Abstract
Background
Immunosuppressed individuals such as kidney transplant recipients (KTR) and hemodialysis patients (DP) show impaired immune responses to COVID-19 vaccination. Plasma Torque Teno Virus (TTV) DNA load is used as surrogate for the individual degree of immunosuppression. We now assessed the association of TTV load at time of COVID-19 vaccination with humoral and cellular immune response rates to vaccination in KTR, DP, and healthy medical personnel (MP).
Methods
A total of 100 KTR, 115 DP and 54 MP were included. All were SARS-CoV-2 seronegative at the time of vaccination with either BNT162b2 or mRNA-1273. Plasma TTV loads were assessed at the time of first vaccination. After two-dose vaccination, seroconversion (de novo detection of SARS-CoV-2 S1-IgA and/or IgG) was determined. In addition, cellular responses as assessed by interferon γ release and neutralizing antibodies were assessed in a subset of participants. ROC analyses were performed to define TTV load cut-offs predicting specific immune responses to vaccination.
Results
Plasma TTV loads at the time of first vaccination were negatively associated with seroconversion after two-dose vaccination in KTR (OR 0.87, 95% CI 0.76–0.99). TTV loads were significantly lower in KTR who developed humoral and cellular immune responses to vaccination compared to non-responders (p = 0.0411 and 0.0030, respectively). Of patients with TTV loads above 106 copies/ml, none developed cellular immune responses against SARS-CoV-2, and only 2 of 17 (12%) seroconverted in response to vaccination.
Conclusion
Plasma TTV loads at the time of first vaccination in immunosuppressed individuals may be useful to predict individual vaccine-specific immune responses.
Keywords: Torque Teno virus, TTV, Kidney transplant recipients, Dialysis patients, COVID-19 mRNA vaccine
1. Introduction
One year after the onset of the SARS-CoV-2 pandemic, the first COVID-19 vaccines became available [1,2]. These elicited efficient humoral and cellular immune responses in immunocompetent persons and protected against severe coronavirus disease 2019 (COVID-19) [1], [2], [3]. However, this protective effect was limited in immunocompromised patients, such as solid organ transplant recipients (SOTR) [4,5]. After vaccination of kidney transplant recipients (KTR) with different COVID-19 mRNA vaccines, seroconversion as measured by binding and neutralizing antibodies were clearly lower than in healthy individuals [6,7]. Cellular immune responses, which play a crucial role in the defense against SARS-CoV-2, were also reduced in SOTR in response to vaccination [5,7,8]. Also patients on renal replacement therapy with hemodialysis (DP) showed an overall reduced immune response to COVID-19 vaccination [6,7,[9], [10], [11]].
There is, however, considerable variation in the immune response to the same dosage of COVID-19 vaccines [4,8,10,12] elicited in immunocompromised individuals. This likely reflects an individually different level of immunosuppression, including post-transplant immunosuppressive therapy and other factors including patient age, sex or comorbidities [5,8,10,12]. A surrogate marker reflecting the degree of individual immunosuppression could help predict immune responses to vaccination.
Within the last years, the level of Torque Teno virus (TTV) DNA in blood was increasingly considered as a marker for the extent of drug-induced immunosuppression after organ transplantation [13], [14], [15]. TTV is a single strand DNA virus of the Anelloviridae family. It is highly prevalent and detectable in about 70% of the overall population and in up to 100% of transplant patients [16], [17], [18], but is not associated with any human disease [16]. TTV replication is limited by host immune responses [17,18] and the TTV DNA load increases with initiation of post-transplant immunosuppression [13,[19], [20], [21]]. Elevated TTV loads have also been detected in hemodialysis patients, which might reflect immunosuppression in end-stage renal disease. [22]
It is assumed that the level of immunosuppression at time of COVID-19 vaccination may be associated with the extent of the humoral immune response elicited by the vaccines, and this is supported by recent studies in SOTR [23], [24], [25]. In the present study, we investigated whether a certain level of plasma TTV load at the time of vaccination may predict not only humoral but also cellular immune responses to COVID-19 vaccination, including two different mRNA vaccines, in KTR and DP.
2. Methods
2.1. Study cohort
The study was performed on a subset of individuals included in the DIA-VACC study initiated in January 2021 [7], investigating immune responses to SARS-CoV-2 infection and/or COVID-19 vaccination in medical personnel (MP), hemodialysis patients (DP) and kidney transplant recipients (KTR). From the total of 3101 individuals, we now included 360 patients according to pre-defined criteria: vaccination with two doses of either BNT162b2 or mRNA-1273 according to recommended vaccination schemes between January and March 2021; available plasma samples collected at the day of first vaccination (T0) and eight weeks later (T1; for BNT162b2 five weeks, for mRNA-1273 four weeks after second vaccination); exclusion of infection with SARS-CoV-2 prior to and up to eight weeks post first vaccination, confirmed by undetectable S1-specific IgA and IgG at T0 and undetectable nucleocapsid-specific IgG at T0 and T1. Exclusion criteria were mTor inhibitor and belatacept based immunosuppression in KTR, as these agents may directly affect TTV load [26], and immunosuppressive therapy in DP and MP. Five patients with discordant results in humoral immune responses (negative S1-IgA/-IgG versus positive neutralizing antibodies) were further excluded from the study cohort.
The study flow is shown in Fig. 1.
Fig. 1.
Selection of study cohort. TTV, torque teno virus; mTOR, mechanistic target of rapamycin; nAb, neutralizing antibodies; IGRA, interferon gamma release assay.
