Abstract
Electron systems with strong geometrical frustrations have flat bands, and their unusual band dispersions are expected to induce a wide variety of physical properties. However, for the emergence of such properties, the Fermi level must be pinned within the flat band. In this study, we performed first-principles calculations on pyrochlore oxide PbSbO and theoretically clarified that the self-doping mechanism induces pinning of the Fermi level in the flat band in this system. Therefore, a very high density of states is realized at the Fermi level, and the ferromagnetic state transforms into the ground state via a flat band mechanism, although the system does not contain any magnetic elements. This compound has the potential to serve as a new platform for projecting the properties of flat band systems in the real world.
Subject terms: Materials science, Physics
Introduction
Systems that exhibit unusual band structures (dispersion relations) have attracted considerable attention in recent years in condensed matter physics. Among these, systems with a dispersion-free band, known as the flat band, are theoretically predicted to exhibit several novel properties, such as perfect ferromagnetism and high-temperature superconductivity, owing to their unique band structures1–9. Furthermore, such systems have attracted considerable attention from a topological perspective. Combined with the special band structure, interesting physical properties such as the integer/fractional quantum Hall effect and fractional Chern insulator phase are theoretically expected to appear10–13. Recently, a flat band device utilizing these special properties has also been proposed14.
A flat band appears when isotropic hopping occurs between nearest neighbor lattice points in a lattice model with strong geometrical frustration. In other words, the band dispersion vanishes owing to the quantum destructive interference of the wavefunction resulting from strong geometric frustration. Numerous lattices with geometric frustrations have been described in Ref.5. Among these, the most extensively studied is the kagome lattice for two-dimensional (2D) systems and the pyrochlore lattice for three-dimensional (3D) systems. This is because these lattices can achieve flat bands without requiring special conditions for inter-site hopping.
Numerous attempts have been made to realize the flat band model in real-world systems. In 2D systems, quantum wire networks15, photonic crystals16,17, laser cooling of atoms18, metal–organic frameworks (MOFs)19, and twisted bilayer graphene20 belong to this category. However, few attempts have been made to develop 3D systems. Pyrochlore ABO (A = Sn, Pb, Tl; B = Nb, Ta)21,22 and WN23 have recently been developed as 3D systems with nearly flat bands; however, the ferromagnetic behavior predicted by first-principles calculations is yet to be confirmed experimentally. This could be attributed to several factors, but the most critical one is the difficulty of carrier doping into the flat bands.
The concept of geometric frustration has also been applied to localized spin systems to explain several unusual magnetic properties. In numerous pyrochlore oxides, magnetic elements with localized spins are arranged on a pyrochlore lattice, and the geometrical frustration of the lattice creates unusual physical properties, such as quantum spin liquids, spin ice, and magnetic monopoles24–28. Lattices with strong geometric frustration are more generally defined as line graphs on bipartite lattices2, and kagome and checkerboard lattices, as well as pyrochlore lattices, fall in this category. Previous studies have also suggested that magnetic ions form a kagome lattice in Herbertsmithite ZnCu(OH)Cl and possess the properties of a quantum spin liquid29–32. Evidence supporting quantum spin liquids is also found in KCuAlBiO(SO)Cl, which has a geometrically frustrated square-kagome lattice structure33.
Flat bands also appear in localized spin systems. Magnon excitations in frustrated spin systems have flat magnon bands, and the ground state near the saturation field can be understood as the crystalline state of localized magnon excitations5,6. Because the interaction between the two localized spins is isotropic and short range in most cases (e.g., the Heisenberg model), the appearance of a flat band is expected. However, for itinerant systems, this situation is more complicated. Interorbital hopping is isotropic if the related orbitals are s orbitals; however, for other orbitals (for example, p, d, or f orbitals), interorbital hopping is not necessarily isotropic. Therefore, there exist only few compounds in which flat bands appear in itinerant systems. Exceptionally, the s orbitals of the A site in pyrochlore ABO (A = Sn, Pb, Tl; B = Nb, Ta)21,22 and the p orbitals of the N atom in WN34 form quasi-flat bands near the Fermi level. Note that none of these are normally magnetic orbitals, such as d or f orbitals. Nevertheless, first-principles calculations predict that these compounds have a ferromagnetic ground state owing to the large density of states (DOS) derived from the flat band if they are doped appropriately.
PbSbO is popular for its use in ancient Egypt as a yellow pigment, called Naples yellow. Some studies have reported that it has a pyrochlore structure34,35, whereas other studies have reported that it has a weberite structure36–38. The structure of the produced compound also differs depending on the synthesis conditions. Recently, a first-principles calculation of PbSbO was reported34. PbSbO can be synthesized using NaCl flux to produce a compound partially containing Na34. The authors attributed the lack of formation of Na-free PbSbO in this environment to the presence of “highly localized states near the Fermi level”. In Na-free PbSbO, as indicated in Ref.34, there exist “highly localized states near the Fermi level,” which represents the “holy grail” for our analysis, i.e., a naturally carrier-doped flat band. Very recently, a paper titled “Catalogue of flat band stoichiometric materials” was published; here, PbSbO was briefly introduced as an example of a flat band material39.
