or post-COVID conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Updated December 16, 2022. Accessed November 24, 2022.
- 2.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlis RH, Santillana M, Ognyanova K, et al. Prevalence and correlates of long COVID symptoms among U.S. adults. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.38804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger Z, Altiery DE Jesus V, Assoumou SA, Greenhalgh T. Long COVID and health inequities: the role of primary care. Milbank Q. 2021;99(2):519–541. doi: 10.1111/1468-0009.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oladele CR, McKinney TL, Tolliver D, Tuckson R, Dawes D, Nunez-Smith M. The Black Coalition Against Covid; Washington, DC: 2022. The state of Black America and COVID-19: a two-year assessment.https://blackcoalitionagainstcovid.org/wp-content/uploads/2022/03/2022-Report-State-of-Black-America-and-COVID-19-A-Two-Year-Assessment-3292022.pdf [Google Scholar]
- 8.Knudsen J, Chokshi DA. Covid-19 and the safety net — moving from straining to sustaining. N Engl J Med. 2021;385(24):2209–2211. doi: 10.1056/NEJMp2114010. [DOI] [PubMed] [Google Scholar]
- 9.Trick WE, Badri S, Doshi K, et al. Epidemiology of COVID-19 vs. influenza: differential failure of COVID-19 mitigation among Hispanics, Cook County Health, Illinois. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0240202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overview of Variants in Countries. CoVariants. https://covariants.org/per-country?region=United+States. Updated March 30, 2023. Accessed November 31, 2022.
- 11.National Health Service Newcastle post-COVID syndrome follow-up screening questionnaire. 2021 https://postcovidsyndromebsol.nhs.uk/images/Content/Newcastle_post_Covid_Screening_Tool.pdf Published. [Google Scholar]
- 12.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Clinical spectrum of SARS-CoV-2 infection. NIH, COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Updated March 6, 2023. Accessed November 24, 2022.
- 14.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.QuickFacts: Cook County, Illinois; Chicago city, Illinois; United States. U.S. Census Bureau. https://www.census.gov/quickfacts/fact/table/cookcountyillinois,chicagocityillinois,US/PST045219. Updated March 30, 2023 Accessed October 6, 2021.
- 20.Yomogida K, Zhu S, Rubino F, Figueroa W, Balanji N, Holman E. Post-acute sequelae of SARS-CoV-2 infection among adults aged ≥18 years — Long Beach, California, April 1–December 10, 2020 [published correction appears in MMWRMorb Mortal Wkly Rep. 2021;70(39):1390] MMWR Morb Mortal Wkly Rep. 2021;70(37):1274–1277. doi: 10.15585/mmwr.mm7037a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booker QS, Austin JD, Balasubramanian BA. Survey strategies to increase participant response rates in primary care research studies. Fam Pract. 2021;38(5):699–702. doi: 10.1093/fampra/cmab070. [DOI] [PubMed] [Google Scholar]
- 22.Olson K, Smyth JD, Horwitz R, et al. Transitions from telephone surveys to self-administered and mixed-mode surveys: AAPOR Task Force Report. J Surv Stat Methodol. 2021;9(3):381–411. doi: 10.1093/jssam/smz062. [DOI] [Google Scholar]
- 23.Hendra R, Hill A. Rethinking response rates: new evidence of little relationship between survey response rates and nonresponse bias. Eval Rev. 2019;43(5):307–330. doi: 10.1177/0193841X18807719. [DOI] [PubMed] [Google Scholar]