Patients and controls gave written informed consent prior to study initiation. This study was approved by the ethical institutional review boards at Technische Universität Dresden (BO-EK-45012021), the University of Leipzig (046/21-lk) and the Saxon Medical Association (Sächsische Landesärztekammer) (EK-BR-10/21-1).
2.2. Quantification of TTV loads
TTV loads were measured in plasma by TaqMan real-time PCR as described previously [27]. TTV DNA level was quantified in the linear range from 102 to 1010 copies/ml. The limit of detection was 102 copies/ml.
2.3. Assessment of humoral immune response
SARS-CoV-2 specific IgA and IgG antibodies against S1 of the spike protein were measured using commercial immunoassays (Anti-SARS-CoV-2-ELISA (IgA) and Anti-SARS-CoV-2-QuantiVac-ELISA (IgG), respectively; both Euroimmun, Lübeck, Germany). Neutralizing antibodies (nAb) against SARS-CoV-2 were determined by an RBD-based surrogate neutralization assay (NeutraLISA). For the identification of previously SARS-CoV-2 infected individuals, a nucleocapside-specific immunoassay (Anti-SARS-CoV-2-NCP-ELISA (IgG)) was performed (both Euroimmun, Lübeck, Germany).
2.4. Assessment of cellular immune response
For the determination of cellular immune responses after two-dose vaccination, an interferon gamma (IFNγ) release assay (IGRA) (Quan-T-Cell SARS-CoV-2 & Quan-T-Cell-ELISA, Euroimmun, Lübeck, Germany) was applied as previously described [7]. A cut-off of 100 mIE/ml was defined as a positive IFNγ release response.
2.5. Statistical analysis
Demographic data are shown in tabular form. Non-parametric data between cohorts were compared by Mann Whitney U, Fisher's exact or χ2 test, and parametric data with unpaired t-test and ANOVA, where applicable. Generalized linear models were used to estimate the effect size of the association between plasma TTV load and seroconversion at T1. The effect size was displayed as odds ratio (OR) and 95% confidence interval (95% CI). Potential confounders of the effect size of the association between TTV and seroconversion to COVID-19 vaccination were assessed using bivariate analysis. Potential effect modifiers were assessed using Mantel Haenszel strata. A change in the effect size of >10% was defined as significant confounding or effect modification. For multivariate analysis, co-variables were selected based on clinical relevance. For the multivariate model, a backward elimination was used, and the ‘rule of 10′ was applied to define the maximum number of the variables in the model. A p-value <0.05 was the defined limit of significance. Log-normally distributed variables were log-transformed. For the determination of a TTV load cut-off for the optimal prediction of seroconversion and IFNγ release at T1 in KTR, a receiver operating curve (ROC) analysis was performed, including determination of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the respective cut-offs, and identification of the optimal classifier by determination of the Youden index. Statistical analyses were performed in IBM SPSS Statistics 24.0 (SPSS Inc., USA), STATA 15 (StataCorp, USA) and GraphPad Prism Version 9.2.0. Graphs were created in GraphPad Prism Version 9.2.0.
3. Results
3.1. Description of the study cohort
In this study, we included 360 individuals, selected from the DIA-VACC cohort (n = 3101) [7], according to pre-defined criteria (Fig. 1 ). No participant had prior infection with SARS-CoV-2 as confirmed serologically. In all patients, plasma TTV loads were determined at the day of first vaccination (T0). In 269 individuals, TTV DNA was detectable at T0, and TTV DNA negative persons (n = 91) were excluded from further analyses. The final study cohort consisted of 54 MP, 115 DP and 100 KTR, and is described in detail in Table 1 .
Table 1.
Characteristics of study groups. Demographics and clinical data of the KTR group included in the current study were compared to the KTR cohort of the DIA-VACC study by Mann-Whitney U test or χ2 test, where applicable. MP, medical personnel; DP, dialysis patients; KTR, kidney transplant recipients; IQR, interquartile range; eGFR, estimated glomerular filtration rate; CNI, calcineurin inhibitors; MMF/MPA, mycophenolate mofetil/myocophenolic acid; n.a., not available; ns, not significant.
