Abstract
Background & Aims
Liver macrophage-mediated inflammation contributes to the pathogenesis of the nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Odd skipped-related 1 (Osr1) is a putative transcription factor previously reported to be involved in NASH progression; however, the underlying mechanisms remain unknown. The current study focused on the role of Osr1 in macrophage polarization and metabolism and its associated functions in the inflammation-induced pathogenesis of NASH.
Methods
OSR1/Osr1 expression patterns were compared in normal and NASH patients and mouse livers. NASH was established and compared between hepatocyte-specific Osr1 knockout (Osr1ΔHep), macrophage-specific Osr1 knockout (Osr1ΔMφ), and wild-type (Osr1F) mice fed with 3 different chronic obesogenic diets and methionine choline-deficient diet. Using genetic and therapeutic strategies in vitro and in vivo, the downstream targets of Osr1 and the associated mechanisms in inflammation-induced NASH were established.
Results
Osr1 was expressed in both hepatocytes and macrophages and exhibited different expression patterns in NASH. In NAFLD and NASH murine models, deleting Osr1 in myeloid cells (Osr1ΔMφ), but not hepatocytes, aggravated steatohepatitis with pronounced liver inflammation. Myeloid Osr1 deletion resulted in a polarization switch toward a pro-inflammatory phenotype associated with reduced oxidative phosphorylation activity. These inflamed Osr1ΔMφ macrophages promoted steatosis and inflammation in hepatocytes via cytokine secretion. We identified 2 downstream transcriptional targets of Osr1, c-Myc, and PPARγ and established the Osr1-PPARγ cascade in macrophage polarization and liver inflammation by genetic study and rosiglitazone treatment in vivo. We tested a promising intervention strategy targeting Osr1-PPARγ by AAV8L-delivered Osr1 expression or rosiglitazone that significantly repressed NAFLD/NASH progression in Osr1F and Osr1ΔMφ mice.
Conclusions
Myeloid Osr1 mediates liver immune homeostasis and disrupting Osr1 aggravates the progression of NAFLD/NASH.
Keywords: Inflammation, Macrophages, Metabolism, NASH, Osr1
Graphical abstract
Summary.
Osr1 regulates macrophage-mediated liver inflammation during nonalcoholic steatohepatitis development by modulating cell polarization and metabolisms. Targeting macrophage Osr1 can be a promising treatment strategy for nonalcoholic steatohepatitis.
Nonalcoholic fatty liver disease (NAFLD) is associated with metabolic syndrome, diabetes, obesity, and hyperlipidemia; it has become one of the most common chronic liver diseases, affecting around 25% of the global population.1,2 One-third of NAFLD develops into a more inflammatory subtype, nonalcoholic steatohepatitis (NASH), characterized by hepatic inflammation and steatosis with or without fibrosis.3 More recent analyses show an overall NASH prevalence of 59% in NAFLD-biopsied patients.4 Among those without an NAFLD diagnosis, 3% to 5% of all adults are estimated to have NASH.5 The classic “2-hit” hypothesis proposed that lipotoxicity-induced oxidative stress, endoplasmic reticulum stress, and increased inflammation drive hepatic injury in NASH.6 Immune imbalance accompanied by dietary and metabolic factors and genetic susceptibility contribute to NAFLD pathogenesis. The increasingly accepted “multiple parallel hit” model considers environmental factors, genetic and epigenetic influences, and variations in the crosstalk between multiple tissues and organs.7 In both theories, inflammation involving macrophage actions is the central mechanism, suggesting targeting macrophages for promising therapeutic strategies.
In the liver, macrophages are classified into 2 major subsets, liver-resident Kupffer cells (KCs) and recruited monocyte-derived macrophages from peripheral blood. Following hepatic injury, KCs recruit additional monocytes that undergo macrophage metabolic reprogramming correlated with their functional state. Although several subpopulations were identified during liver injury, the classical M1/M2 theory remains fundamental. In high-fat diet (HFD) and or methionine- and choline-deficient diet (MCD)-fed mice, there is macrophage infiltration with a dominant M1 phenotype that relies on glycolysis to sustain phagocytic activity and cytokine production; the result is pronounced inflammation-induced hepatic injury.8 Differentiation toward alternatively activated macrophages (M2 type), dependent on oxidative phosphorylation (OXPHOS) and fatty acid oxidation (FAO),9 is associated with hepatic injury attenuation and improved insulin sensitivity.10 These findings suggest that targeting macrophage metabolism is a promising way to address macrophage-associated inflammation. Nevertheless, it remains unclear how macrophage metabolism is regulated to alter macrophage polarization (or vice versa), contributing to NASH pathophysiology.
Odd skipped-related 1 (Osr1) encodes a putative transcription factor containing 4 C2H2-type zinc finger motifs.11 Osr1 was essential for developing significant organs in a murine model, including the heart, lung, and kidney.12,13 Osr1 is a tumor suppressor gene and a potential prognostic biomarker in many cancers.14, 15, 16 We recently reported that Osr1 is involved in NAFLD progression.17,18 Osr1+/- mice displayed liver injury during NAFLD induction with overactivated JNK and NF-κB signaling and elevated hepatic expression levels of pro-inflammatory cytokine genes. The current study investigated the cell-specific role of Osr1 in macrophage polarization and metabolism, providing a better mechanistic understanding of how macrophage-associated inflammation drives NASH progression.
Results
OSR1/Osr1 was Highly Expressed in Macrophages During NASH
The expression pattern of OSR1/Osr1 was examined in the liver tissues of humans and mice. In humans, strong OSR1 expression was observed in several cell types in healthy livers (Figure 1A). Hepatocyte OSR1 expression was predominantly found in the cytosol, which formed clusters (Figure 1A). By contrast, expression of OSR1 in non-parenchymal cells (NPCs) was observed in the nucleus (Figure 1A, green arrow). Interestingly, although OSR1 staining in the NASH liver was significantly reduced in the hepatocytes, it was maintained in the NPCs of increased numbers (Figure 1A, red arrow).
Figure 1.
OSR1/Osr1 expression exhibits heterogeneity in hepatocytes and macrophages during human and murine NASH. (A) Representative immunohistochemistry (IHC) staining of OSR1 in normal and NASH patient liver. (B) Relative expression of Osr1 in normal chow, HFD or MCD diet induced liver and associated NPCs in mice. (C) Representative IHC staining of Osr1 in murine normal and NASH liver. (D) Immunofluorescence staining of Osr1 and F4/80 in murine normal and NASH liver. For IHC staining, macrophages were stained in brown, and the total number of cells occupied was measured for each section with the same magnificent. Numeric data are means ± standard error. n = 4. Significant difference: ∗∗P < .01.
In the mouse liver, the expression pattern of Osr1 was similar to that of the human liver. In the NASH liver, Osr1 expression was significantly decreased in the hepatocytes but maintained in the NPCs with increased abundance (Figure 1B and Figure 1C, red arrow). Using co-immunofluorescence (IF) staining of Osr1 and F4/80, we found that Osr1 was expressed in a subset of F4/80+ cells. However, the staining of Osr1 was not co-labeled with Clec4f, the KC-specific marker (Figure 1D). These results suggest that monocyte-derived macrophages are a source of Osr1-expressing cells.
