Abstract
Background:
In 2021, WHO launched the Global Breast Cancer Initiative to improve breast cancer survival. Comprehensive breast cancer management needs critical attention in Sub-Saharan Africa (SSA), however detailed profiles of treatment regimens received are lacking. We examined treatment initiation, guideline concordance, and abandonment among patients with non-metastatic breast cancer in SSA using data from the hospital-based African Breast Cancer - Disparities in Outcomes (ABC-DO) prospective cohort.
Methods:
During 10 September 2014 to 31 December 2017, ABC-DO recruited 2226 women with newly diagnosed breast cancer (90% histologically confirmed) aged 18+ years in five SSA countries (Namibia, Nigeria, Uganda, South Africa and Zambia). Data on surgery, radiation and systemic therapies were obtained from medical records and self-reports via three-monthly follow-up phone calls. Among 1716 women with non-metastatic disease at diagnosis, initiation and completion of mono- and combined therapy regimens were examined overall, by country-race groups, and by clinical factors relevant for guideline-based treatment.
Findings:
After a median follow-up time of 5.2 years (interquartile range: 4·6-5·9), surgery and systemic therapy with/without radiotherapy was initiated for 36% (370/1028)/38% (386/1028) of women with localised disease (T1-T3N0-N1M0) and 23% (156/688)/24% (167/688) with locally-advanced (T4N2-N3M0) disease. Of women requiring chemotherapy, timely treatment was initiated in 66% (1013/1530) and of these, adequately and marginally completed by 35% (359/1013) and 28% (284/1013), respectively. Abandonment percentages were up to 38% (37/97) in Nigeria. Initiated endocrine therapy was/is ongoing for over three years in 40% (367/921) of women. Over 30% of patients did not undergo surgery. Treatment disparities between country-race groups were substantial for all therapy regimens.
Interpretation:
High proportions of breast cancer patients did not initiate, or abandoned a curative scheme of surgery, systemic therapy and/or radiotherapy, highlighting the need for urgent action to improve treatment quality in SSA to ultimately prevent premature deaths.
Introduction
Breast cancer is the most common cancer, and cause of cancer death, amongst women worldwide, responsible for almost 2·3 million new cases and 700,000 deaths globally in 2020.1 Survival is particularly low in sub-Saharan Africa (SSA) where only 40-70% of women are alive after three years, contrasted by up to 90% after five years being alive in high income countries.2–4 To reduce global breast cancer mortality, WHO recently launched the Global Breast Cancer Initiative (GBCI).5 One of its three strategic pillars is comprehensive breast cancer management with the goal of at least 80% of patients completing stage-appropriate multimodality treatment without abandonment. Crucially, GBCI emphasises that the concept of “abandonment” is two-sided and that not only the patient can be to blame for incomplete treatment but also the system can “abandon” the patient. The assessment of causes for treatment abandonment must begin with the collection of detailed data on treatment, and its quality, for benchmarking and priority setting; in SSA these data are scarce.
Early diagnosis coupled with stage-appropriate treatment were identified as the major determinants of survival in the African Breast Cancer-Disparities in Outcomes (ABC-DO) study – a prospective breast cancer cohort spanning hospitals in five SSA countries.2 It was previously reported that 20% of newly diagnosed breast cancer patients did not initiate treatment (systemic, surgery and/or radiotherapy) within 12 months of diagnosis, detailed assessment about completion of treatment is essential.6 If less than 85% of the total cumulative chemotherapy regimen is completed, patients may experience adverse side effects, but will not or just minimally achieve survival benefit.7, 8
To provide guidance for breast cancer treatment in SSA, the National Comprehensive Cancer Network (NCCN) Harmonized Guidelines™ define tailored strategies adapted to available treatment resources in the region, but concordance to these guidelines remains under-evaluated.9
Herein, we provide a comprehensive profile of current breast cancer management for non-metastatic breast cancer in SSA settings using the prospective ABC-DO cohort. This report focuses on breast cancer surgery and systemic therapy (defined as chemotherapy and/or ET). Because radiotherapy is unavailable in most participating hospitals, and in SSA in general, only basic radiotherapy information is presented. Specifically, we (i) describe initiation of surgery and/or systemic therapy and observe patterns of treatment abandonment in hospital settings across five SSA countries; (ii) compare surgery and systemic therapy concordance with NCCN Harmonized Guidelines™ recommendations; (iii) identify treatment gaps and opportunities for systematic improvements in care.
