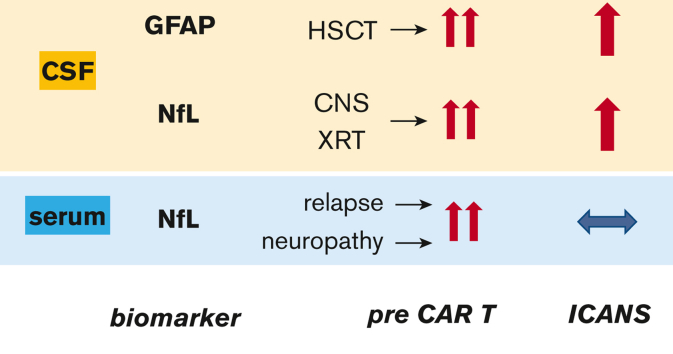
Key Points
-
•
cGFAP and cNfL levels increase in pediatric patients during CAR T-cell–associated neurotoxicity, indicating injury to astrocytes and neurons.
-
•
Nervous system injury from prior therapy correlates with high baseline GFAP and NfL levels in pediatric patients eligible for CAR T cells.
Visual Abstract
Abstract
There is a need for biomarkers to predict and measure the severity of immune effector cell–associated neurotoxicity syndrome (ICANS). Glial fibrillary acidic protein (GFAP) and neurofilament light chain (NfL) are well-validated biomarkers of astroglial and neuronal injury, respectively. We hypothesized that pretreatment GFAP and NfL levels can predict the risk of subsequent ICANS and that increases in GFAP and NfL levels during treatment reflect ICANS severity. We measured cerebrospinal fluid GFAP (cGFAP) and NfL (cNfL) along with serum NfL (sNfL) levels at pretreatment and day 7 to 10 after chimeric antigen receptor (CAR) T-cell infusion in 3 pediatric cohorts treated with CD19- or CD19/CD22-directed CAR T cells. cGFAP and cNfL levels increased during grade ≥1 ICANS in patients treated with CD19-directed CAR T cells but not in those who received CD19/CD22-directed CAR T cells. The sNfL levels did not increase during ICANS. Prelymphodepletion cGFAP, cNfL, and sNfL levels were not predictive of subsequent ICANS. Elevated baseline cGFAP levels were associated with a history of transplantation. Patients with prior central nervous system (CNS) radiation had higher cNfL levels, and elevated baseline sNfL levels were associated with a history of peripheral neuropathy. Thus, cGFAP and cNfL may be useful biomarkers for measuring the severity of CNS injury during ICANS in children. Elevated baseline levels of cGFAP, cNfL, and sNfL likely reflect the cumulative injury to the central and peripheral nervous systems from prior treatment. However, levels of any of the 3 biomarkers before CAR T-cell infusion did not predict the risk of ICANS.
Introduction
Neurotoxicity remains a common but incompletely understood adverse event in cancer immunotherapy.1 Chimeric antigen receptor (CAR) T-cell therapy for hematologic malignancies poses a particularly high risk of neurologic toxicity, affecting 30% to 60% of patients.2 The most common manifestation of immune effector cell–associated neurotoxicity syndrome (ICANS) is a delirium-like encephalopathic state that is often accompanied by language dysfunction. This can progress to an altered level of alertness and, in severe cases, seizures and coma. Rarely, patients develop rapidly-progressive global cerebral edema, which can be fatal.
Biomarker development for ICANS serves 1 or more of the following purposes: (1) predicting toxicity risk for an individual patient, enabling targeted counseling and possible preventive treatment; (2) measuring acute brain injury; and (3) measuring long-term brain injury. In addition, we may gain additional insights into the mechanism of neurotoxicity. Biomarkers for ICANS should ideally be highly specific to the nervous system and should reflect the hypothesized injury mechanisms in ICANS. We selected glial fibrillary acidic protein (GFAP) and neurofilament light chain (NfL), as candidates that meet these criteria well.
GFAP is an intermediate filament of astrocytes, and its elevations in the blood or cerebrospinal fluid (CSF) are specific markers of astrocyte activation and/or injury that occur during traumatic brain injury and other neurologic disorders.3,4 Astrocytes are critical for the maintenance of the neurovascular unit.5 Because blood-brain barrier injury is hypothesized to play an important role in ICANS pathogenesis,6 GFAP may be a good marker of both susceptibility to ICANS and severity of ICANS. We have previously found that CSF GFAP (cGFAP) levels increase during ICANS in pediatric patients treated with CD19-directed CAR T cells.7 We now prospectively validate GFAP as a biomarker of ICANS in an extended cohort of CD19-CAR T-cell– and CD19/CD22-CAR T-cell–treated pediatric patients.
NfL has emerged as an excellent biomarker of neuronal injury in many neurologic conditions, including multiple sclerosis, dementia, stroke, and amyotrophic lateral sclerosis.8,9 In adult patients treated with CD19-directed CAR T cells, serum NfL (sNfL) levels were higher at baseline and during peak neurotoxicity in patients who developed grade ≥2 ICANS than those with grade 1 or no ICANS.10 It remains to be resolved whether the rise in neurofilament levels is specific to ICANS and whether this also occurs in pediatric patients.
The primary objectives of this study were to determine whether (1) NfL and GFAP levels predict the incidence and severity of ICANS before CAR T-cell infusion and (2) NfL and GFAP levels increase during ICANS. Additional exploratory objectives were to determine which clinical factors contribute to the abnormal baseline NfL and GFAP levels.
Methods
Subject selection and ethical review
Patients aged 1 to 26 years with relapsed/refractory leukemia or lymphoma were enrolled in open-label clinical trials to receive lymphodepletion chemotherapy and CAR T cells directed against CD19 (PLAT-02 phase 1/2, #NCT02028455)11 or CD19/CD22 (PLAT-05 phase 1, #NCT03330691).12,13 Cohort size was determined by the prespecified enrollment targets for each phase of the clinical trials. All studies were approved by the Seattle Children’s Research Institute Institutional Review Board.
