Abstract
BACKGROUND
The increased detection of small-sized peripheral non–small-cell lung cancer (NSCLC) has renewed interest in sublobar resection in lieu of lobectomy.
METHODS
We conducted a multicenter, noninferiority, phase 3 trial in which patients with NSCLC clinically staged as T1aN0 (tumor size, ≤2 cm) were randomly assigned to undergo sublobar resection or lobar resection after intraoperative confirmation of node-negative disease. The primary end point was disease-free survival, defined as the time between randomization and disease recurrence or death from any cause. Secondary end points were overall survival, locoregional and systemic recurrence, and pulmonary functions.
RESULTS
From June 2007 through March 2017, a total of 697 patients were assigned to undergo sublobar resection (340 patients) or lobar resection (357 patients). After a median follow-up of 7 years, sublobar resection was noninferior to lobar resection for disease-free survival (hazard ratio for disease recurrence or death, 1.01; 90% confidence interval [CI], 0.83 to 1.24). In addition, overall survival after sublobar resection was similar to that after lobar resection (hazard ratio for death, 0.95; 95% CI, 0.72 to 1.26). The 5-year disease-free survival was 63.6% (95% CI, 57.9 to 68.8) after sublobar resection and 64.1% (95% CI, 58.5 to 69.0) after lobar resection. The 5-year overall survival was 80.3% (95% CI, 75.5 to 84.3) after sublobar resection and 78.9% (95% CI, 74.1 to 82.9) after lobar resection. No substantial difference was seen between the two groups in the incidence of locoregional or distant recurrence. At 6 months postoperatively, a between-group difference of 2 percentage points was measured in the median percentage of predicted forced expiratory volume in 1 second, favoring the sublobar-resection group.
CONCLUSIONS
In patients with peripheral NSCLC with a tumor size of 2 cm or less and pathologically confirmed node-negative disease in the hilar and mediastinal lymph nodes, sublobar resection was not inferior to lobectomy with respect to disease-free survival. Overall survival was similar with the two procedures. (Funded by the National Cancer Institute and others; CALGB 140503 ClinicalTrials.gov number, NCT00499330.)
IN 1995, THE LUNG CANCER STUDY GROUP reported the results of a randomized trial comparing lobectomy with sublobar resection in patients with clinical T1N0 non–small-cell lung cancer (NSCLC).1 The frequency of local recurrence was three times as high with sublobar resection as with lobectomy, and lung cancer–related mortality was 50% higher with sublobar resection. These results established lobectomy as the standard of surgical care for patients with clinical T1N0 NSCLC. In the decades since, advances in imaging and staging methods have allowed the detection of smaller and earlier tumors, a situation that has rekindled interest in sublobar resection for patients with clinical stage IA NSCLC who might otherwise be candidates for lobectomy.2–5 Japanese investigators recently reported the results of a large, randomized trial (JCOG0802) comparing lobectomy with anatomical segmentectomy in patients with clinical stage IA NSCLC with a tumor size of 2 cm or less.6 After a median follow-up of approximately 7 years, anatomical segmentectomy was superior to lobectomy for overall survival (primary end point) and noninferior to lobectomy for relapse-free survival. Here, we report the results of a randomized international trial comparing sublobar resection (wedge resection or segmentectomy) with lobectomy in patients with clinical stage IA NSCLC with a tumor size of 2 cm or less.
METHODS
TRIAL DESIGN AND PATIENTS
Cancer and Leukemia Group B (CALGB) 140503 was a multicenter, international, randomized, noninferiority, phase 3 trial involving patients with NSCLC clinically staged as T1aN0. CALGB is now part of the Alliance for Clinical Trials in Oncology (hereafter referred to as the Alliance). Clinical staging was based on the seventh edition of the tumor–node–metastasis staging system. Patients were recruited from 83 academic and community-based institutions in the United States, Canada, and Australia. Patients were registered to the trial if they had met the preoperative eligibility criteria, and they underwent randomization after meeting the intraoperative eligibility criteria. Preoperative eligibility criteria included the presence of a peripheral lung nodule with a solid component measuring 2 cm or less on preoperative computed tomography (CT) that was presumed or confirmed to be NSCLC; a center of the tumor, as seen on CT, that was located in the outer third of the lung and a tumor location that was suitable for either sublobar resection (wedge or segment) or lobar resection; an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0, 1, or 2 (on a 5-point scale in which higher numbers indicate greater disability); no malignant disease within the past 3 years other than nonmelanoma skin cancer, superficial bladder cancer, or carcinoma in situ of the cervix; no previous chemotherapy or radiation therapy for the index lung cancer; no evidence of locally advanced or metastatic disease; and an age of 18 years or older. Patients with pure ground-glass opacities or pathologically confirmed N1 or N2 disease were not eligible.