| Variable | MP | DP | KTR |
KTR DIA-VACC1 |
p-value2 |
|---|---|---|---|---|---|
| n /% | n /% | n /% | n /% | ||
| number | 54 / 100 | 115 / 100 | 100 / 100 | 532 / 100 | ns |
| Age in years (median [IQR]) | 46 [39–57] | 70 [58–80] | 59 [50–67] | 59 [49–67] | ns |
| Female sex | 42 / 78 | 36 / 31 | 33 / 33 | 188 / 35 | ns |
| BMI in kg/m2 (median [IQR]) | 25.4 [22.6–28.5] | 26.3 [23.5–31.1] | 25.9 [23.3–29.2] | 25.5 [23.0–29.0] | ns |
| Drug treated comorbidities | 11 / 20 | 107 / 93 | 86 / 86 | 475 / 89 | ns |
| Diabetes mellitus | 0 / 0 | 34 / 30 | 19 / 19 | 99 / 19 | ns |
| Cardiovascular disease | 9 / 17 | 101 / 88 | 80 / 80 | 460 / 87 | ns |
| Lung disease | 3 / 6 | 10 / 9 | 6 / 6 | 33 / 6 | ns |
| Liver cirrhosis | 0 / 0 | 1 / 1 | 2 / 2 | 5 / 1 | ns |
| Cancer | 0 / 0 | 9 / 8 | 3 / 3 | 17 / 3 | ns |
| None | 43 / 80 | 8 / 7 | 14 / 14 | 57 / 11 | ns |
| eGFR (median [IQR]) | n.a. | n.a. | 47.6 [34.5–69.7] | 47.7 [34.6–61.8] | ns |
| Time on dialysis in years (median [IQR]) | n.a. | 5 [2–8] | 6 [3–11]3 | 5 [2–9]3 | ns |
| Time after transplantation in years (median [IQR]) | n.a. | n.a. | 7 [3–14] | 8 [4–14] | ns |
| On immunosuppressive therapy | 0 / 0 | 0 / 0 | 100 / 100 | 526 / 99 | ns |
| Corticosteroids | 0 / 0 | 0 / 0 | 46 / 46 | 249 / 47 | ns |
| CNI | 0 / 0 | 0 / 0 | 98 / 98 | 467 / 88 | 0.0011 |
| MMF/MPA | 0 / 0 | 0 / 0 | 87 / 87 | 408 / 77 | 0.024 |
| Other | 0 / 0 | 0 / 0.0 | 1 / 1 | 9 / 2 | ns |
| Type of vaccine | 0.0001 | ||||
| BNT162b2 | 24 / 45 | 57 / 50 | 55 / 55 | 182 / 34 | |
| mRNA-1273 | 30 / 55 | 58 / 50 | 45 / 45 | 349 / 66 |
all KTR included in the DIA-VACC study.
p-value for comparison between KTR group of the current study and all KTR included in the DIA-VACC study.
before kidney transplantation.
3.2. Immune response to vaccination
In all study participants, de novo humoral immune responses (S1-IgA, -IgG) were determined after vaccination with two doses of a COVID-19 mRNA vaccine at eight weeks after the first vaccination (T1). The results are presented in Supplementary Figure 1A-B. IgA and IgG seroconversion rates were lowest in KTR (χ2 test, both: p=<0.0001). Neutralizing antibodies and cellular immune responses against SARS-CoV-2 as assessed by specific IFNγ release were determined at T1 in 209 and 156 participants, respectively. Both responses were also lowest in KTR (χ2 test, both: p=<0.0001) as presented in Supplementary Table and Supplementary Figure 1C-D.
Humoral and cellular immune responses were further analyzed in regard of the given vaccine. In the DP cohort vaccination with mRNA-1273 resulted in higher seroconversion rates than vaccination with BNT162b2 (Supplementary Figure 1A-B). In KTR this difference was observed only for S1-IgA seroconversion. IFNγ release rates in DP and KTR were not different between patients vaccinated with BNT162b2 or mRNA-1273, respectively (Supplementary Figure 1D).
3.3. Association between TTV load and seroconversion after vaccination
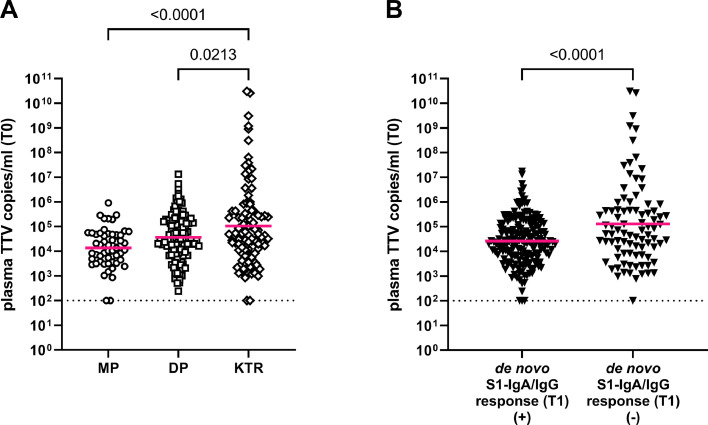
Plasma TTV loads on the day of first COVID-19 vaccination (T0) differed between the patient groups ( Fig. 2 A), with KTR showing the highest median TTV load (5.0 × 104 copies/ml) as compared to DP (3.1 × 104 copies/ml) and MP (1.3 × 104 copies/ml) (ANOVA, p = 0.0001). TTV loads were further correlated to the participants’ seroconversion status after two-dose vaccination (T1) (Fig. 2 B). Patients displaying seroconversion showed significantly lower TTV loads at T0 (median 2.4 × 104 copies/ml) compared to vaccine non-responders (median 6.2 × 104 copies/ml; unpaired t-test; p=<0.0001). Using a generalized linear regression model, a negative association between plasma TTV loads at T0 and seroconversion after two-dose vaccination (de novo detection of S1-specific IgA and/or IgG at T1) was calculated for the total cohort (OR 0.82, 95% CI 0.75–0.91; p<0.001). When the groups were analyzed separately, this association was still observed only in KTR (OR 0.87, 95% CI 0.76–0.99; p = 0.047). Thus, further analyses on TTV and vaccination response were performed in the KTR cohort only.
Fig. 2.
Plasma TTV load at the time of vaccination (T0). Plasma TTV load at the time of vaccination (T0) is stratified according to group (A; medical personnel [MP], dialysis patients [DP] and kidney transplant recipients [KTR]), and (B) humoral vaccine response. Humoral vaccine response was defined as de novo detection of SARS-CoV-2 S1-IgA and/or -IgG (seroconversion) eight weeks after first vaccination (T1). For statistical analysis, TTV loads were log-transformed and means compared by ANOVA or unpaired t-test. Bars indicate mean. Dotted line indicates limit of detection.