Specifically Deleting Osr1 in Myeloid Cells Promoted HFD and MCD-induced Hepatic Steatosis and Inflammation
To identify the cell sources in which Osr1 contributes to the repression of NAFLD/NASH, we determined whether deleting Osr1 in hepatocytes or macrophages would promote NAFLD/NASH progression.
Osr1F, Osr1ΔHep/+, and Osr1ΔHep mice were fed with either chow diet (CD) or 60% HFD for 12 weeks (n = 8). By the end of week 12, the Osr1ΔHep mice had a similar weight and liver/body weight ratio to the Osr1F mice, regardless of sex and dietary treatment. All groups had similar intraperitoneal glucose tolerance test results and developed similar levels of steatosis (Figure 2A–D). These results suggest that hepatocyte Osr1 deletion was not the major contributor to NAFLD progression.
Figure 2.
Deleting Osr1 in hepatocytes did not affect the HFD-induced NAFLD phenotype in either female or male mice. (A) The body weight change, the liver/body weight ratio, intraperitoneal glucose tolerance test (IPGTT), and representative hematoxylin and eosin (H&E) staining in female Osr1F, Osr1ΔAlb/+, and Osr1ΔAlb/-mice fed with CD for 12 weeks. (B) The body weight change, the liver/body weight ratio, IPGTT, and representative H&E staining in female Osr1F, Osr1ΔAlb/+, and Osr1ΔAlb/-mice fed with HFD for 12 weeks. (C) The body weight change, the liver/body weight ratio, IPGTT, and representative H&E staining in male Osr1F, Osr1ΔAlb/+, and Osr1ΔAlb/-mice fed with CD for 12 weeks. (D) The body weight change, the liver/body weight ratio, IPGTT, and representative H&E staining in male Osr1F, Osr1ΔAlb/+, and Osr1ΔAlb/-mice fed with HFD for 12 weeks (n = 8). Numeric values are presented as means ± standard error.
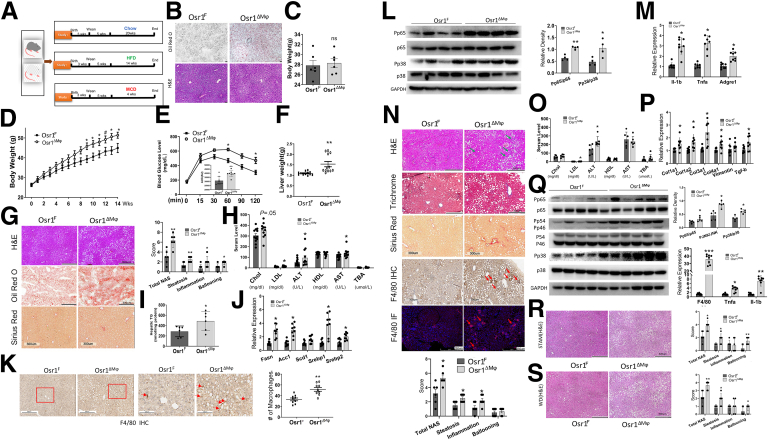
With myeloid-specific Osr1 deletion, when fed with CD for 20 weeks (Figure 3A), the Osr1ΔMφ and control mice had similar body weight, and hepatic steatosis was not developed; however, there was more lipid deposition in the liver of the Osr1ΔMφ mice (Figure 3B–C).
Figure 3.
Deletion of macrophage Osr1 aggravated HFD, and the MCD diet-induced hepatic steatosis and inflammation in mice. (A) Osr1F and Osr1ΔMφ mice were treated with CD for 20 weeks, HFD for 14 weeks, or MCD for 4 weeks. (B) Oil red O staining in frozen sections and hematoxylin and eosin (H&E) staining in paraffin embedded sections upon CD. (C) The mean body weight upon CD. (D) Body weight gain upon HFD. (E) Intraperitoneal glucose tolerance test (IPGTT) upon HFD. (F) Liver weight upon HFD. (G) Representative images of H&E, Oil Red-O, or Sirius red staining, and NAS of indicated groups under HFD treatment. (H) Lipid metabolism and liver damage serum markers. (I) Liver triglyceride and de novo lipogenesis-related genes (J) in the liver upon HFD. (K) Quantification of liver macrophages. (L) liver pro-inflammatory signaling and inflammation-related genes upon HFD (M). (N) Representative images of H&E, Tri-Chrome Masson, Sirius Red, F4/80 immunohistochemistry, and IF staining. NAS of indicated groups under MCD diet treatment. (O) Lipid metabolism and liver damage serum markers. (P) Liver fibrosis-related genes under MCD diet treatment. (Q) Liver pro-inflammatory signaling and genes under MCD diet treatment. (R) Representative images of H&E staining of Osr1F and Osr1ΔMφ liver induced with STAM model and associated NAS scoring. (S) Representative images of H&E staining of Osr1F and Osr1ΔMφ liver under WD treatment for 20 weeks and associated NAS scoring. For (K) and (N) macrophages were stained in brown, and the total area occupied by these cells was measured. Numeric data are means ± standard error. n = 8–12. Significant differences between Osr1F and Osr1ΔMφ are indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Fed with HFD (Figure 3A), significantly higher body weight was observed in the Osr1ΔMφ male (Figure 3D) but not female mice (Figure 4A–B), accompanied by worsening glucose intolerance (Figure 3E) and heavier liver (Figure 3F). The Osr1ΔMφ male liver exhibited enhanced steatosis with a higher NAFLD activity score (NAS) (Figure 3G), consistent with the higher serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and low-density lipoprotein (LDL) (Figure 3H), and the increased hepatic triglyceride (TG) content (Figure 3I). The increased steatosis in the Osr1ΔMφ mice was accompanied by the increased expression of lipogenesis genes (Figure 3J). In addition, Osr1ΔMφ male liver showed increased macrophage infiltration (Figure 3K), associated with overactivated pro-inflammatory signaling (Figure 3L) and higher pro-inflammatory cytokine mRNA levels (Figure 3M), suggesting more hepatic inflammation in Osr1ΔMφ mice.
Figure 4.
Deleting macrophage Osr1 did not affect the experimental NASH phenotype in female mice. (A) The mean body weight in female Osr1F and Osr1ΔMφ mice fed with HFD for 12 weeks. (B) Representative images of hematoxylin and eosin (H&E) staining and NAS score for liver sections from female Osr1F and Osr1ΔMφ mice under HFD treatment. (C) Representative images of H&E staining and NAS score for liver sections from female Osr1F and Osr1ΔMφ mice under MCD diet treatment for 4 weeks. Numeric data is presented as means ± standard error.