Intraoperative eligibility criteria included histologic confirmation of NSCLC (if not already obtained) and confirmation of N0 status by means of frozen-section examination (for tumors on the right side, node levels 4, 7, and 10; for tumors on the left side, node levels 5 or 6, 7, and 10). Nodes that were previously sampled by means of mediastinoscopy, endobronchial ultrasonography, or endoscopic ultrasonography within 6 weeks before the definitive surgical procedure did not need to be resampled.
TRIAL OVERSIGHT
The trial was conducted according to the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. The protocol was approved by the CALGB/Alliance central institutional review board and the institutional review board at each participating institution and is available with full text of this article at NEJM.org. All the patients provided written informed consent before trial enrollment. Since activation of CALGB/Alliance 140503, the trial has been monitored by the Alliance data and safety monitoring board twice a year.
The first two authors developed the trial design, had full access to the raw data, and analyzed the data. The first author wrote the first draft of the manuscript. All the authors had the opportunity to revise the manuscript and vouch for the completeness and accuracy of the data and for the adherence of the trial to the protocol. The primary funder (National Cancer Institute) approved the trial design but had no role in the collection, interpretation, or analysis of the data or in the writing of the manuscript. There were no agreements concerning confidentiality of the data between the primary funder and the authors or the participating institutions.
RANDOMIZATION AND PROCEDURES
Eligible patients were preregistered to the trial with the use of the Oncology Patient Enrollment Network registration system, a Web-based system for patients’ enrollment into National Cancer Institute–sponsored cooperative group clinical trials. Once intraoperative eligibility (as described above) was confirmed, patients underwent randomization (in a 1:1 ratio) to either sublobar resection or lobar resection on the basis of a permuted-block randomization scheme with stratification according to radiographic tumor size (<1 cm, 1 to 1.5 cm or >1.5 to 2.0 cm), histologic type (squamous-cell carcinoma, adenocarcinoma, or other), and smoking status (never, former, or current). Trial-group assignments were not concealed to patients, surgeons, nurses, data managers, or statisticians. The type of sublobar resection (wedge resection or segmentectomy) and the choice of surgical approach (thoracotomy vs. video- or robotic-assisted thoracoscopic surgery) was at the surgeon’s discretion.
END POINTS
The primary end point was disease-free survival, defined as the time between randomization and disease recurrence or death from any cause, whichever occurred first. The primary objective was to determine whether sublobar resection (segmentectomy or wedge resection) is noninferior to lobectomy with respect to disease-free survival among patients with small NSCLC (tumor size, ≤2 cm) exclusive of second primary lung cancer.
Secondary end points were overall survival, locoregional and systemic recurrence, and expiratory flow rates 6 months postoperatively. Overall survival was defined as the time between randomization and death from any cause. Locoregional recurrence was defined as recurrent disease in the lung or the hilar nodes of the index lobe. Regional recurrence was defined as isolated mediastinal nodal recurrence. All other recurrence was deemed to be systemic.
STATISTICAL ANALYSIS
The trial was designed to have approximately 80% power with 351 events of disease recurrence or death to reject the null hypothesis that the hazard ratio after sublobar resection as compared with after lobectomy is less than 1.306 by stratified log-rank test for noninferiority at a one-sided significance level of 5% when the true hazard ratio is 1. With a prespecified noninferiority margin of 1.306, there is a 5% chance that the null hypothesis will be rejected when the hazard ratio after sublobar resection is 30.6% higher than after lobectomy. A justification of the noninferiority margin is provided in the Supplementary Appendix, available at NEJM.org. Interim analyses with early stopping boundaries were planned for noninferiority (i.e., early evidence that sublobar resection is not inferior to lobectomy) and futility (i.e., low probability of showing that sublobar resection is not inferior to lobectomy at the planned final analysis). The critical values of early stopping for noninferiority were calculated on the basis of a Lan–DeMets alpha-spending function for O’Brien–Fleming–like boundaries.7,8 The trial enrolled 697 patients from June 2007 through March 2017. On the basis of interim analyses conducted up to November 2021 and a validation analysis in March 2022, the Alliance data and safety monitoring board recommended unanimously to release the data and terminate further monitoring of the trial by the data and safety monitoring board, noting that there was minimal chance that the trial may yield a different conclusion at the planned final analysis.