Samples
Serum and CSF samples from patients treated with CAR T cells were collected according to the clinical trial protocol. The time points included prelymphodepletion (CSF from all patients), day 1 before CAR T-cell infusion (serum from all patients), and days 7 to 10 (serum from all patients and CSF scheduled for PLAT-05). Additional samples were obtained during adverse events as specified by the clinical trial protocols or clinical judgment. All samples were stored at −80 °C.
Toxicity grading
“Neurotoxicity” and “ICANS” are used interchangeably in this article to refer to the constellation of neurologic signs and symptoms occurring acutely after CAR T-cell treatment. All new or worsening neurologic signs and symptoms, including headache, occurring ≤28 days after CAR T-cell infusion, were prospectively classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 (ctep.cancer.gov). Daily and overall neurotoxicity grades were determined by the most severe CTCAE grade of any neurologic symptom during that period with the following exceptions: headache as the only symptom above grade 2 was assigned overall neurotoxicity grade 2, and new-onset seizure was assigned neurotoxicity of at least grade 3. We graded neurotoxicity on the basis of CTCAE criteria as specified by the clinical trial protocols, which were developed before the publication of the 2018 American Society for Transplantation and Cellular Therapy (ASTCT) CAR T-cell therapy toxicity criteria.14 However, there is excellent concordance between CTCAE and ASTCT ICANS grading,15 making it unlikely that our conclusions would be significantly altered if the current standard ASTCT toxicity grading system had been used. Cytokine release syndrome (CRS) was graded as none, mild, or severe as previously described.11 Severe CRS was defined as a requirement for pressors, inotropes, or respiratory support. Compared with the 2018 ASTCT criteria,14 this grading scheme assigns severe CRS to patients requiring only low-flow oxygen, which is equivalent to ASTCT grade 2 CRS. Otherwise, our “mild” criteria are equivalent to ASTCT grades 1 to 2, and the “severe” criteria reflects ASTCT grade ≥3 CRS.
GFAP and NfL quantification
All measurements were performed on the Meso Scale Discovery platform (Meso Scale Diagnostics, Rockville, MD). For cGFAP quantification, CSF was diluted 1:2 and the R-PLEX Human GFAP Antibody Set (Meso Scale Diagnostics) was used according to the manufacturer’s instructions. For NfL quantification, serum samples were diluted at 1:2 and CSF samples were diluted at 1:6. The capture antibody (UD1; Uman Diagnostics, Umeå, Sweden) was used at 1.25 μg/mL, and the detection antibody (UD3; Uman Diagnostics) was used at 0.5 μg/mL.16 All samples from individual patients were run on the same plate. Levels below the limit of quantification were designated at 50% of the limit of quantification. The intra-assay coefficient of variability was <10%.
Statistical analyses
Descriptive statistics such as mean and/or range for continuous variables and percent for categorical variables were summarized for baseline and demographic information overall and by each study, respectively. For continuous outcomes (eg, log10 of GFAP and NfL levels), linear regression was used to test the association between a covariate of interest and a continuous endpoint (if the endpoint followed a normal distribution). Log10-transformed biomarker levels were used in the univariate linear regression models. For binary endpoints (eg, ICANS grade 0-2 vs grade 3 and above), we performed logistic regression to test the association between a covariate of interest and a binary endpoint. Nonparametric Wilcoxon signed-rank tests were used to test pairwise comparisons of pretreatment and acute biomarker levels and the Kruskal-Wallis test with Dunn’s post-test for groupwise comparisons. Stepwise regression was used for variable selection in multivariate analysis. P values < .05 were considered statistically significant, and adjustments for multiple comparisons were performed when appropriate. Analyses were performed in R studio (version 3.6.2) and GraphPad PRISM (version 9; GraphPad Software Inc, La Jolla, CA).
Results
Patient characteristics
A total of 141 patients from 3 clinical trial cohorts were included in the study. Cohort 1 (N = 43) received CD19-directed CAR T cells in the PLAT-02 trial, whose results have been previously published.7,11 Cohort 2 (N = 75) was enrolled in the expansion phase of PLAT-02 and received the same CAR construct as cohort 1, made with a slightly different manufacturing process.17 Cohort 3 (N = 23) received mixed populations of CD19- and CD22-directed CAR T cells on the PLAT-05 trial. Patient clinical data are presented in Table 1.
Table 1.
Patient demographics and medical history
| Cohort | PLAT-02 #1 | PLAT-02 #2 | PLAT-05 | All |
|---|---|---|---|---|
| Diagnosis (%) | ALL (100) | ALL (85), lymphoma (15) | ALL (100) | |
| Treatment | SCRI-CAR19v1 | SCRI-CAR19v1.5 | SCRI-CAR19x22v1 | |
| N | 43 | 75 | 23 | 141 |
| Female, % | 51 | 33 | 39 | 40 |
| Mean age at CAR T cells (range) | 11.9 (1-25) | 12.2 (1-26) | 12.4 (1-25) | 12.2 |
| Mean relapses | 1.7 | 1.5 | 1.5 | 1.5 |
| Mean HSCTs | 0.7 | 0.4 | 0.5 | 0.5 |
| CNS involvement, % | 20.9 | 20.8 | 21.7 | 21.0 |
| Neuro comorbidities, % | 65.0 | 64.0 | 47.8 | 61.7 |
| Peripheral neuropathy, % | 16.3 | 18.7 | 8.7 | 16.3 |
| TBI (no cranial boost), % | 48.9 | 19.4 | 26.3 | 29.7 |
| Cranial boost, % | 14.0 | 16.7 | 15.8 | 15.2 |
| Mean ICANS grade | 1.1 | 1.0 | 0.6 | 1.0 |
| Mean CRS grade (0-2) | 1.1 | 1.0 | 0.9 | 1.0 |
| Any ICANS, % | 44.2 | 51.4 | 39.1 | 47.1 |
| Grade ≥3 ICANS, % | 20.9 | 16.2 | 4.3 | 15.7 |
ALL, acute lymphoblastic leukemia; HSCTs, hematopoietic stem cell transplants; TBI, total body irradiation.
