Abstract
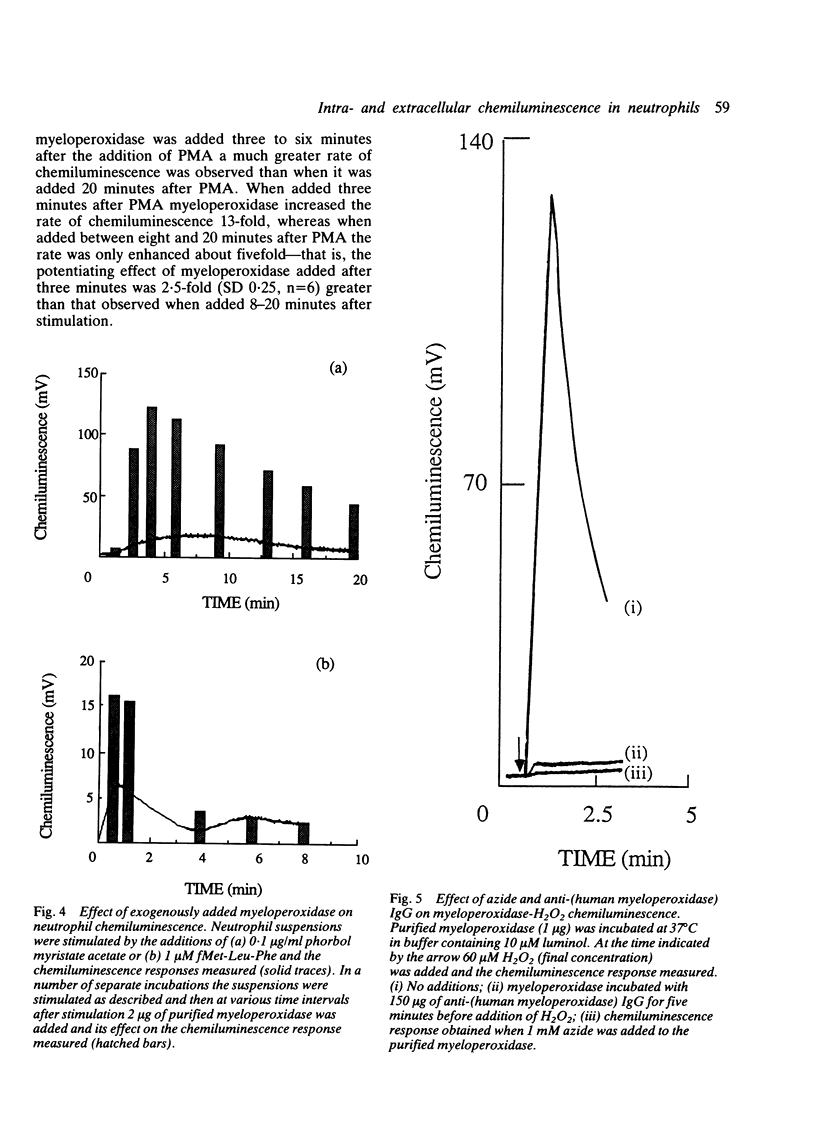
Activated polymorphonuclear leucocytes (neutrophils) can generate both intracellular and extracellular luminol dependent chemiluminescence. As luminol dependent chemiluminescence largely measures the activity of the myeloperoxidase-H2O2 system, and as the extracellular activity of this enzyme may be responsible for the tissue damage associated with inflammatory conditions such as rheumatoid arthritis, the aim of this work was to distinguish between intracellular and extracellular chemiluminescence so that the extracellular activity of this enzyme could be evaluated. Azide was used as a non-specific inhibitor of both intracellular and extracellular chemiluminescence, whereas anti-(human myeloperoxidase) IgG was used to inhibit specifically the extracellular activity of myeloperoxidase. Thus this IgG is a useful analytical tool for studying the extracellular activity of the myeloperoxidase-H2O2 system in the pathology of rheumatoid arthritis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Bender J. G., Van Epps D. E. Analysis of the bimodal chemiluminescence pattern stimulated in human neutrophils by chemotactic factors. Infect Immun. 1983 Sep;41(3):1062–1070. doi: 10.1128/iai.41.3.1062-1070.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Winyard P., Lunec J., Williams A., Good P. A., Crewes S. J., Gutteridge J. M., Rowley D., Halliwell B., Cornish A. Cerebral and ocular toxicity induced by desferrioxamine. Q J Med. 1985 Jul;56(219):345–355. [PubMed] [Google Scholar]
- Briheim G., Stendahl O., Dahlgren C. Intra- and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1984 Jul;45(1):1–5. doi: 10.1128/iai.45.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C., Aniansson H., Magnusson K. E. Pattern of formylmethionyl-leucyl-phenylalanine-induced luminol- and lucigenin-dependent chemiluminescence in human neutrophils. Infect Immun. 1985 Jan;47(1):326–328. doi: 10.1128/iai.47.1.326-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C. Difference in extracellular radical release after chemotactic factor and calcium ionophore activation of the oxygen radical-generating system in human neutrophils. Biochim Biophys Acta. 1987 Aug 19;930(1):33–38. doi: 10.1016/0167-4889(87)90152-2. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Hughes V., Barlow J., Bucknall R. Immunological detection of myeloperoxidase in synovial fluid from patients with rheumatoid arthritis. Biochem J. 1988 Feb 15;250(1):81–85. doi: 10.1042/bj2500081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W. Luminol- and lucigenin-dependent chemiluminescence of neutrophils: role of degranulation. J Clin Lab Immunol. 1987 Jan;22(1):35–39. [PubMed] [Google Scholar]
- Edwards S. W., Nurcombe H. L., Hart C. A. Oxidative inactivation of myeloperoxidase released from human neutrophils. Biochem J. 1987 Aug 1;245(3):925–928. doi: 10.1042/bj2450925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Swan T. F. Regulation of superoxide generation by myeloperoxidase during the respiratory burst of human neutrophils. Biochem J. 1986 Jul 15;237(2):601–604. doi: 10.1042/bj2370601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Gale R., Bertouch J. V., Bradley J., Roberts-Thomson P. J. Direct activation of neutrophil chemiluminescence by rheumatoid sera and synovial fluid. Ann Rheum Dis. 1983 Apr;42(2):158–162. doi: 10.1136/ard.42.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Two distinct mechanisms for stimulation of oxygen-radical production by polymorphonuclear leucocytes. Biochem J. 1983 Nov 15;216(2):459–465. doi: 10.1042/bj2160459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Blake D. Metal ions and oxygen radical reactions in human inflammatory joint disease. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):659–671. doi: 10.1098/rstb.1985.0171. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Polson R. J., Jawad A. S., Bomford A., Berry H., Williams R. Treatment of rheumatoid arthritis with desferrioxamine. Q J Med. 1986 Dec;61(236):1153–1158. [PubMed] [Google Scholar]
- Polson R. J., Jawed A., Bomford A., Berry H., Williams R. Treatment of rheumatoid arthritis with desferrioxamine: relation between stores of iron before treatment and side effects. Br Med J (Clin Res Ed) 1985 Aug 17;291(6493):448–448. doi: 10.1136/bmj.291.6493.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]


