Abstract
Liver cancer (hepatocellular carcinoma) is a common cancer worldwide. It is an aggressive cancer, with high rates of tumor relapse and metastasis, high chemoresistance, and poor prognosis. Liver tumor-initiating cells (LTICs) are a distinctive subset of liver cancer cells with self-renewal and differentiation capacities that contribute to intratumoral heterogeneity, tumor recurrence, metastasis, and chemo-drug resistance. LTICs, marked by different TIC markers, have high plasticity and use diverse signaling pathways to promote tumorigenesis and tumor progression. LTICs are nurtured in the tumor microenvironment (TME), where noncellular and cellular components participate to build an immunosuppressive and tumor-promoting niche. As a result, the TME has emerged as a promising anticancer therapeutic target, as exemplified by some successful applications of tumor immunotherapy. In this review, we discuss the plasticity of LTICs in terms of cellular differentiation, epithelial–mesenchymal transition, and cellular metabolism. We also discuss the various components of the TME, including its noncellular and cellular components. Thereafter, we discuss the mutual interactions between TME and LTICs, including recently reported molecular mechanisms. Lastly, we summarize and describe new ideas concerning novel approaches and strategies for liver cancer therapy.
Keywords: Hepatocellular Carcinoma, Liver Tumor Initiating Cells, Tumor Microenvironment, Immune Milieu, Interactions
Summary.
Liver tumor initiating cells (LTICs) are a subpopulation of liver cancer cells that play important roles in tumor initiation, recurrence, metastasis, and chemo-drug resistance. LTICs and their surrounding tumor microenvironment have been under intense research. In this review, we discuss LTIC plasticity, tumor microenvironment components, and the dynamic interaction between LTICs and the tumor microenvironment and their underlying molecular mechanisms. We also highlight potential therapeutic targets and strategies related to their mutual interactions.
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer mortality globally. It is a heterogeneous disease and has high rates of tumor relapse and metastasis, high chemoresistance, and poor prognosis.1,2 Liver tumor-initiating cells (LTICs) are a distinctive subgroup of HCC cells with self-renewal and differentiation capacities3 that contribute significantly to intratumoral heterogeneity, tumor recurrence, metastasis, and chemo-drug resistance.4 New lines of evidence have shown that LTICs, as defined by specific TIC markers, activate diverse signaling pathways to regulate tumorigenesis and progression.5 This characteristic of LTICs, which is attributed to their phenotypic plasticity, is now a critical area of research that allows a better understanding of the dynamics of TICs.6
Historically, LTICs were identified via functional assays of sorted tumor cells, such as in vitro sphere formation and in vivo xenotransplantation assays, the latter typically performed in immunodeficient mice. In the in vitro sphere formation assay, LTICs are capable of generating 3-dimensional (3D) spheroids with attachment-independent growth in an undifferentiating culture media lacking fetal bovine serum. The xenotransplantation studies were limiting dilution assays that assess the tumorigenicity of sorted marker-positive LTICs compared with the marker-negative tumor cells. With these approaches, several different LTIC-enriched cell surface markers have been discovered, such as epithelial cell adhesion molecule (EpCAM),7 CD133,8 CD90,9 CD44,9 CD24,10 and CD13.9 At the same time, several signaling pathways have been shown to be critical in inducing stemness of HCC and in promoting self-renewal, tumorigenicity, and chemoresistance. Examples include the Wingless/Integrated (Wnt)/β-catenin pathway in EpCAM+ LTICs,11 the Phosphatase and tensin homolog (Pten)-Protein kinase B (AKT)-ATP-binding cassette super-family G member 2 (ABCG2) pathway in CD133+ LTICs,12,13 the transforming growth factor-β (TGF-β)/interleukin (IL)6/signal transducer and activator of transcription (STAT)3 pathway in hepatic progenitor cells with an abnormal differentiation pattern,14 CD133+ LTICs,15 and chemoresistant EpCAM+ LTICs.16
Collectively, these studies have laid the foundation for understanding how the concept of liver cancer cell stemness can be used to elucidate molecular mechanisms underlying the dynamic transit between non-TIC and TIC states, or across various phenotypes of TIC subsets.17
The tumor microenvironment (TME) also has emerged as a factor in modulating LTIC plasticity.18,19 The TME provides a niche that is critical in maintaining TIC plasticity in HCC.20 Within this niche, the noncellular component (extracellular matrix [ECM]), the cellular components (including the immune cells, cancer-associated fibroblasts [CAFs], and other stromal cells), and the secretome and exosomes derived from these cells, participate in providing an immunosuppressive and tumor-promoting environment to drive the phenotypic transition of LTICs.21 Because of this, TME has been identified as a promising therapeutic target, as exemplified by some successful applications of tumor immunotherapy.22,23
In this review, we discuss the plasticity of LTICs and the various components of the TME. Then, we discuss the mutual interactions between LTICs and the TME (Figure 1), while highlighting recently reported molecular mechanisms. Lastly, we summarize and outline new insights into the existing therapeutic approaches and design in potential strategies for liver cancer therapy (Figure 2).
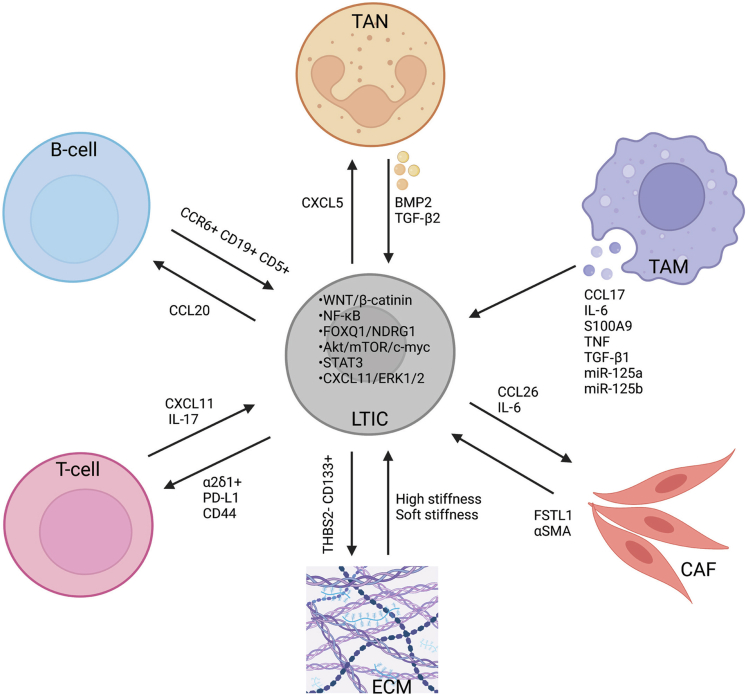
Figure 1.
Mutual communication between LTICs and ECM, CAFs, and infiltrating immune cell populations in the liver cancer tumor microenvironment. Regulation of LTICs by their niche operates through cell–cell interactions, secreted factors, cell–matrix interactions, and the biophysical properties of the niche. Biological factors known to influence interactions between liver TICs and immune cells are shown. Such communication facilitates the activation of TICs and reprograms the immune response, thereby facilitating immune evasion by the tumor. LTICs also modify their local ECM, while the tumor-associated stroma has an important role in regulating LTICs. α-SMA, α-smooth muscle actin; FOXQ1, forkhead box Q1; mTOR, mammalian target of rapamycin. Figure was created with BioRender.com.
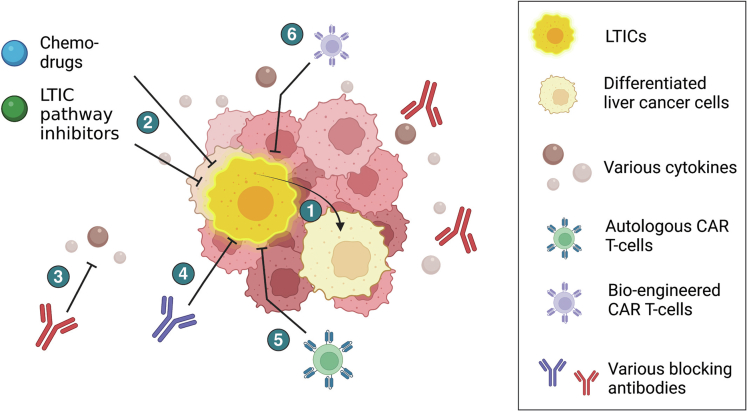
Figure 2.