In the MCD-induced NASH model (Figure 3A), male (Figure 3N) but not female Osr1ΔMφ mice (Figure 4C) exhibited more advanced NASH progression, characterized by more macrovesicular steatosis and elevated serum ALT and LDL levels (Figure 3O). Trichrome Masson and Sirius Red staining revealed more collagen deposition in the Osr1ΔMφ livers (Figure 3N) with enhanced expression of collagen-producing gene expression (Figure 3P). We observed more inflammation in the Osr1ΔMφ livers, characterized by more microgranulomas (Figure 3N, green arrow), F4/80+ macrophages, hepatic crown-like structures (hCLS) (Figure 3N, red arrow), and increased expression of pro-inflammatory cytokine genes (Tnf-α and Il-1β) (Figure 3Q). Western blots demonstrated overactivated pro-inflammatory signaling in the Osr1ΔMφ liver (Figure 3Q). To recapitulate human NASH, we further adapted 2 additional NASH models, a relatively rapid STAM model and a chronic Western diet (WD) model, to investigate the role of macrophage Osr1 in NASH pathogenesis (Figure 3R–S). In both models, myeloid-specific Osr1 deletion resulted in more severe NASH. The histological investigation revealed more steatosis and ballooning in Osr1ΔMφ livers, confirmed by higher total NAS scores (Figure 3R–S). These results suggest that myeloid Osr1 deletion induces severe NAFLD/NASH progression, with advanced steatosis, fibrosis, and aggravated inflammatory responses in male mice.
Deleting Osr1 in Myeloid Cells Skewed Macrophage Polarization
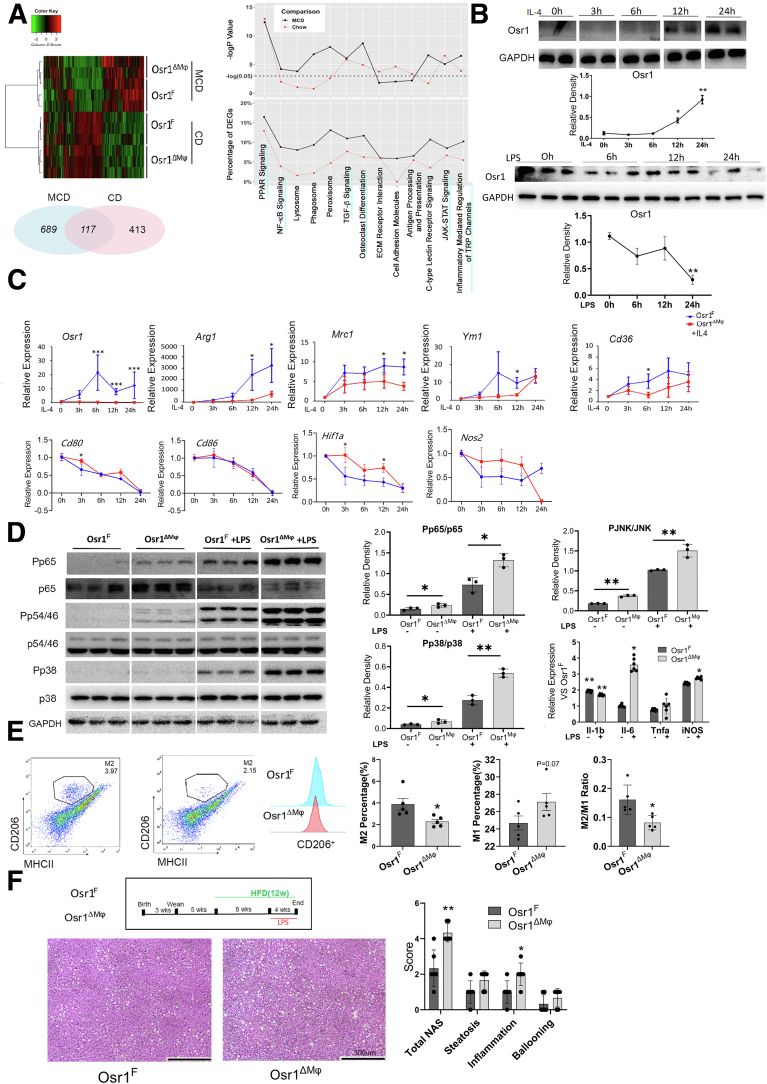
To determine how Osr1 deletion promotes NASH, RNA sequencing (RNA-seq) analysis was performed on the Osr1F and Osr1ΔMφ mice fed with CD or MCD (Figure 5A). Gene ontology (GO) analysis identified distinct Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways associated with macrophage plasticity and polarization, including NF-κB, PPAR, JAK-STAT, and osteoclast differentiation signaling (Figure 5A), suggesting a critical role of Osr1 in macrophage differentiation and polarization.
Figure 5.
Osr1 was required for macrophage alternative M2 polarization in vivo and in vitro. (A) Heat map and pie chart indicated differentially expressed genes (DEGs). The GO analysis and distinct KEGG pathways associated with Osr1 level on CD or MCD. Upper panel, any –logP value higher than the dotted line was identified as significant (P < .05). Lower panel, the y-axis indicates the percentage of DEGs. (B) Relative expression of Osr1 in resting macrophages (M0) and after exposure to IL-4 or LPS. (C) Expression of M1 and M2 markers relative to M0 after exposed to IL-4 in the presence or absence of Osr1. (D) Pro-inflammatory signaling and cytokine production. (E) Quantification for M1 and M2 macrophages. (F) Representative hematoxylin and eosin staining and the associated NAS for Osr1F and Osr1ΔMφ mice liver when exposed to LPS under 12 weeks of HFD treatment (n = 6). Results were shown as means ± standard error of n = 8 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
To test the hypothesis that Osr1 regulates macrophage polarization, bone marrow-derived macrophages (BMDMs) were isolated from Osr1F and Osr1ΔMφ mice and induced to differentiate in vitro. Osr1 expression was relatively weak initially (considered M0), and sustained induction of Osr1 was observed after exposure to interleukin (IL)-4 (Figure 5B). However, the expression pattern of Osr1 was opposite when exposed to lipopolysaccharide (LPS) (Figure 5B). With Osr1 deletion, M2-specific genes, including Arg1, Mrc1, Ym1, and CD36 failed to upregulate upon IL-4 treatment for 24 hours, whereas expression fluctuation was noted on M1-related genes Cd80, Hif1a, and Nos2 (Figure 5C). Consistently, Osr1 deletion resulted in activation of pro-inflammatory p38, JNK, NF-κB p65 (Figure 5D), and overexpression of pro-inflammatory cytokines genes under basal conditions (M0) or LPS induction (Figure 5D). Similar Osr1-associated polarization switches were noted in vivo. MCD-fed mice showed peak inflammatory cytokine production at week 4.19 A significantly fewer percentage of M2 macrophages was observed in the Osr1ΔMφ liver upon 4-week MCD feeding, resulting in a significantly decreased M2/M1 ratio (Figure 5E).
Considering a strong response of Osr1ΔMφ BMDMs to LPS, we further explored this phenomenon in vivo. Osr1F and Osr1ΔMφ mice were exposed to HFD for 12 weeks and were intraperitoneally injected with LPS (100 ug/kg/day) for the last 4 weeks of HFD treatment. Liver histology indicated that Osr1ΔMφ mice exhibited more advanced NASH progression with increased inflammation levels (Figure 5F), which is consistent with our in vitro study.
