Abstract
Introduction
Postoperative impaired sleep quality and pain are associated with adverse outcomes. Stellate ganglion block (SGB) has shown promising results in enhancing sleep quality and alleviating neuropathic pain. This study aimed to investigate the effects of ultrasound-guided SGB on postoperative sleep quality and pain in patients undergoing breast cancer surgery.
Methods
This study is a parallel-group randomized controlled clinical trial with two groups: SGB and control. Fifty female patients undergoing breast cancer surgery were randomized in a 1:1 ratio to receive preoperative ultrasound-guided single-injection SGB (SGB group) or just an ultrasound scan (control group). All participants were blinded to the group assignment. The primary outcome was postoperative sleep quality, assessed by the St. Mary’s Hospital Sleep Questionnaire and actigraphy 2 days postoperatively. The secondary outcome was postoperative pain, measured by the visual analog scale.
Results
A total of 48 patients completed the study, with 23 patients in the control group and 25 in the SGB group. The postoperative St. Mary’s Hospital Sleep Questionnaire scores were significantly higher in the SGB group than in the control group on 1 day postoperative (30.88 ± 2.44 versus 27.35 ± 4.12 points, P = 0.001). The SGB also increased the total sleep time and sleep efficiency (main actigraphy indicators) during the first two postoperative nights. Compared with the control group, preoperative SGB reduced postoperative pain and the incidence of breast cancer-related lymphedema (20% versus 52.2%, P = 0.02, odds ratio 0.229, 95% confidence interval 0.064–0.821). There were no adverse events related to SGB.
Conclusion
Preoperative ultrasound-guided SGB improves postoperative sleep quality and analgesia in patients undergoing breast cancer surgery. SGB may be a safe and practical treatment to enhance the postoperative quality of life in patients with breast cancer.
Trial Registration
The study was registered in the Chinese Clinical Trial Registry (ChiCTR2100046620, principal investigator: Kai Zeng, date of registration: 23 May 2021).
Keywords: Breast cancer, Postoperative pain, Sleep, Stellate ganglion block
Key Summary Points
| Why carry out this study? |
| Most patients with breast cancer develop pain and sleep dysfunction after surgery, which is detrimental to recovery. |
| Stellate ganglion block is an effective analgesic intervention that improves postoperative pain relief and sleep quality. |
| This study aimed to investigate the effects of stellate ganglion block on postoperative analgesia and sleep quality in patients undergoing breast cancer surgery. |
| What was learned from the study? |
| Preoperative stellate ganglion block improves postoperative sleep quality and analgesia in patients with breast cancer. |
| A comprehensive investigation of sleep quality requires both subjective and objective methods. |
| Patients with breast cancer benefit from stellate ganglion block as a safe and effective analgesic treatment. |
Introduction
Breast cancer has now become the most frequently diagnosed cancer globally and the leading cause of cancer-related mortality among females [1]. Breast cancer survivors are confronted with a range of long-term symptoms such as sleep dysfunction (SD), chronic pain, lymphedema, and depression, which impair their quality of life and mental health [2–4]. Specifically, approximately 80% of patients with breast cancer reported sleep disorders, such as sleep onset insomnia, impaired sleep maintenance, and early wakeup [5]. Additional evidence has suggested that SD lowers pain thresholds and increases spontaneous pain symptoms [6, 7]. In general, SD has been seen as strongly associated with all-cause morbidity and mortality [8].
Sleep assessment typically includes both objective and subjective measurements [9]. Actigraphy is the most widely used accelerometer for measuring sedentary time, physical activity, and sleep/wake parameters [10]. Due to the high correlation in different sleep phases with polysomnography, actigraphy has become a feasible alternative to self-reporting assessment [11]. As for subjective measurement, the St. Mary’s Hospital Sleep Questionnaire (SMHSQ) is a 14-item questionnaire designed to evaluate sleep and wakefulness behaviors in the 24 h preceding assessment, which includes assessments of sleep quality, daytime function, sleep onset, and night awakening frequency [12, 13]. SMHSQ has been used successfully to detect changes in sleep patterns in hospitalized patients [14].
Stellate ganglion block (SGB) has been safely performed for more than 70 years [15]. Evidence has shown that SGB is an effective analgesic intervention that improves perioperative pain relief and reduces narcotic needs [16]. Moreover, SGB is of great importance in decreasing hot flushes and sleep insufficiency in female patients with breast cancer [17]. However, among the existing investigations correlating with SGB, there is a lack of evidence of the effect of SGB on postoperative sleep quality or analgesia, as well as the underlying molecular mechanisms. Despite the safety and possible efficacy of SGB, more prospective clinical studies are necessary for solid validation [18–21].
Therefore, the aim of the present study was to investigate the effect of preoperative ultrasound-guided SGB on postoperative sleep quality and analgesia in patients with breast cancer, and the effect on serum inflammatory factor levels, providing an applicable clinical approach to accelerate the postoperative recovery of patients with breast cancer.