Translational and therapeutic strategies targeting LTICs. (1) Differentiation strategies to promote differentiation of LTICs to improve chemosensitivity; (2) combination of chemo-drugs such as sorafenib and regorafenib with multiple LTIC-related pathway inhibitors (eg, STAT3, NANOG, SH2-containing protein tyrosine phosphatase 2, or stearoyl-CoA desaturase-1 inhibitors); (3) antibodies targeting various secreted molecules or cytokines (eg, IL8, annexin A3, and IL6); (4) antibodies targeting various LTIC surface markers (eg, CD47, CD13) to promote chemosensitivity of LTICs; (5) autologous CAR T cells from the same patient with T-cell receptors targeting the various LTIC markers (eg, CD133, EpCAM); and (6) engineered CAR T cells from healthy donors (eg, tandem CAR T cells and universal tricistronic transgene CAR T cells) with bispecific T-cell receptors and improved CAR T cell functions and persistence to target LTICs. Figure was created with BioRender.com.
Plasticity of LTICs
Plasticity is defined as phenotypic transformability, which includes the dedifferentiation of differentiated cells into stem/progenitor cells, and the conversion of cells into various differentiated cell states. Herein, we discuss some examples of plasticity associated with tumor initiation and progression, including differentiation, epithelial–mesenchymal transition (EMT), and plasticity of cell metabolism.
Plasticity of Differentiation
Cell plasticity includes the retrodifferentiation and transdifferentiation of differentiated cells. It facilitates the supplementation of the TIC pool with non-TICs. Accumulating evidence suggests that an inflammatory environment promotes the retrodifferentiation of tumor cells into stem/progenitor cells.24 Notably, it has been shown that nuclear factor-κB (NF-κB), a key inflammatory regulator, is involved in the retrodifferentiation of tumor-derived, hepatocyte-like cells into hepatic progenitor cells.25 Inhibition of the NF-κB pathway with the inhibitor of nuclear factor-κB (IκB) kinase (IKK) inhibitor, curcumin, restrains the stem-like features. The retrodifferentiation of tumor-derived, hepatocyte-like cells into stem/progenitor cells also can be promoted by cytokines such as tumor necrosis factor-α (TNF-α), IL6, CC-motif chemokine ligand (CCL)22, and TGF-β.25 IL6 increases the sphere-forming ability and the expression of stemness-related genes in many human HCC cell lines and contributes to the retrodifferentiation of HCC cells into LTICs through the Janus kinase 1–STAT3–Octamer-binding transcription factor 4 (OCT4) pathway. Other cytokines, such as TGF-β, TNF-α, IL1β, IL8, and IL11 also can promote the retrodifferentiation of tumor-derived, hepatocyte-like cells into stem/progenitor cells.25 The retrodifferentiated cells continue to produce the aforementioned cytokines to maintain the TIC-like phenotype. The findings suggest that a cytokine-fueled inflammatory environment can trigger retrodifferentiation of non-TICs into LTICs.
Wang et al26 recently showed that HCC, intrahepatic cholangiocarcinoma (ICC), and combined HCC-CC could originate from mature hepatocytes in 3 mouse liver cancer models using hydrodynamic transfection methodology, suggesting that mature hepatocytes can transdifferentiate into hepatic progenitor cells in a specific TME before differentiating into various cell types of liver cancer. On the other hand, findings from another recent study showed that a necroptosis-associated hepatic cytokine microenvironment could determine the transdifferentiation of mature hepatocytes into ICC, whereas an apoptotic microenvironment could promote the transdifferentiation of mature hepatocytes into HCC.24 In fact, Yamashita et al16 reported that oncostatin M, an IL6-related cytokine, was able to induce the hepatocytic differentiation of EpCAM+ LTICs. Similarly, it has been reported that overexpression of hepatocyte growth factor 4α promoted the differentiation of HCC cells into hepatocytes and significantly reduced the percentage of CD133+ LTICs.27 High-dose exogenous bone morphogenetic protein 4 also promotes CD133+ LTIC differentiation and inhibits their tumorigenic capacity.28 Given the emerging role of differentiation plasticity in the maintenance of the TIC-like phenotype, inducing TIC differentiation into non-TICs, it has been suggested that differentiating agents have therapeutic potential in HCC by attenuating cancer cell stemness. Future studies to identify novel and effective differentiation agents are needed urgently because they conceivably could be used to eradicate LTICs.
Plasticity of EMT
EMT is a complex biological process that involves both cellular and molecular reprogramming. In EMT, epithelial cells dedifferentiate and acquire mesenchymal properties, including invasiveness and motility. EMT has been shown to serve as a vital regulator of cancer cells that show TIC-like features. For example, Mitra et al29 reported that mislocalization of vimentin, an intracellular EMT marker, to the cell surface (csVim) could be used to isolate cancer stem-like cells. They found that csVim+CD133- cells had stem-like features similar to those of the csVim-CD133+ population.29 On the other hand, reversal of EMT, via CD44 down-regulation, prevented lung metastasis in a mouse metastasis model by inhibiting the extracellular signal-regulated kinase (ERK)/Snail pathway.30 In addition, promoting mesenchymal–epithelial transition (MET), the reverse process of EMT, has been proposed as a strategy to eradicate mesenchymal TICs by inducing their transition to epithelial cells that lack stemness properties. As an example, activation of protein kinase A could induce MET in TICs in a histone demethylase PHD Finger Protein 2 (PHF2)-dependent manner, leading to the loss of tumor-initiating ability in TICs.31 These results suggest that induction of MET may serve as a potential differentiation therapy for LTICs.
Plasticity of Cellular Metabolism of LTICs
Changes in glucose metabolism are prominent in LTICs. As an example, glucose uptake is increased markedly in liver TICs via the preferential expression of glucose transporters, such as Glucose transporter-1 (GLUT1) and GLUT3, whose inhibition increases the sensitivity to chemotherapy in vivo.32 In addition, low levels of total and phosphorylated AMP-activated protein kinase (AMPK), which is a low-energy sensor that favors oxidative phosphorylation (OXPHOS), were detected in sorafenib-resistant, stem-like HCC cells and promoted the expression of stemness-related genes by up-regulating hypoxia-inducible factor 1-α.33 These suggest that liver TICs are highly dependent on glycolysis. Mitophagy also has been shown to regulate the stemness of liver TICs34 and exert dual function in modulating the sensitivity of chemotherapy in HCC: mild mitophagy increases drug resistance by degrading chemotherapy-damaged mitochondria whereas excessive mitophagy exacerbates chemotherapy-induced apoptosis.35,36 A sirtuin 1/mitochondrial ribosomal protein S5 axis bridges stemness properties and aerobic metabolism of LTIC with mitochondrial ribosomal protein S5 acetylation status functioning as a switch from OXPHOS to glycolysis in response to hypoxia.34 Collectively, mitochondrial function and OXPHOS are involved in the regulation of LTIC-mediated chemoresistance. Indeed, energy addiction is a striking characteristic of liver TICs, featured by both addicted glycolysis and sustained OXPHOS.
In addition to glucose metabolism, fatty acid addiction and enhanced fatty acid oxidation have been implicated as important alternative energy sources in LTICs that were observed during β-catenin–mutated HCC tumorigenesis.37 Fatty acid oxidation can be activated by Nanog Homeobox (NANOG) to support the self-renewal ability of TICs and chemotherapy resistance.38 Glutamate oxidation can serve as another energy source for OXPHOS in HCC cells under conditions of aglycemia.39 Overall, these findings showed the plasticity of energy metabolism in liver TICs and their contribution to chemotherapy resistance. NANOG, MYC, and β-catenin are key proteins regulating the crosstalk of cancer stemness, energy metabolism, and chemotherapy resistance in HCC.