The primary analysis of efficacy end points was based on the intention-to-treat population, which included all the patients who had undergone randomization according to their randomly assigned treatments. In the analysis of disease-free survival, data for patients who were alive without disease recurrence were censored at the time of the last follow-up. In the analysis of overall survival, data for patients who were alive were censored at the time of the last follow-up. Survival end points were characterized with the use of the Kaplan–Meier estimator. The P value for testing the noninferiority of sublobar resection to lobar resection for disease-free survival was obtained from a stratified log-rank test with tumor size, histologic type, and smoking status as stratification factors. Hazard ratios and their confidence intervals were estimated with the use of stratified Cox proportional-hazards models. Violation of the proportional-hazards assumption was evaluated by the method of Schoenfeld residuals. We calculated 90% confidence intervals for disease-free survival and its derived variables so that they are consistent with the one-sided significance level of 5% used for the primary noninferiority test of sublobar resection as compared with lobectomy.
After randomization and on review of source documents, 27 patients were deemed to have not met all intraoperative eligibility criteria (15 assigned to sublobar resection and 12 assigned to lobar resection). In addition, 5 patients were converted from their assigned lobar resection to sublobar resection and 10 from their assigned sublobar resection to lobar resection. Therefore, in addition to the intention-to-treat analysis, we conducted a sensitivity per-protocol analysis based only on patients who met all intraoperative eligibility and who had undergone their assigned surgical procedure. A post hoc analysis on the heterogeneity of treatment effects for disease-free and overall survival across patient subgroups, including race, sex, age group, ECOG performance-status score, tumor location, tumor size, histologic type, and smoking status, was summarized with forest plots, and the hazard ratios and confidence intervals therein were estimated from unstratified Cox proportional-hazards models fitted to the subgroups. To examine consistency of treatment effect across trial sites, we classified sites on the basis of total enrollment into high-enrolling sites (>30 patients), medium-enrolling sites (10 to 30 patients), and low-enrolling sites (<10 patients). We obtained site-adjusted hazard ratios and confidence intervals with the use of a Cox proportional-hazards mixed-effects model, with trial sites as a random effect. In another post hoc analysis, we explored the treatment effect on recurrence-free survival (for which all deaths were censored) and on lung cancer–related death as compared with other causes of death, with cumulative incidence functions estimated with the use of the Gray method9 and the associated hazard ratios and confidence intervals estimated by means of the Fine–Gray subdistribution hazard model.10
The incidences of disease recurrence were summarized according to treatment group, and the confidence intervals of the differences in incidences were estimated with the use of the Miettinen and Nurminen method.11 The changes in pulmonary functions (forced expiratory volume in 1 second [FEV1] and forced vital capacity [FVC]) between baseline and 6 months postoperatively were summarized according to treatment group, and the confidence intervals of median difference were estimated with the use of the bootstrap bias-corrected and accelerated method with 2000 bootstrapped samples.12 Other than the confidence interval of the primary end point, all reported confidence intervals were computed at a 95% confidence level. The widths of confidence intervals were not adjusted for multiple testing and may not be used in place of hypothesis testing. Short-term morbidity and mortality for this trial had been reported previously.13