In this study, we demonstrate that pyrochlore oxide PbSbO exhibits ferromagnetism without carrier doping by first-principles calculations and that similar to SnNbO, this system has a flat band (FB1) mainly consisting of Pb-s orbitals at the top of the valence band (VBM). The most striking feature of this system is the overlap of another flat band (hereafter referred to as FB2), which mainly consists of Sb-s orbitals. This causes some of the electrons in FB1 to flow into FB2, resulting in the hole doping of FB1.
The remainder of this paper is organized as follows. The calculation methods and results are described in “Methods and results” section. Conclusions are given in “Conclusions” section.
Methods and results
We computed the electronic structure of PbSbO from first-principles calculations based on the density functional theory40,41. Details on these calculations can be found in Supplemental Material for details of the calculation, which includes Refs.40–42.
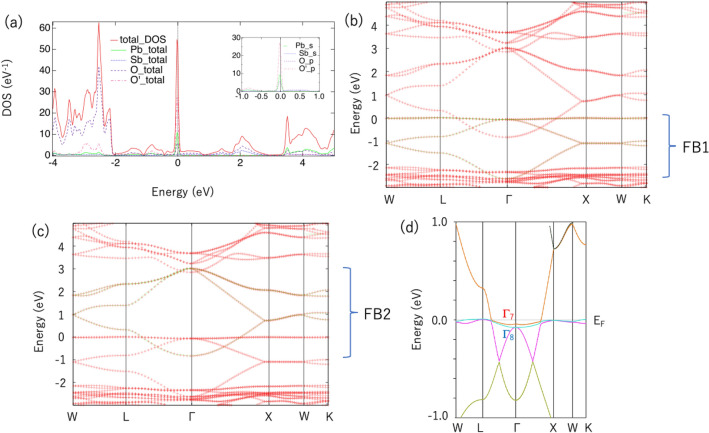
Figure 1a presents the DOS curves of PbSbO in the nonmagnetic state. A clear flat band can be observed at the Fermi level (). This flat band has a similar origin to the flat band in SnNbO; that is, it is mainly composed of s orbitals of Pb atoms located in the pyrochlore lattice and p orbitals of O atoms (O sites) surrounded by Pb atoms.
Figure 1.
(a) DOS curve of PbSbO from first-principles calculations. The inset shows a zoomed-in view of the DOS, including the Pb-s, Sb-s, O-p, and O-p components. (b) Energy dispersion of PbSbO. Red points are obtained from our first-principles calculations, and green points (FB1) are obtained from maximally localized Wannier function (MLWF) fitting with four “Pb-s” orbitals. (c) Red points are the same as in (b), and green points (FB2) are obtained by MLWF fitting with four “Sb-s” orbitals. (d) Energy dispersion of PbSbO near , including spin-orbit interaction. is set to 0 eV or the VBM. The parameters used in (b,c) are listed in Table 1 and in Supplemental Material for details of the calculation, which includes Refs.40–42.
We can construct a four-orbital tight-binding model with four s orbitals centered at Pb atoms (“Pb-s” orbitals) in the primitive unit cell (PUC) of PbSbO using the MLWF42 (see Supplemental Material for details of the calculation, which includes Refs.40–42). The original valence band can be perfectly reproduced, as shown in Fig. 1b. The four largest parameters are listed in Table 1. The number of bands can be easily counted along the X–Z–W axis because all bands are doubly degenerate on this axis owing to the non-symmorphic space group. Similarly, the conduction bands can be reproduced using four MLWFs centered at Sb atoms (“Sb-s” orbitals) in the same PUC of PbSbO. The results are depicted in Fig. 1c. When we ignore parameters other than the first-nearest hopping , these bands convert into two perfect flat bands. Therefore, we refer to these bands as FB1 and FB2, respectively. We can clearly observe that the top of FB1 is nearly flat, whereas the top of FB2 is significantly bent.
Table 1.
Tight-binding parameters for pyrochlore PbSbO and SnNbO.
| PbSbO | SnNbO | |||||
|---|---|---|---|---|---|---|
| FB1 | FB2 | FB | ||||
| Distance (a) | Distance (a) | Distance (a) | ||||
| − 0.586 | On-site | 1.539 | On-site | − 0.7037 | On-site | |
| − 0.266 | 0.3536 | − 0.344 | 0.3536 | − 0.2764 | 0.3536 | |
| − 0.025 | 0.7071 | − 0.049 | 0.6124 | − 0.037 | 0.7071 | |
| − 0.009 | 1.0607 | − 0.043 | 0.7071 | 0.013 | 0.6124 | |
(a) Four-orbital MLWF model centered with the Pb site for FB1 of PbSbO, (b) four-orbital MLWF model centered with the Sb site for FB2 of PbSbO, and (c) four-orbital MLWF model centered with the Sn site for the flat band of SnNbO. Details on the notation are presented in Supplemental Material for details of the calculation, which includes Refs.40–42. All energy units are electron volt. The distance is expressed in units of a, the lattice constant of each compound.