Components of the TME in HCC: Niche of the LTICs
ECM and Physical Components of the Environment
The ECM is a collection of biochemical molecules, including proteins, glycoproteins, proteoglycans, and polysaccharides, which together constitute the basement membrane and interstitial matrix. In normal tissue, the composition of the ECM is tightly regulated by controlling the expression and activities of the ECM enzymes at the transcriptional and translational and post-translational levels, respectively.40 ECM dynamics are deranged in HCC. For instance, during tumor formation of HCC, there is increased deposition of various collagens, including collagen I, collagen II, collagen III, collagen V, and collagen IX, which provide the 3D scaffold within which tumor-associated cells organize to form complex structures characteristic of the TME.41,42 As the tumor continues to grow, its blood flow cannot supply sufficient oxygen and nutrient supplies to meet metabolic demands. This results in enhanced tumor cell glycolysis and lactate production, which in turn generates a more hypoxic and acidic environment that further exacerbates detrimental metabolic states. HCCs that arise within a cirrhotic background also can be impacted by mechanical components of the TME, such as fluid shear stress and tissue stiffness, which directly impact tumor cells. Altogether, ECM deposition, tissue architecture and stiffness, vasculature, interstitial fluid flow, and fluid shear pressure shape the physical context of the TME.43
Immune Milieu
The immune milieu in the liver is dominated by immune cells and cytokines that foster a natural physiologically tolerogenic niche.44 The key cells with major immunosuppressive roles implicated in HCC immune evasion, as shown by multi-omics and single-cell analyses, include tumor-associated macrophages (TAMs), regulatory T cells, and myeloid-derived suppressor cells (MDSCs).45,46 The enhanced ability of TICs to initiate tumors suggests that TICs probably can evade immune detection. Indeed, accumulating evidence supports the important roles of several immune cell types (TAMs and other immune cells) and lymphatic endothelial cells (LECs) in driving TIC expansion and TIC-specific avoidance of immune detection and destruction.22 In addition to the ability of LTICs to evade immune detection, secretion of TGF-β, IL10, indoleamine 2,3-dioxygenase, and/or arginase by various cell types in the TME further promotes immune suppression.44 Conversely, it should be noted that proinflammatory cytokines, such as IL2, interferon-γ, chemokine (C-X-C motif) ligand (CXCL)10, and CXCL9, can attract cells that drive antitumor immune response.44
CAF, Other Stromal Cells, and Exosomes
Most HCCs arise in the setting of chronic inflammation and cirrhosis and are associated with abundant activated fibroblasts, and some of these fibroblasts gain entry into the liver tumor as CAFs.22 Together with other stromal cells, such as endothelial cells and hepatic stellate cells, CAFs shape the TME to promote the development of HCC. The interplay between these cells of TME and HCC cells are impacted by secreted chemokines and exosomes,47 which alter the signaling pathways and cellular behavior of HCC cells, contributing to the tumor’s chemoresistance and various aggressive phenotypes.
Interactions Between TME and LTICs in Promoting Liver Cancer Stemness
Enhancement of LTICs by ECM and Physical Components
Increasing evidence suggests that the liver ECM serves as a niche in maintaining LTICs.48,49 High ECM stiffness has been reported to promote stemness maintenance in HCC cells by activating integrin β1/Akt/mammalian target of rapamycin (mTOR)/Sex determining region Y-box 2 (SOX2) signaling.50 On the other hand, soft matrix stiffness also has been reported to contribute to tumor outgrowth. For example, the local soft spot TME formed by thrombospondin 2–deficient CD133+ LTICs by collagen degradation and matrix metalloproteinase activity may increase the stemness and drug resistance of LTICs and provide an escape route for metastasis.51 Based on the earlier-described reports that both hard and soft ECM stiffness can contribute to liver cancer progression through LTICs, we speculate that the function of ECM stiffness on LTICs may depend on the stages of tumor development. In the early stage of HCC, higher ECM stiffness may enhance LTIC proliferation and self-renewal, whereas in the later stage of HCC, a softer ECM may facilitate metastasis. With recent progress in biomechanical technologies such as microfluidic devices and atomic force microscopy, additional studies are needed to assess the effects of tumor matrix stiffness on stemness maintenance, LTIC morphology, metabolic phenotypes, and the underlying mechanisms of how LTICs respond toward the stress signals from the tumor matrix.52
The variable ECM stiffness in HCC reflects the dynamic nature of TME, which often is modified and regulated by various enzymes. One such enzyme is heparinase, a heparan sulfate endoglycosidase, and it plays an important role in stimulating tumor initiation by modifying the extracellular environment. Heparin’s overexpression in transgenic mouse models resulted in enhanced tumorigenicity in the 7,12-dimethylbenz[a]anthracene–induced mouse tumor models.53 In that study, host-derived heparanase in the transgenic heparanase-overexpressing mice modified the target niche to facilitate secondary tumor formation, as shown by the increased number of lung metastases formed upon tail-vein injection of cancer cells in a heparanase-overexpressing host.
Besides the physical components of TME, the environmental conditions of the TME also control LTICs. Hypoxia is an important factor that frequently is present in the TME of HCC. As an example, knockout of the Facilitate Chromatin Transcription complex abolished cellular adaptation to hypoxia and subsequent tumor initiation in a p53-knockout/cellular Myc (c-Myc)–overexpression mouse HCC model.54 This was attributed to the requirement of Facilitate Chromatin Transcription in glycolytic flux maintenance and lactic acid extrusion to prevent intracellular lactate acidification and subsequent apoptosis. This mechanism argues that adaptation to hypoxic stress helps facilitate tumor initiation in metastasis.
Acidification of TME also affects HCC tumor initiation, as shown in a diethylnitrosamine (DEN)-induced HCC model in which mice treated with 200 mmol/L alkaline NaHCO3 had fewer liver tumor nodules formed.55 It was further found that exposure to acidic TME activated phosphorylated AKT (p-AKT) signaling to increase the expression of stearoyl-CoA desaturase-1 and its binding to peroxisome proliferator-activated receptor-α to cause lipid accumulation in HCC cells. This in turn fueled HCC initiation and progression. In another study, alkalization to neutralize lactic acidosis was performed by infusing bicarbonate and anticancer drug through the tumor-feeding artery to the HCC patients’ tumors via the targeting intratumoral lactic acidosis–transarterial chemoembolization (TACE) procedure.56 Normal TACE without bicarbonate infusion was used for comparison. Significantly, the embolized area, as shown by magnetic resonance imaging, showed clear necrosis of the tumor tissue and improved efficacy as compared with conventional TACE that did not target intratumoral lactic acidosis.
Lastly, fluid shear stress recently was suggested to promote tumor initiation. One study showed that HepG2 cells, when subjected to fluid shear stress, showed increased tumorigenesis by inducing a greater number of tumor nodules formed, upon either intraperitoneal or intrahepatic injection of the cells, as compared with the statically cultured HepG2 cell counterpart.57 Further investigation showed that the stress could induce cytoskeletal rearrangement to trigger the release of Yes-associated protein (YAP) from its binding partner, integrin β, at the cell membrane. The nuclear-translocated YAP was able to activate the expression of EMT-related genes. Supporting this idea, knockdown of YAP reduced liver tumor nodule formation.