In a next step, we assessed possible confounders and effect modifiers of the association between plasma TTV loads and seroconversion rate in KTR using bivariate analysis and Mantel Haenszel strata. As shown in Table 2 , neither patient sex, age, time since transplantation, type of vaccine, BMI, history of diabetes mellitus and estimated glomerular filtration rate (eGFR) nor intake of calcineurin inhibitors (CNI), mycophenolate mofetil (MMF) and corticosteroids at the time of inclusion affected the association between plasma TTV loads and seroconversion after vaccination. In a multivariate model including sex, age and time since transplantation, no change in the effect size of the association between plasma TTV load and seroconversion after vaccination could be found (OR 0.88, 95% CI 0.76–1.00) (Table 2).
Table 2.
Unadjusted and adjusted effect size of the association between plasma TTV load at the time of first vaccination (T0) and seroconversion after two-dose COVID-19 mRNA vaccination (T1) in kidney transplant recipients (KTR). BMI, body-mass-index; DM, diabetes mellitus; CNI, calcineurin inhibitor; MMF, mycophenolate-mofetil; MPA, mycophenolic acid; eGFR, estimated glomerular filtration rate; KT, kidney transplantation.
| Co-variables | Odds ratio | 95% Confidence Interval | |
| Unadjusted (KTR) | 0.87 | 0.76–0.99 | |
| Adjusted | Sex | 0.87 | 0.76–1.00 |
| Age | 0.87 | 0.76–0.99 | |
| BMI | 0.87 | 0.76–0.99 | |
| DM | 0.87 | 0.76–1.00 | |
| CNI intake | 0.88 | 0.76–1.02 | |
| MMF/MPA intake | 0.88 | 0.76–1.01 | |
| Corticosteroide intake | 0.89 | 0.77–1.02 | |
| eGFR | 0.85 | 0.73–0.99 | |
| Time post KT | 0.87 | 0.76–1.00 | |
| Type of vaccine | 0.87 | 0.77–1.02 | |
| age group* | <59 years | 0.84 | 0.69–1.03 |
| >59 years | 0.90 | 0.75–1.09 | |
| sex | female | 0.92 | 0.74–1.14 |
| male | 0.85 | 0.71–1.01 | |
| Multivariate model | Sex/age/time post KT | 0.88 | 0.76–1.00 |
stratified according to median.
Demographic and clinical data compared between vaccine responders and non-responders are shown in Table 3 . Only MMF intake differed between the groups and occurred at higher frequency in non-responders, but this effect did not influence the association between plasma TTV levels and seroconversion after vaccination (Table 2).
Table 3.
Characteristics of kidney transplant recipients (KTR) stratified according to seroconversion after vaccination. Comparison between groups was performed via Fisher's exact test for binomial variables and Mann Whitney U test or unpaired t-test for continuous variables. IQR, interquartile range; eGFR, estimated glomerular filtration rate; CNI, calcineurin inhibitors; MMF/MPA, mycophenolate mofetil/myocophenolic acid; ns, not significant.
| Variable | KTR | |||
|---|---|---|---|---|
| total | de novo S1-IgA/IgG positive | de novo S1-IgA/IgG negative | p-value | |
| n /% | n /% | n /% | ||
| number | 100 / 100 | 31 / 31 | 69 / 69 | |
| Age in years (median [IQR]) | 59 [50–67] | 58 [49–65] | 59 [50–70] | ns |
| Female Sex | 33 / 33 | 8 / 26 | 25 / 36 | ns |
| BMI in kg/m2 (median [IQR]) | 25.9 [23.3–29.2] | 25.4 [24.0–29.0] | 26.0 [23.1–29.3] | ns |
| Drug treated comorbidities | 86 / 86 | 27 / 87 | 59 / 86 | ns |
| Diabetes mellitus | 19 / 19 | 8 / 26 | 10 / 15 | ns |
| Cardiovascular disease | 80 / 80 | 26 / 84 | 54 / 78 | ns |
| Lung disease | 6 / 6 | 2 / 7 | 4 / 6 | ns |
| Liver cirrhosis | 2 / 2 | 0 / 0 | 2 / 3 | ns |
| Cancer | 3 / 3 | 2 / 7 | 1 / 1 | ns |
| eGFR (median [IQR]) | 47.6 [34.5–69.7] | 52.0 [42.6–58.9] | 44.7 [31.1–60.1] | ns |
| Time on dialysis in years (median [IQR]) | 6 [3–11]1 | 7 [3–12]1 | 5 [2–11]1 | ns |
| Time on transplantation in years (median [IQR]) | 7 [3–14] | 7 [3–17] | 7 [3–12] | ns |
| On immunosuppressive therapy | 100 / 100 | 100 / 100 | 100 / 100 | ns |
| Corticosteroids | 46 / 46 | 12 / 39 | 34 / 50 | ns |
| CNI | 98 / 98 | 29 / 94 | 69 / 100 | ns |
| MMF/MPA | 87 / 87 | 23 / 74 | 64 / 93 | 0.0207 |
before kidney transplantation.