Methods
Study Design and Participants
A parallel-group randomized controlled clinical trial involving two groups: SGB and control, was conducted at the First Affiliated Hospital of Fujian Medical University in China, from 26 May 2021 to 23 March 2022. This study was approved by the ethics committee of The First Affiliated Hospital of Fujian Medical University (21 May 2021, FMU|2021|200), and was registered in the Chinese Clinical Trial Registry (ChiCTR2100046620) on 23 May 2021. The study protocol was performed in accordance with the Declaration of Helsinki. A total of 58 female patients aged 18–65 years with ASA I–II who were scheduled for modified radical mastectomy for breast cancer were included in this study. The exclusion criteria included rejection of participation, allergic to anesthetics, history of insomnia, abnormal coagulation disorders, and anatomical deformities of the neck or shoulders. Written informed consent was obtained from all enrolled participants.
Participants were randomized to receive preoperative single-injection SGB under ultrasound guidance (SGB group) or just an ultrasound scan (control group). A list of randomization sequences was generated in a 1:1 ratio by the statistical consultant using the random number table method and then sealed in opaque, sequentially numbered envelopes. All participants, anesthesiologists, outcome collectors, and data analysts were blinded to the group assignment throughout the whole observation period, including all postoperative follow-ups.
Stellate Ganglion Block Procedure
A skilled anesthesiologist who was blinded to group allocation performed all SGBs under sterile conditions using real-time ultrasound (Model Edge; Fujifilm SonoSite, Bothell, Washington, USA). The single-injection SGB began 15 min after the induction of anesthesia in the operating room. In the SGB procedure, patients were placed in the supine position with their necks slightly rotated to the left side. A linear transducer (6–13 MHz) was placed on the neck to achieve a clear visualization of the carotid artery, internal jugular vein, vertebral artery, vagus, thyroid gland, esophagus, longus colli, and anterior scalene at the C6 level. A 48-mm, 20-gauge needle (BD Medical, Sandy, UT, USA) was then inserted into the prevertebral fascia of the longus colli muscle in an “in-plane” direction [22]. Once the needle tip penetrated the prevertebral fascia with a negative aspiration, 3 mL of 0.5% ropivacaine was administered, followed by the spread of the local anesthetic being visualized under ultrasound.
General Anesthesia
After entering the operating room, all patients were monitored with electrocardiogram, invasive blood pressure, peripheral pulse oximetry, and bispectral index (BIS). General anesthesia was administered with midazolam 2 mg, propofol 1.5–2 mg kg−1, sufentanil 0.4–0.6 μg kg−1, and cisatracurium 0.2–0.4 mg kg−1. Flurbiprofen 1.5–2.5 mg kg−1 and tropisetron 5 mg were administered 30 min before the end of the surgery. The BIS values were maintained in the range of 40–60. Using pressure-controlled mechanical ventilation, an end-tidal carbon dioxide partial pressure of 35–45 mmHg was maintained. After the removal of the laryngeal mask/tracheal tube, the patients were transferred to the postanesthesia care unit (PACU) for further monitoring, where the first follow-up visit was carried out to assess Horner’s syndrome and adverse events. A successful sympathetic block can be diagnosed by the presence of Horner’s syndrome on the blockage side (i.e., fascial flush, enophthalmos, ptosis, miosis, and conjunctival congestion) [17]. During the first postoperative 48 h, patients received patient-controlled analgesia (PCA) pump, which consisted of flurbiprofen 4 mg kg−1, tropisetron 0.3 mg kg−1, and sufentanil 2 μg kg−1.
Outcomes
The primary outcome was postoperative sleep quality. The Pittsburgh Sleep Quality Index was used to assess patients’ baseline subjective sleep quality 1 day before the surgery. The SMHSQ and actigraphy (ActiGraph, Pensacola, FL, USA) were used to record subjective and objective sleep quality for 3 consecutive days (1 day preoperative, surgery day, and 1 day postoperative), respectively. The actigraphy was worn on the contralateral wrists of the patients to assess sleep efficiency (SE), sleep onset latency (SOL), total sleep time (TST), number of awakenings (NA), and wake after sleep onset (WASO) through a computer program. The SMHSQ scores from 6 to 38 were used to indicate how well the patient slept.
The secondary outcome was postoperative pain intensity. Cumulative analgesic consumption and numbers of PCA administration were documented. Rest pain intensity was evaluated at 6, 24, and 48 h after surgery using the visual analog scale (VAS).
A 5-mL sample of blood was drawn before surgery and 24 h after surgery to determine the serum concentrations of melatonin, cortisol, serotonin, tumor necrosis factor-α, and interleukin-6. Intraoperative vital signs, Horner’s syndrome, adverse events (i.e., postoperative delirium, cognitive dysfunction, nausea and vomiting, arrhythmia, nerve block complications), the incidence of breast cancer-related lymphedema (BCRL), and postoperative hospital stays were documented.
Statistical Analysis
The sample size calculation for this trial was based on the SE recorded by actigraphy on surgery day. The SE (mean ± standard deviation) of the control group and the SGB group in our pilot study (n = 5) was 80.60 ± 21.06 (%) and 93.88 ± 5.69 (%), respectively. On the basis of our power analysis (α = 0.05 and β = 0.1), a sample size of 23 participants per group was required. To allow for a 10% dropout rate, a sample size of 50 cases was determined.