Enhancement of LTICs by the Immune Milieu
TAMs within the TME
Increasing evidence has shown the importance of crosstalk between TAMs and LTICs in HCC. As an example, polarized M2 TAMs that secrete CCL17 have been shown to promote the migration, sphere formation, expression of stemness markers, and a side population of cancer stemness of HCC cells.58 Moreover, Wan et al59 showed that HCC TAMs promoted expansion of CD44+ LTICs and accelerated sphere production. They further found that IL6 secreted by HCC TAMs promoted TIC expansion in human HCCs by activating STAT3 signaling, resulting in a feed-forward loop that contributed to LTIC self-renewal.59 In addition, it has been reported that TAMs secreted S100 calcium-binding protein A9, an inflammatory microenvironment-related secretory protein, and enhanced the self-renewal of LTICs.60 TAMs also produce TNF, which promotes EMT and cancer stemness through Wnt–β-catenin signaling.61 TAMs also can promote TIC-like properties through TGF-β1–induced EMT in HCC.62 A decrease in the levels of exosomal microRNA (miR)-125a or miR-125b secreted by TAMs has been found to exert TIC-promoting effects in HCC cells through the modulation of CD90 expression.63
Other immune cells within the TME
The other immune cells in the TME that play an important role in the initiation and development of HCC include tumor-associated neutrophils (TANs), MDSCs, T cells, and B cells. TANs may secrete bone morphogenetic protein 2 and TGF-β2, both members of the TGF-β superfamily,64 which in turn increase miR-301b-3p expression in HCC cells with a stem-like phenotype. The increased miR-301b-3p expression inhibits the gene expression of the limbic system–associated membrane protein and cylindromatosis lysine 63 deubiquitinase (CYLD) lysine 63 deubiquitinase, leading to hyperactivation of the NF-κB pathway, increased CXCL5 secretion, and increased TAN infiltration.65,66 Such interaction between TANs and cancer stem-like cells controls the progression of HCC, indicating that TANs may be a target for the elimination of HCC cells with a stem-like phenotype.
As for T cells, it has been reported that autologous T cells could preferentially kill CD133+ LTICs in HCC.67 Many studies have shown that LTICs are able to enhance immune evasion from T cells via, for example, low expression of co-stimulatory molecules and increased expression of T-cell inhibitory molecules, such as Programmed death ligand-1 (PD-L1).68 LTICs also are able to promote the expansion of protumorigenic immune phenotypes, for example, by impairing the proliferation of effector T cells and promoting the expansion of regulatory T cells and by enhancing macrophage polarization toward the M2 immunosuppressive phenotype.68 How the genetic backgrounds of HCC tumors affect their response to immunotherapy has been investigated in mouse models.69 These studies showed that β-catenin activation down-regulated the expression of several cytokines, including CCL5, in LTICs. This, in turn, abolished the recruitment of antigen-specific CD8+ T cells and, ultimately, attenuated the exposure of LTICs to immunosurveillance.69
In another study using hydrodynamic tail-vein and orthotopic injection mouse models with Hepa1-6 mouse HCC cells, IL23 overexpression resulted in increased numbers of liver tumor nodules formed as compared with the control cells in the immunocompetent mice.70 Similarly, hydrodynamic tail-vein injection of IL23-encoding plasmid into DEN-treated mice also induced a greater number of tumors formed as compared with the vector control group. It was found that IL23 promoted IL17 production from CD4 and CD8 T cells. Knockout of IL17 abrogated the HCC formation induced by IL23. This indicates the critical role of IL17 in this IL23-induced HCC. Further investigation of the IL23-overexpressing Hepa1-6 cells in the mouse tumors identified that the source of IL17 production was from the innate lymphoid cells (ILCs), which were characterized to be CD3– cells with a CD19-CD11b-NKL1-NKp46- CD127+CD4-Sca-1+RORγt+ phenotype. Intriguingly, this ILC subpopulation, when isolated from tumor-bearing mice, could stimulate the tumor-forming ability of Hepa1-6 cells upon hydrodynamic injection into immunocompetent mice as compared with the control phosphate-buffered saline–treated Hepa1-6 cells. This study highlights the contributing roles of particular ILC populations and the cytokine IL17 in the TME to drive IL23-induced HCC formation.
In another study on the immune TME in the steatotic liver of Pten knockout (Pten-null) mice, it was found that a high-fat diet increased HCC tumor incidence.71 It was further found that Wnt/β-catenin signaling was up-regulated in the steatotic Pten-null mice, suggesting a crosstalk between Akt and Wnt/β-catenin signaling pathways. Interestingly, blockade of steatosis in the Pten-null model inhibited TIC expansion, as shown by a reduced number of CD133+ cells. Steatosis blockade also reduced Wnt production by the infiltrating macrophages in Pten-null mouse HCC. The finding suggests a positive role of macrophages in the steatotic TME in promoting HCC induced by Pten deficiency.
MDSCs also can promote liver cancer cell stemness by impairing dendritic cell function and suppressing T-cell infiltration into HCC. Drug-resistant HCC cells, widely reported to be enriched with TIC features, enhanced the expansion and immunosuppressive function of MDSCs through preferential secretion of IL6.72 Depletion of MDSCs, by either administration of anti-Lymphocyte antigen 6 complex locus G6D (Ly6G)/Lymphocyte antigen 6 complex locus C (Ly6C) (anti–Gr-1) antibody or blockade of IL6 signaling, sensitized LTICs to 5-fluorouracil chemo-drug treatment.72
Enhancement of LTICs by CAFs and Other Stromal Cells
CAFs are a well-characterized component of the TME. In a recent study, CAFs were able to induce tumor-initiating properties of HCC cells, resulting in increased tumor incidence and expression of TIC markers (eg, CD44, Myc, and Oct4).73 The co-implanted CAFs also increased forkhead box Q1 expression, which, in turn, promoted the transcription of NDRG1 to drive HCC initiation. The forkhead box Q1/NDRG1 axis also promoted CCL26 production via STAT6 signaling to attract additional CAFs to HCC. The findings showed a positive feedback relationship between CAFs and TICs in HCC.
In another study, follistatin-like 1 (FSTL1) was correlated positively with the expression of α-smooth muscle actin, a fibroblast marker, in multiple liver and HCC mouse models (partial hepatectomy model, neuroblastoma RAS viral oncogene homolog (NRAS)/AKT hydrodynamic tail vein injection and DEN/carbon tetrachloride–induced mouse HCC models).74 HCC cells exposed to conditioned medium from FSTL1-overexpressing CAFs showed greater tumorigenicity in limiting dilution assays and this was abolished upon treatment with ant-FSTL1 blocking antibody. In this CAF FSTL1–driven tumor initiation, mechanistically, Toll-like receptor 4 (TLR4) was enhanced in the target HCC cells to activate the Akt/mTOR/Eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4EBP1)/c-myc signaling, and the enhanced tumorigenicity induced by CAF could be abrogated by TLR4 inhibitor. This study nicely showed the positive role of CAFs in tumor-initiation promotion.
Endothelial cells within the TME also play roles in LTIC regulation. Conditioned medium from endothelial cells was shown to enhance CD133 expression in Huh-7 cells transfected with hepatitis B virus X protein (HBx) and that this was mediated by TGF-β signaling.75 LECs, which are another important component of the TME, have been reported to promote tumor cell proliferation by secreting lympho-angiocrine factors and preferentially interact with CD133+ LTICs through direct interaction between high mannose N-glycans and mannose receptors.76 This interaction then up-regulated IL17A signaling in LECs and helped LTICs self-renew and escape immune attack.76 Of note, neutralization of the IL17A pathway inhibits the self-renewal of LTICs,76 implicating IL17A, a promising therapeutic target for liver cancer.
Enhancement of LTICs by Exosomes
Exosomes are secreted extracellular vesicles that can carry nucleic acids, proteins, and cell metabolites. A previous study found that serum exosomes containing p120-catenin protein, which was underexpressed in liver cancer cells,77 suppressed HCC growth and metastasis in an orthotopic injection model. Interestingly, exosomes from p120-catenin–overexpressing cells, as compared with control cells, also suppressed sphere formation, expression of the stemness genes BMI1, Nanog, Oct4, and Sox-2, and HCC cancer stem cell markers CD133, CD24, CD90, and EpCAM in HCC cell lines. It was further found that exosomal p120-catenin decreased STAT3 phosphorylation, thereby suppressing STAT3 signaling. Supporting this, the STAT3 inhibitor S3i-210 could suppress HCC sphere formation, while overexpression of STAT3 reversed the sphere formation suppression induced by exosomal p120-catenin. This study suggests that exosomes in TME can affect the TIC properties of HCC cells.