3.4. TTV load cut-off predicting seroconversion to COVID-19 vaccination in KTR
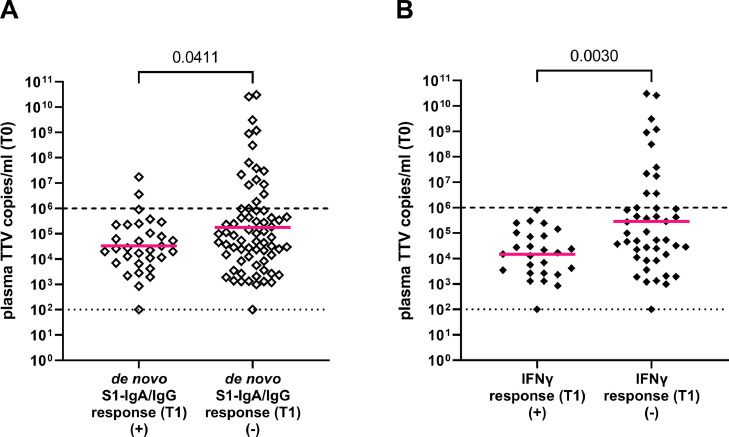
To examine whether a specific plasma TTV load at the time of vaccination could predict seroconversion to vaccination in KTR, we performed a ROC analysis and, based on specific TTV load cut-offs, calculated the respective sensitivity, specificity, PPV and NPV for the prediction of seroconversion (Table 4 ). Only two patients with TTV loads above a cut-off of 106 copies/ml prior to vaccination seroconverted within eight weeks after COVID-19 vaccination (2/17; 12%) (Fig. 3 A).
Table 4.
Plasma TTV cut-off values at the time of first vaccination (T0) for the prediction of seroconversion after two-dose vaccination (T1).
| Copies/ml TTV | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) |
| < 103 | 6.5 (1.2–20.7) | 97.1 (90.0–99.5) | 50.0 (8.9–91.1) | 69.8 (60.0–78.1) |
| < 104 | 25.8 (13.7–43.3) | 75.4 (64.0–84.0) | 32.0 (17.2–51.6) | 69.3 (58.2–78.6) |
| < 105 | 71.0 (53.4–83.9) | 47.8 (36.5–59.4) | 37.9 (26.6–50.8) | 78.6 (64.1–88.3) |
| < 4.0 × 105* | 90.3 (75.1–96.7) | 33.3 (23.4–45.1) | 37.8 (27.7–49.2) | 88.5 (71.0–96.0) |
| < 106 | 93.6 (79.3–98.9) | 21.7 (13.6–32.8) | 34.9 (25.6–45.7) | 88.2 (65.7–97.9) |
| < 107 | 96.7 (83.8–99.8) | 15.9 (9.1–26.3) | 34.1 (25.0–44.5) | 91.7 (64.6–99.6) |
optimal classifier as determined by Youden's index.
Fig. 3.
Plasma TTV load in kidney transplant recipients (KTR) at the time of vaccination (T0). Plasma TTV load in KTR is stratified according to humoral (A) and cellular (B) immune response to vaccination. Humoral and cellular vaccine response were defined as de novo detection of SARS-CoV-2 S1-IgA and/or -IgG (seroconversion) and detection of IFNγ release eight weeks after first vaccination (T1). For statistical analysis, TTV loads were log-transformed and means compared by unpaired t-test. Bars indicate mean. Dotted line indicates limit of detection. Dashed line indicates clinically defined cut-off for reliable prediction of humoral and cellular immune response after vaccination.
3.5. Association between TTV load and cellular immune response to vaccination in KTR
We finally investigated the association between cellular immune responses to vaccination and TTV load at T0 in KTR. As shown in Fig. 3 B, TTV loads were lower in KTR showing IFNγ release response at T1 after vaccination (unpaired t-test; p = 0.003). None of the patients showing plasma TTV loads higher than the cut-off of 106 copies/ml at time of first vaccination developed a cellular immune response to vaccination. Thus, this cut-off predicted especially cellular immune responses to COVID-19 vaccination (Table 5 ).
Table 5.
Plasma TTV cut-off values at the time of first vaccination (T0) for the prediction of cellular immune response after two-dose vaccination (T1).
| Copies/ml TTV | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) |
| < 103 | 7.9 (13.7–24.1) | 95.6 (85.2–99.2) | 50.0 (8.9–91.1) | 64.2 (52.2–74.6) |
| < 104 | 42.3 (25.5–61.1) | 77.8 (63.7–87.5) | 52.4 (32.4–71.7) | 70.0 (56.3–80.9) |
| < 105 | 76.9 (58.0–89.0) | 49.0 (35.0–63.0) | 46.5 (32.5–61.1) | 78.6 (60.5–89.8) |
| < 2.6 × 105* | 92.3 (75.9–98.6) | 46.7 (32.9–63.6) | 50.0 (36.4–63.6) | 91.3 (73.2–98.5) |
| < 106 | 100.0 (87.1–100.0) | 24.4 (14.2–38.7) | 43.3 (31.6–55.9) | 100.0 (74.1–100.0) |
| < 107 | 100.0 (87.1–100.0) | 20.0 (10.9–33.8) | 41.9 (30.5–54.3) | 100.0 (70.1–100.0) |
optimal classifier as determined by Youden's index.
4. Discussion
In the present study we show that there is a significant association between plasma TTV loads at time of first COVID-19 mRNA vaccination and humoral as well as cellular immune responses following two-dose vaccination in kidney transplant recipients.
