Abstract
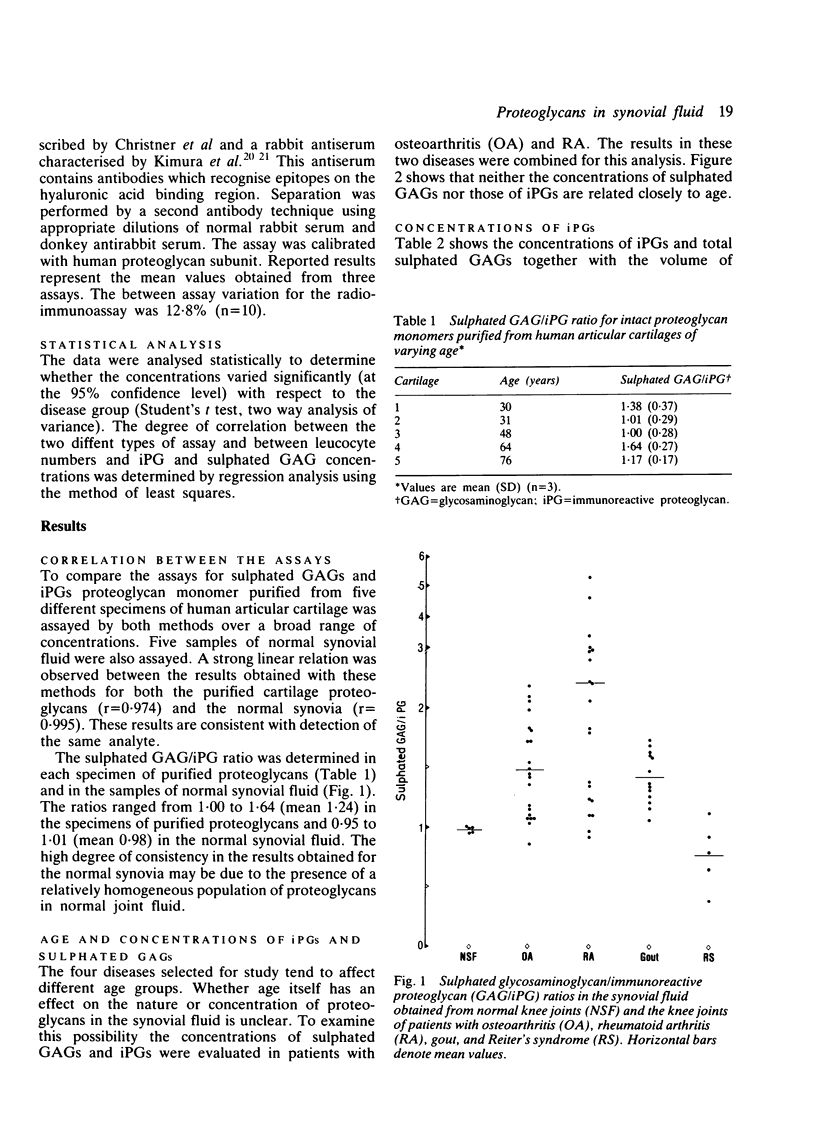
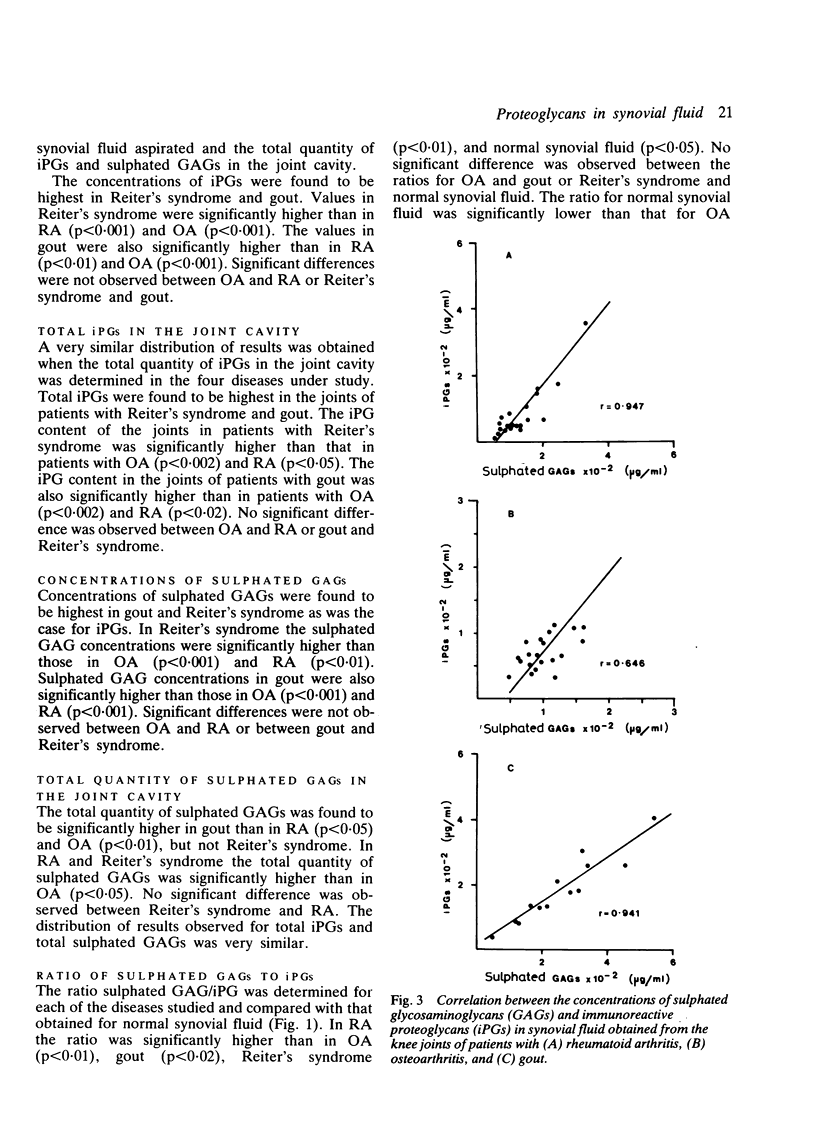
Immunoreactive proteoglycans (iPGs) and sulphated glycosaminoglycans (GAGs) were assayed in synovial fluid obtained from 22 patients with osteoarthritis (OA), 21 with rheumatoid arthritis (RA), 13 with gout, and five with Reiter's syndrome. A strong positive linear correlation was observed between concentrations of sulphated GAGs and iPGs in RA (r = 0.95) and gout (r = 0.94). A linear correlation was also observed in OA (r = 0.65). Patients with gout and Reiter's syndrome had significantly higher concentrations of sulphated GAGs and iPGs than patients with OA or RA. Patients with gout also had significantly higher total quantities of sulphated GAGs and iPGs in the knee joint cavity than patients with OA or RA. In all four diseases similar profiles were observed when comparisons were made between the total quantities and concentrations of sulphated GAGs and iPGs in synovial fluid. These results indicate that the observed differences in concentrations are not simply a function of dilution. The concentrations of sulphated GAGs and iPGs did not correlate closely with the type or number of inflammatory cells in the synovial fluid. Considerable variation was noted in the sulphated GAG/iPG ratios, suggesting that different mechanisms may be contributing to the release of proteoglycans in the diseases studied.
Full text
PDF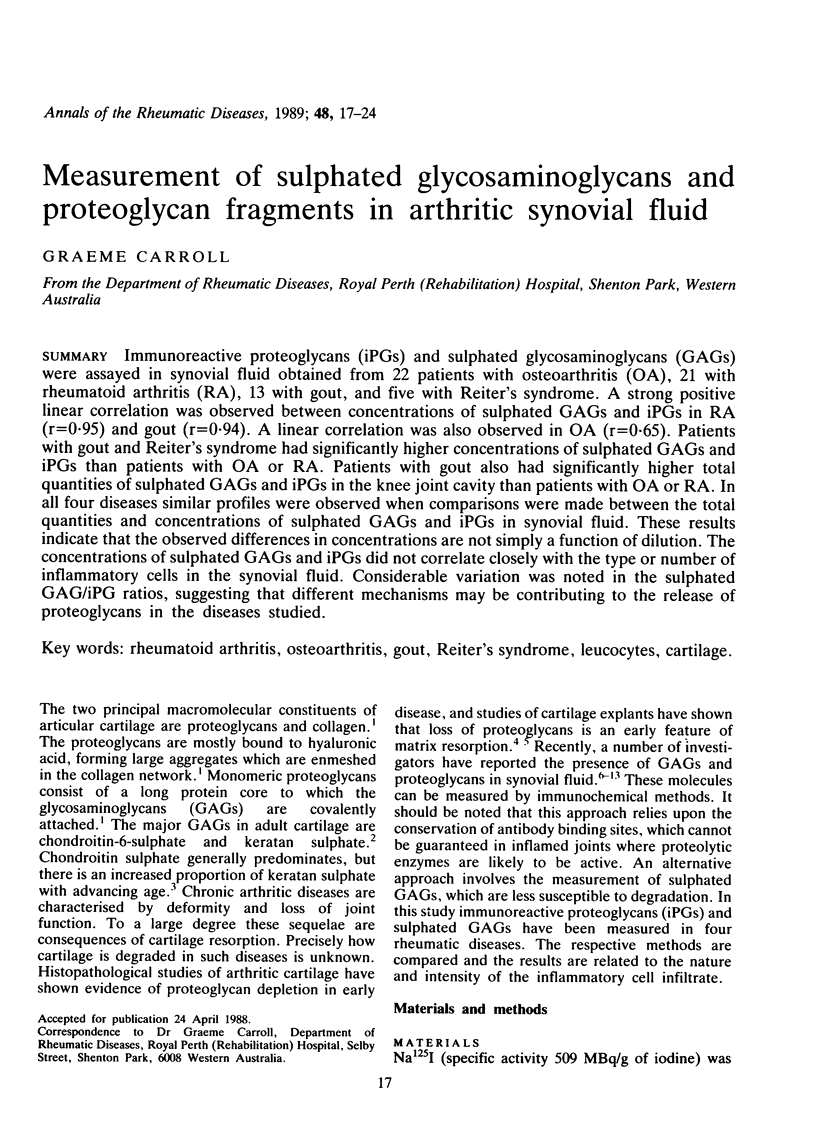



