Abstract
The results of antinuclear antibody tests using the indirect immunofluorescence technique may be reported as a description of the pattern and the intensity of fluorescence obtained at a certain dilution. If quantitative results are required titration is necessary. Such titrations may vary greatly between different laboratories. The present study involving 26 laboratories shows an improvement of interlaboratory comparability for the homogeneous fluorescence pattern when a common reference serum is used. Cultured cells as substrate appear to give better quantitative agreement than rat liver sections. National reference sera should be standardised in items of the appropriate WHO reference preparation.
Full text
PDF

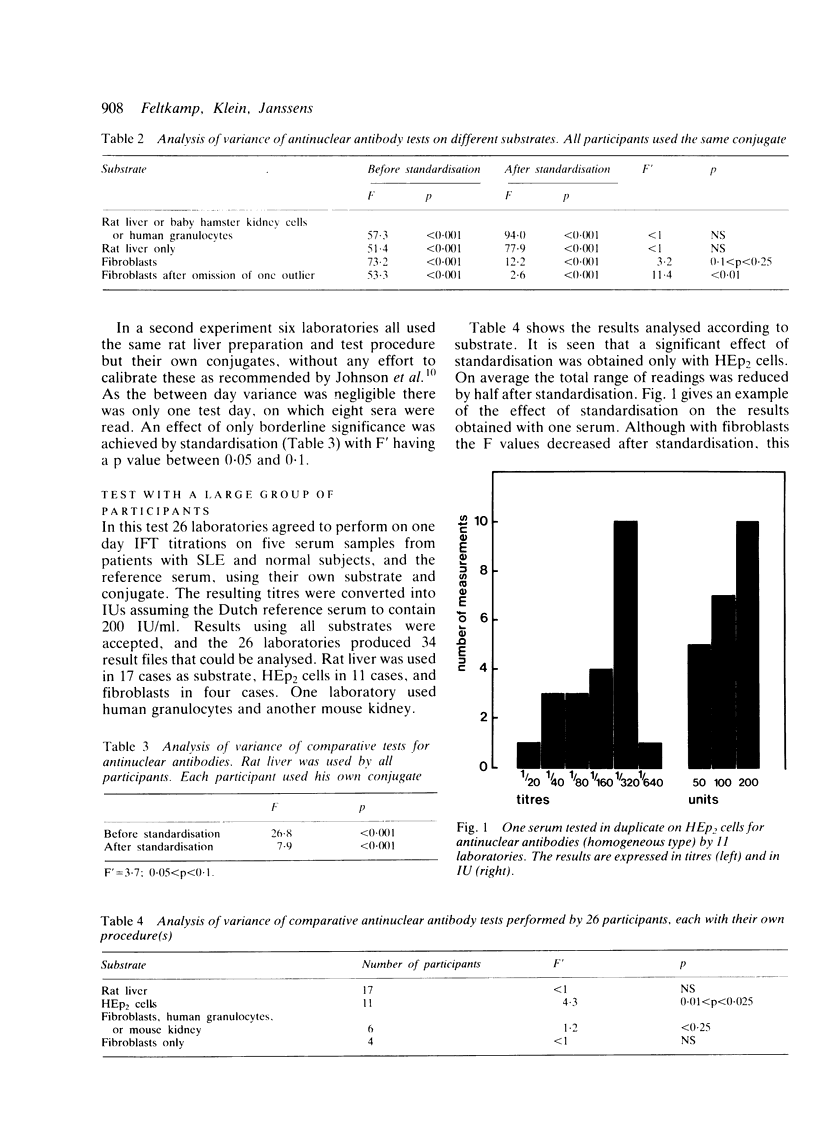

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. G., Addison I. E., Dixon H. G. Antinuclear-factor serum (homogeneous): an international collaborative study of the proposed research standard 66-233. Ann N Y Acad Sci. 1971 Jun 21;177:337–345. doi: 10.1111/j.1749-6632.1971.tb35063.x. [DOI] [PubMed] [Google Scholar]
- Anderson S. G., Bentzon M. W., Houba V., Krag P. International reference preparation of rheumatoid arthritis serum. Bull World Health Organ. 1970;42(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Bonifacio E., Hollingsworth P. N., Dawkins R. L. Antinuclear antibody. Precise and accurate quantitation without serial dilution. J Immunol Methods. 1986 Jul 24;91(2):249–255. doi: 10.1016/0022-1759(86)90486-2. [DOI] [PubMed] [Google Scholar]
- Klein F., Janssens M. B. Standardisation of serological tests for rheumatoid factor measurement. Ann Rheum Dis. 1987 Sep;46(9):674–680. doi: 10.1136/ard.46.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molden D. P., Nakamura R. M., Tan E. M. Standardization of the immunofluorescence test for autoantibody to nuclear antigens (ANA): use of reference sera of defined antibody specificity. Am J Clin Pathol. 1984 Jul;82(1):57–66. doi: 10.1093/ajcp/82.1.57. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Rippey J. H. Quality assurance and proficiency testing for autoantibodies to nuclear antigen. Arch Pathol Lab Med. 1985 Feb;109(2):109–114. [PubMed] [Google Scholar]
- Seligmann M., Cannat A., Hamard M. Studies on antinuclear antibodies. Ann N Y Acad Sci. 1965 Jun 30;124(2):816–832. doi: 10.1111/j.1749-6632.1965.tb19004.x. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Fritzler M. J., McDougal J. S., McDuffie F. C., Nakamura R. M., Reichlin M., Reimer C. B., Sharp G. C., Schur P. H., Wilson M. R. Reference sera for antinuclear antibodies. I. Antibodies to native DNA, Sm, nuclear RNP, and SS-B/La. Arthritis Rheum. 1982 Aug;25(8):1003–1005. doi: 10.1002/art.1780250814. [DOI] [PubMed] [Google Scholar]