LTICs Modulate the TME to Promote Tumor Progression
The impact on tumor progression by LTIC-TME interactions was shown by a recent study that evaluated an anticancer vaccine derived from spheroid culture-enriched TIC.78 Here, TICs of various tumor types were grown and enriched in 3D spheroid culture before being rendered growth-defective by 150 Gy radiation to create cancer vaccines. The effect of vaccination on the tumor growth and the thymus rejuvenation in tumor-bearing mice then was determined. Cells maintained in 2-dimensional culture that were not enriched in cancer-initiating cells were used as a control for the earlier-described vaccine. It was found that the TIC-enriched, 3D-spheroid–derived cell vaccine led to thymic renewal in terms of increase in thymic size and expansion of Vγ4γδT-cell subsets bearing T-cell receptor repertoire targeting the 3D culture-derived TIC, which in turn led to tumor regression and eradication. This study argues that TIC may suppress host T-cell immunity to create an immunopermissive TME, and that this can be blocked by vaccination against enriched TICs.
In another example, it was found that PD-L1 Y112 phosphorylation was required for liver tumor formation by Hepa1-6 cells in immunocompetent but not in immunodeficient mice.79 The phosphorylation of PD-L1 Y112, which stabilized PD-L1 via its glycosylation, was mediated by Janus kinase, which in turn was activated by TIC-derived IL6. PD-L1 stabilization suppressed the T-cell cytotoxic efficacy against the tumor cells, thus showing that TIC could influence the interplay with the tumor immune microenvironment in tumor initiation.
To address how the immunosuppressive features of the TME evolved during initiation and progression of HCC, another study examined CD8+ T cells in a conditional HCC mouse model.80 This showed that liver CD8+ T cells expressed immune-inhibitory receptors Programmed cell death 1 (PD-1) and Lymphocyte Activation Gene 3 (LAG3) at the premalignant stage, thus indicating immune dysfunction. In late-stage HCC, the CD8+ T cells could not be rescued by PD-1 blockade, indicating an irreversible immunosuppressive state triggered by the presence of tumor antigens. Transcriptome analyses showed that T-cell exhaustion was owing to chronic tumor antigen exposure. Interestingly, the non–tumor-specific CD8 T cells (T-cell–receptor restricted to another antigen) isolated from the same tumor did not show up-regulation of immune-inhibitory receptors, indicating the T-cell dysfunction in tumor initiation is tumor antigen-specific and mainly owing to continuous tumor antigen exposure rather than the other microenvironment factors. This study suggests LTICs continuously model T-cell function within the TME.
Immunosuppression by HCC cells with expression of PD-L1 was shown to be modulated by messenger RNA (mRNA) translation in another study. Here, combined transgenic overexpression of c-Myc and Kristen Rat Sarcoma Viral oncogene homolog (KRAS)G12D promoted HCC formation and lung metastasis, as compared with c-Myc overexpression on its own.81 Further investigation showed the presence of immune evasion of the Myc/KRASG12D HCC cells was mediated by increased expression of PD-L1 protein (despite a background of increased CD8+ T cells). Intriguingly, the increased PD-L1 protein expression was not owing to an increase in PD-L1 mRNA expression but to more efficient translation of PD-L1 mRNA to PD-L1 protein in the c-Myc/KRASG12D cells. Translational repression at the upstream open-reading frame of the PD-L1 mRNA was bypassed by the phosphorylated eukaryotic translation initiation factor 2A (eIF2α) that favors the translation of the main open-reading frame instead of the upstream open-reading frame of the PD-L1 mRNA and thus improving translation. This study further argues that the more aggressive behavior of Myc/KRASG12D TIC arose from their ability to evade the immune surveillance of the tumor microenvironment.
It also has been shown that HCC cells secrete chemokines within the TME that recruit immune cells that promote angiogenesis and tumor initiation. CXCL11 was reported to selectively recruit activated T cells to inflammatory sites during hepatic inflammation.82 Up-regulated CXCL11 promoted sphere formation and tumorigenicity by LTICs positive for the cell surface calcium channel subunit α2δ1 via CXCR3/ERK1/2 signaling. These findings argue that CXCL11 may mediate communication between activated T cells and α2δ1+ LTICs.83 In addition, LTIC-derived CCL20, the selective chemokine ligand for CCR6, was reported to recruit CD19+ CD5+ B cells expressing CCR6.84 CCL20 is one of the CC chemokines highly expressed in Tumor Protein p53 (TP53)-mutated HCC LTICs with an obvious stemness trait. The interaction of CCL20 and CCR6 shows bidirectional communication between B lymphocytes and LTICs.84
Besides immune milieu modification, LTICs may enhance liver tumor formation by promoting hepatic fibrosis. Hepatic progenitor cells (HPCs), which are regarded as a source of LTICs, are reported to differentiate into matrix-secreting myofibroblasts upon lipopolysaccharide stimulation.85 Intrasplenic co-injection of the HPC cell line enhanced tumorigenesis in DEN-treated rats, and this occurred in the context of a pronounced inflammatory and fibrotic response. Further investigation showed that primary Oval Cell Marker Antibody (OV-6) (OV6)+ HPCs isolated from 2-acetylaminofluorene and partial hepatectomized rats promoted liver tumor formation in DEA-treated animals and that this too was associated with liver fibrosis. HPC with tumor-initiating properties differentiated into myofibroblasts when activated by lipopolysaccharide/TLR4 signaling, which led to the production of IL6 and TNF-α. This, in turn, promoted HPC proliferation and created a tumorigenic fibrotic environment. These findings highlight the positive role of liver fibrosis as a target of TME modification by LTICs.
In addition to the DEN-induced HCC model, another group used YAPS127A-induced liver tumorigenesis86 to identify interactive tumor-vascular signaling hubs that sensitized cells to migration. The YAP/Transcriptional enhancer factor TEF-3 (TEAD4) signaling axis in the YAP-mutated TICs triggered osteopontin production to stimulate c-Met signaling in endothelial cells that induced promigratory properties. In human liver cancer samples, endothelial c-Met expression was associated with poor clinical outcome. This study indicates the LTIC subset carrying the YAP mutation can modify vascular endothelial cells within the TME that promote tumor progression.
Lastly, it was found that exosomes from TIC-enriched spheroids promoted resistance of HCC cells to the multikinase inhibitor regorafenib compared with cells grown in 2-dimensional culture.87 Regorafenib chemoresistance was dependent on Ras-Related Protein Rab-27A (RAB27A), which regulates exosome secretion. RAB27A knockout suppressed TIC expression of the stemness gene Nanog, as well as sphere formation, and sensitized subcutaneous HCC xenografts to regorafenib. More importantly, Nanog expression in adherent non-TIC HCC cells was suppressed upon treatment with exosomes derived from RAB27A-knockout spheroids as compared with those receiving treatment of exosomes derived from control cells. This indicates that TICs release exosomes that modulate stemness gene expression and chemoresistance in non-TIC tumor cells, thus identifying a TIC-dependent paracrine stimulation pathway in the TME.
Translational Applications and Therapeutic Perspectives
Multiple therapeutic strategies targeting LTICs have been suggested and are under further investigation.
Targeting LTIC Dormancy
Antiproliferative chemotherapy or radiotherapy eradicates proliferating tumor cells and induces senescence, leading to tumor growth arrest.23,88 Induction of mitotic quiescence in the surviving LTICs induces a dormant metabolic state that is reversible, implicating cell-cycle arrest but not necessarily growth arrest.88 In this setting, LTIC plasticity combined with senescence escape and stemness-related pathway activation may trigger tumor relapse and metastasis.23,88 In a related study, ischemic HCC cells that survive treatment of transarterial chemoembolization (±chemotherapy) were shown to be reliant on autophagy to sustain quiescence/dormancy and that an autophagy inhibitor promoted tumor cell death.89 Therefore, autophagy inhibitors may be a potential tool for co-treatment with antiproliferative tyrosine kinase inhibitors such as sorafenib and regorafenib.
