Abstract
Twelve new 2,2′-bi(1,2,4-oxadiazole) derivatives containing heterocyclic and long-chain substituents have been synthesized by the condensation of aldehyde azines and oximes. In silico studies of these compounds revealed their good drug likeness and drug score. Some of the synthesized compounds showed moderate to excellent larvicidal activity.
Keywords: aldehyde azines, oximes, bis-oxadiazoles, in silico studies, larvicidal activity
INTRODUCTION
Many clinically used drugs contain N,O-heterocyclic groups [1, 2]. Bis-oxadiazole derivatives were reported to inhibit the activity of γ-GT enzymes [3]. Among seventeen 1,3,4-oxadiazole derivatives, five compounds showed excellent antibacterial activity [4]. Ghanwat et al. [5] synthesized a series of novel 2,5-disubstituted 1,3,4-oxadiazoles which exhibited anti-inflammatory and antioxidant activities [5]. 1,3,4-Oxadiazoles containing a fluoropyridine moiety were evaluated against Echinochloa cruss-galli, Avena fatua, and Sorgum halepense weeds [6]. Isoquinoline-based 1,3,4-oxadiazoles were shown to be potent thymidine phosphorylase inhibitors [7].
New oxadiazoles with trihydroxyphenyl and 4-hydroxyquinoline groups were reported to have better anti-COVID activity than existing potent drugs, and computational studies supported the results of in vitro anti-COVID-19 assays [8]. 3,5-Diaryl-1,2,4-oxadiazoles were tested as new apoptosis inducers and potential anticancer agents [9, 10]. Two cytotoxic alkaloids, phidianidines A and B, containing a 1,2,4-oxadiazole ring linked to indole were isolated from the marine opisthobranch mollusk Phidiana militaris [11–13]. Some 1,4-benzoxazine–1,2,4-oxadiazole hybrids showed a promising in vitro anticancer activity against four cancer cell lines in comparison to etoposide as reference drug [14]. Bis(5-aryl-3-benzoyl-2,3-dihydro-1,3,4-oxadiazoles) synthesized from hydrazones and acetic anhydride/benzoyl chloride showed insecticidal, herbicidal, and nematicidal activities [15]. Twenty-four 1,3,4-oxadiazole derivatives with chloro, methoxy, and hydroxy substituents were synthesized and tested for their antibacterial activity in comparison to amoxicillin and cefixime [16]. Figure 1 shows the structures of some medically relevant oxadiazole derivatives.
Fig. 1.
Structures of some medically relevant oxadiazole derivatives.
Herein we describe the synthesis of new 2,2′-bi(1,2,4-oxadiazole) derivatives containing heterocyclic and long-chain alkyl moieties, in silico prediction of their biological properties, and in vitro larvicidal activity.
RESULTS AND DISCUSSION
Target compounds 1–12 were synthesized in good yields by the condensation of the corresponding aldehyde azines and oximes in the presence of triethylamine and molecular iodine in tetrahydrofuran at 60°C (Scheme 1). Their structure was confirmed by 1H and 13C NMR and mass spectra and elemental analyses (see Experimental and Supplementary Materials).
Scheme.
1.
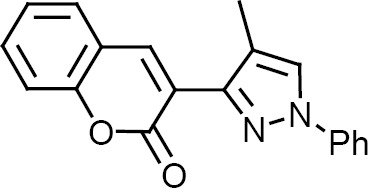
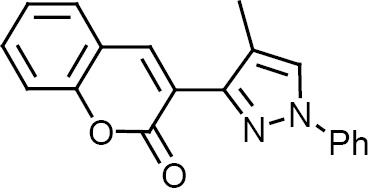
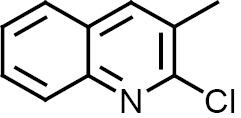
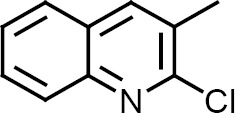
Compounds 1–12 were subjected to in silico analysis using OSIRIS and PASS Online software, and the results are summarized in Table 1. Compounds 1–6 were evaluated for their larvicidal activity in the concentration range from 10 to 100 μg/mL (Tables 2, 3). Compounds 1 and 4 showed moderate activity, compounds 3 and 6 exhibited good activity, and 2-butyl-4-chloro-1H-imidazol-5-yl (2c) and 2-chloroquinolin-3-yl (5c) derivatives showed excellent activity.