All statistical analyses were performed using SPSS 26.0 software (IBM Corp., Armonk, New York, USA). The normality of the distribution of quantitative variables was assessed via the Shapiro–Wilk test and Q–Q plots. Normally distributed variables are represented as mean ± standard deviation and analyzed by using the independent-samples t-test. Non-normally distributed variables are represented as median [IQR] and analyzed using the Mann–Whitney U test. Categorical variables are summarized as number (%) and analyzed using the chi-square or Fisher’s exact test, as appropriate. Actigraphy performances, VAS scores, and serological parameters were evaluated using a two-way (between-group comparisons with time and group as two factors to be analyzed) repeated-measures ANOVA or generalized linear mixed model analysis. Correlation analysis was performed using Pearson’s or Spearman’s correlation. In addition, relative risk (RR) with 95% confidence intervals (CIs) was used to compare the incidence of BCRL between groups. P < 0.05 (two-tailed) was considered statistically significant.
Results
Patient Characteristics
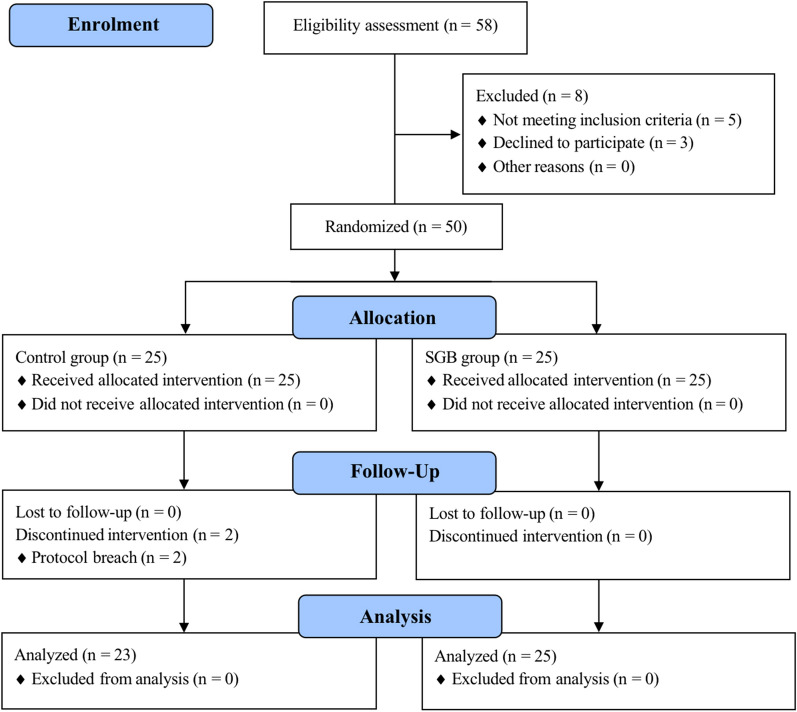
In total, 58 female patients were assessed for eligibility for this study from May 2021 to March 2022. Among them, five patients did not meet the inclusion criteria and three patients refused to participate. The remaining 50 patients were randomized into either the SGB group or the control group accordingly. An additional two patients from the control group were excluded from the analysis due to protocol breach (not wearing actigraphy; actigraphy data error). Consequently, 48 patients completed the full study protocol. The CONSORT flowchart for all participants is shown in Fig. 1. The baseline demographic data of the patients are listed in Table 1 and are well balanced between the SGB and control group. All patients in the SGB group presented Horner’s syndrome in PACU. There were no differences in adverse events and postoperative hospital stays between the two groups (Table 1).
Fig. 1.
CONSORT diagram of study flow
Table 1.
Patient demographic data
| Variables | Control group (n = 23) | SGB group (n = 25) | P value |
|---|---|---|---|
| Age (years) | 48.61 ± 8.19 | 47.72 ± 7.90 | 0.704 |
| BMI (kg m−2) | 23.03 ± 2.98 | 23.87 ± 3.24 | 0.358 |
| ASA I/II | 5/18 | 2/23 | 0.237 |
| History of chemotherapy, n | 3 (13.04%) | 2 (8.00%) | 0.660 |
| Education, n | 0.783 | ||
| Lower than primary school | 13 (56.52%) | 12 (48.00%) | |
| Middle school to high school | 8 (34.78%) | 9 (36.00%) | |
| Junior college to university | 2 (8.70%) | 4 (16.00%) | |
| Pittsburgh sleep quality index scores | 8.52 ± 4.06 | 7.64 ± 3.97 | 0.450 |
| Surgical laterality (left/right) | 10/13 | 9/16 | 0.769 |
| Axillary lymph node dissection, n | 11 (47.83%) | 7 (28.00%) | 0.234 |
| Duration of surgery (min) | 113.65 ± 3.86 | 113.00 ± 25.26 | 0.949 |