Targeting the LTIC Niche in TME
Targeting the LTIC niche is another promising therapeutic strategy for HCC that targets LTIC.90 However, a better understanding of how the niche controls LTIC dormancy, resistance to chemotherapy and immunotherapeutics, and stemness property maintenance, still is needed. Nonetheless, immunologic approaches that target interplay between LTICs and tumor immune milieu recently were explored. These include vaccination against LTIC-specific antigens, adoptive transfer of chimeric antigen receptor (CAR) T cells, treatment with immune checkpoint inhibitors, and antibody-based LTIC marker blockade.23
One approach involved vaccination of dendritic cells loaded with LTIC-specific antigens with the aim of stimulating immune-mediated elimination of LTICs.22 There are ongoing clinical trials that use CAR T cells to target other LTIC markers/antigens in HCC patients, include CD133-targeting CAR T cells (NCT02541370), and EpCAM-directed CAR T cells in primary (NCT03013712) and relapsed HCC patients (NCT02729493).23 The LTIC-directed CAR T cells in the earlier-described clinical trials were generated in an autologous manner from the same patient.
Recently, tandem CAR T cells and universal tricistronic transgene CAR T cells have come under intense research. Tandem CAR T cells bear bispecific chimeric antigen receptors, targeting 2 or more antigens to increase antitumor immunogenic efficacy. Universal tricistronic transgene CAR T cells are generated from allogenic CAR T cells from healthy individuals by gene editing, with the aim of minimizing graft-versus-host disease, and thus enhancing persistence and activity of the CAR T cells in cancer patients.91 Last but not least, blocking CD47, a do-not-eat-me signal, with the use of anti-CD47 antibodies to enhance the phagocytosis of CD47-expressing LTICs, is another new and promising HCC treatment strategy.22
Targeting the LTICs
Targeting redox balance
Redox balance and signaling have been reported to be pivotal in generating cancer cell multidrug resistance.92 For example, a previous study screened a library of small-molecule compounds that disrupt the cellular redox balance for their ability to block colony and sphere formation of ICC cell lines. This led to the identification of a compound called Hinokitiol, that also blocked expression of cancer stem cell–related genes, such as Sox2, Oct4, Nanog, CD133, EpCAM, and c-myc.92 The compound promoted apoptosis and markedly suppressed ERK1/2 and p38 phosphorylation of the mitogen-activated protein kinase pathway. Further studies using colony formation and sphere formation assays as well as mouse tumoroid models showed that Hinokitol sensitized ICC cells to the inhibitory effects of palbociclib, a Cyclin-dependent kinase 4/6 (CDK4/6) inhibitor. This study showed that large-scale drug screening for redox-related compounds may be a useful way to identify lead compounds targeting LTICs.
Targeting various LTIC surface markers and signaling pathways
Targeting LTIC surface markers is another strategy that can be used to disrupt LTICs.22 One study showed that a CD13-targeting peptide conjugated to an antitumor agent enhances this agent’s efficacy against TICs in HCC. The CD13 inhibitor ubenimex93 also was shown to exert anticancer effects synergistically with multiple chemo-drugs in HCC. An anti-CD47 antibody also was found to sensitize HCC cells to sorafenib treatment in patient-derived mouse xenografts (PMID: 25902734). Clinical trials using anti-CD47 antibodies already have been performed in various solid tumors other than the liver,22,94 and trials in HCC patients are eagerly awaited. Another strategy under consideration is the targeting of LTIC-related signaling pathways such as the Wnt/β-catenin, Akt, and Hippo pathways.22,93 A STAT3 inhibitor (napabucasin), a NANOG inhibitor (amcasertib), a SH2-containing protein tyrosine phosphatase 2 inhibitor (SHP099, TNO155, RMC-4630, and RLY-1971), and a stearoyl-CoA desaturase-1 inhibitor (SSI-4, GSK1940029) have been tested either alone or in combination with the anti-HCC agent sorafenib in multiple clinical trials.22 Targeting fucosylation by specific inhibitors has been shown to promote the efficacy of sorafenib through a mechanism thought to inhibit LTIC stemness.93 Together, these tools against LTICs underline the therapeutic potential of targeting LTIC surface markers and the related signaling pathways.
Other strategies targeting LTICs
Various secretory molecules, such as IL8, annexin A3, IL6, oncostatin M, insulin-like growth factor, fibroblast growth factor, angiopoietin-like protein 1, and cathepsin S, are known to promote cancer stemness in HCC.22 Inhibiting these secretory molecules is considered to be another potential means to target LTICs. Supporting this idea, an annexin A3–targeting antibody was shown to suppress LTIC self-renewal and sensitize HCC cells to cisplatin and sorafenib/regorafenib.95
Conclusions
LTICs play significant roles in the initiation, progression, and metastasis stages of HCC development, however, the optimal time and method for targeting LTICs requires further investigation.22 This is especially important for LTIC-related immunotherapy and combinations of different targeting strategies. Biopsy-driven detection of LTICs for precise diagnosis, post-treatment monitoring, and prognostication also urgently are required because LTICs have great plasticity with regard to differentiation, EMT status, and metabolism. Finally, interpatient heterogeneity of LTICs96 will necessitate a personalized approach to targeting different LTIC subtypes and/or subpopulations.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was supported by the Hong Kong Research Grants Council Theme-based Research Scheme (T12-704/16-R, T12-716/22-R), an Innovation and Technology Commission grant to the State Key Laboratory of Liver Research (ITC PD/17-9), the National Natural Science Foundation of China (81872222 and 82203234), and the University Development Fund of The University of Hong Kong. Irene Oi-Lin Ng is a Loke Yew Professor in Pathology.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Sia D., Villanueva A., Friedman S.L., Llovet J.M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 3.Nio K., Yamashita T., Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer. 2017;16:4. doi: 10.1186/s12943-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng H., Pomyen Y., Hernandez M.O., et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology. 2018;68:127–140. doi: 10.1002/hep.29778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita T., Honda M., Nakamoto Y., et al. Discrete nature of EpCAM+ and CD90+ cancer stem cells in human hepatocellular carcinoma. Hepatology. 2013;57:1484–1497. doi: 10.1002/hep.26168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashita T., Wang X.W. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123:1911–1918. doi: 10.1172/JCI66024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita T., Ji J., Budhu A., et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma S., Chan K.W., Hu L., et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Ji J., Wang X.W. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012;39:461–472. doi: 10.1053/j.seminoncol.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee T.K., Castilho A., Cheung V.C., et al. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita T., Budhu A., Forgues M., Wang X.W. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 12.Lee T.K., Castilho A., Cheung V.C., et al. Lupeol targets liver tumor-initiating cells through phosphatase and tensin homolog modulation. Hepatology. 2011;53:160–170. doi: 10.1002/hep.24000. [DOI] [PubMed] [Google Scholar]
- 13.Ma S., Lee T.K., Zheng B.J., et al. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 14.Tang Y., Kitisin K., Jogunoori W., et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci U S A. 2008;105:2445–2450. doi: 10.1073/pnas.0705395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You H., Ding W., Rountree C.B. Epigenetic regulation of cancer stem cell marker CD133 by transforming growth factor-beta. Hepatology. 2010;51:1635–1644. doi: 10.1002/hep.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita T., Honda M., Nio K., et al. Oncostatin m renders epithelial cell adhesion molecule-positive liver cancer stem cells sensitive to 5-fluorouracil by inducing hepatocytic differentiation. Cancer Res. 2010;70:4687–4697. doi: 10.1158/0008-5472.CAN-09-4210. [DOI] [PubMed] [Google Scholar]