Table 1.
In-silico studies of compounds 1–12
| Compd. no. | Ar | R | Drug likeness/ drug score | PASS online activity |
|---|---|---|---|---|
| 1 |
|
|
3.22/0.12 |
(1) Histidine kinase inhibitor (2) Aldehyde oxidase inhibitor |
| 2 |
|
|
2.18/0.08 |
(1) Imidazoline I1 receptor agonist (2) Nicotinic alpha6beta3beta4alpha5 receptor antagonist |
| 3 |
|
|
4.46/0.14 |
(1) CYP2A11 substrate (2) Antineoplastic |
| 4 |
|
|
5.32/0.06 |
(1) CYP2A11 substrate (2) Nootropic |
| 5 |
|
|
2.24/0.07 |
(1) Nicotinic alpha2beta2 receptor antagonist (2) CF transmembrane conductance regulator agonist |
| 6 |
|
|
4.14/0.21 |
(1) Anaphylatoxin receptor antagonist (2) Complement factor D inhibitor |
| 7 |
|
(C16H33)2NCH2 | – |
(1) Histidine kinase inhibitor (2) Aldehyde oxidase inhibitor |
| 8 |
|
(C16H33)2NCH2 | – |
(1) Imidazoline I1 receptor agonist (2) Nicotinic alpha6beta3beta4alpha5 receptor antagonist |
| 9 |
|
(C16H33)2NCH2 | – |
(1) CYP2A11 substrate (2) Antineoplastic |
| 10 |
|
(C16H33)2NCH2 | – |
(1) CYP2A11 substrate (2) Nootropic |
| 11 |
|
(C16H33)2NCH2 | – |
(1) Nicotinic alpha2beta2 receptor antagonist (2) CF transmembrane conductance regulator agonist |
| 12 |
|
(C16H33)2NCH2 | – |
(1) Anaphylatoxin receptor antagonist (2) Complement factor D inhibitor |
Table 2.
In-vitro larvicidal activity (mortality rate, %) of compounds 1–6 at different concentrations
| Compound no. | 10 μg/mL | 25 μg/mL | 50 μg/mL | 75 μg/mL | 100 μg/mL |
|---|---|---|---|---|---|
| 1 | 19.2 | 31.5 | 43.8 | 52.1 | 63.2 |
| 2 | 33.1 | 47.4 | 62.8 | 74.6 | 88.3 |
| 3 | 22.3 | 36.8 | 47.5 | 56.2 | 69.6 |
| 4 | 15.2 | 27.8 | 39.5 | 49.2 | 55.4 |
| 5 | 30.9 | 42.5 | 57.3 | 69.6 | 75.5 |
| 6 | 27.1 | 38.2 | 52.6 | 63.1 | 71.8 |
Table 3.
Average mortality rates, standard deviations, and LC50 of compounds 1–6
| Compound no. | Average larvicidal activity (mortality rate, %) | Standard deviation | LC50, μg/mL |
|---|---|---|---|
| 1 | 41.96 | 17.21 | 61.96 |
| 2 | 61.24 | 21.77 | 42.46 |
| 3 | 46.48 | 18.08 | 55.93 |
| 4 | 37.42 | 16.22 | 69.48 |
| 5 | 55.16 | 18.54 | 47.13 |
| 6 | 50.56 | 18.13 | 51.42 |
EXPERIMENTAL
Chemicals were purchased from Aldrich, SD Fine, and Avra Chemicals. Brine shrimp eggs (Artemia cysts) were obtained Sumi Pets and Aquarium (Pattabiram, Tamil Nadu). The 1H and 13C NMR spectra were recorded on a Bruker Avance spectrometer at 400 and 100 MHz, respectively. The mass spectra (electrospray ionization) were recorded on an Agilent Technologies QTOF 6530 instrument. Elemental analysis was performed with an Elementar Vario Micro Cube CHNS analyzer.