PPARγ and c-Myc are Direct Targets of Osr1 for Regulating Macrophage Alternative M2 Polarization
Our RNA-seq analysis identified differential expression of c-Myc and Pparγ in the Osr1ΔMφ vs Osr1F liver under both CD and MCD. Confirmed in the BMDMs, PPARγ and c-Myc expression were associated with Osr1 level during the phenotype switch between M1 and M2 (Figure 6A). However, PPARγ and c-Myc expression no longer responded to IL-4 induction upon Osr1 deletion (Figure 6B). Osr1 overexpression in RAW264.7 cells resulted in increased expression of PPARγ and c-Myc (Figure 6C). These results suggest that PPARγ and c-Myc expression depends on Osr1.
Figure 6.
Osr1 directly transactivated PPAR-γ and c-Myc in macrophages. (A) The expression level of Osr1, PPARγ, and c-MYC upon M1 induction, M2 induction, M1-M2 switch (M1 switch to M2 induction), and M2-M1 switch (M2 switch to M1 induction). (B) Expression of the PPARγ and c-MYC in M0 and after exposure to IL-4 in Osr1F and Osr1ΔMφ BMDMs. (C) PPARγ and c-MYC expression in indicated RAW264.7 cells. (D) Chromatin immunoprecipitation-qPCR of Osr1 in c-MYC and PPARγ promoters. (E) Relative luciferase activities compared with control. (F) M2 or M1 marker mRNA levels relative to M0 in 1 μM rosiglitazone. BMDMs were obtained from bone marrow and induced to M0 macrophages with 50 ng/mL M-CSF. The induced macrophages were further stimulated with lipopolysaccharide (LPS, 0.1 μg/mL) and interferon-gamma (IFN-γ, 20 ng/mL) or with IL-4 (20 ng/mL) to induce polarization toward M1 or M2 phenotypes, respectively. Results are displayed as means ± standard error. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
To determine whether Osr1 regulates the transactivation of PPARγ and c-Myc, we performed a bioinformatically incorporative analysis. We identified potential genomic regions of c-Myc and PPARγ that were further determined by chromatin immunoprecipitation quantitative real-time polymerase chain reaction (qPCR) (Figure 6D) and luciferase reporter assay (Figure 6E). To establish the functional Osr1-PPARγ regulation in macrophage polarization, Osr1F or Osr1ΔMφ BMDMs were treated with IL-4 in the presence of rosiglitazone, a PPARγ agonist. Although rosiglitazone failed to fully rescue the expression of Mrc1 and c-Myc in Osr1ΔMφ BMDMs, it rescued the typical response to IL-4, a signature of enhanced expression of Arg1, Ym1, and CD36 (Figure 6F). Simultaneously, the expression level of the 4 M1 markers except for Hif1awere similar in Osr1F and Osr1ΔMφ BMDMs (Figure 6F). These findings suggest that PPARγ and c-Myc are downstream targets of Osr1 in macrophage polarization.
Osr1 is Required for Palmitate Oxidation, and Deleting Osr1 Shifted Macrophage Metabolism Toward a Glycolysis-dependent ATP Production Profile
Cellular metabolism reprogramming is a hallmark of macrophage polarization. With glucose and pyruvate as substrates, the total ATP production rates were similar in Osr1F and Osr1ΔMφ macrophages; however, deleting Osr1 caused a 25% reduction in the OXPHOS rate (Figure 7A) (66.53% ± 1.43% vs 59.38% ± 2.55%; P < .001) and a 21.4% increase in the glycolysis rate (Figure 7A) (33.47% ± 1.43% vs 40.6% ± 2.55%; P < .001), resulting in an increased ratio in glycolysis vs OXPHOS (Figure 7A). Similarly, deleting Osr1 significantly reduced the OXPHOS rate (Figure 7B) and increased the rate of glycolysis during M2 induction (Figure 7B). With the presence of rosiglitazone, the skewed glycolysis and OXPHOS in Osr1ΔMφ BMDMs were recovered (Figure 7B). Deleting Osr1 led to an impaired response to PA, evidenced by about 30% lower basal and maximal oxygen consumption rate (OCR), which were recovered by rosiglitazone (Figure 7C). These findings suggest that Osr1 helps maintain FAO, which depends on PPARγ.
Figure 7.
Osr1 deletion shifted the macrophage to glycolysis ATP production profile and disrupted the mitochondrial palmitate oxidation during M2 polarization. (A–B) Real-time APT rate analysis in Osr1F or Osr1ΔMφ BMDMs during M1 (A) or M2 (B) polarization. (C) Mitochondrial respiration function assessed by Seahorse Mito Stress test using palmitate (BSA-conjugated palmitate) as substrates. OXPHOS parameters were assessed by recording the OCR values after sequential OM, FCCP, and Rot+AA injection. (D) Representative images of converted NAD(P)H and FAD under FLIM. The calculated intensity of the bounded form of NAD(P)H and mitochondrial FLIRR was plotted with indicated induction in bar graphs (right 2 panels). AA, Antimycin A; FCCP, carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; OM, oligomycin; Ros, rosiglitazone; Rot, rotenone. The number of cells analyzed for FLIM: 62, 75, 240, 154, 133 for M0, M0+PA, M1, M2, M2+PA, respectively, in Osr1F group, and 102, 79, 112, 39, 65, 254 for M0, M0+PA, M1, M2, M2+PA, M2+PA+Ros, respectively, in Osr1ΔMφ BMDMs. The analysis is completed using R programming. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
The OXPHOS activity consumes NADH (increased NADH-enzyme-bound fraction) and produces FAD (decreased FAD enzyme-bound fraction).20 To determine whether Osr1 targets mitochondrial OXPHOS, we applied fluorescence lifetime imaging microscopy (FLIM) to track the mitochondrial NAD(P)H and FAD at the single-cell level (Figure 7D). Osr1 deletion resulted in a decreased enzyme-bound NAD(P)H intensity in the M2 but not M0 and M1 BMDMs (Figure 7D). From the mitochondrial fluorescence lifetime redox ratio (FLIRR), defined as the fraction of bound NAD(P)H (α2) divided by the fraction of bound FAD (α1),20 we observed significantly decreased FLIRR in the Osr1ΔMφ M2 but not the M0 and M1 (Figure 7D). These results suggest an impaired mitochondrial OXPHOS in the Osr1ΔMφ M2 BMDMs. When PA was given to M2 macrophages, Osr1ΔMφ M2 BMDMs exhibited decreased enzyme-bound NAD(P)H intensity and FLIRR, entirely recovered by rosiglitazone treatment (Figure 7D). A lower FLIRR value was also found in the Osr1ΔMφ M0 BMDMs under PA treatment. These findings suggest that deleting Osr1 significantly disrupts OXPHOS in M2 BMDMs.