Data quality was ensured by review of data by the Alliance Statistical and Data Management Center (SDMC) and the trial chairperson (first author), in accordance with Alliance policies. The analyses of the efficacy end points, including disease-free and overall survival, have been independently validated by an Alliance SDMC statistician, who is not associated with the trial. All statistical analyses were conducted by the trial statisticians and statistical programmers with the data locked on June 21, 2022. Data management and statistical analysis were performed with SAS software, version 9.4, and graphs were generated in R software, version 3.6.3.
RESULTS
PATIENTS
Between June 15, 2007, and March 13, 2017, a total of 1080 patients with suspected or confirmed T1aN0 NSCLC were preregistered to the trial by 125 surgeons at 83 participating institutions. A total of 697 patients (64.5%) met preoperative and intraoperative eligibility criteria and were randomly assigned to undergo either sublobar resection (340 patients) or lobar resection (357 patients) (Fig. S1 in the Supplementary Appendix). Of the 340 patients assigned to sublobar resection, 201 (59.1%) underwent wedge resection and 129 (37.9%) underwent an anatomical segmental resection. In a previously reported subgroup analysis, failure to proceed with intraoperative randomization was attributable to undiagnosed benign disease (50.0%), a higher stage of NSCLC that was discovered at the time of surgery (22.6%), or malignant disease other than NSCLC (7.7%).14 The demographic and clinical characteristics of the randomly assigned patients at baseline are shown in Table 1.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Sublobar Resection (N = 340) | Lobar Resection (N = 357) | Total (N = 697) |
|---|---|---|---|
|
| |||
| Age — yr | |||
| MediaN | 68.3 | 67.6 | 67.9 |
| RaNge | 37.8–89.7 | 43.2–88.9 | 37.8–89.7 |
| Race — no. (%)† | |||
| White | 314 (92.4) | 313 (87.7) | 627 (90.0) |
| Black | 16 (4.7) | 29 (8.1) | 45 (6.5) |
| AsiaN | 2 (0.6) | 4 (11) | 6 (0.9) |
| Other | 8 (2.4) | 11 (31) | 19 (2.7) |
| Sex — no. (%) | |||
| Male | 150 (44.1) | 147 (41.2) | 297 (42.6) |
| Female | 190 (55.9) | 210 (58.8) | 400 (57.4) |
| ECOG performaNce-status score — no. (%)‡ | |||
| 0 | 263 (77.4) | 250 (70.0) | 513 (73.6) |
| 1 | 72 (21.2) | 102 (28.6) | 174 (25.0) |
| 2 | 5 (1.5) | 5 (14) | 10 (1.4) |
| SmokiNg status — no. (%) | |||
| Never | 28 (8.2) | 35 (9.8) | 63 (9.0) |
| Former | 172 (50.6) | 177 (49.6) | 349 (50.1) |
| CurreNt | 140 (41.2) | 145 (40.6) | 285 (40.9) |
| Tumor size — no. (%) | |||
| <1.0 cm | 28 (8.2) | 30 (8.4) | 58 (8.3) |
| 1.0—1.5 cm | 174 (51.2) | 180 (50.4) | 354 (50.8) |
| >1.5–2.0 cm | 138 (40.6) | 147 (41.2) | 285 (40.9) |
| Histologic type — no. (%) | |||
| AdeNocarciNoma | 218 (64.1) | 226 (63.3) | 444 (63.7) |
| Squamous-cell carciNoma | 45 (13.2) | 53 (14.8) | 98 (14.1) |
| Other | 77 (22.6) | 78 (21.8) | 155 (22.2) |
Percentages may not total 100 because of rounding.
Race was reported by the patient.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
SURVIVAL
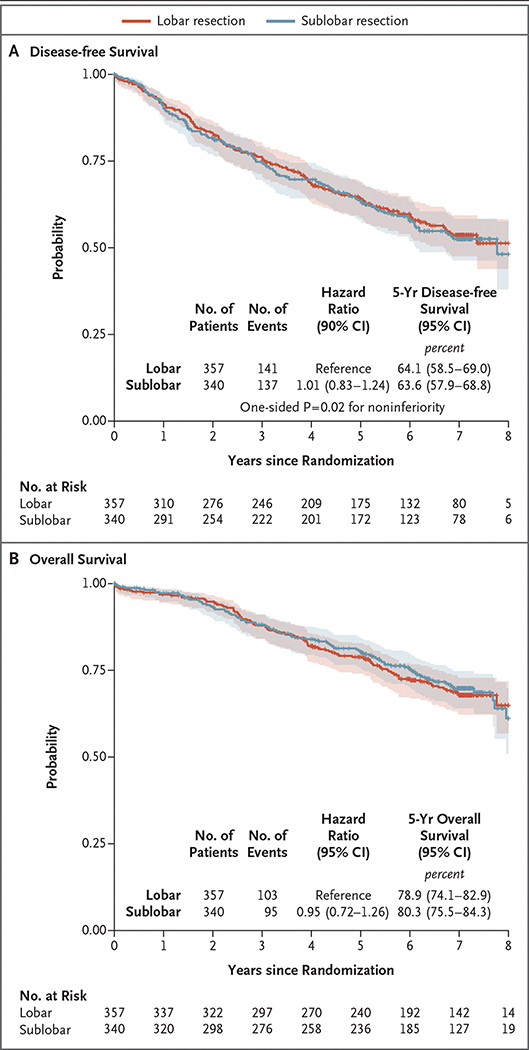
After a median follow-up of 7 years, sublobar resection was not inferior to lobectomy for disease-free survival (hazard ratio for disease recurrence or death, 1.01; 90% confidence interval [CI], 0.83 to 1.24). The 5-year disease-free survival was 63.6% (95% CI, 57.9 to 68.8) after sublobar resection and 64.1% (95% CI, 58.5 to 69.0) after lobar resection (Fig. 1A). The treatment effect was similar across trial sites (Table S2), with a hazard ratio of 0.99 (90% CI, 0.80 to 1.21) after adjustment for trial sites as a random effect. In a post hoc exploratory analysis, results were generally consistent between the overall analysis and subgroup analyses defined by key demographic and clinical variables, including age group, sex, tumor location, histologic type, smoking history, tumor size, and ECOG performance-status score (Fig. 2). Overall survival (key secondary end point) was similar in the sublobar-resection group and the lobar-resection group (hazard ratio for death, 0.95; 95% CI, 0.72 to 1.26). The 5-year overall survival was 80.3% (95% CI, 75.5 to 84.3) after sublobar resection and 78.9% (95% CI, 74.1 to 82.9) after lobectomy (Fig. 1B). The per-protocol sensitivity analysis yielded similar findings to the intention-to-treat analysis for both disease-free and overall survival (Fig. S2). The post hoc subgroup analysis showed no substantial between-group difference in overall survival across all key demographic and clinical variables (Fig. S3).