General procedure for the synthesis of compounds 1–12. A mixture of the corresponding aldehyde (0.04 mol), hydrazine hydrate (0.02 mol), and one drop of acetic acid in 15 mL of ethanol was stirred at 60–70°C for 2 h to obtain aldehyde azine A. Oxime B was prepared from 0.01 mol of the same aldehyde (for 1–6) or 2-(dihexadecylamino)acetaldehyde (for 7–12) and 0.01 mol of hydroxylamine hydrochloride at 0–5°C. A mixture of A and B, triethylamine, and iodine (catalyst) in tetrahydrofuran was stirred at 60°C for 6 h. The mixture was poured into cold water, and the solid product was filtered off and dried.
3,3′,3″,3′′′-{5H,5′H-[2,2′-Bi(1,2,4-oxadiazole)]-3,3′,5,5′-tetrayl}tetrakis(4H-chromen-4-one) (1). Yield: 92%, Rf 0.4 (hereinafter, EtOAc–hexane, 50:50 by volume). 1H NMR spectrum (DMSO-d6), δ, ppm: 5.1 s (2H, (OCHN), 6.7 s (2H, =CH), 7.1 s (2H, =CH), 7.4 m (4H, Harom), 7.5–7.6 m (8H, Harom), 8.08 m (4H, Harom). 13C NMR spectrum (DMSO-d6), δC, ppm: 183.0, 177.5, 160.6, 157.2, 152.6, 150.6, 135.2, 125.7, 125.4, 123.9, 123.4, 116.1, 114.2, 83.7. Mass spectrum: m/z 803.3 [M + DMSO-d6]+. Found, %: C 66.05; H 3.89; N 7.28; O 22.78. C40H22N4O10. Calculated, %: C 66.85; H 3.09; N 7.80; O 22.26.
3,3′,5,5′-Tetrakis(2-butyl-4-chloro-1H-imidazol-5-yl)-5H,5′H-2,2′-bi(1,2,4-oxadiazole) (2). Yield 94%, Rf 0.5. 1H NMR spectrum (CDCl3), δ, ppm: 0.9 t (12H, CH3), 1.31 m (8H, CH2), 1.6 m (8H, CH2), 2.88 t (8H, 2′-CH2), 5.84 s (2H, OCHN), 11.1 s (1H, NH), 11.3 s (1H, NH), 12.5 s (1H, NH), 13.0 s (1H, NH). 13C NMR spectrum (DMSO-d6), δC, ppm: 157.9, 148.2, 147.9, 136.5, 134.2, 122.9, 116.1, 84.6, 51.5, 30.6, 27.6, 27.9, 22.3, 14.1. Mass spectrum: m/z 798 [M + CH3OH]+. Found, %: C 50.55; H 5.45; Cl 18.51; N 21.17; O 4.32. C32H42Cl4N12O2. Calculated, %: C 50.01; H 5.51; Cl 18.45; N 21.87; O 4.16.
3,3′,3″,3′′′-{5H,5′H-[2,2′-bi(1,2,4-oxadiazole)]-3,3′,5,5′-tetrayltetrakis(1H-pyrazole-4,3-diyl)}tetrakis(2H-chromen-2-one) (3). Yield 90%, Rf 0.4. 1H NMR spectrum (DMSO-d6), δ, ppm: 5.6 s (2H, NCHO), 7.4 m (8H, Harom), 7.5 m (4H 5′-H), 7.6 m (4H, Harom), 7.8 m (4H, Harom), 8.1 m (4H, =CH), 12.6 m (4H, NH). 13C NMR spectrum (DMSO-d6), δC, ppm: 161.9, 158.9, 153.0, 146.1, 138.9, 132.5, 132.1, 129.4, 128.3, 127.9, 125.4, 120.9, 116.1, 112.8, 85.7. Mass spectrum: m/z 982 [M]+. Found, %: C 63.28; H 3.08; N 17.24; O 16.40. C52H30N12O10. Calculated, %: C 63.54; H 3.08; N 17.10; O 16.28.