- 17.Takebe N., Miele L., Harris P.J., et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau E.Y., Lo J., Cheng B.Y., et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016;15:1175–1189. doi: 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez M.M., Fiore E., Bayo J., et al. 4Mu decreases CD47 expression on hepatic cancer stem cells and primes a potent antitumor T cell response induced by interleukin-12. Mol Ther. 2018;26:2738–2750. doi: 10.1016/j.ymthe.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N.Z., Wang S.S., Li M.Y., et al. Cancer stem cells in hepatocellular carcinoma: an overview and promising therapeutic strategies. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918816287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y., Zhang J., Zhang X., et al. Cancer stem cells: a potential breakthrough in HCC-targeted therapy. Front Pharmacol. 2020;11:198. doi: 10.3389/fphar.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee T.K., Guan X.Y., Ma S. Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2022;19:26–44. doi: 10.1038/s41575-021-00508-3. [DOI] [PubMed] [Google Scholar]
- 23.Walcher L., Kistenmacher A.K., Suo H., et al. Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. 2020;11:1280. doi: 10.3389/fimmu.2020.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seehawer M., Heinzmann F., D'Artista L., et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562:69–75. doi: 10.1038/s41586-018-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois-Pot-Schneider H., Fekir K., Coulouarn C., et al. Inflammatory cytokines promote the retrodifferentiation of tumor-derived hepatocyte-like cells to progenitor cells. Hepatology. 2014;60:2077–2090. doi: 10.1002/hep.27353. [DOI] [PubMed] [Google Scholar]
- 26.Wang G., Wang Q., Liang N., et al. Oncogenic driver genes and tumor microenvironment determine the type of liver cancer. Cell Death Dis. 2020;11:313. doi: 10.1038/s41419-020-2509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin C., Lin Y., Zhang X., et al. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology. 2008;48:1528–1539. doi: 10.1002/hep.22510. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L., Sun H., Zhao F., et al. BMP4 administration induces differentiation of CD133+ hepatic cancer stem cells, blocking their contributions to hepatocellular carcinoma. Cancer Res. 2012;72:4276–4285. doi: 10.1158/0008-5472.CAN-12-1013. [DOI] [PubMed] [Google Scholar]
- 29.Mitra A., Satelli A., Xia X., et al. Cell-surface vimentin: a mislocalized protein for isolating csVimentin(+) CD133(-) novel stem-like hepatocellular carcinoma cells expressing EMT markers. Int J Cancer. 2015;137:491–496. doi: 10.1002/ijc.29382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y., Ruan B., Liu W., et al. Knockdown of CD44 inhibits the invasion and metastasis of hepatocellular carcinoma both in vitro and in vivo by reversing epithelial-mesenchymal transition. Oncotarget. 2015;6:7828–7837. doi: 10.18632/oncotarget.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattabiraman D.R., Bierie B., Kober K.I., et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science. 2016;351:aad3680. doi: 10.1126/science.aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H.L., Wang M.D., Zhou X., et al. Blocking preferential glucose uptake sensitizes liver tumor-initiating cells to glucose restriction and sorafenib treatment. Cancer Lett. 2017;388:1–11. doi: 10.1016/j.canlet.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Bort A., Sanchez B.G., Mateos-Gomez P.A., et al. Targeting AMP-activated kinase impacts hepatocellular cancer stem cells induced by long-term treatment with sorafenib. Mol Oncol. 2019;13:1311–1331. doi: 10.1002/1878-0261.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Z., Jia J., Heng G., et al. Sirtuin-1/mitochondrial ribosomal protein S5 axis enhances the metabolic flexibility of liver cancer stem cells. Hepatology. 2019;70:1197–1213. doi: 10.1002/hep.30622. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Chen H.N., Wang K., et al. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 2019;70:66–77. doi: 10.1016/j.jhep.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Song J., Zhao W., Lu C., Shao X. LATS2 overexpression attenuates the therapeutic resistance of liver cancer HepG2 cells to sorafenib-mediated death via inhibiting the AMPK-Mfn2 signaling pathway. Cancer Cell Int. 2019;19:60. doi: 10.1186/s12935-019-0778-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Senni N., Savall M., Cabrerizo Granados D., et al. Beta-catenin-activated hepatocellular carcinomas are addicted to fatty acids. Gut. 2019;68:322–334. doi: 10.1136/gutjnl-2017-315448. [DOI] [PubMed] [Google Scholar]
- 38.Chen C.L., Uthaya Kumar D.B., et al. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016;23:206–219. doi: 10.1016/j.cmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jezek J., Plecita-Hlavata L., Jezek P. Aglycemic HepG2 cells switch from aminotransferase glutaminolytic pathway of pyruvate utilization to complete Krebs cycle at hypoxia. Front Endocrinol (Lausanne) 2018;9:637. doi: 10.3389/fendo.2018.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu G., Liu R., Shan Y., Sun C. Marine bacterial exopolysaccharide EPS11 inhibits migration and invasion of liver cancer cells by directly targeting collagen I. J Biol Chem. 2021;297 doi: 10.1016/j.jbc.2021.101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaniotis G., Rayes R.F., Qi S., et al. Collagen IV-conveyed signals can regulate chemokine production and promote liver metastasis. Oncogene. 2018;37:3790–3805. doi: 10.1038/s41388-018-0242-z. [DOI] [PubMed] [Google Scholar]
- 43.Lee H.J., Diaz M.F., Price K.M., et al. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat Commun. 2017;8 doi: 10.1038/ncomms14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ringelhan M., Pfister D., O'Connor T., et al. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 45.Cancer Genome Atlas Research Network Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–1341 e1323. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q., He Y., Luo N., et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829–845 e820. doi: 10.1016/j.cell.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Li X., Li C., Zhang L., et al. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol Cancer. 2020;19:1. doi: 10.1186/s12943-019-1085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nallanthighal S., Heiserman J.P., Cheon D.J. The role of the extracellular matrix in cancer stemness. Front Cell Dev Biol. 2019;7:86. doi: 10.3389/fcell.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.You Y., Zheng Q., Dong Y., et al. Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells. Oncotarget. 2016;7:32221–32231. doi: 10.18632/oncotarget.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng K.Y., Shea Q.T., Wong T.L., et al. Chemotherapy-enriched THBS2-deficient cancer stem cells drive hepatocarcinogenesis through matrix softness induced histone H3 modifications. Adv Sci (Weinh) 2021;8 doi: 10.1002/advs.202002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian B., Luo Q., Ju Y., Song G. A soft matrix enhances the cancer stem cell phenotype of HCC cells. Int J Mol Sci. 2019;20:2831. doi: 10.3390/ijms20112831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lv J., Liu Y., Cheng F., et al. Cell softness regulates tumorigenicity and stemness of cancer cells. EMBO J. 2021;40 doi: 10.15252/embj.2020106123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang G.L., Gutter-Kapon L., Ilan N., et al. Significance of host heparanase in promoting tumor growth and metastasis. Matrix Biol. 2020;93:25–42. doi: 10.1016/j.matbio.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen J., Yang C., Zhang M.S., et al. Histone chaperone FACT complex coordinates with HIF to mediate an expeditious transcription program to adapt to poorly oxygenated cancers. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110304. [DOI] [PubMed] [Google Scholar]
- 55.Ding M., Zhang S., Guo Y., et al. Tumor microenvironment acidity triggers lipid accumulation in liver cancer via SCD1 activation. Mol Cancer Res. 2022;20:810–822. doi: 10.1158/1541-7786.MCR-21-0699. [DOI] [PubMed] [Google Scholar]
- 56.Ying C., Jin C., Zeng S., et al. Alkalization of cellular pH leads to cancer cell death by disrupting autophagy and mitochondrial function. Oncogene. 2022;41:3886–3897. doi: 10.1038/s41388-022-02396-6. [DOI] [PubMed] [Google Scholar]