The first two parameters and determine the position and total bandwidth of FB (), respectively. The bandwidth of the highest band of FB (= ) is determined by other parameters, such as and . Because the bandwidth of FB1 is extremely narrow, the next-nearest neighbor hopping parameter is very small: eV. Further, is also smaller in PbSbO than in SnNbO. For FB2, and have larger absolute values than those in FB1. This corresponds to a larger in FB2.
However, contrary to the expectation of the ionic configuration PbSbO, PbSbO becomes metallic. This is because the energy of the Sb-s orbital forming the conduction band is lower than that of the Nb-d orbital in the conduction band of SnNbO; thus, the energy gap collapses.
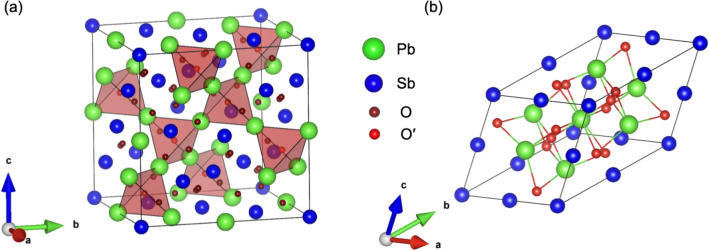
This behavior can be explained by considering the crystal structure of PbSbO, which is shown with a cubic pyrochlore structure in Fig. 2. Pyrochlore oxide ABO can also be expressed as ABOO owing to the presence of two oxygen sites. ABOO can be decomposed into a corner-shared AO tetrahedral network (AO unit) and corner-shared BO octahedral network (BO unit)21. Let us consider only the A-s and B-s orbitals. In this case, the pyrochlore lattices of the A and B atoms form separate flat bands (FB1 and FB2, respectively). For the AO unit, the inclusion of O-p orbitals does not affect FB1 because of symmetry21. Conversely, for the BO unit, the inclusion of O-p orbitals severely affects FB2, and the topmost band of FB2 is significantly bent.
Figure 2.
Crystal structure of pyrochlore PbSbO for the (a) conventional unit cell and (b) PUC. A 3D corner-sharing network of PbO tetrahedra is highlighted. The illustration of the crystal structures was produced using VESTA43. The internal atomic positions in the PUC were generated using cif2cell44.
The flatness of FB1 is described by the ratio of the width of the entire FB1 band (W) to that of the flat band () at the top of FB1, that is, . This ratio is an important parameter that represents the strength of the property as a flat band. The value of r is approximately 0.1 for SnNbO and 0.025 for PbSbO. Note that this r value is even lower than the r value of 0.043 for a typical 2D MOF45.
Because the flat band system may cause interesting topological behavior owing to spin-orbit interaction (SOI)6, we also performed a band calculation with SOI (see Supplemental Material for details of the calculation, which includes Refs.40–42). Figure 1d presents the energy dispersion of PbSbO near including SOI. Without SOI, the quasi-flat band touches the dispersive band at the point46,47. In PbSbO, the state at this point is specified by the threefold (sixfold if spin is included) degenerated irreducible representation without SOI. When SOI is included, this state splits into twofold and fourfold states, as shown in Fig. 1d, for PbSbO.
Because this energy splitting is sufficiently small with respect to the bandwidth of FB1 (= W), we can apply the Guo and Franz model, which describes a 3D topological insulator on a pyrochlore lattice47. The spin-orbit coupling parameter is described as , where denotes the energy splitting at the point. In PbSbO, eV is positive, which follows a general trend for ABO (A = Tl, Pb; B = Nb, Ta)22,48.
The high DOS in (= high ) destabilizes the system and induces various phase transitions; the most typical is the phase transition to ferromagnetism. In particular, previous studies have rigorously proved that slight on-site Coulomb repulsion U changes the ferromagnetic state to the ground state in an ideal flat band system1,4. Even in a system with quasi-flat bands (owing to electron hopping other than to the nearest neighbor site), a numerical study has demonstrated that a ferromagnetic ground state is realized under a sufficiently large U49. The criterion for U to separate the ferromagnetic and paramagnetic ground states is , where represents the width of the quasi-flat band. Due to the expanded nature of Wannier orbitals, quantitatively estimating U on “Pb-s” Wannier orbitals is not an easy task50. However, in this case, it is plausible to expect U to be larger than eV. We also obtain the exact result wherein the ferromagnetic ground state is stable to small flat band bending51.