Deleting Macrophage Osr1 Aggravated the Inflammation and Fat Deposition in Hepatocytes via Cytokine Production
To determine how inflamed Osr1ΔMφ macrophages disrupt lipid homeostasis and promote inflammation in hepatocytes, 3 sets of macrophages and hepatocytes co-culture were conducted. First, when primary hepatocytes were co-cultured with the Osr1ΔMφ M2 BMDMs, the cell mixture had significantly higher Pp65/p65 and Pp38/p38 ratios than the cell mixture co-cultured with the Osr1F M2 BMDMs (Figure 8A). Similarly, the cell co-culture of Osr1ΔMφ M1 BMDMs and hepatocytes displayed overactivation of p38 signaling, supported by an increased Pp38/p38 ratio (Figure 8A). Second, using a transwell system, we observed higher ratios of Pp65/p65 (P < .05) and Pp38/p38 (P = .06) in the hepatocytes co-cultured with M1 Osr1ΔMφ BMDM than those with Osr1F M1 BMDMs (Figure 8B). The hepatocytes co-cultured with M2 Osr1ΔMφ BMDMs significantly increased the Pp38/p38 ratio (Figure 8B). Third, hepatocytes cultured with Osr1ΔMφ-conditioned medium significantly increased lipid deposition under BSA and PA treatment (Figure 8C) associated with elevated mRNA levels of pro-inflammatory cytokines (Tnfa, Il-6, and Il-1β) and de novo lipogenesis genes (Figure 8D). These findings suggest that deleting macrophage Osr1 aggravates hepatocyte inflammation and fat deposition by modulating cytokine production.
Figure 8.
Osr1 disruption in macrophages aggravated hepatocyte inflammation and fat deposition via cytokine excretion. (A) Pro-inflammatory signaling in macrophage-hepatocyte co-cultured cell lysates. (B) Pro-inflammatory signaling in hepatocytes transwell co-cultured with macrophages. (C) Representative hepatocyte fat deposition in conditioned medium from Osr1F and Osr1ΔMφ macrophages. (D) The mRNA levels of lipogenesis and pro-inflammatory cytokines from hepatocytes cultured in the conditioned medium. Numeric data are presented as means ± standard error (n = 6). Significant differences are indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Pharmacological Activation of PPARγ Signaling Prevented HFD-induced Steatohepatitis in Osr1ΔMφ Mice
To determine the functional role of Osr1-PPARγ regulation in NASH progression, we performed genetic and in vivo pharmacological studies using rosiglitazone. In the genetic study, compound heterozygotes of Osr1 and PPARγ (Osr1ΔMφ/+PPARγΔMφ/+) and their littermate control mice were induced to develop NAFLD. The NAFLD progression of the Osr1ΔMφ/+ and the PPARγΔMφ/+ livers were similar. However, the Osr1ΔMφ/+PPARγ ΔMφ/+ livers displayed more advanced NASH with higher NAS, with higher scores in steatosis, inflammation, and hepatocyte ballooning than their littermate controls (Figure 9A–B).
Figure 9.
Osr1 and PPARγ functionally interacted in macrophages during the pathogenesis of NAFLD/NASH. (A) Hematoxylin and eosin and Sirius red staining in the indicated mice. (B) NAS scoring in the indicated mice during HFD treatment for 12 weeks. Numeric data are presented as means ± standard error. Significant differences are indicated as follows: ∗P < .05, ∗∗P < .01.
Rosiglitazone treatment significantly reduced the body weight gain (Figure 10A–B) and improved glucose intolerance at week 14 in Osr1ΔMφ mice (Figure 10C), whereas the liver weight or liver/body weight ratio remained the same (Figure 10D). Further analysis in liver lysates indicated more sensitized insulin signaling in rosiglitazone-treated Osr1ΔMφ mice (Figure 10E).
Figure 10.
Pharmacological PPARγ agonist rosiglitazone prevented HFD induced liver injury and inflammation in Osr1ΔMφmice. (A) Schematic diagram for the study design with HFD and rosiglitazone. DMSO or rosiglitazone was administered to Osr1F and Osr1ΔMφ mice at 10 mg/kg/day during the final 4 weeks of the total 14 weeks of HFD treatment. (B) Body weight gain during the rosiglitazone/DMSO treatment. (C) Intraperitoneal glucose tolerance test (IPGTT) results and the area under the curve (AUC) in indicated groups after the rosiglitazone treatment. (D) Liver weight and liver/body weight ratio in indicated mice. (E) Prior to harvest, mice were intraperitoneally injected with insulin or DMSO. Insulin signaling of the extracted proteins from the Osr1ΔMφ liver were inspected. (F) Lipid metabolism and liver damage serum markers. (G) NAS score, representative hematoxylin and eosin (H&E) staining, Oil Red O staining in perfused hepatocytes and associated quantification, and TG level in the liver (H). (I) Quantification of macrophage numbers in Osr1ΔMφ liver. (J) Pro-inflammatory signals in Osr1ΔMφ liver. For F4/80 immunohistochemical (IHC) staining, macrophages were stained brown, and the total number of cells occupied was measured for each section with the same magnificent. Numeric data are presented as means ± standard error (n = 6–8). Significant differences are indicated as follows: ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001. DMSO, Dimethyl sulfoxide; Ros, rosiglitazone.
Significantly lower serum ALT and LDL levels (Figure 10F), improved NAFLD phenotype (Figure 10G), and reduced TG content in perfused hepatocytes and liver (Figure 10H) were observed in the Osr1ΔMφ mice treated with rosiglitazone. The Osr1ΔMφ mice livers revealed lower numbers of infiltrated macrophages (Figure 10I) and deactivated pro-inflammatory signaling (Figure 10J) under rosiglitazone treatment. A treatment effect of rosiglitazone was also noticed in the Osr1F liver as indicated by a lower NAS score, less steatosis and ballooning hepatocytes (Figure 10G).
Inducing Osr1 Expression Therapeutically Improved the NAFLD/NASH of the Osr1ΔMφ Mice
In mouse models, we assessed the feasibility of targeting Osr1 for NAFLD/NASH treatment (Figure 11A). AAV8L transduction efficiency was confirmed by fluorescence-activated cell sorting showing that about 17% of the F4/80+ population was infected by AAV8L after AAV8L-GFP injection for 4 weeks (Figure 11B) and before the special diet treatment. After HFD treatment for 14 weeks, wild-type mice injected with AAV8L-Osr1 had about a 40% increase in Osr1 expression in hepatic NPCs by comparing with the control group (Figure 11C). The NPC expression of PPARγ was also increased upon AAV8L-Osr1 transduction (Figure 11C). The co-IF staining further indicated that the AAV8L-Osr1 efficiently replenished the Osr1 expression in Osr1ΔMφ mice macrophages (Figure 11D, yellow arrow).
Figure 11.
AAV8L-delivered Osr1 expression rescued NAFLD/NASH of the Osr1ΔMφmice. (A) Schematic diagram for the study design with special diet treatment and AAV infection. Mice were injected with pAAV8L-control or pAAV8L-Osr1 at the dosage of 1.0 × 1010 gc/mouse at wean (3 weeks of age) through retro-orbital venous sinus delivery, followed by HFD or MCD treatment for either 14 or 4 weeks. The time point when the fluorescence-activated cell sorting (B), Western blot (C), and IF staining (D) were conducted were indicated. (E) Body weight at sacrifice. Mice were fed with HFD for 14 weeks. (F) Intraperitoneal glucose tolerance test (IPGTT) results at 12 weeks of HFD and associated quantification. (G) Liver weight upon HFD treatment. (H) Representative hematoxylin and eosin (H&E) staining and NAS scoring under HFD and MCD treatment. (I) Representative IF staining of F4/80 and quantification. (J) Macrophage polarization in the liver. (K) Pro-inflammatory cytokines mRNA level. M1 and M2 macrophages were identified as CD45+F4/80+CD11b+MHCII+ CD206- and CD45+F4/80+CD11b+CD206+ MHCII-, respectively. Results are presented as means ± standard error of n = 5 independent experiments. Significant differences are indicated as follows: ∗P < .05; ∗∗P < .01.