Baseline GFAP and NfL levels did not correlate with neurotoxicity risk
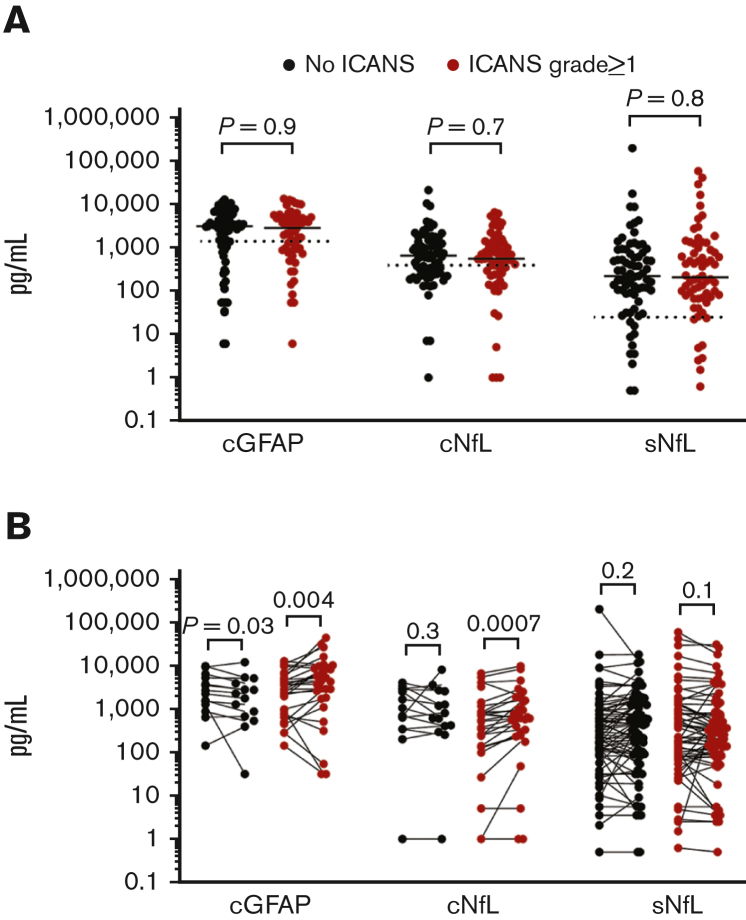
The first prespecified aim of this study was to determine whether patients with subsequent neurotoxicity have higher baseline levels of cGFAP, cNfL, and/or sNfL. Both CSF and serum baseline samples were available for 137 of 141 patients. For 3 patients, prelymphodepletion CSF samples were unavailable, and 1 patient lacked a day 1 serum sample. The median prelymphodepletion cGFAP level across all patients was 2978 pg/mL (interquartile range [IQR], 904-5213 pg/mL), the median prelymphodepletion cNfL level was 600 pg/mL (IQR, 246-1329 pg/mL), and the median day 1 sNfL level was 217 pg/mL (IQR, 78-874 pg/mL). The range of values for each analyte was very wide across patients, with or without neurotoxicity (Figure 1A). There was no association between baseline biomarker levels and subsequent ICANS in univariate analysis (Table 2) or groupwise comparisons between patients with and without ICANS in the individual or combined cohorts (Table 3). There was a poor skill for baseline cGFAP, cNfL, or sNfL in discriminating patients with subsequent grade ≥1 ICANS from those without ICANS, with the area under the receiver operating characteristic curve of 0.5044, 0.5162, and 0.5144, respectively. We also tested groupwise comparisons between patients with ICANS 0 to 2 vs ICANS ≥3 or ICANS 0 to 1 vs ICANS ≥2 and again found no statistically significant associations between baseline biomarkers and ICANS severity. The only variable that was associated with the risk of subsequent ICANS was CRS grade, both in univariate and multivariate regression analysis (Table 2).
Figure 1.
GFAP and NfL levels are elevated at baseline and increase in the CSF during ICANS. (A) Baseline biomarker measurements. Each point represents the data from 1 patient, all 3 cohorts are combined. Bars show the median. CSF samples were obtained prelymphodepletion and serum samples are from day 1 before CAR T-cell infusion. The dotted lines show the upper bound of published reference levels (1386 pg/mL for cGFAP, 380 pg/mL for cNfL, 25 pg/mL for sNfL). Groups were compared by Mann-Whitney test. (B) Pairwise comparisons of biomarker levels show rises of cGFAP and cNfL, but not sNfL during ICANS. Note that cGFAP decreased in patients without ICANS. Each linked set of data points is from 1 patient. The “pre” time point for cGFAP and cNfL represents the prelymphodepletion sample, the “acute” time points represent the sample obtained on days 5 to 15 post CAR T-cell infusion. For sNfL, “pre” represents samples obtained on day 1 and “post” represents samples obtained from days 7 to 10. Data were analyzed by Wilcoxon matching-pairs signed-rank test.
Table 2.
ICANS predictors
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Prelymphodepletion cGFAP | 1.10 | 0.69-1.79 | .7 |
| Prelymphodepletion cNfL | 0.81 | 0.49-1.31 | .4 |
| Day 1 sNfL | 1.09 | 0.77-1.57 | .6 |
| Cohort 1 | |||
| Cohort 2 | 1.33 | 0.63-2.86 | .5 |
| Cohort 3 | 0.81 | 0.28-2.26 | .7 |
| Age at CAR T-cell infusion | 1.05 | 0.99-1.10 | .086 |
| Sex | 0.95 | 0.48-1.88 | .9 |
| No. of relapses | 0.96 | 0.68-1.35 | .8 |
| Hematopoietic stem cell transplant, n | 0.74 | 0.41-1.31 | .3 |
| No radiation | |||
| TBI | 0.92 | 0.42-1.97 | |
| TBI + cranial boost | 1.76 | 0.65-4.95 | .4 |
| CNS 1 | |||
| CNS 2 | 0.69 | 0.28-1.62 | .4 |
| CNS 3 | 1.10 | 0.04-28.2 | >.9 |
| Neurocomorbidities | 1.35 | 0.68-2.69 | .4 |
| Peripheral neuropathy | 1.57 | 0.64-3.95 | .3 |
| No CRS | |||
| Mild CRS | 17.3 | 3.36-318 | .006 |
| Severe CRS | 34.0 | 5.33-679 | .002 |
Univariate model, P values < .05 are in bold.