| Adverse events, n | |||
| Hot flushes | 0 | 3 (12%) | 0.235 |
| Dizziness | 2 (8.70%) | 0 | 0.224 |
| Bradycardia | 1 (4.35%) | 0 | 0.479 |
| Numbers of PCA administration, n | 0.17 ± 0.65 | 0.12 ± 0.60 | 0.766 |
| Postoperative hospital stays (days) | 6.22 ± 2.32 | 6.72 ± 2.97 | 0.519 |
Data are expressed as mean ± standard deviation, median [IQR], or number (%) where appropriate. The P value was calculated by the chi-square test, Fisher exact test, or Mann–Whitney U test
SGB stellate ganglion block, BMI body mass index, ASA American Society of Anesthesiologists, PCA patient-controlled analgesia
Stellate Ganglion Block Improves Postoperative Sleep Quality
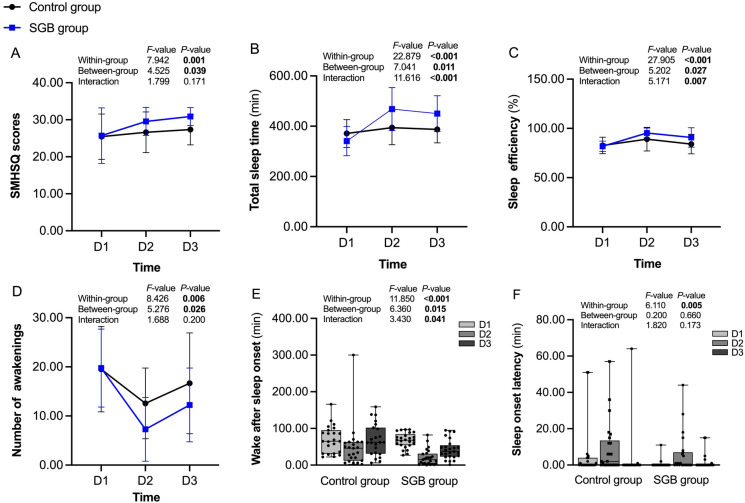
The SMHSQ and actigraphy were used to assess the subjective and objective sleep quality of patients with breast cancer, respectively (Fig. 2). The postoperative SMHSQ scores were significantly higher in the SGB group than in the control group, especially on 1 day postoperative (30.88 ± 2.44 versus 27.35 ± 4.12 points, P = 0.001). There were significant differences in TST and SE between the SGB group and the control group during the first two postoperative nights, especially on 1 day postoperative (P = 0.001 and 0.007, respectively), whereas lower NA and WASO were observed in the patients who received SGB on surgery day (P = 0.010 and 0.029, respectively), In addition, significant interactions between time and group in WASO, TST, and SE were found (all P < 0.05).
Fig. 2.
The SMHSQ (A) and actigraphy (B–F) performance from 1 day preoperative to 1 day postoperative in the control and SGB group. SMHSQ St. Mary’s hospital sleep questionnaire, SGB stellate ganglion block, D1 1 day preoperative, D2 surgery day, D3 1 day postoperative
The correlation between SMHSQ and actigraphy was also analyzed (Fig. 3). Further analysis showed that the SMHSQ scores were positively correlated with SE (r = 0. 226, P < 0.01) and TST (r = 0.279, P < 0.01), but negatively correlated with WASO (r = − 0.169, P < 0.05).
Fig. 3.
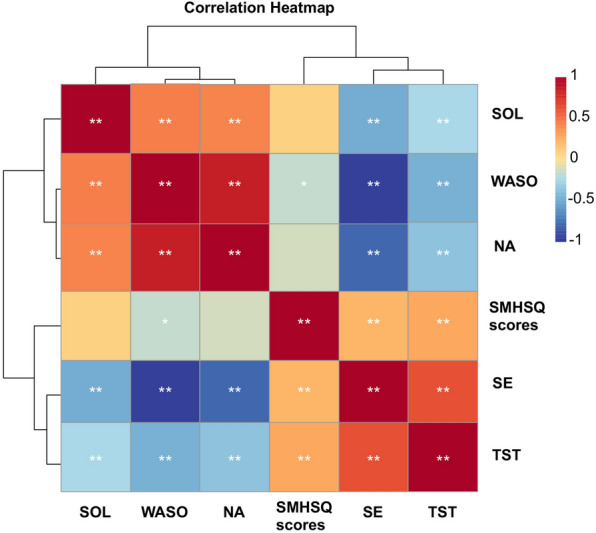
Correlation analysis of SMHSQ and actigraphy indicators. SMHSQ, St. Mary’s hospital sleep questionnaire, SE sleep efficiency, SOL sleep onset latency, TST total sleep time, NA number of awakenings, WASO wake after sleep onset; *P < 0.05, **P < 0.01
Stellate Ganglion Block Alleviates Postoperative Pain
Postoperative pain at rest was measured at 6, 24, and 48 h after surgery by the VAS (Fig. 4). The VAS pain scores were significantly lower at each timepoint in the SGB group than in the control group (P < 0.001). However, no significant differences in the VAS scores were observed between the time points from 6 to 48 h within the SGB and control group of patients (P = 0.735). As shown in Table 1, there was no significant difference in the number of PCA administrations between the two groups (P = 0.766).
Fig. 4.