3,3′,3″,3′′′-{5H,5′H-[2,2′-bi(1,2,4-oxadiazole)]-3,3′,5,5′-tetrayltetrakis(1-phenyl-1H-pyrazole-4,3-diyl)}tetrakis(2H-chromen-2-one) (4). Yield 91%, Rf 0.6. 1H NMR spectrum (DMSO-d6), δ, ppm: 5.6 s (2H, NCHO), 7.4 m (12H, Harom), 7.5 m (8H, Harom), 7.6 m (12H, Harom), 7.8 m (4H, Harom), 8.1 m (4H, =CH), 8.4 s (4H, 5′-H). 13C NMR spectrum (DMSO-d6), δC, ppm: 161.9, 153.0, 146.1, 140.9, 139.7, 129.4, 129.3, 128.3, 127.9, 126.2, 125.4, 120.9, 119.9, 118.4, 116.1, 114.0, 86.0. Mass spectrum: m/z 1286 [M]+. Found, %: C 70.45; H 3.91; N 13.24; O 12.40. C76H46N12O10. Calculated, %: C 70.91; H 3.60; N 13.06; O 12.43.
3,3′,5,5′-Tetrakis(2-chloroquinolin-3-yl)-5H,5′H-2,2′-bi(1,2,4-oxadiazole) (5). Yield 94%, Rf 0.4. 1H NMR spectrum (DMSO-d6), δ, ppm: 5.6 s (2H, NCHO), 7.6 m (4H, Harom), 7.7 m (4H, Harom), 7.9 d (4H, Harom), 8.0 d (4H, Harom), 8.2 s (2H, Harom), 9.06 s (2H, Harom). 13C NMR spectrum (DMSO-d6), δC, ppm: 158.3, 152.0, 151.9, 149.7, 148.8, 145.4, 136.3, 134.6, 131.0, 130.9, 129.9, 128.1, 127.8, 127.5, 127.3, 127.2, 127.0, 126.8, 126.6, 126.2, 86.1. Mass spectrum: m/z 786 [M]+. Found, %: C 60.78; H 2.91; Cl 17.99; N 14.24; O 4.08. C40H22Cl4N8O2. Calculated, %: C 60.93; H 2.81; Cl 17.99; N 14.21; O 4.06.
3,3′,5,5′-Tetrakis(1,3-thiazol-2-yl)-5H,5′H-2,2′-bi(1,2,4-oxadiazole) (6). Yield 91%, Rf 0.4. 1H NMR spectrum (DMSO-d6), δ, ppm: 5.64 s (2H, OCHN)), 7.3 d.d (2H, Harom), 7.5 d.d (2H, Harom), 7.65 d.d (4H, Harom). 13C NMR spectrum (DMSO-d6), δC, ppm: 168.5, 164.6, 157.6, 143.9, 141.9, 132.7, 118.7. Mass spectrum: m/z 473.98. Found, %: C 40.39; H 2.22; N 23.71; O 6.64; S 27.04. C16H10N8O2S4. Calculated, %: C 40.49; H 2.12; N 23.61; O 6.74; S 27.03.
3,3′-{3,3′-Bis[(dihexadecylamino)methyl]-5H,5′H-[2,2′-bi(1,2,4-oxadiazole)]-5,5′-diyl}bis(4H-chromen-4-one) (7). Yield 94%, Rf 0.7. 1H NMR spectrum (CDCl3), δ, ppm: 0.90 t (12H, CH3), 1.26 m (56H, CH2), 1.29 m (40H, CH2)), 1.30 m (8H, CH2, 1.36 m (8H, CH2), 2.46 t (8H, NCH2CH2), 2.48 s (4H, NCH2C), 3.90 s (2H, OCHN), 7.20 s (2H, 2′-H), 7.47 t (2H, Harom), 7.55 t (4H, Harom), 8.08 d (2H, Harom). 13C NMR spectrum (DMSO-d6), δC, ppm: 183.1, 166.0, 157.4, 150.9, 135.9, 125.7, 123.4, 116.9, 116.1, 57.1, 53.0, 32.0, 29.7, 29.2, 28.1, 27.4, 22.7, 14.1. Found, %: C 76.05; H 10.96; N 6.69; O 6.30. C88H148N6O6. Calculated, %: C 76.25; H 10.76; N 6.06; O 6.93.