CNS, central nervous system.
Table 3.
Levels of prelymphodepletion cGFAP/cNfL and day 1 sNfL do not correlate with risk of subsequent ICANS
| PLAT-02, cohort 1 |
PLAT-02, cohort 2 |
PLAT-05 |
All patients |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (pg/mL) | P value | N | Median (pg/mL) | P value | N | Median (pg/mL) | P value | N | Median (pg/mL) | P value | N | |
| cGFAP prelymphodepletion | ||||||||||||
| No ICANS | 5798 | 24 | 2210 | 36 | 1838 | 14 | 3027 | 74 | ||||
| ICANS | 5012 | .9479 | 17 | 1661 | .3240 | 37 | 899.6 | .4485 | 9 | 2738 | .9305 | 63 |
| cNfL prelymphodepletion | ||||||||||||
| No ICANS | 294.5 | 24 | 860.7 | 36 | 674.5 | 14 | 632.2 | 74 | ||||
| ICANS | 532.4 | .1749 | 17 | 670.6 | .7358 | 37 | 96.65 | .0982 | 9 | 538.2 | .7460 | 63 |
| sNfL | ||||||||||||
| Day 1 | ||||||||||||
| No ICANS | 380.2 | 24 | 122.2 | 36 | 288.3 | 13 | 215.5 | 73 | ||||
| ICANS | 356.5 | >.9999 | 19 | 164.3 | .5964 | 38 | 131.3 | .8811 | 9 | 203.0 | .7709 | 66 |
The ICANS group comprises all patients with any grade of neurotoxicity. Mann-Whitney test was used for comparing the ICANS and no ICANS groups within each cohort and for each biomarker, no adjustment was made for multiple comparisons.
cGFAP and cNfL levels increase during ICANS but sNfL levels did not
The second aim of the study was to determine whether ICANS is associated with an increase in cGFAP, cNfL, and/or sNfL levels. Given the heterogeneity of baseline levels of all 3 biomarkers, we used pairwise comparisons of pretreatment and acute samples from the same patients to detect interval changes.
CSF was prospectively collected on day 10 in cohort 3, whereas in cohorts 1 and 2, lumbar punctures in the acute setting after CAR T-cell infusion were performed only in patients with acute ICANS or for other clinical indications. The total number of paired samples analyzed in each group are shown in Table 4. In patients with ICANS, acute CSF samples were obtained at a median of 3 days after ICANS onset (range, 1 day before to 9 days after) and a median of 1 day after peak ICANS (range, 3 days before to 5 days after).
Table 4.
Biomarker changes over time
| PLAT-02, cohort 1 |
PLAT-02, cohort 2 |
PLAT-05 |
All patients |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute-baseline (pg/mL) | P value | N | Acute-baseline (pg/mL) | P value | N | Acute-baseline (pg/mL) | P value | N | Acute-baseline (pg/mL) | P value | N | |
| cGFAP | ||||||||||||
| ICANS 0 | ∗ | 1 | ∗ | 1 | −363.7 | .0923 | 12 | −437.8 | .0295 | 14 | ||
| All ICANS | 3087 | .0210 | 12 | 622.7 | .0313 | 7 | −108.3 | .5703 | 9 | 623.6 | .0041 | 28 |
| cNfL | ||||||||||||
| ICANS 0 | ∗ | 1 | ∗ | 1 | −0.1 | .3203 | 12 | −0.1 | .2734 | 14 | ||
| All ICANS | 257 | .0049 | 12 | 261.3 | .0781 | 7 | 46.8 | .3125 | 9 | 143.2 | .0007 | 28 |
| sNfL | ||||||||||||
| ICANS 0 | 73.7 | .0249 | 24 | −25.6 | .0984 | 36 | 116.2 | .0195 | 13 | 0 | .1727 | 73 |
| All ICANS | −57.9 | .0420 | 11 | 11.1 | .8462 | 37 | −55.5 | .0078 | 9 | −19.5 | .1228 | 57 |
Pairwise comparisons were performed between acute samples (CSF, days 5-15 after CAR T-cell infusion for cohorts 1 and 2, day 10 for cohort 3; serum days 7-10 for all patients) and baseline (prelymphodepletion for CSF; day 1 for serum) samples. Measurements represent the difference calculated by subtracting the baseline level from the acute level; the median is shown for each group. P values were calculated by Wilcoxon matched-pairs signed-rank test; P values <.05 are in bold.
Insufficient number of samples for statistical analysis.
Paired serum samples were available from 139 of 141 patients (Figure 1B; Table 4). The acute serum samples were obtained at a median of 3 days after ICANS onset (range, 6 days before to 10 days after) and at a median of 2 days after ICANS peak (range, 8 days before to 9 days after).
To ensure that the acute samples truly reflected the changes attributable to ICANS, we excluded samples from this analysis if the acute sample was obtained before ICANS onset from a patient who subsequently developed ICANS. Thus, 1 CSF sample from cohort 1, 8 serum samples from cohort 1, and 1 serum sample from cohort 2 were excluded from this study. We also performed the same analyses with all the included samples (supplemental Table 1) and all conclusions remained unchanged.
We have previously shown that cGFAP levels were higher in cohort 1 patients during acute neurotoxicity than at baseline.7 This correlation was maintained in pairwise comparison in this cohort (mean increase, 3087 pg/mL; P = .0210; Wilcoxon matched-pairs signed-rank test). Cohort 2 patients with ICANS also showed an increase in cGFAP (mean increase, 623 pg/mL; P = .0313). cNfL levels were increased in cohort 1 patients with ICANS (mean increase, 257 pg/mL; P = .0049), but the change was not statistically significant in cohort 2 (mean increase, 261 pg/mL; P = .0781). In contrast, cohort 3 patients with or without ICANS showed no significant change in cGFAP or cNfL levels from pretreatment to day 10 after CAR T-cell infusion (Table 4). cGFAP levels were decreased in patients without ICANS across all cohorts (Table 4).