Figure 1. Disease-free and Overall Survival.
The shaded areas indicate 95% confidence intervals.
Figure 2. Exploratory Subgroup Analysis of Disease-free Survival.
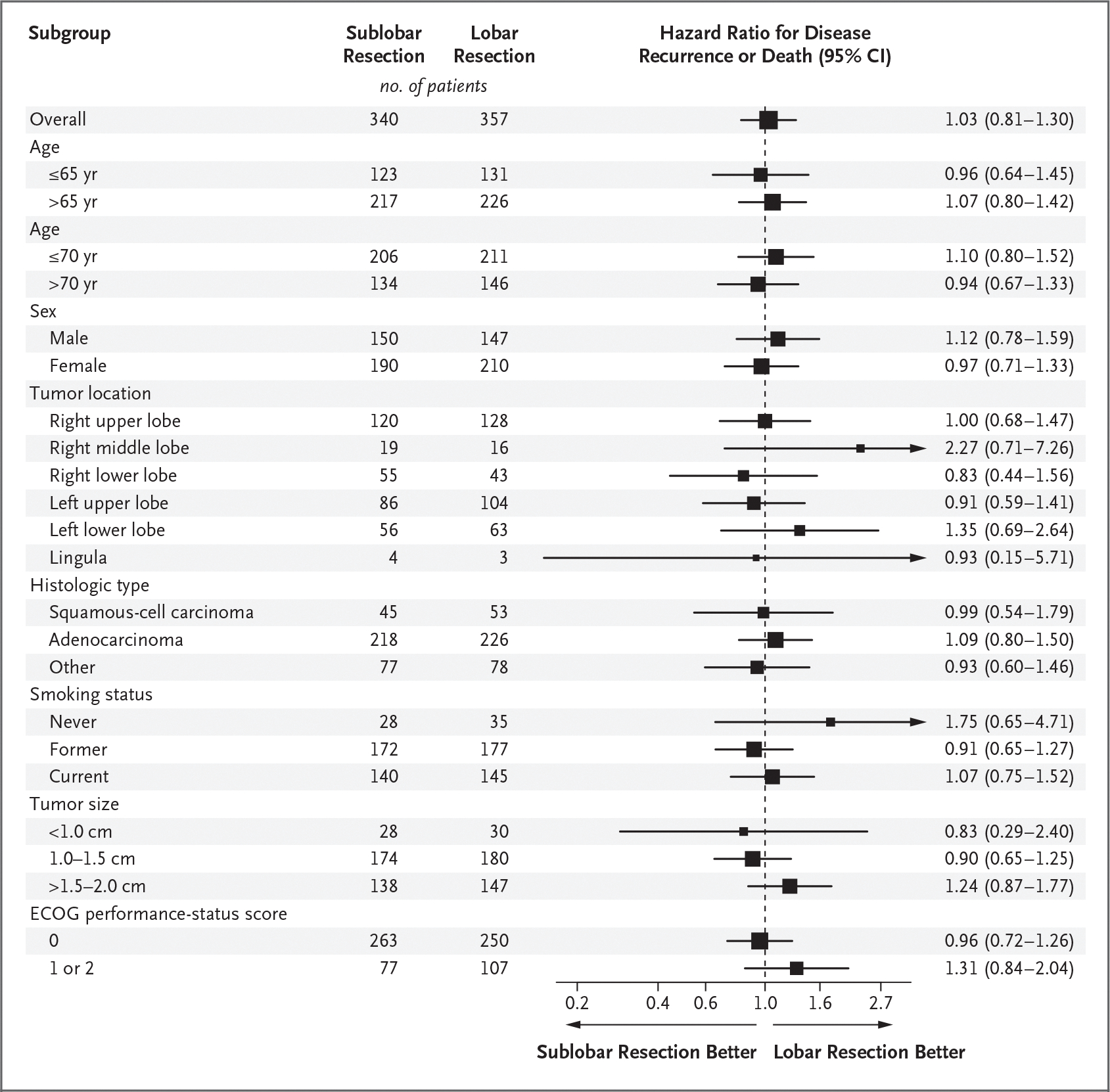
Hazard ratios and 95% confidence intervals were estimated with the use of unstratified Cox proportional-hazards models. The size of the squares indicating the hazard ratios is proportional to the number of patients included in the analysis. Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
RECURRENCE
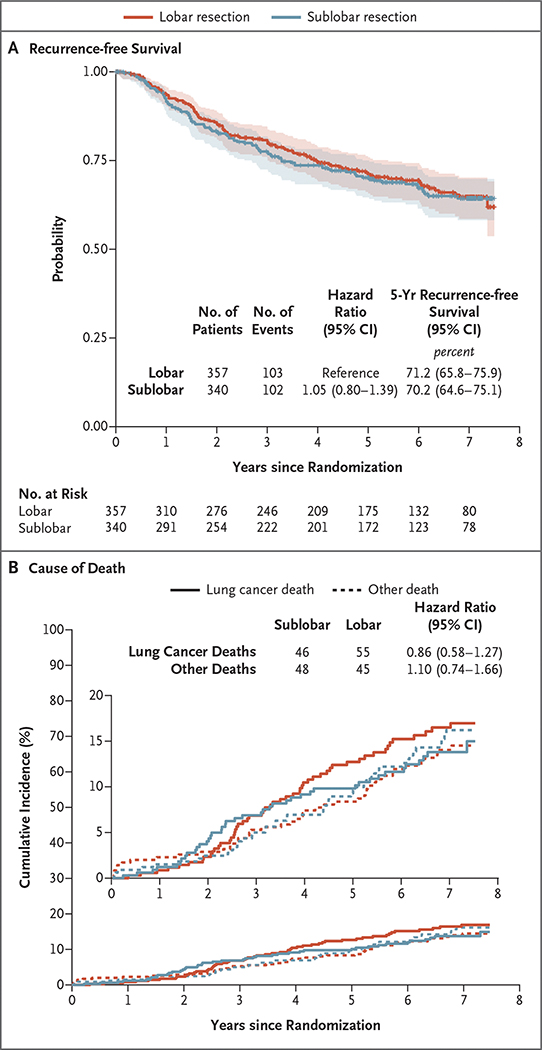
After the exclusion of 10 patients (4 in the sublobar-resection group and 6 in the lobar-resection group) who had died of treatment-related events within 90 days after their surgical procedure, 687 patients were available for assessment of disease recurrence (336 in the sublobar-resection group and 351 in the lobar-resection group). Disease recurrence developed in 102 patients (30.4%) after sublobar resection and 103 (29.3%) after lobectomy (Table 2). Locoregional recurrence occurred in 45 patients (13.4%) after sublobar resection and 35 (10.0%) after lobectomy. More than 50% of the recurrences in each group were systemic in nature. In a post hoc exploratory analysis, recurrence-free survival was similar in the sublobar-resection group and the lobar-resection group (hazard ratio for disease recurrence, 1.05; 95% CI, 0.80 to 1.39) (Fig. 3A). The 5-year recurrence-free survival was 70.2% (95% CI, 64.6 to 75.1) after sublobar resection and 71.2% (95% CI, 65.8 to 75.9) after lobar resection. A total of 101 lung cancer–related deaths were noted (46 in the sublobar-resection group and 55 in the lobar-resection group), as were 93 deaths from other causes (48 and 45 in the respective groups). The cumulative incidence of deaths from lung cancer and other causes of death was similar in the two groups (Fig. 3B).