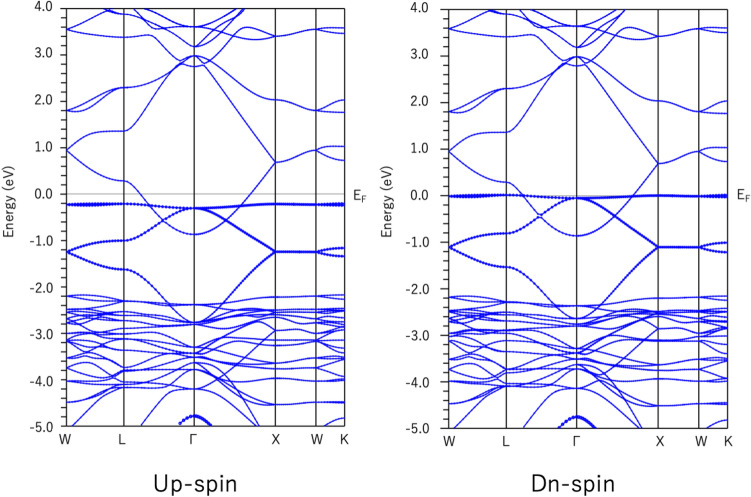
Because pyrochlore PbSbO is already metallic, carriers need not be doped to achieve a ferromagnetic state. Further, spin-polarized first-principles calculations were performed. The band structures of the spin-up and spin-down states are shown in Fig. 3. An exchange splitting of approximately 0.2 eV occurs between the up-spin and down-spin bands. FB1 is completely occupied by the up-spin band but partially occupied by the down-spin band. That is, is pinned at the topmost band of FB1 for the down-spin band. The total magnetic moment is 0.307 per PbSbO. The ground state is not half metallic, unlike doped SnNbO, because FB2 is nearly equally occupied with up- and down-spins.
Figure 3.
Energy dispersion of ferromagnetic PbSbO for up-spin and down-spin.
Next, we discuss the phase stability of pyrochlore PbSbO. As presented above, a large value may induce instability. We have already demonstrated that a ferromagnetic transition lowers the total energy; however, structural phase transitions may also occur. Furthermore, a previous report has suggested that the pyrochlore phase may be unstable36. Therefore, we also computed the electronic structure of weberite PbSbO and compared the total energies of the pyrochlore phase (P-phase) and weberite phase (W-phase). At this time, the calculation is performed ensuring that the calculation conditions are almost identical. Consequently, the W-phase is more stable by approximately 0.549 eV per PbSbO. Because the ferromagnetic stabilization energy in the P-phase is approximately 0.014 eV, we can conclude that the W-phase is more stable than the P-phase under normal conditions. The W-phase is an insulator with a bandgap of eV. This result is consistent with the experimental observation that Naples yellow is yellow in color. For the W-phase, we did not find any feature of a flat band in the energy dispersion (see Supplemental Material for details of the calculation, which includes Refs.40–42).
Another report suggested that PbSbO adopts a pyrochlore structure but is rhombohedrally distorted35. In this regard, the band structure of TlNbO with a similar small strain is computed52; however, the flat band itself is preserved only by changing the fine structure. Therefore, PbSbO may retain the flat band, even if a small distortion is applied.
To confirm this point, we performed a first-principles calculation with an imposed rhombohedral distortion. Details of the calculations are described in Supplemental Material for details of the calculation, which includes Refs.40–42; however, note that we relaxed both crystal lattice and internal atomic positions. We concluded that the loss of symmetry displaced the oxygen atoms considerably, and the electronic state changed accordingly. Figure 4 shows the DOS and band dispersion at the optimized atomic positions. The DOS peak near , which was singular in the cubic crystal, is split into two. The band dispersion is also partially split for the FB. Despite the flat band splitting, we found that was sufficiently high to accept ferromagnetic transition. The spin-polarized calculation showed that the total magnetic moment was 0.37 per PbSbO, which is higher than that in the cubic phase. This result shows the robustness of the flat band against the rhombohedral distortion.
Figure 4.
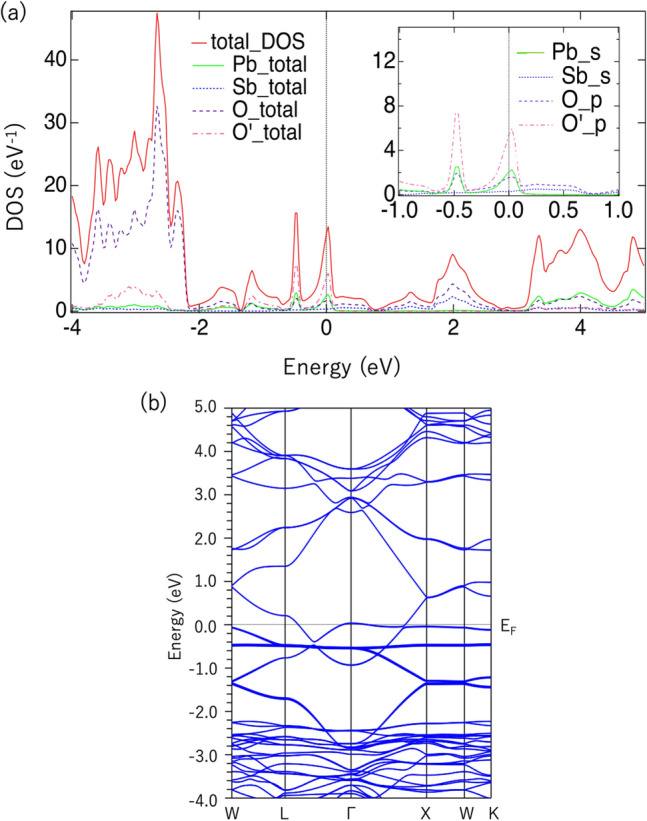
(a) DOS and (b) energy dispersion of PbSbO with rhombohedral distortion.