When fed with HFD, the body weights of Osr1ΔMφ mice with AAVL-Osr1 were significantly lower than those with AAVL-control treatment. Similarly, the Osr1F mice with AAVL-Osr1 had marginally lower body weights than those with AAVL-control (Figure 11E). AAV8L-Osr1 administration reversed the glucose intolerance in Osr1ΔMφ mice during HFD treatment compared with Osr1F mice (Figure 11F). The Osr1ΔMφ mice with AAVL-Osr1 presented similar liver weight with that of AAV8L-Osr1F mice (Figure 11G). Regarding liver histology, Osr1 replenishment improved liver steatosis and inflammation in the Osr1ΔMφ mice (Figure 11H). Osr1 replenishment rescued liver inflammation in the Osr1ΔMφ mice by reduced macrophage infiltration (Figure 11I) with recovered M2 sub-populations and M2/M1 ratios. Interestingly, Osr1 overexpression also improved the NASH score, enlarged the M2 macrophage sub-population, and increased the M2/M1 ratio in Osr1F mice (Figure 11J). We also observed reduced proinflammatory cytokine mRNA levels during AAV8L-Osr1 administration (Figure 11K). These findings suggest that rescuing Osr1 expression improves NAFLD/NASH and liver inflammation.
Discussion
We established the role of Osr1 in macrophage metabolism and polarization and elucidated its associated functions in the inflammation-induced pathogenesis of NASH (Figure 12). Using mouse models with myeloid Osr1 deletion, we observed aggravated NAFLD/NASH progression induced by HFD or MCD, suggesting the protective role of macrophage Osr1 in NAFLD/NASH pathogenesis. Deleting Osr1 in macrophages resulted in the glycolysis-dependent energy metabolism and a polarization switch toward an inflammatory M1 phenotype. We identified c-Myc and PPARγ as the direct downstream targets of Osr1 in macrophage polarization. With a genetic study using myeloid-specific Osr1 and PPARγ compound heterozygote mice and a pharmaceutical PPARγ activation study, we elucidate a functional macrophage Osr1-PPARγ transcriptional cascade in liver inflammation and the associated NAFLD/NASH. Most importantly, by improving liver inflammation and recovering the macrophage M2/M1 ratio, AAV8L-Osr1 replenishment/overexpression inhibited liver inflammation, contributing to improved NASH.
Figure 12.
Osr1 regulates macrophage-mediated liver inflammation in non-alcoholic fatty liver disease progression. Schematic diagram of the mechanisms of NASH induced in Osr1ΔMφ mice. The Osr1-PPARγ cascade is a potential driver for macrophage M2 polarization by regulating cellular OXPHOS. Deleting Osr1 induced metabolic imbalance of glycolysis/OXPHOS in macrophages, promoting pro-inflammatory responses and steatosis in the liver.
Osr1 has been widely studied for embryonic development12,13,21 and tumorigenesis.14,15,22 However, its role in metabolism and inflammation has never been reported, even though it has been decades since the Odd, Osr1 homolog in Drosophila was found to mark plasmatocyte (Drosophila macrophage).23 In HFD/MCD diet-induced NAFLD/NASH models, myeloid-specific Osr1 deletion resulted in decreased M2 percentiles and M2/M1 ratios associated with elevated pro-inflammatory responses in the liver. These results suggest that Osr1 regulates macrophage polarization, probably by maintaining the M2 phenotype. Our study provides solid evidence to support this finding. First, Osr1 expression increased during macrophage M2 commitment, with decreased expression when switching to M1. Second, the Osr1 expression level correlated with M2 markers during downregulation or overexpression. Finally, Osr1-responsive genomic regions of PPARγ and c-Myc were identified. PPARγ plays a pivotal role in promoting the M2 phenotype switch by upregulating CD206 and CD16324 and modulating Kupffer M1/M2 polarization.25 Similarly, c-Myc resolved inflammation and drove macrophage M2 polarization.26 In our study, rosiglitazone rescued M2 polarization both in vitro and in vivo, demonstrating the functional role of the Osr1-PPARγ axis in this process. Thus, we identified a novel hierarchical network led by Osr1 (involving PPARγ and c-Myc) in modulating macrophage plasticity toward M2.
Macrophage infiltration of both M1 and M2 types is a signature of liver inflammation. Osr1 expression was found in a subpopulation of, but not all F4/80+ cells in the MCD-induced NASH model. In vitro, strong Osr1 expression was observed in the M2 and the transitional type (M1-M2) of macrophages. These data suggested that Osr1+ macrophages might be characterized by an intrinsic ability to switch plasticity during the progression of liver inflammation. Unfortunately, our data did not further address the exact phenotype of Osr1+ macrophages in the MCD-induced NASH model. However, we excluded that Osr1+ macrophages were a source of or derived from KCs, because Osr1 did not co-express with the KC marker, Clecf4. Future studies will need to further characterize the plasticity of Osr1-expressing macrophages under both physiological and pathophysiological status.
Macrophage polarization is accompanied by metabolic reprogramming, switching from an OXPHOS-based aerobic profile to a glycolysis-based anaerobic one and vice versa.27 Alternative macrophage polarization relies on the transcription factor PPAR-γ, its coactivator PGC1β, and its downstream target CD36,28 which promote FAO and mitochondrial OXPHOS. A potential role of Osr1 in metabolic reprogramming was highlighted by identifying its role in transactivating PPAR-γ. In our study, deleting Osr1 resulted in an increased ratio of glycolysis to OXPHOS in M1 and M2 BMDMs. During M2 polarization, deleting Osr1 significantly blocked OXPHOS, suggesting that Osr1 maintains OXPHOS in M2 BMDMs. Metabolic changes were pronounced under PA treatment. PA is elevated in NAFLD patients’ blood and induces metabolic inflammation by activating NF-κB signaling in metabolically-activated macrophages.29,30 In contrast, PA activates the anti-inflammatory PPARγ by unknown mechanisms.24,30 In our study, deleting Osr1 blocked the PA oxidation and the anti-inflammatory effects of PPARγ, supporting the notion that the anti-inflammatory effect in macrophages is mediated by Osr1. Our FLIM data did not determine whether Osr1 regulates glycolysis because FLIM does not resolve cytoplasmic enzyme-bound NAD(P). Osr1 may inhibit the inflammatory response of M1 BMDMs via c-Myc signaling, considering the negative role of c-Myc in macrophage glycolysis during the early stage of M1 polarization.31 Further information, such as Osr1-associated lactate production, needs to be elucidated.