Methods
Study design
The ongoing ABC-DO prospective breast cancer cohort recruited all adult women (≥18 years at interview) newly diagnosed with invasive breast cancer (90% histologically confirmed, 10% clinical) in seven public and one private hospitals across five SSA countries (Namibia, Nigeria, Uganda, South Africa, Zambia; appendix p 3). The protocol of ABC-DO has previously been published.10 In short, the prospective ABC-DO design consisted of a baseline patient interview at first presentation to the participating hospital and thereafter a personal three-monthly follow-up call. Treatment, histology, pathology and staging data were recorded (details below). Trained fieldworkers entered data into an mHealth application with automatic encrypted upload to a cloud server.11 To capture the actual breast cancer journey of patients in SSA, ABC-DO was intentionally observational without additional capacities for diagnosis and treatment provided. The ABC-DO patients from South Africa are a subset of the on-going SABCHO (South African Breast Cancer and HIV) cohort, whose data were harmonized as closely as possible.12 The ABC-DO study was approved by all institutional ethics committees (appendix p 2), and all women provided written or thumbprint informed consent.
Study sample
We analysed the treatments received by women who were classified as non-metastatic (M0) at the initial presentation (Figure 1) . Palliative care was beyond the scope of this article and will be reported separately.
Figure 1:
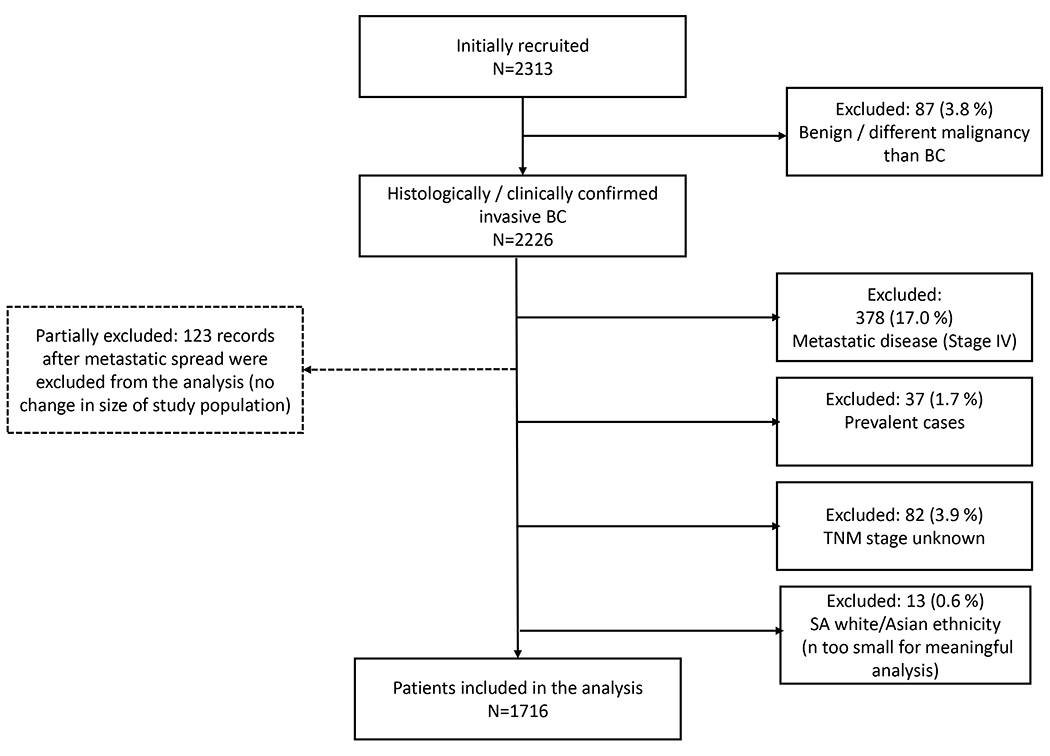
Flow chart of study sample determination
Demographic and clinical data
To account for ethnic and sociodemographic differences, the South African and Namibian participants were subdivided into black and mixed-race (South Africa) or non-black (Namibia) resulting in seven population groups. In South Africa 13 White and Asian women were not included as they tend to have better treatment access, but were too few to be being analysed separately. This approach was also recently applied in the three year survival analysis.2 Factors collected in the baseline questionnaire included: education, relationship status, residency, socioeconomic status, HIV status and other comorbidities (appendix p 4). Breast cancer stage according to the American Joint Committee on Cancer (AJCC) TNM staging system (8th edition), and histology-confirmed tumour grade were entered (appendix p 5 and 6).13 To assess correlation with the NCCN Harmonised Guidelines™, TNM stage was dichotomized into localised (T1-3, N0-1, M0) vs locally advanced/inoperable tumours (T4,N2-3,M0). Immunohistochemistry (IHC) was routinely done in Namibia and South Africa and not in the other countries: oestrogen (ER), progesterone (PR) and human epidermal growth factor 2 receptors (HER2) were determined, the latter with florescent in-situ hybridization (FISH) for equivocal results. ER and PR were considered positive with >1% staining, and HER2+ if FISH was positive or the HER2 score was 3. Receptor defined subtypes were classified into HR+ (oestrogen and/or progesterone positive)/HER2+, HR+/HER2−, HR− /HER2+ and HR−/HER2− (triple negative). Patients with distant metastasis (imaging or clinical confirmation) were excluded, lack of any metastasis mentioned was considered as M0 according to TNM classification (for n=415 (24%) of patients).