N,N′-{(5,5′-Bis(2-butyl-4-chloro-1H-imidazol-5-yl)-5H,5′H-[2,2′-bi(1,2,4-oxadiazole)]-3,3′-diyl)bis(methylene)}bis(N-hexadecylhexadecan-1-amine) (8). Yield 92%, Rf 0.8. 1H NMR spectrum (CDCl3), δ, ppm: 0.89 t (18H, CH3), 1.26 m (56H, CH2), 1.29 m (40H, CH2), 1.31 m (12H, CH2), 1.36 m (8H, CH2), 1.60 m (4H, CH2), 2.46 t (8H, NCH2CH2), 2.48 s (4H, NCH2C), 2.87 t (4H, CH2N), 4.3 s (2H, OCHN), 13.0 s (2H, NH). 13C NMR spectrum (DMSO-d6), δ, ppm: 166.0, 147.4, 136.4, 120.7, 90.1, 57.1, 52.9, 31.9, 30.6, 29.7, 29.2, 28.1, 27.9, 27.3, 22.7, 22.3, 14.1. Found, %: C 71.45; H 11.34; Cl 5.05; N 9.86; O 2.30. C84H158Cl2N10O2. Calculated, %: C 71.50; H 11.29; Cl 5.02; N 9.93; O 2.27.
3,3′-{3,3′-Bis[(dihexadecylamino)methyl]-5H,5′H-[2,2′-bi(1,2,4-oxadiazole)]-5,5′-diylbis(1H-pyrazole-4,3-diyl)}bis(2H-chromen-2-one) (9). Yield 90%, Rf 0.7. 1H NMR spectrum (CDCl3), δ, ppm: 0.90 t (12H, CH3), 1.26 m (56H, CH2)), 1.29 m (40H, CH2), 1.30 m (8H, CH2), 1.36 m (8H, CH2), 2.46 t (8H, NCH2CH2), 2.48 s (4H, NCH2C), 3.9 s (2H, OCHN), 7.42 t (4H, Harom), 7.55 t (2H, Harom), 7.65 t (2H, Harom), 7.84 t (2H, Harom), 8.08 d (2H, 5′-H), 12.62 s (2H, NH). 13C NMR spectrum (DMSO-d6), δC, ppm: 164.7, 161.9, 153.2, 146.2, 132.1, 129.4, 128.3, 127.9, 125.4, 122.4, 120.9, 116.1, 112.8, 58.1, 55.1, 49.0, 32.0, 31.4, 29.7, 29.2, 28.1, 27.2, 22.7, 14.1. Found, %: C 74.33; H 10.12; N 9.25; O 6.30. C94H152N10O6. Calculated, %: C 74.36; H 10.09; N 9.23; O 6.32.
3,3′-{3,3′-Bis[(dihexadecylamino)methyl]-5H,5′H-[2,2′-bi(1,2,4-oxadiazole)]-5,5′-diylbis(1-phenyl-1H-pyrazole-4,3-diyl)}bis(2H-chromen-2-one) (10). Yield 91%, Rf 0.4. 1H NMR spectrum (CDCl3), δ, ppm: 0.90 t (12H, CH3), 1.26 m (56H, CH2), 1.29 m (40H, CH2), 1.30 m (8H, CH2), 1.36 m (8H, CH2), 2.46 t (8H, NCH2CH2), 2.48 s (4H, NCH2C)), 4.6 s (2H, OCHN), 7.42 t (4H, Harom), 7.45 t (2H, Harom), 7.55 t (4H, Harom), 7.64 t (6H, Harom), 7.84 t (2H, Harom), 8.08 d (2H, 5′-H), 8.2 s (2H, Harom). 13C NMR spectrum (DMSO-d6), δC, ppm: 166.0, 161.9, 158.0, 153.0, 146.2, 140.9, 139.8, 134.1, 128.4, 127.9, 129.5, 126.3, 125.4, 122.4, 120.8, 119.8, 116.3, 91.4, 57.0, 55.1, 52.9, 49.3, 32.0, 31.4, 29.7, 29.2, 28.1, 27.9, 27.2, 22.7, 14.1. Found, %: C 76.26; H 9.40; N 8.47; O 5.87. C106H160N10O6. Calculated, %: C 76.21; H 9.65; N 8.38; O 5.75.