Our attempts to interfere with NASH/NAFLD progression by targeting Osr1-mediated macrophage inflammation are promising. Mice with AAV8L-delivered Osr1 expression reduced liver inflammation with less macrophage infiltration and corrected M2/M1 ratios, significantly improving NAFLD/NASH phenotype. We could not exclude a possible synergic effect of Osr1-overexpression in various cell types (especially hepatocytes) on protecting NAFLD/NASH, although we showed that deleting hepatic Osr1 did not change the NAFLD/NASH progression. Targeting PPARγ, rosiglitazone treatment blocked NAFLD/NASH progression and improved insulin sensitivity, consistent with previous reports.32,33 Rosiglitazone acts on many cells, including adipocytes, hepatocytes, and macrophages. The Osr1ΔMφ mice showed reduced body weight gain upon rosiglitazone, possibly because of the effects on adipocytes24; however, inhibited body weight gain was not observed in the rosiglitazone treated Osr1F mice that also improved NASH. The therapeutic effects of rosiglitazone to reduce hepatic steatosis may be offset by their actions to enhance PPARγ expression on hepatocyte function.32 Thus, the amelioration of NAFLD in our study highlights a working mechanism of rosiglitazone targeting macrophage-mediated inflammation and insulin sensitization. Our findings suggest a promising treatment for NASH by targeting the macrophage-centered inflammation mediated by the Osr1-PPARγ axis.
Materials and Methods
Human Liver Samples
De-coded human liver samples came from the Tongji Hospital, Huazhong University of Science and Technology (Wuhan, China). The Osr1 immunohistochemistry staining was performed at the clinical pathology laboratory of Tongji Hospital (Wuhan, China).
Animals and Treatments
The Osr1fl/fl mice were generated as described.34,35 Mice were maintained in a C57BL/6 background on a 12-hour light/dark cycle. Hepatocyte and myeloid cell-specific Osr1-disrupted mice were generated using the Cre-LoxP strategy. Briefly, control Osr1fl/+ or Osr1fl/fl (Osr1F), heterozygous AlbCre+Osr1fl/+(Osr1ΔHep/+) and LysMcre/+Osr1fl/+(Osr1ΔMφ/+), and homozygous AlbCre+Osr1fl/fl (Osr1ΔHep) and LysMcre/+Osr1fl/fl (Osr1ΔMφ) mice were treated with regular chow diet, HFD (60% fat calories, 14 weeks), MCD (4 weeks) or Western diet (WD, 40% fat calories, 20% fructose and 2% cholesterol, 20 weeks) at the age of 8 weeks. STAM models were induced by a single subcutaneous injection of 200 ug streptozocin (Sigma) 2 days after birth and feeding with HFD for 4 weeks.
In a separate experiment, male Osr1F or Osr1ΔMφ mice were given daily intraperitoneal injections of either vehicle (dimethyl sulfoxide) or rosiglitazone (10 mg/kg/day) for 4 weeks after 10 weeks of HFD treatment. For the in vivo AAV rescue study, mice were treated with pAAV8L-control or pAAV8L-Osr1 through the retro-orbital venous sinus at wean, followed by 14 weeks of HFD or 4 weeks of MCD. All animal experiments were completed according to the protocols reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Intraperitoneal Glucose Tolerance Test
Mice were fasted overnight by transferring them to clean cages with no food in the upper or bottom sections of the cage. Mice were weighed and injected intraperitoneally with 20% glucose solution (2 g/kg body weight glucose). Blood from the tail vein was obtained at 0, 15, 30, 60, 90, and 120 minutes after the injection to determine blood glucose level with a glucose meter.
RNA Sequencing
RNA quantification was performed on a bioanalyzer (Agilent Technologies, Santa Clara, CA). One nanogram was used as input for library preparation using Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA) and Nextera XT Index Kit (Illumina, San Diego, CA). Libraries were quantified, normalized to 4 nM, pooled, and diluted to be sequenced on a NextSeq (Illumina) using 75 bp paired-end sequencing.
Real-time PCR
qPCR was performed using SYBR Green Supermix (Bio-Rad, Hercules, CA) on the CFX384 real-time system (Bio-Rad). After the cycling program, melting curve analysis was performed immediately after amplification to confirm primer specificity. Three or more biological replicates were used for each condition, and 2 technical replicates were performed for each sample. Quantification data were analyzed using methods derived from the comparative CT method. For gene expression analysis, genes of interest were normalized to Cyclophilin, and data were expressed as fold change against Cyclophilin (± standard error of the mean).
Fluorescence Lifetime Imaging of Macrophages and Imaging Processing
The NAD(P)H and FAD fluorescence lifetime images of macrophages were acquired using an inverted multi-photon fluorescence microscopy (Marianas, 3i) coupled to a 40X water-immersion objective (1.1 NA). NAD(P)H and FAD fluorescence were stimulated at 750 nm and 890 nm, respectively, using a titanium:sapphire femtosecond laser (COHERENT, Chameleon). The laser power was set at 16 mW for NAD(P)H fluorescence excitation and 30 mW for FAD fluorescence excitation; 400- to 480-nm and 500- to 580-nm bandpass filters were used to isolate NAD(P)H and FAD fluorescence, respectively. Two photomultiplier tubes (Hamamatsu) were used to detect the fluorescence for each channel. Fluorescence lifetime images were obtained using time-correlated single-photon counting electrons (SPC-150N, Becker, and Hickl). Each 256 × 256-pixel fluorescence lifetime image was obtained with a pixel dwell time of 50 μs and 5 frame repeats. The instrument response function was measured using the NAD(P)H channel from the second harmonic generation of urea crystals excited at 900 nm.
The fluorescence lifetime analysis was performed using SPCImage (Becker and Hickl). Fluorescence lifetime components were obtained by deconvolving the instrument response function and fitting the decay result to a 2-component exponential decay model (, where I(t) is the fluorescence intensity as a function of time t, and are the short and long lifetimes, respectively, and are their corresponding fractions (), and C accounts for the background noise. To obtain the lifetime values of each cell, cell masks were generated by segmenting NAD(P)H intensity images into individual cells using a semi-automated pipeline in CellProfiler. A mean fluorescence lifetime (), lifetime redox ratio (NAD(P)H α2/FAD α1), and mean lifetime component values for each cell were calculated in MATLAB based on the cell masks.
Statistical Analysis
Data were expressed as means ± standard deviations or means ± standard errors. Statistical significance was assessed using the unpaired, 2-tailed Student t tests or one-way analysis of variance. Significant difference was indicated as: ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Acknowledgments
CRediT Authorship Contributions
Lin Liu, MD, PhD (Conceptualization: Equal; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Software: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Yi Zhou, MD, PhD (Data curation: Supporting)
Zhimin Liu, MD, PhD (Data curation: Supporting; Investigation: Supporting)
Jiangyuan Li, Graduate Student (Data curation: Supporting; Methodology: Supporting; Software: Supporting)
Linghao Hu, Graduate Student (Data curation: Supporting; Investigation: Supporting)
Leya He, MD, PhD (Data curation: Supporting)
Guannan Gao, MS (Data curation: Supporting)
Brian Kidd, Graduate Student (Data curation: Supporting)
Alexandra Walsh, PhD (Data curation: Supporting; Validation: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Rulang Jiang, PhD (Resources: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Chaodong Wu, MD, PhD (Resources: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Ke Zhang, PhD (Data curation: Supporting; Formal analysis: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Linglin Xie, MD, PhD (Conceptualization: Lead; Formal analysis: Supporting; Funding acquisition: Lead; Investigation: Lead; Project administration: Lead; Supervision: Lead; Validation: Lead; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This project is supported by a National Institutes of Health R01 grant (1R01DK112368-01, PI: Linglin Xie).