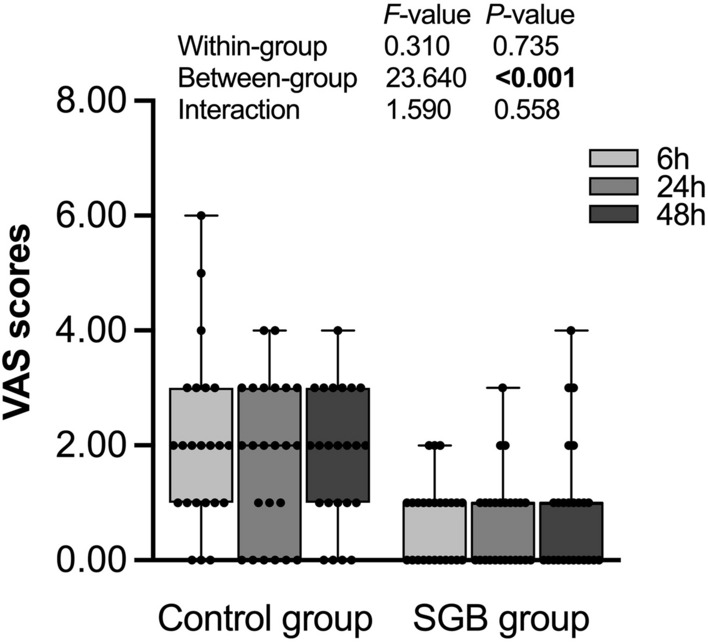
VAS pain scores at postoperative 6, 24, and 48 h in the control group and SGB group. SGB stellate ganglion block, VAS visual analog scale
Serological Indicators
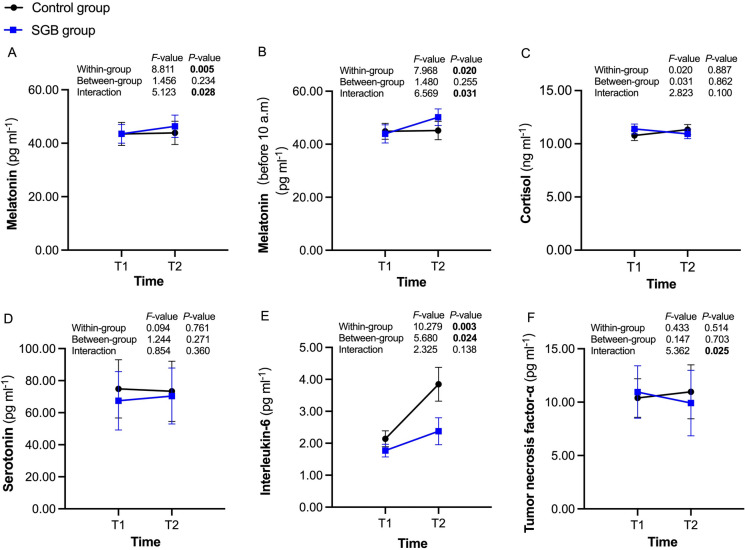
As shown in Fig. 5, the serum concentrations of melatonin from preoperative to 24 h postoperative have increased significantly in the SGB group (43.52 ± 3.48 versus 46.34 ± 4.18 pg ml−1, P = 0.005). Furthermore, considering the effect of the circadian rhythm of human serum melatonin secretion, data from patients who underwent surgery before 10 a.m. were selected for further analysis; the results remained the same in this subset of patients (43.91 ± 3.43 versus 50.20 ± 3.13 pg ml−1, P = 0.020). Moreover, the serum concentrations of interleukin-6 at 24 h postoperative were significantly lower in patients who received SGB than those in the control group (P = 0.024), with a significant difference in the times from preoperative to 24 h postoperative within the same group of patients (P = 0.003).
Fig. 5.
Concentrations of serological indicators at preoperative and 24 h postoperative in the control group (black circles) and SGB group (blue squares). SGB stellate ganglion block, T1 preoperative, T2 24 h postoperative
Stellate Ganglion Block Reduces the Incidence of Breast Cancer-Related Lymphedema
The incidence of BCRL was documented during the first 2 postoperative days. As shown in Table 2, compared with the control group, preoperative SGB significantly reduced the incidence of BCRL (20% versus 52.2%, P = 0.020, odds ratio 0.229, 95% confidence interval 0.064–0.821).
Table 2.
Incidence of breast cancer-related lymphedema in both groups
| Group | Breast cancer-related lymphedema# | Edema& | ||||
|---|---|---|---|---|---|---|
| Non-edema | Edema | P value | Ipsilateral edema | Contralateral edema | P value | |
| Control group | 11 (47.8%) | 12 (52.2%) | 0.020* | 5 (41.7%) | 7 (58.3%) | 0.686 |
| SGB group | 20 (80.0%) | 5 (20.0%) | 2 (40.0%) | 3 (60.0%) | ||
Data are expressed as the number (%) of patients. The P value was calculated by the chi-square test (#) or Fisher exact test (&)
SGB stellate ganglion block
*P < 0.05
Also, contralateral edema occurred more often than ipsilateral edema in both groups; however, the difference was not statistically significant (P = 0.686).
Right-Sided Stellate Ganglion Block Exerts Similar Effects on Both Sides
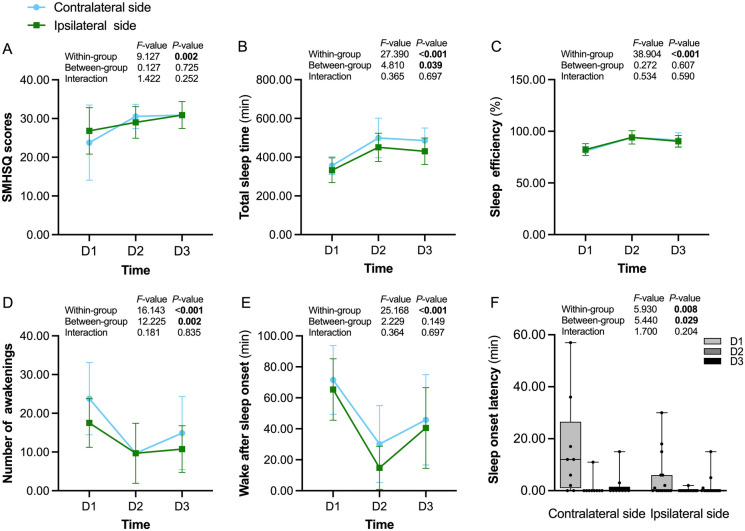
The effects of the right-sided SGB on the contralateral side (left side) and ipsilateral side (right side) were further analyzed. The results suggested that, of all subjective and objective parameters assessed, only NA (P = 0.002), SL (P = 0.029), and TST (P = 0.039) were statistically different in patients with ipsilateral breast cancer in comparison with patients with contralateral breast cancer (Fig. 6). Moreover, no significant differences in the serological indicators, VAS scores, or incidence of BCRL were observed between the two sides (all P > 0.05).