- 57.Yu H., He J., Su G., et al. Fluid shear stress activates YAP to promote epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Oncol. 2021;15:3164–3183. doi: 10.1002/1878-0261.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huch M., Dolle L. The plastic cellular states of liver cells: are EpCAM and Lgr5 fit for purpose? Hepatology. 2016;64:652–662. doi: 10.1002/hep.28469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan S., Zhao E., Kryczek I., et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei R., Zhu W.W., Yu G.Y., et al. S100 calcium-binding protein A9 from tumor-associated macrophage enhances cancer stem cell-like properties of hepatocellular carcinoma. Int J Cancer. 2021;148:1233–1244. doi: 10.1002/ijc.33371. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y., Wen H., Zhou C., et al. TNF-alpha derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/beta-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. 2019;378:41–50. doi: 10.1016/j.yexcr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Fan Q.M., Jing Y.Y., Yu G.F., et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Wang B., Xiao S., et al. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J Cell Biochem. 2019;120:3046–3055. doi: 10.1002/jcb.27436. [DOI] [PubMed] [Google Scholar]
- 64.Pickup M., Novitskiy S., Moses H.L. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou S.L., Yin D., Hu Z.Q., et al. A positive feedback loop between cancer stem-like cells and tumor-associated neutrophils controls hepatocellular carcinoma progression. Hepatology. 2019;70:1214–1230. doi: 10.1002/hep.30630. [DOI] [PubMed] [Google Scholar]
- 66.Trompouki E., Hatzivassiliou E., Tsichritzis T., et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 67.Pan Q.Z., Pan K., Wang Q.J., et al. Annexin A3 as a potential target for immunotherapy of liver cancer stem-like cells. Stem Cells. 2015;33:354–366. doi: 10.1002/stem.1850. [DOI] [PubMed] [Google Scholar]
- 68.Ho D.W., Tsui Y.M., Sze K.M., et al. Single-cell transcriptomics reveals the landscape of intra-tumoral heterogeneity and stemness-related subpopulations in liver cancer. Cancer Lett. 2019;459:176–185. doi: 10.1016/j.canlet.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Ruiz de Galarreta M., Bresnahan E., Molina-Sanchez P., et al. Beta-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y., Song Y., Lin D., et al. NCR(-) group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development. EBioMedicine. 2019;41:333–344. doi: 10.1016/j.ebiom.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Debebe A., Medina V., Chen C.Y., et al. Wnt/beta-catenin activation and macrophage induction during liver cancer development following steatosis. Oncogene. 2017;36:6020–6029. doi: 10.1038/onc.2017.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu M., Zhao Z., Song J., et al. Interactions between interleukin-6 and myeloid-derived suppressor cells drive the chemoresistant phenotype of hepatocellular cancer. Exp Cell Res. 2017;351:142–149. doi: 10.1016/j.yexcr.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Luo Q., Wang C.Q., Yang L.Y., et al. FOXQ1/NDRG1 axis exacerbates hepatocellular carcinoma initiation via enhancing crosstalk between fibroblasts and tumor cells. Cancer Lett. 2018;417:21–34. doi: 10.1016/j.canlet.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 74.Loh J.J., Li T.W., Zhou L., et al. FSTL1 secreted by activated fibroblasts promotes hepatocellular carcinoma metastasis and stemness. Cancer Res. 2021;81:5692–5705. doi: 10.1158/0008-5472.CAN-20-4226. [DOI] [PubMed] [Google Scholar]
- 75.Rawal P., Siddiqui H., Hassan M., et al. Endothelial cell-derived TGF-beta promotes epithelial-mesenchymal transition via CD133 in HBx-infected hepatoma cells. Front Oncol. 2019;9:308. doi: 10.3389/fonc.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei Y., Shi D., Liang Z., et al. IL-17A secreted from lymphatic endothelial cells promotes tumorigenesis by upregulation of PD-L1 in hepatoma stem cells. J Hepatol. 2019;71:1206–1215. doi: 10.1016/j.jhep.2019.08.034. [DOI] [PubMed] [Google Scholar]
- 77.Cheng Z., Lei Z., Yang P., et al. Exosome-transmitted p120-catenin suppresses hepatocellular carcinoma progression via STAT3 pathways. Mol Carcinog. 2019;58:1389–1399. doi: 10.1002/mc.23022. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Yang H., Li Q., et al. Three-dimensional ameliorated biologics elicit thymic renewal in tumor-bearing hosts. J Immunol. 2018;201:1975–1983. doi: 10.4049/jimmunol.1701727. [DOI] [PubMed] [Google Scholar]
- 79.Chan L.C., Li C.W., Xia W., et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J Clin Invest. 2019;129:3324–3338. doi: 10.1172/JCI126022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schietinger A., Philip M., Krisnawan V.E., et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity. 2016;45:389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Y., Poggio M., Jin H.Y., et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat Med. 2019;25:301–311. doi: 10.1038/s41591-018-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Helbig K.J., Ruszkiewicz A., Semendric L., et al. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004;39:1220–1229. doi: 10.1002/hep.20167. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y., Zhao W., Li S., et al. CXCL11 promotes self-renewal and tumorigenicity of alpha2delta1(+) liver tumor-initiating cells through CXCR3/ERK1/2 signaling. Cancer Lett. 2019;449:163–171. doi: 10.1016/j.canlet.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 84.He H., Wu J., Zang M., et al. CCR6(+) B lymphocytes responding to tumor cell-derived CCL20 support hepatocellular carcinoma progression via enhancing angiogenesis. Am J Cancer Res. 2017;7:1151–1163. [PMC free article] [PubMed] [Google Scholar]
- 85.Liu W.T., Jing Y.Y., Gao L., et al. Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts constitutes the hepatocarcinogenesis-associated microenvironment. Cell Death Differ. 2020;27:85–101. doi: 10.1038/s41418-019-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomann S., Weiler S.M.E., Marquard S., et al. YAP orchestrates heterotypic endothelial cell communication via HGF/c-MET signaling in liver tumorigenesis. Cancer Res. 2020;80:5502–5514. doi: 10.1158/0008-5472.CAN-20-0242. [DOI] [PubMed] [Google Scholar]
- 87.Huang H., Hou J., Liu K., et al. RAB27A-dependent release of exosomes by liver cancer stem cells induces Nanog expression in their differentiated progenies and confers regorafenib resistance. J Gastroenterol Hepatol. 2021;36:3429–3437. doi: 10.1111/jgh.15619. [DOI] [PubMed] [Google Scholar]
- 88.Ashokachakkaravarthy K., Pottakkat B. Mitotic quiescence in hepatic cancer stem cells: an incognito mode. Oncol Rev. 2020;14:452. doi: 10.4081/oncol.2020.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gade T.P.F., Tucker E., Nakazawa M.S., et al. Ischemia induces quiescence and autophagy dependence in hepatocellular carcinoma. Radiology. 2017;283:702–710. doi: 10.1148/radiol.2017160728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ju F., Atyah M.M., Horstmann N., et al. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res Ther. 2022;13:233. doi: 10.1186/s13287-022-02904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin H., Cheng J., Mu W., et al. Advances in universal CAR-T cell therapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.744823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bai P., Ge C., Yang H., et al. Screening a redox library identifies the anti-tumor drug Hinokitiol for treating intrahepatic cholangiocarcinoma. Front Biosci. 2022;27:18. doi: 10.31083/j.fbl2701018. [DOI] [PubMed] [Google Scholar]
- 93.Man K.F., Ma S. Mechanisms of resistance to tyrosine kinase inhibitors in liver cancer stem cells and potential therapeutic approaches. Essays Biochem. 2022;66:371–386. doi: 10.1042/EBC20220001. [DOI] [PubMed] [Google Scholar]
- 94.Jiang Z., Sun H., Yu J., et al. Targeting CD47 for cancer immunotherapy. J Hematol Oncol. 2021;14:180. doi: 10.1186/s13045-021-01197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tong M., Che N., Zhou L., et al. Efficacy of annexin A3 blockade in sensitizing hepatocellular carcinoma to sorafenib and regorafenib. J Hepatol. 2018;69:826–839. doi: 10.1016/j.jhep.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 96.Ho D.W., Tsui Y.M., Chan L.K., et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun. 2021;12:3684. doi: 10.1038/s41467-021-24010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]