Table 2.
Patterns of Recurrence.
| Type of Recurrence | Sublobar Resection (N = 336) | Lobar Resection (N = 351) | Difference (95% CI)* |
|---|---|---|---|
| number (percent) | percentage points | ||
| Overall | 102 (30.4) | 103 (29.3) | 1.0 (−5.8 to 7.9) |
| Locorégional recurrence | 45 (13.4) | 35 (10.0) | 3.4 (1.0 to 8.3) |
| Regional recurrence only | 6 (1.8) | 9 (2.6) | −0.8 (−3.2 to 1.6) |
| Any distant recurrence | 51 (15.2) | 59 (16.8) | −1.6 (−7.1 to 3.9) |
| New primary lung cancer | 60 (17.9) | 52 (14.8) | 3.0 (−2.5 to 8.6) |
The widths of the confidence intervals have not been adjusted for multiplicity and may not be used in place of hypothesis testing.
Figure 3. Recurrence-free Survival and Cause of Death.
Panel A shows recurrence-free survival in the intention-to-treat population. Recurrence-free survival was defined as the time between randomization and the occurrence of locoregional or distant recurrence; all other events, including death from any cause, were censored at the occurrence of these events. The shaded areas indicate 95% confidence intervals. Panel B shows cumulative-incidence functions for death related to lung cancer as compared with death not related to lung cancer; four patients with an unknown cause of death (three in the lobar-resection group and one in the sublobar-resection group) were excluded from the analysis. In both panels, the widths of the confidence intervals have not been adjusted for multiplicity and may not be used in place of hypothesis testing.