Fig. 6.
Sleep performances of the contralateral and ipsilateral sides from 1 day preoperative to 1 day postoperative in the SGB group. SMHSQ St. Mary’s Hospital Sleep Questionnaire, SGB stellate ganglion block, D1 1 day preoperative, D2 surgery day, D3 1 day postoperative
Discussion
The current study demonstrated that preoperative ultrasound-guided SGB can enhance subjective and objective postoperative sleep quality in patients undergoing breast cancer surgery. Further, this effect became gradually significant over time. Preoperative single-injection SGB also provided superior analgesic effects and reduced the incidence of BCRL. In addition, patients in the SGB group had significantly higher serum concentrations of melatonin and lower serum concentrations of interleukin-6 24 h postoperative than preoperative. These findings indicated that SGB is a potentially effective intervention for patients with breast cancer.
The basic mechanism of sleep regulation involves a balance among the autonomic nervous system, homeostatic sleep drive, and circadian sleep–wake rhythms [23]. Cheng et al. [24] constructed a whole-brain functional connectivity network using the Shen atlas, suggesting that the brain regions associated with sleep quality have extensive transneuronal connections with stellate ganglion [25, 26]. Besides, stellate ganglion block also mediates melatonin rhythm disorders resulting from increased sympathetic nerve tone [27] and increases the serum concentration of melatonin overnight [28], which is consistent with the results of our study. Both findings from the current study and previous studies have demonstrated the effect of SGB on improving sleep quality, as well as the possibility of interrupting the transmission of stellate ganglion signals to the autonomic nervous system, central brain, and neuroendocrine system through the SGB.
Several studies have suggested that subjective and objective measures should be combined to assess sleep quality comprehensively [29, 30]. The golden standard of sleep assessment is the polysomnography [31]. In a previous study assessing the correlation between actigraphy and polysomnography, actigraphy was found to overestimate TST and SE, and underestimate SOL and WASO [32]. Despite these differences, actigraphy still presents prominently reliability and sensibility in the diagnosis of sleep patterns and evaluation of treatment effects [33–35]. The present study demonstrated that preoperative SGB displayed excellent performance at improving both the actigraphy performances (i.e., TST, SE, and WASO) and the SMHSQ scores. Further, the high relevance of these two measures and the elevated serum melatonin concentrations also indicated high-quality sleep after the administration of SGB.
Preoperative SGB showed superior pain management by reducing the VAS pain scores at rest in the first 48 h after surgery. Studies showed that SGB blocks the activity of the sympathetic nervous system, which can be triggered by trauma or surgery [36, 37]. Sermeus et al. [37] found that the signal transmissions from the sensory and thermal fibers of many deep somatic elements (C or A-δ) were impaired after SGB, as measured by quantitative sensory testing. Additionally, our study found that the serum interleukin-6 concentrations decreased significantly in the SGB group than in the control group. Evidence has indicated the importance of interleukin-6 in mediating neuropathic pain [38]. Prior studies have also suggested that multimodal analgesia (a combination of different opioid and non-opioid analgesics as well as regional anesthesia) is the key to enhanced recovery [39]. Hence, the application of SGB could be a promising component of multimodal analgesia approach.
Previous studies have found that injecting saline into the stellate ganglion exerts a certain degree of sympathetic nerve block [40]. Hence, to avoid unnecessary trauma, a control group with only an ultrasound scan was chosen instead of a placebo injection. Nerve block treatments with ropivacaine typically last 4–6 h, so Horner’s syndrome can still be diagnosed under PACU observation. Furthermore, SGB-associated complications, such as local anesthetic systemic toxicity, brachial plexus block, hoarseness, and hematoma, were not observed in our study. One possibility might be that the nerve block-related complications might be covered by the anesthesia state and eventually elapsed after surgery.
In contrast to the traditional concept that the ipsilateral nerve block exerts a superior analgesic effect than the contralateral, our study demonstrated that the right-sided SGB had similar effects on sleep, pain, serological indicators, and the incidence of BCRL between the contralateral and ipsilateral sides. This provides solid evidence to guide clinical practice while avoiding the risks associated with left-sided SGB.
Nevertheless, there are several limitations to the current study. First, the concentration and dose of ropivacaine have been fixed; given the effectiveness of SGB, experiments should be set up to study the dose–response curve of ropivacaine. Second, the sample size was calculated on the basis of the primary outcome; it is possible that the current study was underpowered and subject to type-II error in terms of secondary outcome metrics, and therefore, larger-size trials are required in the future. Third, we collected only VAS scores at rest, which may not fully assess patients’ pain; comprehensive assessments should be used in future studies.