N,N′-{(5,5′-Bis(2-chloroquinolin-3-yl)-5H,5′H-[2,2'-bi(1,2,4-oxadiazole)]-3,3′-diyl)bis(methylene)}bis(N-hexadecylhexadecan-1-amine) (11). Yield 91%, Rf 0.7. 1H NMR spectrum (CDCl3), δ, ppm: 0.90 t (12H, CH3), 1.26 m (56H, CH2), 1.29 m (40H, CH2), 1.30 m (8H, CH2), 1.36 m (8H, CH2), 2.46 t (8H, NCH2CH2), 2.48 s (4H, NCH2C), 4.5 s (2H, OCHN), 7.60 t (2H, Harom), 7.75 t (2H, Harom), 7.91 d (2H, Harom), 8.04 d (2H, Harom), 8.30 s (2H, Harom). 13C NMR spectrum (DMSO-d6), δC, ppm: 166.0, 151.8, 145.2, 136.8, 130.9, 130.1, 127.9, 127.6, 127.0, 126.3, 91.4, 57.0, 52.8, 32.0, 31.4, 29.7, 29.2, 28.1, 27.9, 27.2, 22.7, 14.1. Found, %: C 74.30; H 10.58; Cl 4.95; N 7.84; O 2.33. C88H148Cl2N8O2. Calculated, %: C 74.38; H 10.50; Cl 4.99; N 7.89; O 2.25.
N,N′-{5,5′-Bis(1,3-thiazol-2-yl)-5H,5′H-[2,2′-bi(1,2,4-oxadiazole)]-3,3′-diylbis(methylene)}bis(N-hexadecylhexadecan-1-amine) (12). Yield 95%, Rf 0.4. 1H NMR spectrum (CDCl3), δ, ppm: 0.90 t (12H, CH3), 1.26 m (56H, CH2), 1.29 m (40H, CH2), 1.30 m (8H, CH2), 1.36 m (8H, CH2), 2.46 t (8H, NCH2CH2), 2.48 s (4H, NCH2C), 4.5 s (2H, OCHN), 7.31 d (2H, 5′-H), 7.53 d (2H, 4′-H). 13C NMR spectrum (DMSO-d6), δC, ppm: 166.0, 165.8, 141.8, 118.9, 96.8, 57.1, 53.0, 32.0, 29.7, 29.2, 28.1, 27.9, 27.2, 22.7, 14.1. Found, %: C 72.31; H 11.23; N 8.82; O 2.54; S 5.10. C76H142N8O2S2. Calculated, %: C 72.21; H 11.32; N 8.86; O 2.53; S 5.07.
Larvicidal activity. A sterilized test tube was charged with 25 mL of distilled water, a small amount of sodium chloride was added, and a required pH value was adjusted by adding sodium hydrogen carbonate. Magnesium sulfate was added to obtain an alkaline solution, Artemia cysts were added, and the tube was incubated at 27°C for 24 h to produce larvae. Solutions of each compound 1–6 DMSO with concentrations of 10, 25, 50, 75, and 100 μg/mL were prepared in test tubes, 10 larvae were taken in each test tube, the test tubes were incubated at 27°C for 24 h, and the number of dead larvae was counted to determine percent mortality.
CONCLUSIONS
Bis-oxadiazoles containing heterocyclic groups and long chains have been synthesized in high yields and evaluated for their larvicidal activity. The compounds substituted with heterocyclic groups showed higher activity than those with long-chain substituents. The biological potential of the synthesized compounds has been estimated by in silico studies.