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Age-related changes in the composition and structure of human articular-cartilage proteoglycans. Biochem J. 1978 Dec 15;176(3):683–693. doi: 10.1042/bj1760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll G. J. Spectrophotometric measurement of proteoglycans in osteoarthritic synovial fluid. Ann Rheum Dis. 1987 May;46(5):375–379. doi: 10.1136/ard.46.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Page Thomas D. P., Hazleman B. The role of cytokines in arthritic diseases: in vitro and in vivo measurements of cartilage degradation. Int J Tissue React. 1987;9(4):349–354. [PubMed] [Google Scholar]
- Fife R. S., Myers S. L. Evidence for an interaction between canine synovial cell proteoglycans and link proteins. Biochim Biophys Acta. 1985 Dec 13;843(3):238–244. doi: 10.1016/0304-4165(85)90144-8. [DOI] [PubMed] [Google Scholar]
- Gysen P., Franchimont P. Radioimmunoassay of proteoglycans. J Immunoassay. 1984;5(3-4):221–243. doi: 10.1080/01971528408063009. [DOI] [PubMed] [Google Scholar]
- Gysen P., Malaise M., Gaspar S., Franchimont P. Measurement of proteoglycans, elastase, collagenase and protein in synovial fluid in inflammatory and degenerative arthropathies. Clin Rheumatol. 1985 Mar;4(1):39–50. doi: 10.1007/BF02032316. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Smith C., Keiser H. D., Craig R. Glycosaminoglycans produced by human synovial cell cultures. Coll Relat Res. 1982 Jul;2(4):313–329. doi: 10.1016/s0174-173x(82)80023-x. [DOI] [PubMed] [Google Scholar]