Our data reveal that the plasma TTV load at the time of vaccination is inversely correlated with seroconversion in response to mRNA vaccination in KTR. This is in agreement with earlier findings in SOTR, similarly showing an association between higher TTV loads and decreased humoral immune response rates to COVID-19 mRNA vaccination [[23], [24], [25],28,29]. In our KTR cohort, we could demonstrate that with each log level increase in TTV load, the odds of seroconversion decreased by 13%. This association was independent of confounding variables or effect modifiers.
In addition, we newly show that in KTR the plasma TTV load at the time of first vaccination is negatively associated with cellular responses elicited by COVID-19 mRNA vaccines. This is of special importance, as previous studies have indicated that the cellular immune response to COVID-19 mRNA vaccination is of high importance for the protection against SARS-CoV-2 [30], but is in overall impaired in SOTR [7,31,32].
Our present investigations reveal that seroconversion after 2-dose vaccination in KTR was highly unlikely when TTV loads exceeded 106 copies/ml at T0, while it occurred in 35% of KTR with TTV levels below this threshold. Median TTV loads and the cut-off predicting seroconversion in our KTR cohort were higher than those found by others [28]. This discrepancy is possibly due to the use of different TTV DNA quantification assays, as recently described [33]. Remarkably, the development of cellular immune responses to vaccination in KTR was even more reliably predicted by TTV load. In none of the patients exceeding a TTV cut-off of 106 copies/ml plasma at the time of first vaccination there was evidence of vaccine-specific IFNγ release. Taken together, our data implicate that the plasma TTV load is not only a valuable surrogate marker predicting the individual risk of infection or allograft dysfunction after SOT [13,14], but when above 106 copies/ml may also be useful to predict humoral and cellular immune responses to COVID-19 mRNA vaccination. Assessing TTV loads can thus contribute to optimizing vaccination schemes and vaccine-induced immune responses in the individual host. Our data highlight that further studies will be important to assess whether the observed association between plasma TTV loads and vaccine-induced immune responses also applies to other antiviral vaccinations. Besides, further studies are required on patients after hematopoietic stem cell transplantation [34], in whom the significance of TTV loads is less clear [35].
In DP, no association between TTV loads and seroconversion after vaccination was observed. This may be due to their lower immunosuppressive status, and confirms that the TTV load serves as a surrogate marker especially in patients receiving highly potent immunosuppressive therapy.
Our patients were vaccinated with either BNT162b2 or mRNA-1273. So far, higher seroconversion rates were observed mostly after mRNA-1273 vaccination in immunocompetent and immunocompromised individuals, including dialysis patients [7,24,[36], [37], [38]]. Similarly, we found in overall higher seroconversion rates after vaccination with mRNA-1273, which, however, reached statistical significance only in DP. Cellular immune response rates did not differ between BNT162b2 and mRNA-1273 vaccinees. Notably, the type of vaccine did not influence the association between TTV load and probability of seroconversion in KTR.
Among all co-factors considered in our KTR cohort, only MMF/MPA intake was more common in vaccine non-responders, which is in line with previous data [4,7,24,28,31,39]. MMF intake did, however, not affect the association between plasma TTV load and probability of seroconversion.
The presented data are limited for patients receiving standard immunosuppressive schemes, and further investigations on SOTR undergoing alternative treatments are needed.
In summary, the plasma TTV load at the time of vaccination, when elevated above 106 copies/ml, could be a useful predictive marker for seroconversion and cellular immune response to COVID-19 mRNA vaccination in KTR. Further studies will be important to assess the association between TTV levels and the efficacy of other vaccines against SARS-CoV-2 in immunocompromised patients, as well as to predict the patients’ immune responses towards vaccinations against other viruses.
Funding statement
This work was supported by the Center for Virology, Medical University of Vienna, by the Sächsisches Staatsministerium für Wissenschaft, Kultur und Tourismus via Sächsische AufbauBank (SAB), grant number (SAB-Antragsnr.) 100592538., as well as Else Kröner Fresenius Stiftung, Bad Homburg v. d. H., grant number Fördervertrag EKFS 2021_EKSE.27.
CRediT authorship contribution statement
Marianne Graninger: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Julian Stumpf: Conceptualization, Data curation, Formal analysis, Validation, Writing – review & editing. Gregor Bond: Conceptualization, Data curation, Formal analysis, Validation, Writing – review & editing. Irene Görzer: Methodology, Validation, Writing – review & editing. David N. Springer: Formal analysis. Friederike Kessel: Investigation, Methodology. Hannah Kröger: Investigation, Methodology. Kerstin Frank: Investigation, Methodology. Torsten Tonn: Investigation, Methodology. Christian Hugo: Conceptualization, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. Elisabeth Puchhammer-Stöckl: Conceptualization, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare no commercial or other association that might pose a conflict of interest.
Acknowledgements
1. DIA-Vacc- Investigators/Network [7].
2. Medizinische Klinik und Poliklinik III, Universitätsklinikum, Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany:
Patrick Arndt, Florian Gembardt, Jan Sradnick, Anne Steglich
3. Medizinische Fakultät Carl Gustav Carus, Institut für Medizinische Informatik und Biometrie (IMB), Technische Universität, Dresden, Germany:
René Mauer
4. Further supporters:
EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany provided antibody ELISAs and interferon-gamma release assays for this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2023.105428.