Supplementary information
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information
The online version contains supplementary material available at 10.1134/S1070428023010177.
REFERENCES
- 1.Wang P.Y., Shao W.B., Xue H.T., Fang H.S., Zhou J., Wu Z.-B., Song B.A., Yang S. Res. Chem. Intermed. 2017;43:6115. doi: 10.1007/s11164-017-2980-x. [DOI] [Google Scholar]
- 2.De Oliveira C.S., Lira B.F., Barbosa Filho J.M., Lorenzo J.G.F., De Athayde-Filho P.F. Molecules. 2012;17:10192. doi: 10.3390/molecules170910192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomi I.H.R., Al-Qaisi A.H.J., AlQaisi Z.H.J. J. King Saud Univ. 2011;23:23. doi: 10.1016/j.jksus.2010.06.002. [DOI] [Google Scholar]
- 4.Salama, E.E., BMC Chem., 2020, vol. 14, article no. 30. 10.1186/s13065-020-00682-6
- 5.Kashid, B.B., Salunkhe, P.H., Dongare, B.B., More, K.R., Khedkar, V.M., and Ghanwat, A.A., Bioorg. Med. Chem. Lett., 2020, vol. 30, article ID 127136. 10.1016/j.bmcl.2020.127136 [DOI] [PubMed]
- 6.Kalhor M., Dadras A. J. Heterocycl. Chem. 2013;50:220. doi: 10.1002/jhet.950. [DOI] [Google Scholar]
- 7.Zaman, K., Rahim, F., Taha, M., Wadood, A., Ali Shah, S.A., Ahmed, Q.U., Zakaria, Z.A., Sci Rep., 2019, vol. 9, article no. 16015. 10.1038/s41598-019-52100-0 [DOI] [PMC free article] [PubMed]
- 8.Rabie A.M. New J. Chem. 2021;45:761. doi: 10.1039/D0NJ03708G. [DOI] [Google Scholar]
- 9.Zhang H.Z., Kasibhatla S., Kuemmerle J., Kemnitzer W., Ollis-Mason K., Qiu L., Crogan-Grundy C., Tseng B., Drewe J., Cai S.X. J. Med. Chem. 2005;11:5215. doi: 10.1021/jm050292k. [DOI] [PubMed] [Google Scholar]
- 10.Buscemi S., Pace A., Piccionello A.P., Pibiri I., Vivona N., Giorgi G., Mazzanti A., Spinelli D. J. Org. Chem. 2006;71:8106. doi: 10.1021/jo061251e. [DOI] [PubMed] [Google Scholar]
- 11.Carbone M., Li Y., Irace C., Mollo E., Castelluccio F., Di Pascale A., Cimino G., Santamaria R., Guo Y.-W., Gavagnin M. Org. Lett. 2011;13:2516. doi: 10.1021/ol200234r. [DOI] [PubMed] [Google Scholar]
- 12.Vitale R.M., Gatti M., Carbone M., Barbieri F., Felicità V., Gavagnin M., Florio T., Amodeo P. ACS Chem. Biol. 2013;8:2762. doi: 10.1021/cb400521b. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Jiang C.-S., Gao L.-X., Gong J.-X., Wang Z.-H., Li J.-Y., Li J., Li X.-W., Guo Y.-W. Bioorg. Med. Chem. Lett. 2016;26:778. doi: 10.1016/j.bmcl.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 14.Nagaraju A., Kumar Nukala S., Swamy Thirukovela T.N., Manchal R. ChemistrySelect. 2021;6:3318. doi: 10.1002/slct.202100198. [DOI] [Google Scholar]
- 15.Mohammed A.E., Adel Z., Susan M. J. Islamic Acad. Sci. 1991;4:184. [Google Scholar]
- 16.Kamboj, S., Kajal, A., Saini, V., and Prasad, D.N., BioMed Res. Int., 2014, vol. 18, article ID 172791. 10.1155/2014/172791 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.