- Kimura J. H., Thonar E. J., Hascall V. C., Reiner A., Poole A. R. Identification of core protein, an intermediate in proteoglycan biosynthesis in cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Aug 10;256(15):7890–7897. [PubMed] [Google Scholar]
- Larsen A., Dale K., Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 1977 Jul;18(4):481–491. doi: 10.1177/028418517701800415. [DOI] [PubMed] [Google Scholar]
- Marsh J. M., Wiebkin O. W., Gale S., Muir H., Maini R. N. Synthesis of sulphated proteoglycans by rheumatoid and normal synovial tissue in culture. Ann Rheum Dis. 1979 Apr;38(2):166–170. doi: 10.1136/ard.38.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Saxne T., Heinegård D., Wollheim F. A. Cartilage proteoglycans in synovial fluid and serum in patients with inflammatory joint disease. Relation to systemic treatment. Arthritis Rheum. 1987 Sep;30(9):972–979. doi: 10.1002/art.1780300903. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D., Wollheim F. A., Pettersson H. Difference in cartilage proteoglycan level in synovial fluid in early rheumatoid arthritis and reactive arthritis. Lancet. 1985 Jul 20;2(8447):127–128. doi: 10.1016/s0140-6736(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D., Wollheim F. A. Therapeutic effects on cartilage metabolism in arthritis as measured by release of proteoglycan structures into the synovial fluid. Ann Rheum Dis. 1986 Jun;45(6):491–497. doi: 10.1136/ard.45.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace S. L., Robinson H., Masi A. T., Decker J. L., McCarty D. J., Yü T. F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977 Apr;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]