Rhombohedral distortion may lead to another interesting phenomenon: charge disproportionation due to splitting in two of the single Sb sites in the cubic crystal of the rhombohedral crystal. For example, in BaSbO, the valence of Sb is Sb in the high symmetry phase, but when the symmetry decreases, it splits into two types: Sb and Sb. In BaSbO-based superconductors, it has been suggested that this charge fluctuation may assist superconductivity53. To verify this, we calculated the density at the first radial mesh point (RTOxx in WIEN2k) of a given atom as an approximation for the electron density within the nucleus54. We obtained the value (Sb2)/(Sb1) = 1.002 for PbSbO. This is much smaller than the ratio of 1.55 for BaSbO and 1.32 for BaBiO53, which means that almost no charge disproportionation occurs in PbSbO. The reason for this is unclear at this stage, but we suspect that the possible Fermi surface nesting in the (111) direction is imperfect in PbSbO. However, in BaSbO, the entire Fermi surface can disappear by folding the Brillouin zone from the Pmm structure to the Fmm structure. Moreover, the multi-band nature of PbSbO may also prevent the gap opening.
It has already been reported that the P-phase is stable in an Na-containing atmosphere, but the composition of the product becomes (Na,Pb)SbO and does not show a flat band34. Therefore, to synthesize P-phase PbSbO, some ingenuity is required. For example, Cai et al. pointed out that in some ABO compounds, P- and W-phases compete, and a high-pressure P-phase exists in the stability field38. Therefore, high-pressure synthesis may be effective in certain cases. For example, CaSbO and CdSbO form the W-phase under ambient pressure, whereas the P-phase can be synthesized by high-pressure synthesis at 60–65 kbar55. Controlling other conditions, such as the atmosphere and temperature, may also be effective in obtaining a metastable P-phase. Regarding the high pressure, we also performed a first-principles calculation with a changing lattice volume for the cubic P-phase. Details of the calculations are described in Supplemental Material for details of the calculation, which includes Refs.40–42. We confirmed that applying pressure decreased the magnetic moment, but this effect was not major. After applying a pressure of 29.4 GPa, a magnetic moment of 0.14 remained. This also demonstrated the robustness of the flat band to the applied pressure.
Finally, there is a possibility that a unique Weyl point appears in P-phase PbSbO. A recent theoretical study has shown that when electron-doped SnNbO becomes ferromagnetic, it has Weyl points owing to spin-orbit coupling56. These Weyl points may also be observed in PbSbO. However, because the signs of the effective spin-orbit coupling are opposite between SnNbO and PbSbO, the Weyl points appear differently48. Moreover, PbSbO does not become a Weyl semimetal because of the presence of a large Fermi surface derived from FB2. Furthermore, a small rhombohedral distortion may cause band crossing, as in TlNbO52, which may change the topological properties. PbSbO will add a new variety to flat band systems57,58.
Conclusions
In summary, we discovered that the top of the valence band of pyrochlore PbSbO contained a quasi-flat band. This anomalous band dispersion originated from the geometric frustration lattice of the 3D PbO network. Unlike other previously proposed flat band compounds, in PbSbO, the carriers are self-doped by another band. In other words, the problem of carrier doping, which is the greatest hurdle for the metallization of flat band systems, is naturally solved. Although the problem of competition with the weberite phase remains, this material is a strong candidate for realizing doped flat band systems and magnetic materials that do not contain magnetic elements. This ferromagnetism is robust with respect to rhombohedral distortion and pressure application. Novel Weyl points attributed to spontaneous magnetization combined with spin-orbit coupling are also anticipated.
Supplementary Information
Acknowledgements
The authors thank A. Iyo and T. Ikenobe for fruitful discussions. This study was partially supported by KAKENHI (Grant No. JP19K03731) of the Japan Society for the Promotion of Science (JSPS).
Author contributions
I.H. carried out the first-principles calculations; I.H. and Y.H. constructed the tight-binding model. I.H., Y.H., and K.K. contributed to analyzing the data. I.H., Y.H., and H.E. wrote the paper. I.H. and Y.H. prepared figures. All authors reviewed the manuscript.
Data availibility
Any data used to generate the results in this study can be obtained from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-31917-w.