EXPIRATORY FLOW RATES
At 6 months postoperatively, the magnitude of reduction from baseline in the percentage of predicted FEV1 was greater after lobar resection (−6.0; 95% CI, −8.0 to −5.0) than after sublobar resection (−4.0; 95% CI, −5.0 to −2.0) (Table S1). Similarly, the magnitude of reduction in the percentage of predicted FVC was greater after lobectomy (−5.0; 95% CI, −7.0 to −3.0) than after sublobar resection (−3.0; 95% CI, −4.0 to −1.0).
DISCUSSION
In this large, randomized trial, we found that in patients with peripheral clinical stage T1aN0 (≤2 cm) NSCLC, sublobar resection was noninferior to lobectomy with respect to disease-free survival (primary end point). We also found that overall survival (secondary end point) was similar with the two procedures. The results of post hoc exploratory analyses that examined the association between relevant demographic and clinical variables and disease-free and overall survival were consistent with the overall results of the trial. However, given the small sample size and few events in each subgroup, these findings should be interpreted with caution. In addition, no substantial difference between the two groups was seen in the incidences or patterns of disease recurrence. Locoregional recurrences were slightly numerically higher after sublobar resection than after lobectomy (13.4% vs. 10.0%), but the difference was not clinically meaningful. Although we did not mandate the extent of lymph-node dissection beyond sampling of major hilar and two mediastinal nodal stations, regional recurrence occurred in 1.8% of the patients after sublobar resection and 2.6% of the patients after lobectomy.
It is important that these results are interpreted strictly within the constraints of the eligibility criteria mandated by the trial. Specifically, the results are applicable only to a highly selected group of patients with peripherally located NSCLC who are deemed to have clinical T1aN0 disease (tumor size, ≤2 cm) according to imaging criteria and in whom the absence of metastases to hilar and mediastinal lymph nodes is pathologically confirmed. We had previously reported that among patients with clinically node-negative disease who were registered for the trial, 6.4% had positive major hilar or mediastinal nodes precluding randomization.14 These results will become increasingly relevant as the proportion of patients with early-stage lung cancer increases with expanded implementation of lung cancer screening and as the number of older persons with early-stage disease in whom sublobar resection may be the preferred surgical option increases.15,16 We had previously reported 30-day mortality of 0.6% and 90-day mortality of 1.2% after sublobar resection.13 These values compared favorably with 30-day mortality of 1.1% and 90-day mortality of 1.7% after lobectomy. We would note that 80% of all the resections in both groups of this trial were performed in a minimally invasive fashion.
Another proposed benefit of sublobar resection is preservation of pulmonary function as measured by expiratory flow rates. In this trial, we observed a lower decrement in forced expiratory flow after sublobar resection than after lobectomy. However, the absolute difference between the two groups was only 2 percentage points for both FEV1 and FVC. Although this difference is arguably not clinically meaningful in this patient population with normal baseline pulmonary functions, it may be more clinically relevant in patients with compromised pulmonary functions or in those with lower-lobe disease in whom lobar resection may be associated with greater impairment of pulmonary function. In addition, a single measurement at 6 months may not be predictive of potential further reductions or perhaps improvements in flow rates at 12 or 18 months postoperatively. More likely, however, proof of preservation of pulmonary function may be best shown with the use of functional tests, such as the 6-minute walk test or pulmonary exercise testing.
Our trial results are consistent with recently reported results by investigators of the Japanese Clinical Oncology Group. Saji and colleagues reported the results of JCOG0802, a randomized noninferiority trial comparing lobectomy with anatomical segmentectomy in a similar cohort of patients.6 The results showed that anatomical segmentectomy was noninferior to lobar resection for overall and relapse-free survival. Although these results are generally similar to those reported in the current trial, several critical methodologic differences exist between the two trials. An important difference is that anatomical segmentectomy, a procedure considered by most surgeons to be more oncologically sound than wedge resection, was the only method of sublobar resection allowed in the JCOG0802 trial. In the current trial, both anatomical segmentectomy and wedge resection were considered to be acceptable methods of sublobar resection. Wedge resection was allowed in the current trial because it is the most frequently practiced method of sublobar resection in North America and Europe; thus, its inclusion would make the trial more representative of a “real world” setting.17,18 Surgical details of both methods of sublobar resection, including margin status, are being analyzed. Another important difference between the two trials is that in JCOG0802 more than 90% of the patients had adenocarcinoma, of whom 45% had an associated ground-glass component. These part-solid tumors are generally thought to be associated with better survival than a completely solid adenocarcinoma. The high proportion of patients who had part-solid tumors may have contributed to the outstanding 5-year survival of more than 90% reported in each group of that trial. It is also consistent with the reported low incidence of distant metastases, which was 4.8% in the lobectomy group and 4.9% in the segmentectomy group. In contrast, distant metastases developed in 16.0% of all the patients in the current trial, which accounted for more than 50% of all recurrences.