Conclusion
The current study has demonstrated that preoperative ultrasound-guided SGB can effectively improve postoperative quality, alleviate analgesia levels, and reduce the incidence of BCRL in patients with breast cancer.
Acknowledgements
The authors gratefully acknowledge Dr. Jin-hua Chen for his assistance with statistical analysis and also thank the participants of the study.
Funding
This study was supported by the Natural Science Foundation of China (No. 82271238) and Joint Funds for the Innovation of Science and Technology, Fujian province, China (No. 2019Y9013 and 2020Y9029). The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Rui-zhi Yang, Yan-zhen Li: These authors helped with experimental design, data collection, and thesis writing, they contributed equally to this work. Min Liang, Jian-jun Yu, Ming-li Chen, Jin-jia Qiu, Shi-zhu Lin: These authors helped with data collection and statistical analysis. Kai Zeng, Xiao-dan Wu: These authors helped with experimental design, personnel scheduling, and article editing. All authors read and approved the final manuscript.
Disclosures
Rui-zhi Yang, Yan-zhen Li, Min Liang, Jian-jun Yu, Ming-li Chen, Jin-jia Qiu, Shi-zhu Lin, Xiao-dan Wu, and Kai Zeng declare that they have no competing interests.
Compliance with Ethics Guidelines
The study protocol was conducted at the First Affiliated Hospital of Fujian Medical University from May 2021 to March 2022 in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of The First Affiliated Hospital of Fujian Medical University (21 May 2021, FMU|2021|200) and was registered in the Chinese Clinical Trial Registry (ChiCTR2100046620, Principal investigator: Kai Zeng, Date of registration: 2021-5-23). Written informed consent was obtained from all participants in this study.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the Open Science Framework (OSF) repository (osf.io/yugjh/; [https://doi.org/10.17605/OSF.IO/YUGJH]).
Footnotes
Rui-zhi Yang and Yan-zhen Li contributed equally to the work.
Contributor Information
Xiao-dan Wu, Email: wxiaodan@sina.com.
Kai Zeng, Email: fymzk6822@163.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Carreira H, Williams R, Müller M, et al. Associations between breast cancer survivorship and adverse mental health outcomes: a systematic review. J Natl Cancer Inst. 2018;110(12):1311–1327. doi: 10.1093/jnci/djy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreutz C, Schmidt ME, Steindorf K. Effects of physical and mind-body exercise on sleep problems during and after breast cancer treatment: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;176(1):1–15. doi: 10.1007/s10549-019-05217-9. [DOI] [PubMed] [Google Scholar]
- 4.Stan D, Loprinzi CL, Ruddy KJ. Breast cancer survivorship issues. Hematol Oncol Clin North Am. 2013. 10.1016/j.hoc.2013.05.005. [DOI] [PMC free article] [PubMed]
- 5.Fox R, Ancoli-Israel S, Roesch S, et al. Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy. Support Care Cancer. 2020;28(2):845–855. doi: 10.1007/s00520-019-04834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillman DR. Sleep loss in the hospitalized patient and its influence on recovery from illness and operation. Anesth Analg. 2021;132(5):1314–1320. doi: 10.1213/ANE.0000000000005323. [DOI] [PubMed] [Google Scholar]
- 7.Haack M, Simpson N, Sethna N, Kaur S, Mullington J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. 2020;45(1):205–216. doi: 10.1038/s41386-019-0439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hale L, Troxel W, Buysse D. Sleep health: an opportunity for public health to address health equity. Annu Rev Public Health. 2020;41:81–99. doi: 10.1146/annurev-publhealth-040119-094412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Rissling M, Natarajan L, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep. 2012;35(2):237–245. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47(9):1821–1845. doi: 10.1007/s40279-017-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts DM, Schade MM, Mathew GM, Gartenberg D, Buxton OM. Detecting sleep using heart rate and motion data from multisensor consumer-grade wearables, relative to wrist actigraphy and polysomnography. Sleep. 2020;43(7):zsaa045. 10.1093/sleep/zsaa045. [DOI] [PMC free article] [PubMed]
- 12.Leigh TJ, Bird HA, Hindmarch I, Constable PD, Wright V. Factor analysis of the St. Mary's Hospital Sleep Questionnaire. Sleep. 1988;11(5):448–453. doi: 10.1093/sleep/11.5.448. [DOI] [PubMed] [Google Scholar]
- 13.Ellis BW, Johns MW, Lancaster R, et al. The St. Mary's Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4(1):93–97. [DOI] [PubMed]
- 14.Murphy F, Bentley S, Ellis BW, Dudley H. Sleep deprivation in patients undergoing operation: a factor in the stress of surgery. Br Med J. 1977;2(6101):1521–1522. doi: 10.1136/bmj.2.6101.1521-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rae Olmsted KL, Bartoszek M, Mulvaney S, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130–138. doi: 10.1001/jamapsychiatry.2019.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharbel D, Singh P, Blumenthal D, et al. Preoperative stellate ganglion block for perioperative pain in lateralized head and neck cancer: preliminary results. Otolaryngol Head Neck Surg. 2020;162(1):87–90. doi: 10.1177/0194599819889688. [DOI] [PubMed] [Google Scholar]