References
- 1.Mielke A. Ferromagnetic ground states for the Hubbard model on line graphs. J. Phys. A. 1991;24:L73. doi: 10.1088/0305-4470/24/2/005. [DOI] [Google Scholar]
- 2.Mielke A. Ferromagnetism in the Hubbard model on line graphs and further considerations. J. Phys. A. 1991;24:3311. doi: 10.1088/0305-4470/24/14/018. [DOI] [Google Scholar]
- 3.Mielke A. Exact ground states for the Hubbard model on the Kagome lattice. J. Phys. A. 1992;25:4335. doi: 10.1088/0305-4470/25/16/011. [DOI] [Google Scholar]
- 4.Tasaki H. From Nagaoka’s ferromagnetism to flat-band ferromagnetism and beyond: An introduction to ferromagnetism in the Hubbard model. Prog. Theor. Phys. 1998;99:489. doi: 10.1143/PTP.99.489. [DOI] [Google Scholar]
- 5.Derzhko O, Richter J, Maksymenko M. Strongly correlated flat-band systems: The route from Heisenberg spins to Hubbard electrons. Int. J. Mod. Phys. B. 2015;29:1530007. doi: 10.1142/S0217979215300078. [DOI] [Google Scholar]
- 6.Liu Z, Liu F, Wu Y-S. Exotic electronic states in the world of flat bands: From theory to material. Chin. Phys. B. 2014;23:077308. doi: 10.1088/1674-1056/23/7/077308. [DOI] [Google Scholar]
- 7.Kobayashi K, Okumura M, Yamada S, Machida M, Aoki H. Superconductivity in repulsively interacting fermions on a diamond chain: Flat-band-induced pairing. Phys. Rev. B. 2016;94:214501. doi: 10.1103/PhysRevB.94.214501. [DOI] [Google Scholar]
- 8.Hatsugai Y, Maruyama I. ZQ topological invariants for polyacetylene, Kagome and pyrochlore lattices. Europhys. Lett. 2011;95:20003. doi: 10.1209/0295-5075/95/20003. [DOI] [Google Scholar]
- 9.Kurita M, Yamaji Y, Imada M. Topological insulators from spontaneous symmetry breaking induced by electron correlation on pyrochlore lattices. J. Phys. Soc. Jpn. 2011;80:044708. doi: 10.1143/JPSJ.80.044708. [DOI] [Google Scholar]
- 10.Neupart T, Santos L, Chamon C, Mudry C. Fractional quantum Hall states at zero magnetic field. Phys. Rev. Lett. 2011;106:236804. doi: 10.1103/PhysRevLett.106.236804. [DOI] [PubMed] [Google Scholar]
- 11.Sheng DN, Gu Z-C, Sun K, Sheng L. Fractional quantum Hall effect in the absence of Landau levels. Nat. Commun. 2011;2:389. doi: 10.1038/ncomms1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regnault N, Bernevig BA. Fractional Chern insulator. Phys. Rev. X. 2011;1:021014. doi: 10.1103/PhysRevX.1.021014. [DOI] [PubMed] [Google Scholar]
- 13.Bergholtz EJ, Liu Z, Trescher M, Moessner R, Udagawa M. Topology and interactions in a frustrated slab: Tuning from Weyl semimetals to fractional chern insulators. Phys. Rev. Lett. 2015;114:016806. doi: 10.1103/PhysRevLett.114.016806. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Sethi G, Zhang C, Ni X, Liu F. Giant intrinsic circular dichroism of enantiomorphic flat Chern bands and flatband devices. Phys. Rev. B. 2020;102:125115. doi: 10.1103/PhysRevB.102.125115. [DOI] [Google Scholar]
- 15.Shiraishi K, Tamura H, Takayanagi H. Design of a semiconductor ferromagnet in a quantum-dot artificial crystal. Appl. Phys. Lett. 2001;78:3702. doi: 10.1063/1.1376434. [DOI] [Google Scholar]
- 16.Couny F, Benabid F, Light PS. Large pitch Kagome-structured hollow-core photonic crystal fiber. Opt. Lett. 2006;31:3574. doi: 10.1364/OL.31.003574. [DOI] [PubMed] [Google Scholar]
- 17.Mohan P, Nakajima F, Akabori M, Motohisa J, Fukui T. Fabrication of semiconductor Kagome lattice structure by selective area metalorganic vapor phase epitaxy. Appl. Phys. Lett. 2003;83:689. doi: 10.1063/1.1593823. [DOI] [Google Scholar]
- 18.Ruostekoski J. Optical Kagome lattice for ultracold atoms with nearest neighbor interactions. Phys. Rev. Lett. 2009;103:080406. doi: 10.1103/PhysRevLett.103.080406. [DOI] [PubMed] [Google Scholar]
- 19.Yamada MG, Soejima T, Tsuji N, Hirai D, Dincă M, Aoki H. First-principles design of a half-filled flat band of the Kagome lattice in two-dimensional metal–organic frameworks. Phys. Rev. B. 2016;94:081102R. doi: 10.1103/PhysRevB.94.081102. [DOI] [Google Scholar]