The sNfL levels did not increase between day 1 and days 7 to 10 in any of the ICANS subgroups. In fact, in cohorts 1 and 3, patients with ICANS had a statistically significant decrease in sNfL levels, whereas there was an increase in patients without ICANS (Table 4). The increase in cGFAP and cNfL levels correlated with ICANS severity such that patients with more severe ICANS had greater absolute increases in both biomarkers (P = .0254 and P = .0179, respectively; Spearman rank-sum correlation). In contrast, the greater the ICANS severity, the greater the decrease in sNfL levels (P = .0103).
These findings show that cGFAP and cNfL increased during ICANS in patients who were ill enough to warrant a lumbar puncture in an acute setting. More severe ICANS was associated with a greater increase in cGFAP and cNfL levels. Patients with mild or no ICANS showed changes that were too small to detect with our cohort size.
Contributors to elevated baseline levels of GFAP and NfL
The baseline levels of GFAP and NfL were heterogeneous and extremely elevated in many patients in all 3 cohorts. This prompted us to perform exploratory analyses of the demographic and medical history factors that might contribute to the elevation of baseline biomarkers. We hypothesized that measurable GFAP and NfL levels increase in response to prior insults to the CNS, including radiation, CNS leukemic involvement, or preexisting neurologic comorbidities. We performed univariate regression on the following variables: cohort, age, sex, number of relapses, number of HSCTs, history of TBI with or without CNS boost, CNS disease status, history of any preexisting neurologic comorbidities, and history of peripheral neuropathy. Prelymphodepletion cGFAP levels were positively associated with the number of prior HSCTs and a history of TBI (Table 5). Prelymphodepletion cNfL levels were higher in patients with a history of TBI plus a CNS boost and those with preexisting neurologic comorbidities (Table 5). The sNfL levels on day 1 were positively associated with the number of prior relapses, TBI, and a history of peripheral neuropathy (Table 5). Cohort effects were also observed, with higher levels of cGFAP in cohort 1 than in other cohorts, whereas cNfL levels were similar between the cohorts (Table 5). The variables mentioned above retained their best predictive ability in the multivariate models with stepwise variable selection (Table 5). Taken together, these data support the conclusion that prior treatment toxicity is the cause of elevated baseline biomarker levels.
Table 5.
Contributors to elevated baseline biomarker levels
| Variable | cGFAP |
cNfL |
sNfL |
|||
|---|---|---|---|---|---|---|
| β 95% CI | P value | β 95% CI | P value | β 95% CI | P value | |
| Cohort 1 | ||||||
| Cohort 2 | −1.0 to −0.47 | <.001∗ | −0.06 to 0.48 | .13 | −0.62 to 0.09 | .14 |
| Cohort 3 | −0.93 to −0.27 | <.001∗ | −0.63 to 0.09 | .14 | −1.0 to −0.03 | .039 |
| Age at CAR T-cell infusion | −0.02 to 0.02 | >.9 | 0.00 to 0.04 | .053 | −0.02 to 0.03 | .6 |
| Sex | −0.39 to 0.10 | .2 | −0.37 to 0.12 | .3 | −0.31 to 0.34 | >.9 |
| Number of relapses | −0.07 to 0.17 | .4 | −0.05 to 0.19 | .3 | 0.08 to 0.40 | .003∗ |
| Number of HSCTs | 0.06 to 0.47 | .011 | −0.14 to 0.28 | .5 | −0.04 to 0.51 | .093 |
| No radiation | ||||||
| TBI | 0.00 to 0.56 | .048 | −0.09 to 0.44 | .2 | 0.11 to 0.82 | .010 |
| TBI + cranial boost | −0.13 to 0.57 | .2 | 0.36 to 1.0 | <.001∗ | −0.18 to 0.71 | .2 |
| CNS 1 | ||||||
| CNS 2 | −0.19 to 0.42 | .5 | −0.26 to 0.36 | .5 | −0.10 to 0.72 | .14 |
| CNS 3 | −0.22 to 1.4 | .2 | −0.19 to 1.5 | .13 | −0.73 to 1.5 | .5 |
| Neuro comorbidities | −0.12 to 0.38 | .3 | 0.07 to 0.56 | .012∗ | −0.26 to 0.39 | .7 |
| Peripheral neuropathy | −0.23 to 0.44 | .5 | −0.10 to 0.57 | .2 | 0.08 to 0.92 | .020∗ |
Univariate linear regression was performed on log10-transformed measurements. P values < .05 are in bold. β 95% CI of the regression slope.
CI, confidence interval.
Variable selected in stepwise multivariate regression model, P < .05.