- 17.Lipov E, Joshi J, Sanders S, et al. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: a pilot study. Lancet Oncol. 2008;9(6):523–532. doi: 10.1016/s1470-2045(08)70131-1. [DOI] [PubMed] [Google Scholar]
- 18.Chen D, Yin Z, Fang B. Measurements and status of sleep quality in patients with cancers. Support Care Cancer. 2018;26(2):405–414. doi: 10.1007/s00520-017-3927-x. [DOI] [PubMed] [Google Scholar]
- 19.Madsen MT, Huang C, Gögenur I. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: a systematic review. Sleep Med Rev. 2015;20:73–83. doi: 10.1016/j.smrv.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Wen S, Chen L, Wang T-H, et al. The efficacy of ultrasound-guided stellate ganglion block in alleviating postoperative pain and ventricular arrhythmias and its application prospects. Neurol Sci. 2021;42(8):3121–3133. doi: 10.1007/s10072-021-05300-4. [DOI] [PubMed] [Google Scholar]
- 21.Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102(10):3647–3661. doi: 10.1210/jc.2017-01138. [DOI] [PubMed] [Google Scholar]
- 22.Kim MK, Yi MS, Park PG, et al. Effect of stellate ganglion block on the regional hemodynamics of the upper extremity: a randomized controlled trial. Anesth Analg. 2018;126(5):1705–1711. doi: 10.1213/ANE.0000000000002528. [DOI] [PubMed] [Google Scholar]
- 23.TL L-C. Sleep and sleep disorders: an overview. Med Clin N Am. 2004;88(3):xi–xiv. 10.1016/j.mcna.2004.02.005. [DOI] [PubMed]
- 24.Cheng W, Rolls ET, Ruan H, Feng J. Functional connectivities in the brain that mediate the association between depressive problems and sleep quality. JAMA Psychiat. 2018;75(10):1052–1061. doi: 10.1001/jamapsychiatry.2018.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westerhaus M, Loewy A. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res. 2001;903:117–127. doi: 10.1016/s0006-8993(01)02453-2. [DOI] [PubMed] [Google Scholar]
- 26.Lipov E, Gluncic V, Lukić IK, Candido K. How does stellate ganglion block alleviate immunologically-linked disorders? Med Hypoth. 2020;144:110000. 10.1016/j.mehy.2020.110000. [DOI] [PubMed]
- 27.Uchida K, Tateda T, Hino H. Novel mechanism of action hypothesized for stellate ganglion block related to melatonin. Med Hypoth. 2002;59(4):446–449. doi: 10.1016/s0306-9877(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 28.Iwama H, Adachi M, Tase C, Akama Y. Cervical sympathectomy affects adrenocorticotropic hormone and thyroid-stimulating hormone in rats. J Anesth. 1996;10(3):181–184. doi: 10.1007/bf02471387. [DOI] [PubMed] [Google Scholar]
- 29.Kreutz C, Müller J, Schmidt M, Steindorf K. Comparison of subjectively and objectively assessed sleep problems in breast cancer patients starting neoadjuvant chemotherapy. Support Care Cancer. 2021;29(2):1015–1023. doi: 10.1007/s00520-020-05580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barsasella D, Syed-Abdul S, Malwade S, et al. Sleep quality among breast and prostate cancer patients: a comparison between subjective and objective measurements. Healthcare (Basel) 2021;9(7):785. doi: 10.3390/healthcare9070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baglioni C, Nanovska S, Regen W, et al. Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol Bull. 2016;142(9):969–990. doi: 10.1037/bul0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conley S, Knies A, Batten J, et al. Agreement between actigraphic and polysomnographic measures of sleep in adults with and without chronic conditions: a systematic review and meta-analysis. Sleep Med Rev. 2019;46:151–160. doi: 10.1016/j.smrv.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nascimento-Ferreira MV, Collese TS, de Moraes ACF, et al. Validity and reliability of sleep time questionnaires in children and adolescents: a systematic review and meta-analysis. Sleep Med Rev. 2016;30:85–96. doi: 10.1016/j.smrv.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Rentz L, Ulman H, Galster S. Deconstructing commercial wearable technology: contributions toward accurate and free-living monitoring of sleep. Sensors (Basel, Switzerland) 2021;21(15):5071. doi: 10.3390/s21155071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 36.Kumar N, Thapa D, Gombar S, Ahuja V, Gupta R. Analgesic efficacy of pre-operative stellate ganglion block on postoperative pain relief: a randomised controlled trial. Anaesthesia. 2014;69(9):954–660. doi: 10.1111/anae.12774. [DOI] [PubMed] [Google Scholar]
- 37.Sermeus LA, Vanlinthout LE, Hans GH, et al. Effects of stellate ganglion block on analgesia produced by cervical paravertebral block as established by quantitative sensory testing: a randomized controlled trial. Pain Med. 2018;19(11):2223–2235. doi: 10.1093/pm/pny004. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y-Q, Liu Z, Liu Z-H, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflamm. 2016;13(1):141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YYK, Boden KA, Schreiber KL. The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: a narrative review. Anaesthesia. 2021;76(Suppl):1. doi: 10.1111/anae.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei N, Chi M, Deng L, Wang G. Antioxidation role of different lateral stellate ganglion block in isoproterenol-induced acute myocardial ischemia in rats. Reg Anesth Pain Med. 2017;42(5):588–599. doi: 10.1097/aap.0000000000000647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available in the Open Science Framework (OSF) repository (osf.io/yugjh/; [https://doi.org/10.17605/OSF.IO/YUGJH]).