- 20.Lisi S, Lu X, Benschop T, et al. Observation of flat bands in twisted bilayer graphene. Nat. Phys. 2021;17:189. doi: 10.1038/s41567-020-01041-x. [DOI] [Google Scholar]
- 21.Hase I, Yanagisawa T, Aiura Y, Kawashima K. Possibility of flat-band ferromagnetism in hole-doped pyrochlore oxides Sn2Nb2O7 and Sn2Ta2O7. Phys. Rev. Lett. 2018;120:196401. doi: 10.1103/PhysRevLett.120.196401. [DOI] [PubMed] [Google Scholar]
- 22.Hase I, Yanagisawa T. Possible three-dimensional topological insulator in pyrochlore oxides. Symmetry. 2020;12:1076. doi: 10.3390/sym12071076. [DOI] [Google Scholar]
- 23.Ye XB, Tuo P, Pan BC. Flatband in a three-dimensional tungsten nitride compound. J. Chem. Phys. 2020;152:224503. doi: 10.1063/5.0008739. [DOI] [PubMed] [Google Scholar]
- 24.Canals B, Lacroix C. Pyrochlore antiferromagnet: A three-dimensional quantum spin liquid. Phys. Rev. Lett. 1998;80:2933. doi: 10.1103/PhysRevLett.80.2933. [DOI] [Google Scholar]
- 25.Ramirez AP, Hayashi A, Cava RJ, Siddharthan R, Shastry BS. Zero-point entropy in ‘spin ice’. Nature. 1999;399:333. doi: 10.1038/20619. [DOI] [Google Scholar]
- 26.Castelnovo C, Moessner R, Sondhi SL. Magnetic monopoles in spin ice. Nature. 2008;451:42. doi: 10.1038/nature06433. [DOI] [PubMed] [Google Scholar]
- 27.Kadowaki H, Doi N, Aoki Y, Tabata Y, Sato TJ, Lynn JW, Matsuhira K, Hiroi Z. Observation of magnetic monopoles in spin ice. J. Phys. Soc. Jpn. 2009;78:103706. doi: 10.1143/JPSJ.78.103706. [DOI] [Google Scholar]
- 28.Matsuhira K, Paulsen C, Lhotel E, Sekine C, Hiroi Z, Takagi S. Spin dynamics at very low temperature in spin ice DyTiO. J. Phys. Soc. Jpn. 2011;80:123711. doi: 10.1143/JPSJ.80.123711. [DOI] [Google Scholar]
- 29.Shores MP, Nytko EA, Bartlett BM, Nocera DG. A structurally perfect S = 1/2 Kagomé antiferromagnet. J. Am. Chem. Soc. 2005;127:13462. doi: 10.1021/ja053891p. [DOI] [PubMed] [Google Scholar]
- 30.Lee PA. An end to the drought of quantum spin liquids. Science. 2008;321:1306. doi: 10.1126/science.1163196. [DOI] [PubMed] [Google Scholar]
- 31.Mendels P, Bert F. Quantum Kagome antiferromagnet ZnCu(OH)Cl. J. Phys. Soc. Jpn. 2010;79:011001. doi: 10.1143/JPSJ.79.011001. [DOI] [Google Scholar]
- 32.Han T-H, Helton JS, Chu S, Nocera DG, Rodriguez-Rivera JA, Broholm C, Lee YS. Fractionalized excitations in the spin-liquid state of a Kagome-lattice antiferromagnet. Nature. 2012;492:406. doi: 10.1038/nature11659. [DOI] [PubMed] [Google Scholar]
- 33.Fujihala M, Morita K, Mole R, et al. Gapless spin liquid in a square-Kagome lattice antiferromagnet. Nat. Commun. 2020;11:3429. doi: 10.1038/s41467-020-17235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchetti A, Saniz R, Krishnan D, Rabbachin L, Nuyts G, De Meyer S, Verbeeck J, Janssens K, Pelosi C, Lamoen D, Partoens B, De Wael K. Unraveling the role of lattice substitutions on the stabilization of the intrinsically unstable PbSbO pyrochlore: Explaining the lightfastness of lead pyroantimonate artists’ pigments. Chem. Mater. 2020;32:2863. doi: 10.1021/acs.chemmater.9b04821. [DOI] [Google Scholar]
- 35.Brisse F, Stewart DJ, Seidl V, Knop O. Pyrochlores. VIII. Studies of some 2–5 pyrochlores and related compounds and minerals. Can. J. Chem. 1972;50:3648. doi: 10.1139/v72-580. [DOI] [Google Scholar]
- 36.Burchard G, Rüdorff W. Zur Frage nach der Existenz eines kubischen pyrochlors PbSbO. Z. Anorg. Allg. Chem. 1978;447:149. doi: 10.1002/zaac.19784470116. [DOI] [Google Scholar]
- 37.Ivanov SA, Zavodnik VE. Crystal structure of lead antimonate PbSbO. Sov. Phys. Crystallogr. 1990;35:494. [Google Scholar]
- 38.Cai L, Nino JC. Complex ceramic structures. I. Weberites. Acta Cryst. B. 2009;65:269. doi: 10.1107/S0108768109011355. [DOI] [PubMed] [Google Scholar]
- 39.Regnault N, Xu Y, Li M-R, Ma D-S, Jovanovic M, Yazdani A, Parkin SSP, Felser C, Schoop LM, Ong NP, Cava RJ, Elcoro L, Song Z-D, Bernevig BA. Catalogue of flat-band stoichiometric materials. Nature. 2022;603:824. doi: 10.1038/s41586-022-04519-1. [DOI] [PubMed] [Google Scholar]