CSF and serum NfL do not correlate strongly within individual patients
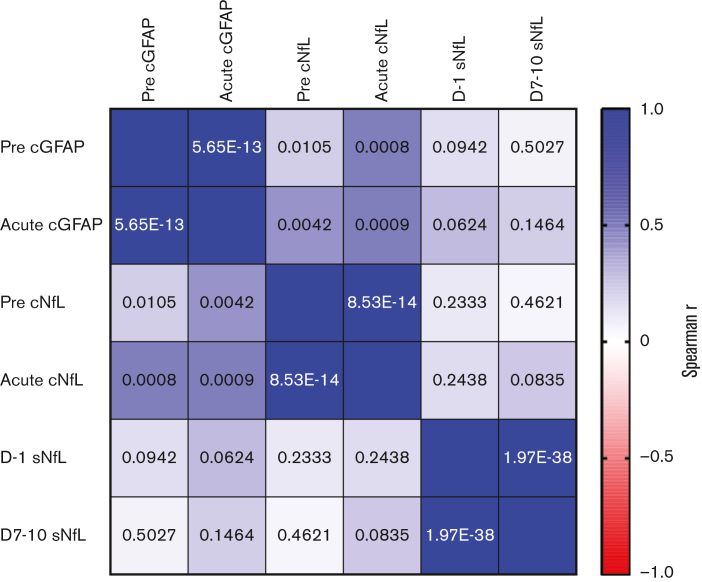
To gain additional insights into the dynamics of GFAP and NfL, we measured the concordance across time within the same biomarker and the concordance of the 3 different biomarkers at each time point within individual patients. Paired samples of the same biomarker (pre- and acute) were strongly correlated in individual patients (Figure 2). There was also a significant correlation between cGFAP and cNfL levels within the same CSF samples. Surprisingly, cNfL and sNfL levels were not highly correlated when measured at the same time point (baseline or acute) in the same patient (Figure 2). Even when only considering CSF and serum samples that were collected within 24 hours of each other, the correlation was not statistically significant (Spearman’s r = 0.335; P = .0480; CSF and serum sample pairs [N = 35]). This suggests that different underlying mechanisms contribute to changes in the CSF and systemic NfL levels.
Figure 2.
Correlation between cGFAP, cNfL, and sNfL levels in individual patients. The color of each box indicates the strength of association between the measurements in individual patients (Spearman’s r). The number in each box shows the P value for the Spearman rank-sum correlation. The “pre” time point for cGFAP and cNfL represents the prelymphodepletion sample, the “acute” time points represent the sample obtained on days 5 to 15 post CAR T-cell infusion. For sNfL, “pre” represents samples obtained on day 1 and “post” represents samples obtained from days 7 to 10.
Discussion
We showed that NfL and GFAP have the potential to be useful biomarkers of neurologic toxicity during CAR T-cell therapy in pediatric patients with hematologic malignancies. In patients with ICANS who received CD19-directed CAR T cells, there was an increase in the NfL and GFAP levels in the CSF, and these increases were higher in patients with more severe neurotoxicity. This suggests that neurons and astrocytes are injured during ICANS, although the underlying mechanisms are not completely understood.1,18 Disruption of the blood-brain barrier has been postulated as an important contributor to ICANS6 and astrocyte injury during this process if plausible given their intrinsic role in maintaining the neurovascular unit.5 In fatal cases of CD19-CAR T-cell−associated neurotoxicity, brain histopathology shows disruption of cerebral microvessels.6,19 Animal models also support an injury mechanism via the neurovascular unit.18,20,21 Gliosis of the cerebral cortex has been shown after recovery from ICANS, but it remains uncertain whether this is a reactive process or if it is directly related to the pathogenesis of ICANS.7 There is also evidence supporting our finding that neurons themselves are injured during ICANS. Altered mentation and depressed consciousness are hallmarks of ICANS and are accompanied by abnormal electroencephalographic patterns, most commonly slowing patterns.22, 23, 24, 25, 26 Quinolinic acid and glutamate, which have been associated with excitotoxicity, were higher in the CSF of patients with acute neurotoxicity than in those obtained before CAR T-cell treatment.27
However, in the patients receiving CD19/CD22-CAR T cells, a rise in cGFAP and cNfL levels was not detected. There are several possible explanations for this discrepancy. It may be that the CD19/CD22 product was less toxic, even though the CD19 construct was identical to that in the cohorts receiving CD19-CAR T cells alone. CD19-CAR ICANS may occur via a mechanism different from that of CD22-CAR ICANS, however, this seems unlikely because we observed an expansion of both CAR T-cell populations in the patients receiving mixed products. In addition, patients in cohorts 1 and 2 with available paired CSF samples had more severe neurotoxicity than those from cohort 3. Acute CSF samples from cohort 3 were also collected later in the course of ICANS at a median of 4 days after peak ICANS, whereas it was 1 day in cohort 1 and 2 days after peak ICANS in cohort 2. However, it is unlikely that NfL and GFAP levels returned to baseline within days. In a study of oxaliplatin neurotoxicity, sNfL normalized within 4 to 6 months after treatment cessation.28 In patients with traumatic brain injury, NfL levels decreased 5 years after the injury, and GFAP levels showed a decrease for the first 6 months but then increased again.29
It was surprising that increases in cNfL levels were not accompanied by increases in sNfL levels, and we even found decreases in sNfL levels over time in cohort 1 for 3 patients with ICANS. Possible explanations for this decrease in the serum include hemodilution because patients with CRS and ICANS were more likely to receive fluid resuscitation. Therapies such as corticosteroids may have been neuroprotective or decreased leakage across the blood-CSF barrier. In contrast to our findings of disparate CSF and serum NfL changes, a recent meta-analysis found a moderately strong correlation between sNfL and cNfL in a variety of conditions.30 One explanation is that other studies have examined a single neurologic condition or risk factor, whereas our patients were exposed to a variety of mechanisms of injury to the central and peripheral nervous system throughout the treatment of relapsed/refractory leukemia and lymphoma. Thus, in pediatric patients with systemic malignancies, measurements of both cNfL and sNfL may be required to accurately reflect the contributions of central and peripheral nervous system injury, and sNfL may not always be a good proxy of cNfL.