References
- 1.Younossi Z.M., Blissett D., Blissett R., Henry L., Stepanova M., Younossi Y., Racila A., Hunt S., Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Siegel A.B., Zhu A.X. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellantani S., Caballeria J., Colombo M., Craxi A., Crespo J., Day C.P., Eguchi Y., Geier A., Kondili L.A., Kroy D.C., Lazarus J.V., Loomba R., Manns M.P., Marchesini G., Nakajima A., Negro F., Petta S., Ratziu V., Romero-Gomez M., Sanyal A., Schattenberg J.M., Tacke F., Tanaka J., Trautwein C., Wei L., Zeuzem S., Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Machado M.V., Diehl A.M. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology. 2016;150:1769–1777. doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Jindal A., Bruzzi S., Sutti S., Locatelli I., Bozzola C., Paternostro C., Parola M., Albano E. Fat-laden macrophages modulate lobular inflammation in nonalcoholic steatohepatitis (NASH) Exp Mol Pathol. 2015;99:155–162. doi: 10.1016/j.yexmp.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Viola A., Munari F., Sanchez-Rodriguez R., Scolaro T., Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1462. doi: 10.3389/fimmu.2019.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odegaard J.I., Ricardo-Gonzalez R.R., Red Eagle A., Vats D., Morel C.R., Goforth M.H., Subramanian V., Mukundan L., Ferrante A.W., Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulter D.E., Swaykus E.A., Beran-Koehn M.A., Goldberg D., Wieschaus E., Schedl P. Molecular analysis of odd-skipped, a zinc finger encoding segmentation gene with a novel pair-rule expression pattern. EMBO J. 1990;9:3795–3804. doi: 10.1002/j.1460-2075.1990.tb07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K.K., Xiang M., Zhou L., Liu J., Curry N., Suner D.H., Garcia-Pavia P., Zhang X., Wang Q., Xie L. Gene network and familial analyses uncover a gene network involving Tbx5/Osr1/Pcsk6 interaction in the second heart field for atrial septation. Hum Mol Genet. 2016;25:1140–1151. doi: 10.1093/hmg/ddv636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q., Lan Y., Cho E.S., Maltby K.M., Jiang R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288:582–594. doi: 10.1016/j.ydbio.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Yuan Y., Liang P., Guo X., Ying Y., Shu X.S., Gao M., Jr., Cheng Y. OSR1 is a novel epigenetic silenced tumor suppressor regulating invasion and proliferation in renal cell carcinoma. Oncotarget. 2017;8:30008–30018. doi: 10.18632/oncotarget.15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J., Liang Q., Cheung K.F., Kang W., Lung R.W.M., Tong J.H.M., To K.F., Sung J.J.Y., Yu J. Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells. Cancer. 2013;119:304–312. doi: 10.1002/cncr.27724. [DOI] [PubMed] [Google Scholar]
- 16.Zong L., Sun Y. OSR1 suppresses acute myeloid leukaemia cell proliferation by inhibiting LGR5-mediated JNK signalling. Autoimmunity. 2021;54:561–568. doi: 10.1080/08916934.2021.1975274. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y., Liu Z., Lynch E.C., He L., Cheng H., Liu L., Li Z., Li J., Lawless L., Zhang K.K., Xie L. Osr1 regulates hepatic inflammation and cell survival in the progression of non-alcoholic fatty liver disease. Lab Invest. 2021;101:477–489. doi: 10.1038/s41374-020-00493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch E.C., Liu Z., Liu L., Wang X., Zhang K.K., Xie L. Disrupting Osr1 expression promoted hepatic steatosis and inflammation induced by high-fat diet in mouse model. PLoS One. 2022;17 doi: 10.1371/journal.pone.0268344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellicoro A., Ramachandran P., Iredale J.P., Fallowfield J.A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 20.Hu L., Wang N., Cardona E., Walsh A.J. Fluorescence intensity and lifetime redox ratios detect metabolic perturbations in T cells. Biomed Opt Express. 2020;11:5674–5688. doi: 10.1364/BOE.401935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L., Liu J., Olson P., Zhang K., Wynne J., Xie L. Tbx5 and Osr1 interact to regulate posterior second heart field cell cycle progression for cardiac septation. J Mol Cell Cardiol. 2015;85:1–12. doi: 10.1016/j.yjmcc.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Iglesias D., Eliopoulos N., El Kares R., Chu L.L., Romagnani P., Goodyer P. A variant OSR1 allele which disturbs OSR1 mRNA expression in renal progenitor cells is associated with reduction of newborn kidney size and function. Hum Mol Genet. 2011;20:4167–4174. doi: 10.1093/hmg/ddr341. [DOI] [PubMed] [Google Scholar]
- 23.Jung S.H., Evans C.J., Uemura C., Bannerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 24.Odegaard J.I., Ricardo-Gonzalez R.R., Goforth M.H., Morel C.R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A.W., Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo W., Xu Q., Wang Q., Wu H., Hua J. Effect of modulation of PPAR-gamma activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7 doi: 10.1038/srep44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pello O.M., De Pizzol M., Mirolo M., Soucek L., Zammataro L., Amabile A., Doni A., Nebuloni M., Swigart L.B., Evan G.I., Mantovani A., Locati M. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–421. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Xu R., Gu H., Zhang E., Qu J., Cao W., Huang X., Yan H., He J., Cai Z. Metabolic reprogramming in macrophage responses. Biomark Res. 2021;9:1. doi: 10.1186/s40364-020-00251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vats D., Mukundan L., Odegaard J.I., Zhang L., Smith K.L., Morel C.R., Wagner R.A., Greaves D.R., Murray P.J., Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korbecki J., Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019;68:915–932. doi: 10.1007/s00011-019-01273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kratz M., Coats B.R., Hisert K.B., Hagman D., Mutskov V., Peris E., Schoenfelt K.Q., Kuzma J.N., Larson I., Billing P.S., Landerholm R.W., Crouthamel M., Gozal D., Hwang S., Singh P.K., Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae S., Park P.S.U., Lee Y., Mun S.H., Giannopoulou E., Fujii T., Lee K.P., Violante S.N., Cross J.R., Park-Min K.H. MYC-mediated early glycolysis negatively regulates proinflammatory responses by controlling IRF4 in inflammatory macrophages. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratziu V., Charlotte F., Bernhardt C., Giral P., Halbron M., Lenaour G., Hartmann-Heurtier A., Bruckert E., Poynard T., LIDO Study Group Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 33.Bouhlel M.A., Derudas B., Rigamonti E., Dievarat R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., Saels B., Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Lan Y., Liu H., Ovitt C.E., Jiang R. Generation of Osr1 conditional mutant mice. Genesis. 2011;49:419–422. doi: 10.1002/dvg.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H., Lan Y., Xu J., Chang C.F., Brugmann S.A., Jiang R. Odd-skipped related-1 controls neural crest chondrogenesis during tongue development. Proc Natl Acad Sci U S A. 2013;110:18555–18560. doi: 10.1073/pnas.1306495110. [DOI] [PMC free article] [PubMed] [Google Scholar]