Appendix. Supplementary materials
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the Bnt162b2 Mrna Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the Mrna-1273 Sars-Cov-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho A., Muecksch F., Schaefer-Babajew D., Wang Z., Finkin S., Gaebler C., et al. Anti-Sars-Cov-2 receptor-binding domain antibody evolution after Mrna vaccination. Nature. 2021;600(7889):517–522. doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky B., Werbel W., Avery R., Tobian A., Massie A., Segev D., et al. Antibody response to 2-dose Sars-Cov-2 Mrna vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holden I.K., Bistrup C., Nilsson A.C., Hansen J.F., Abazi R., Davidsen J.R., et al. Immunogenicity of Sars-Cov-2 Mrna vaccine in solid organ transplant recipients. J. Intern. Med. 2021;290(6):1264–1267. doi: 10.1111/joim.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon-Arevalo H., Choi M., Stefanski A.L., Halleck F., Weber U., Szelinski F., et al. Impaired humoral immunity to Sars-Cov-2 Bnt162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021;6(60):eabj1031. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 7.Stumpf J., Siepmann T., Lindner T., Karger C., Schwobel J., Anders L., et al. Humoral and cellular immunity to Sars-Cov-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using Mrna-1273 or Bnt162b2 Mrna vaccine. Lancet Reg. Health Eur. 2021;9 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucchiari D., Egri N., Bodro M., Herrera S., Del Risco-Zevallos J., Casals-Urquiza J., et al. Cellular and humoral response after Mrna-1273 Sars-Cov-2 vaccine in kidney transplant recipients. Am. J. Transpl. 2021;21(8):2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J.J., Lee T.H., Tian Y.C., Lee C.C., Fan P.C., Chang C.H. Immunogenicity rates after Sars-Cov-2 vaccination in people with end-stage kidney disease: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strengert M., Becker M., Ramos G.M., Dulovic A., Gruber J., Juengling J., et al. Cellular and humoral immunogenicity of a Sars-Cov-2 Mrna vaccine in patients on haemodialysis. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stumpf J.K.A, Mauer R., Steglich A., Gembardt F., Martin H., Glombig G., Frank K., Tonn T., Hugo C. Equivalent humoral and cellular immune response but different side effect rates following Sars-Cov-2 vaccination in peritoneal and haemodialysis patients using messenger Rna vaccines. Nephrol. Dial Transpl. 2022;37(4):796–798. doi: 10.1093/ndt/gfab324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupper A., Rabinowich L., Schwartz D., Schwartz I.F., Ben-Yehoyada M., Shashar M., et al. Reduced humoral response to Mrna Sars-Cov-2 Bnt162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transpl. 2021;21(8):2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doberer K., Schiemann M., Strassl R., Haupenthal F., Dermuth F., Gorzer I., et al. Torque Teno virus for risk stratification of graft rejection and infection in kidney transplant recipients-a prospective observational trial. Am. J. Transpl. 2020;20(8):2081–2090. doi: 10.1111/ajt.15810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaksch P., Kundi M., Gorzer I., Murakozy G., Lambers C., Benazzo A., et al. Torque Teno virus as a novel biomarker targeting the efficacy of immunosuppression after lung transplantation. J. Infect. Dis. 2018;218(12):1922–1928. doi: 10.1093/infdis/jiy452. [DOI] [PubMed] [Google Scholar]
- 15.Jaksch P., Gorzer I., Puchhammer-Stockl E., Bond G. Integrated immunologic monitoring in solid organ transplantation: the road toward torque Teno virus-guided immunosuppression. Transplantation. 2022;106(10):1940–1951. doi: 10.1097/TP.0000000000004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto H. History of discoveries and pathogenicity of Tt viruses. Curr. Top Microbiol. 2009;331:1–20. doi: 10.1007/978-3-540-70972-5_1. [DOI] [PubMed] [Google Scholar]
- 17.Maggi F., Pistello M., Vatteroni M., Presciuttini S., Marchi S., Isola P., et al. Dynamics of persistent Tt virus infection, as determined in patients treated with alpha interferon for concomitant Hepatitis C virus infection. J. Virol. (2001) 75(24):11999–2004. doi: 10.1128/Jvi.75.24.11999-12004.2001. [DOI] [PMC free article] [PubMed]
- 18.Focosi D., Antonelli G., Pistello M., Torquetenovirus Maggi F. The human virome from bench to bedside. Clin. Microbiol. Infec. 2016;22(7):589–593. doi: 10.1016/j.cmi.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Gorzer I., Haloschan M., Jaksch P., Klepetko W. Puchhammer-Stockl E. Plasma DNA levels of torque teno virus and immunosuppression after lung transplantation. J. Heart Lung Transpl. 2014;33(3):320–323. doi: 10.1016/j.healun.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Focosi D., Macera L., Pistello M., Maggi F. Torque Teno virus viremia correlates with intensity of maintenance immunosuppression in adult orthotopic liver transplant. J. Infect. Dis. 2014;210(4):667–668. doi: 10.1093/infdis/jiu209. [DOI] [PubMed] [Google Scholar]
- 21.Gorzer I., Jaksch P., Kundi M., Seitz T., Klepetko W., Puchhammer-Stockl E. Pre-transplant plasma torque Teno virus load and increase dynamics after lung transplantation. PLoS ONE (2015) 10(4). doi: ARTN e0122975 10.1371/journal.pone.0122975. [DOI] [PMC free article] [PubMed]