Strikingly, our study found highly-elevated baseline levels of cGFAP, cNfL, and sNfL, which were far above the expected values for the given age in most patients. Reference values for cGFAP are not available for healthy children, but in a control cohort of children without neurologic conditions, the median cGFAP level was 133 pg/mL, with the highest value at 1386 pg/mL.31 Only 31% of our patients had cGFAP levels below 1386 pg/mL. For cNfL, reference levels are also not available for healthy children. In healthy adults aged 18 to 30 years, the normal cutoff for cNfL has been defined as <380 pg/mL.32 Only 37% of our patients had cNfL levels below 380 pg/mL. The sNfL reference values have been established in several cohorts of healthy children. These range between 4 pg/mL and 5 pg/mL, with the 99th percentile around 25 pg/mL.33,34 Only 15% of the patients in our study had baseline sNfL levels of ≤25 pg/mL. This contradicts with an adult cohort of 96 patients who received CAR T cells, whose baseline levels of sNfL were similar to those in age-matched healthy subjects.10 Patients with a history of CNS involvement were excluded from the adult study, but no other obvious differences between the studies explain why our pediatric cohorts’ baseline sNfL levels were so much more abnormal. Possible reasons include more aggressive treatment regimens in pediatric patients, which include more intrathecal chemotherapy35,36 and higher sensitivity of the developing nervous system to injury related to cancer treatment, particularly radiotherapy.37,38 Indeed, we found an association between increased cGFAP levels and a history of HSCT. HSCT has been identified as a risk factor for decreased neurocognitive function in children.39 Higher cNfL levels were robustly associated with a history of radiation with CNS boost. The long-term adverse effects of CNS radiotherapy in children are well established,37 and we now show that cNfL has the potential to be a readily quantifiable biomarker of CNS radiation injury. A history of peripheral neuropathy was associated with an increase in sNfL. This is consistent with the toxic effects of certain chemotherapy drugs, which primarily affect the peripheral nervous system. For example, an association between peripheral neuropathy and transient increase in sNfL levels has been observed in adults receiving oxaliplatin or paclitaxel.28,40
We did not find an association between baseline cGFAP, cNfL, and sNfL levels with the risk of subsequent neurotoxicity. This finding differs from the reported adult cohort, where higher baseline serum NfL levels were associated with a higher risk of subsequent ICANS.10 It is possible that the high levels of biomarker heterogeneity in our cohort masked a weak association with ICANS risk. We again confirmed CRS as the 1 robustly predictive risk factor for ICANS, a finding which aligns with most of the previous reports.41 Prospectively validated risk-prediction algorithms for ICANS also largely rely on CRS-associated risk factors, biomarkers, and clinical signs.24 Although this does not prove that CRS is required for the development of ICANS, there is likely a mechanistic link between the two.
The limitations of this study include the lack of paired CSF samples in cohorts 1 and 2 patients with mild or no ICANS. This likely caused a bias toward a stronger association of cGFAP and cNfL increases during ICANS than we would have seen if paired CSF samples had been available from all patients. Pretreatment CSF samples were obtained before lymphodepletion chemotherapy, whereas baseline serum samples were obtained after lymphodepletion. Thus, it is possible that some of the changes observed in CSF biomarker levels over time were related to lymphodepletion. However, this is unlikely given our observation that cGFAP levels decreased in patients without ICANS.
Another possible limitation was the fact that we used an electrochemiluminescence assay (ECLIA) to detect GFAP and NfL. Single-molecule array (Simoa) assays have become prevalent for NfL quantification in recent years.42 A direct comparison of Simoa and ECLIA showed similar performance but higher sensitivity in the very low range of values for Simoa.43 Thus, our study may have missed small changes in NfL levels in patients with very low measurements. In addition, the absolute values of NfL were higher on the Simoa platform the ECLIA measurements,43 indicating that the highly-elevated NfL levels in our patients may have been even higher than those measured using Simoa.
We did not study long-term changes in the biomarkers, and it remains unknown whether and when the increases in GFAP and NfL normalize after ICANS resolution. It also remains unknown whether elevated biomarker levels are associated with any measurable neurocognitive changes in the short term and the long term.
In conclusion, cNfL and cGFAP are promising biomarkers for the measurement of acute CNS injury during ICANS. Further research should measure the longitudinal effects of cumulative cancer treatment toxicity on the NfL and GFAP levels and correlate them with neurocognitive outcomes in patients with childhood hematologic malignancies.
Conflict-of-interest disclosure: J.G. has served as a consultant for Johnson & Johnson. R.A.G. serves on a study steering committee for and is an inventor on patents licensed to Juno Therapeutics, a Bristol Myers Squibb company, and has served on advisory boards for Novartis. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank Jonathan Weinstein for foundational ideas for this project.
J.G. received funding through the National Institute of Neurological Disorders and Stroke (NINDS) Child Neurology Career Development Program K-12 award (1K12NS098482-02) and the NINDS Mentored Clinical Scientist Career Development award (5K08NS118138-02). Partial funding for this study was provided by the Stand Up to Cancer and St. Baldrick’s Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113), RO1 CA136551 to 05, Alex’s Lemonade Stand Phase 1/2 Infrastructure Grant, Conquer Cancer Foundation Career Development Award, Washington State Life Sciences Discovery Fund, Ben Towne Foundation, William Lawrence and Blanche Hughes Foundation, and Juno Therapeutics. The study data were collected and managed using REDCap electronic data capture tools hosted at the University of Washington with the Institute of Translational Health Science grant support (UL1TR000423 from the NCRR/NIH).
Authorship
Contribution: J.G., J.R.P., C.E.A., and R.A.G. conceptualized and designed the study; J.G. wrote the manuscript; all authors edited the manuscript for content and accuracy; N.M.T., A.L.S., S.D.R.-R., A.L.W., and J.G. performed data acquisition; and K.D.S., Q.W., and J.G. analyzed the data.