Regardless of these differences in trial design, the concordance of results between the two trials is reassuring. Together, these findings affirm that sublobar resection for patients with clinical T1aN0 disease by either anatomical segmentectomy or wedge resection is an effective management approach for this subgroup of patients with NSCLC.
Supplementary Material
Acknowledgments
We thank Bryce Damman, M.S., and Brandy Jaszewski, B.S., from the Alliance Statistics and Data Management Center (Mayo Clinic) for statistical and data-management contributions.
Supported by the National Cancer Institute of the National Institutes of Health, under award numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology) and UG1CA232760, UG1CA233184, UG1CA233247, UG1CA233253, UG1CA233290, UG1CA233327, and U10CA180863 (to the Canadian Cancer Trials Group) and supported in part by Covidien (formerly Tyco Healthcare) and Ethicon. For a list of entities that have provided support to Alliance for Clinical Trials in Oncology and Alliance Foundation Trials programs, visit https://acknowledgments.alliancefound.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Nasser Altorki, Weill Cornell Medicine, New York–Presbyterian Hospital, New York
Xiaofei Wang, Alliance Statistics and Data Management Center and the Department of Biostatistics and Bioinformatics, Duke University, Durham, NC.
David Kozono, Alliance Protocol Operations Office, Chicago
Colleen Watt, Alliance Protocol Operations Office, Chicago
Rodney Landrenau, University of Pittsburgh Medical Center, Pittsburgh
Dennis Wigle, Mayo Clinic, Rochester, MN
Jeffrey Port, Weill Cornell Medicine, New York–Presbyterian Hospital, New York
David R. Jones, Memorial Sloan Kettering Cancer Center, New York
Massimo Conti, Institut Universitaire de Cardiologie et Pneumologie de Québec, Quebec, Canada
Ahmad S. Ashrafi, Surrey Memorial Hospital Thoracic Group Fraser Valley Health Authority, Surrey, BC, Canada
Moishe Liberman, Centre Hospitalier de l’Université de Montréal, Montreal, QC, Canada
Kazuhiro Yasufuku, University of Toronto, Toronto, Canada
Stephen Yang, Johns Hopkins University, Baltimore
John D. Mitchell, University of Colorado Hospital School of Medicine, Aurora
Harvey Pass, New York University Grossman School of Medicine, New York, New York
Robert Keenan, Moffitt Cancer Center, Tampa, FL
Thomas Bauer, Hackensack Meridian Health System, Edison, NJ
Daniel Miller, Emory University School of Medicine, Atlanta
Leslie J. Kohman, SUNY Upstate Medical University, Syracuse, New York
Thomas E. Stinchcombe, Duke Cancer Institute, Duke University Medical Center, Durham, NC
Everett Vokes, University of Chicago Comprehensive Cancer Center, Chicago
REFERENCES
- 1.Ginsberg RJ, Rubinstein LV; Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg 1995;60:615–23. [DOI] [PubMed] [Google Scholar]
- 2.Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754–64. [DOI] [PubMed] [Google Scholar]
- 3.Onaitis MW, Furnary AP, Kosinski AS, et al. Equivalent survival between lobectomy and segmentectomy for clinical stage IA lung cancer. Ann Thorac Surg 2020;110:1882–91. [DOI] [PubMed] [Google Scholar]
- 4.Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensitymatched analysis. J Clin Oncol 2014;32:2449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491–6. [DOI] [PubMed] [Google Scholar]
- 6.Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607–17. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549–56. [PubMed] [Google Scholar]
- 8.Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–63. [Google Scholar]
- 9.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54. [Google Scholar]
- 10.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 11.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985;4:213–26. [DOI] [PubMed] [Google Scholar]
- 12.Diciccio TJ, Romano JP. A review of bootstrap confidence intervals. J R Statist Soc B 1988;50:338–54. [Google Scholar]
- 13.Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohman LJ, Gu L, Altorki N, et al. Biopsy first: lessons learned from cancer and leukemia group B (CALGB) 140503. J Thorac Cardiovasc Surg 2017;153:1592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamel MK, Lee B, Harrison SW, Port JL, Altorki NK, Stiles BM. Sublobar resection is comparable to lobectomy for screen-detected lung cancer. J Thorac Cardiovasc Surg 2022;163:1907–15. [DOI] [PubMed] [Google Scholar]
- 16.Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (≤ 2 cm) non-small cell lung cancers — a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27–32. [DOI] [PubMed] [Google Scholar]
- 17.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from the Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247–54. [DOI] [PubMed] [Google Scholar]
- 18.Seder CW, Salati M, Kozower BD, et al. Variation in pulmonary resection practices between the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery databases. Ann Thorac Surg 2016;101:2077–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.