- 22.Touinssi M., Gallian P., Biagini P., Attoui H., Vialettes B., Berland Y., et al. Tt virus infection: prevalence of elevated viraemia and arguments for the immune control of viral load. J. Clin. Virol. 2001;21(2):135–141. doi: 10.1016/s1386-6532(01)00157-3. [DOI] [PubMed] [Google Scholar]
- 23.Hoek R.A., Verschuuren E.A., de Vries R.D., Vonk J.M., van Baarle D., van der Heiden M., et al. High torque Tenovirus (Ttv) load before first vaccine dose is associated with poor serological response to Covid-19 vaccination in lung transplant recipients. J. Heart Lung Transplant. 2022;41(6):765–772. doi: 10.1016/j.healun.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallais F., Renaud-Picard B., Solis M., Laugel E., Soulier E., Caillard S., et al. Torque Teno virus DNA load as a predictive marker of antibody response to a three-dose regimen of covid-19 mrna-based vaccine in lung transplant recipients. J. Heart Lung Transplant. 2022;41(10):1429–1439. doi: 10.1016/j.healun.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberto P., Cinti L., Napoli A., Paesani D., Riveros Cabral R.J., Maggi F., et al. Torque Teno virus (Ttv): a gentle spy virus of immune status, predictive marker of seroconversion to Covid-19 vaccine in kidney and lung transplant recipients. J. Med. Virol. 2023 doi: 10.1002/jmv.28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiemann M., Puchhammer-Stockl E., Eskandary F., Kohlbeck P., Rasoul-Rockenschaub S., Heilos A., et al. Torque Teno virus load-inverse association with antibody-mediated rejection after kidney transplantation. Transplantation. 2017;101(2):360–367. doi: 10.1097/TP.0000000000001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maggi F., Pifferi M., Fornai C., Andreoli E., Tempestini E., Vatteroni M., et al. Tt Virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J. Virol. 2003;77(4):2418–2425. doi: 10.1128/jvi.77.4.2418-2425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Querido S., Adragao T., Pinto I., Ormonde C., Papoila A.L., Pessanha M.A., et al. Torquetenovirus viral load is associated with anti-spike antibody response in Sars-Cov-2 Mrna Bnt162b2 vaccinated kidney transplant patients. Clin. Transpl. 2022:e14825. doi: 10.1111/ctr.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Focosi D., Baj A., Azzi L., Novazzi F., Maggi F. Ttv viral load as a predictor of antibody response to Sars Cov-2 vaccination. The J. Heart and Lung Transpl. 2023;42(2):143–144. doi: 10.1016/j.healun.2022.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarke A., Coelho C.H., Zhang Z., Dan J.M., Yu E.D., Methot N., et al. Sars-Cov-2 vaccination induces immunological T cell memory able to cross-recognize variants from alpha to omicron. Cell. 2022;185(5):847–859. doi: 10.1016/j.cell.2022.01.015. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders J.F., Bemelman F.J., Messchendorp A.L., Baan C.C., van Baarle D., van Binnendijk R., et al. The Recovac immune-response study: the immunogenicity, tolerability, and safety of Covid-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation. 2022;106(4):821–834. doi: 10.1097/TP.0000000000003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruether D.F., Schaub G.M., Duengelhoef P.M., Haag F., Brehm T.T., Fathi A., et al. Sars-Cov2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin. Gastroenterol. Hepatol. 2022;20(1) doi: 10.1016/j.cgh.2021.09.003. 162-72 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorzer I., Haupenthal F., Maggi F., Gelas F., Kulifaj D., Brossault L., et al. Validation of plasma torque Teno viral load applying a Ce-certified Pcr for risk stratification of rejection and infection post kidney transplantation. J. Clin. Virol. 2023;158 doi: 10.1016/j.jcv.2022.105348. [DOI] [PubMed] [Google Scholar]
- 34.Ge C., Du K., Luo M., Shen K., Zhou Y., Guo K., et al. Serologic response and safety of Covid-19 vaccination in Hsct or Car T-cell recipients: a systematic review and meta-analysis. Exp. Hematol. Oncol. 2022;11(1):46. doi: 10.1186/s40164-022-00299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redondo N., Navarro D., Aguado J.M., Fernandez-Ruiz M. Viruses, friends, and foes: the case of torque Teno virus and the net state of immunosuppression. Transpl. Infect. Dis. 2022;24(2):e13778. doi: 10.1111/tid.13778. [DOI] [PubMed] [Google Scholar]
- 36.Yau K., Chan C.T., Abe K.T., Jiang Y., Atiquzzaman M., Mullin S.I., et al. Differences in Mrna-1273 (Moderna) and Bnt162b2 (Pfizer-Biontech) Sars-Cov-2 vaccine immunogenicity among patients undergoing dialysis. CMAJ. 2022;194(8):E297–E305. doi: 10.1503/cmaj.211881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narasimhan M., Mahimainathan L., Clark A.E., Usmani A., Cao J., Araj E., et al. Serological response in lung transplant recipients after two doses of Sars-Cov-2 Mrna vaccines. Vaccines (Basel) 2021;9(7):708. doi: 10.3390/vaccines9070708. Pmcid: Pmc8310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z., Mateus J., Coelho C.H., Dan J.M., Moderbacher C.R., Galvez R.I., et al. Humoral and cellular immune memory to four Covid-19 vaccines. Cell. 2022;185(14):2434–2451. doi: 10.1016/j.cell.2022.05.022. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lammert A., Schnuelle P., Rabenau H.F., Ciesek S., Kramer B.K., Gottmann U., et al. Sars-Cov-2 vaccination in kidney transplant recipients-stratified analysis of the humoral immune response. Transpl. Direct. 2022;8(11):e1384. doi: 10.1097/TXD.0000000000001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.