- 40.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 41.Blaha P, Schwarz K, Madsen GKH, Kvashnicka D, Luitz J. WIEN2k, an Augmented Plane Wave Plus Local Orbitals Program for Calculating Crystal Properties. Vienna University of Technology; 2001. [Google Scholar]
- 42.Mostofi AA, Yates JR, Lee Y-S, Souza I, Vanderbilt D, Marzari N. Wannier90: A tool for obtaining maximally localised Wannier functions. Comput. Phys. Commun. 2008;178:685. doi: 10.1016/j.cpc.2007.11.016. [DOI] [Google Scholar]
- 43.Momma K, Izumi F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011;44:1272. doi: 10.1107/S0021889811038970. [DOI] [Google Scholar]
- 44.Björkman T. CIF2Cell: Generating geometries for electronic structure programs. Comput. Phys. Commun. 2011;182:1183. doi: 10.1016/j.cpc.2011.01.013. [DOI] [Google Scholar]
- 45.Liu Z, Wang Z-F, Mei J-W, Wu Y-S, Liu F. Flat Chern band in a two-dimensional organometallic framework. Phys. Rev. Lett. 2013;110:106804. doi: 10.1103/PhysRevLett.110.106804. [DOI] [PubMed] [Google Scholar]
- 46.Bergman DL, Wu C, Balents L. Band touching from real-space topology in frustrated hopping models. Phys. Rev. B. 2008;78:125104. doi: 10.1103/PhysRevB.78.125104. [DOI] [Google Scholar]
- 47.Guo H-M, Franz M. Three-dimensional topological insulators on the pyrochlore lattice. Phys. Rev. Lett. 2009;103:206805. doi: 10.1103/PhysRevLett.103.206805. [DOI] [PubMed] [Google Scholar]
- 48.Hase I, Higashi Y, Yanagisawa T. Quasi-flat-band in s1/s2 pyrochlore oxides and the effect of spin–orbit interaction. J. Phys. Conf. Ser. 2022;2164:012063. doi: 10.1088/1742-6596/2164/1/012063. [DOI] [Google Scholar]
- 49.Kusakabe K, Aoki H. Robustness of the ferromagnetism in flat bands. Physica B. 1994;194–196:215. doi: 10.1016/0921-4526(94)90437-5. [DOI] [Google Scholar]
- 50.Rösner M, Şaşıoğlu E, Friedrich C, Blügel S, Wehling TO. Wannier function approach to realistic Coulomb interactions in layered materials and heterostructures. Phys. Rev. B. 2015;92:085102. doi: 10.1103/PhysRevB.92.085102. [DOI] [Google Scholar]
- 51.Tanaka A, Ueda H. Stability of ferromagnetism in the Hubbard model on the Kagome lattice. Phys. Rev. Lett. 2003;90:067204. doi: 10.1103/PhysRevLett.90.067204. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Luo K, Chen Z, Zhu Z, Yu R, Fang C, Weng H. Topological phases in pyrochlore thallium niobate TlNbO. npj Comput. Mater. 2019;5:105. doi: 10.1038/s41524-019-0245-5. [DOI] [Google Scholar]
- 53.Kim M, Klenner S, McNally GM, Nuss J, Yaresko A, Wedig U, Kremer RK, Pöttgen R, Takagi H. Mixed valence and superconductivity in perovskite antimonates. Chem. Mater. 2021;33:6787. doi: 10.1021/acs.chemmater.1c01362. [DOI] [Google Scholar]
- 54.Spiel C, Blaha P, Schwarz K. Density functional calculations on the charge-ordered and valence-mixed modification of YBaFeO. Phys. Rev. B. 2009;79:115123. doi: 10.1103/PhysRevB.79.115123. [DOI] [Google Scholar]
- 55.Knop O, Demazeau G, Hagenmuller P. Pyrochlores. XI. High-pressure studies of the antimonates ASbO (A = Ca, Sr, Cd) and preparation of the weberite SrBiO. Can. J. Chem. 1980;58:2221. doi: 10.1139/v80-357. [DOI] [Google Scholar]
- 56.Zhou Y, Jin K-H, Huang H, Wang Z, Liu F. Weyl points created by a three-dimensional flat band. Phys. Rev. B. 2019;99:201105R. doi: 10.1103/PhysRevB.99.201105. [DOI] [Google Scholar]
- 57.She J-H, Kim CH, Fennie CJ, Lawler MJ, Kim E-A. Topological superconductivity in metal/quantum-spin-ice heterostructures. npj Quant. Mater. 2017;2:64. doi: 10.1038/s41535-017-0063-2. [DOI] [Google Scholar]
- 58.Armitage NP, Mele EJ, Vishwanath A. Weyl and Dirac semimetals in three-dimensional solids. Rev. Mod. Phys. 2017;90:015001. doi: 10.1103/RevModPhys.90.015001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data used to generate the results in this study can be obtained from the corresponding author upon reasonable request.