Footnotes
Deidentified data are available on request from the corresponding author, Juliane Gust (juliane.gust@seattlechildrens.org).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Shalabi H, Nellan A, Shah NN, Gust J. Immunotherapy associated neurotoxicity in pediatric oncology. Front Oncol. 2022 Feb 21;12:836452. doi: 10.3389/fonc.2022.836452. eCollection 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs. 2018;32(12):1091–1101. doi: 10.1007/s40263-018-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelhak A, Foschi M, Abu-Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18(3):158–172. doi: 10.1038/s41582-021-00616-3. [DOI] [PubMed] [Google Scholar]
- 4.Messing A, Brenner M. GFAP at 50. ASN Neuro. 2020;12 doi: 10.1177/1759091420949680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 6.Gust J, Hay KA, Hanafi L-A, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gust J, Finney OC, Li D, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. 2019;86(1):42–54. doi: 10.1002/ana.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barro C, Chitnis T, Weiner HL. Blood neurofilament light: a critical review of its application to neurologic disease. Ann Clin Transl Neurol. 2020;7(12):2508–2523. doi: 10.1002/acn3.51234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 10.Schoeberl F, Tiedt S, Schmitt A, et al. Neurofilament light chain serum levels correlate with the severity of neurotoxicity after CAR T-cell treatment. Blood Adv. 2022;6:3022–3026. doi: 10.1182/bloodadvances.2021006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annesley C. SCRI-CAR19x22v2 T Cell Product Demonstrates Bispecific Activity in B-ALL. Blood. 2021;138:470. [Google Scholar]
- 13.Gardner RA, Annesley C, Wilson A, et al. Efficacy of SCRI-CAR19x22 T cell product in B-ALL and persistence of anti-CD22 activity. J Clin Oncol. 2020;38(suppl15):3035. [Google Scholar]
- 14.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 15.Pennisi M, Jain T, Santomasso BD, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4(4):676–686. doi: 10.1182/bloodadvances.2019000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalla Costa G, Martinelli V, Sangalli F, et al. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology. 2019;92:e733–e741. doi: 10.1212/WNL.0000000000006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceppi F, Wilson AL, Annesley CE, et al. Modified manufacturing process modulates CD19CAR T-cell engraftment fitness and leukemia-free survival in pediatric and young adult subjects. Cancer Immunol Res. 2022;10:856–870. doi: 10.1158/2326-6066.CIR-21-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faulhaber LD, Phuong AQ, Hartsuyker KJ, et al. Brain capillary obstruction during neurotoxicity in a mouse model of anti-CD19 chimeric antigen receptor T-cell therapy. Brain Commun. 2022;4(1):fcab309. doi: 10.1093/braincomms/fcab309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torre M, Solomon IH, Sutherland CL, et al. Neuropathology of a case with fatal CAR T-cell-associated cerebral edema. J Neuropathol Exp Neurol. 2018;77(10):877–882. doi: 10.1093/jnen/nly064. [DOI] [PubMed] [Google Scholar]
- 20.Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709. doi: 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taraseviciute A, Tkachev V, Ponce R, et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8(6):750–763. doi: 10.1158/2159-8290.CD-17-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belin C, Devic P, Ayrignac X, et al. Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-76055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gust J, Annesley CE, Gardner RA, Bozarth X. EEG correlates of delirium in children and young adults with CD19-directed CAR T cell treatment-related neurotoxicity. J Clin Neurophysiol. 2021;38(2):135–142. doi: 10.1097/WNP.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin DB, Al Jarrah A, Li K, et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 2020;77(12):1536–1542. doi: 10.1001/jamaneurol.2020.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokolov E, Karschnia P, Benjamin R, et al. Language dysfunction-associated EEG findings in patients with CAR-T related neurotoxicity. BMJ Neurol Open. 2020;2(1) doi: 10.1136/bmjno-2020-000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strati P, Nastoupil LJ, Westin J, et al. Clinical and radiologic correlates of neurotoxicity after axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(16):3943–3951. doi: 10.1182/bloodadvances.2020002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. doi: 10.1158/2159-8290.CD-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S-H, Choi MK, Park NY, et al. Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci Rep. 2020;10(1):7995. doi: 10.1038/s41598-020-64511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahim P, Politis A, van der Merwe A, et al. Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology. 2020;95(6):e623–e636. doi: 10.1212/WNL.0000000000009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alagaratnam J, Widekind S von, Francesco DD, et al. Correlation between CSF and blood neurofilament light chain protein: a systematic review and meta-analysis. BMJ Neurol. Open. 2021;3(1) doi: 10.1136/bmjno-2021-000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jany PL, Agosta GE, Benko WS, et al. CSF and blood levels of GFAP in Alexander disease. eNeuro. 2015;2(5) doi: 10.1523/ENEURO.0080-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jessen Krut J, Mellberg T, Price RW, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One. 2014;9(2):e154395. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitz E, Smitka M, Schallner J, et al. Serum neurofilament light chain in pediatric spinal muscular atrophy patients and healthy children. Ann Clin Transl Neurol. 2021;8(10):2013–2024. doi: 10.1002/acn3.51449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinert M-C, Benkert P, Wuerfel J, et al. Serum neurofilament light chain is a useful biomarker in pediatric multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e749. doi: 10.1212/NXI.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel SE, Stock W, Johnson RH, et al. Pediatric-inspired treatment regimens for adolescents and young adults with Philadelphia chromosome–negative acute lymphoblastic leukemia: a review. JAMA Oncol. 2018;4(5):725–734. doi: 10.1001/jamaoncol.2017.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neaga A, Jimbu L, Mesaros O, et al. Why do children with acute lymphoblastic leukemia fare better than adults? Cancers (Basel) 2021;13(15):3886. doi: 10.3390/cancers13153886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krull KR, Hardy KK, Kahalley LS, Schuitema I, Kesler SR. Neurocognitive outcomes and interventions in long-term survivors of childhood cancer. J Clin Oncol. 2018;36(21):2181–2189. doi: 10.1200/JCO.2017.76.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung YT, Sabin ND, Reddick WE, et al. Leukoencephalopathy and long-term neurobehavioral, neurocognitive and brain imaging outcomes in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol. 2016;3(10):e456–e466. doi: 10.1016/S2352-3026(16)30110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parris KR, Russell KM, Triplett BM, Phipps S. Neurocognitive functioning in long-term survivors of pediatric hematopoietic cell transplantation. Bone Marrow Transplant. 2021;56(4):873–882. doi: 10.1038/s41409-020-01125-5. [DOI] [PubMed] [Google Scholar]
- 40.Huehnchen P, Schinke C, Bangemann N, et al. Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight. 2022;7(6) doi: 10.1172/jci.insight.154395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T cell–associated neurotoxicity. Front Immunol. 2020;11:577027. doi: 10.3389/fimmu.2020.577027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400. doi: 10.1038/s41467-021-23620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54(10):1655–1661. doi: 10.1515/cclm-2015-1195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.