Treatment data
Treatment data up to 15th July 2021 (median follow-up time of 5·2 years (interquartile range: 4·6-5·9) were obtained from (i) hospital medical records ; (ii) women’s self-reports retrieved from a treatment follow-up questionnaire at six months post-diagnosis, (iii) regular three-monthly follow-up calls via the question “Are you currently receiving any medical treatment for breast cancer?”, and (iv) baseline questionnaire information on treatment received before hospital presentation. Extracted data were for surgery: date, surgery type (mastectomy, breast conserving surgery (BCS)), and lymph node dissection (axillary sampling, sentinel node biopsy, complete axillary node dissection, unknown/missing) ; for chemotherapy: first and last cycle dates, therapy timing (adjuvant vs neoadjuvant), number of cycles, drug regimen (combinations or monotherapies from cyclophosphamide (C), doxorubicin (Adriamycin; A), Fluorouracil (F), Methotrexate (M) and Taxane (T), and targeted therapy (i.e. Trastuzumab)); for oral ET : first and last treatment dates, therapy timing (neoadjuvant vs adjuvant), drugs prescribed (tamoxifen, aromatase inhibitor) ; for surgical ET : ovarian ablation performed and date. If multiple treatment dates were available, the earliest date was chosen. For radiotherapy, only “initiated yes/no” was extracted.
Statistical analysis
Descriptive statistics were used throughout, without the use of any inferential statistics. Combining information from medical and self-reports, binary indicators (yes/no) of treatment ever initiated for each treatment modality were created. Crude mono- and combined therapy initiation frequencies and percentages were calculated overall, by population group, and by localised/locally advanced tumours.
Treatment quality assessment was guided by NCCN Harmonized Guidelines™ for SSA. (i) A reference patient group for chemotherapy was defined as women having at least one of the following risk factors: HER2+, HR−, N+ (≥two nodes affected), ≥T2, G3 and young age (<35 years) at diagnosis. Chemotherapy initiation was considered guideline concordant if any treatment started within three months after baseline. To account for differences in chemotherapy regimen quality, two levels of completion were defined : “Marginally completed” was defined as at least five cycles of FAC or equivalent regimen administered within 20 weeks (recommended are 15) or four cycles of AC (evidence category 2B) within the recommended 12 weeks; “adequately completed” (i.e. >85%) was defined as at least five cycles of FAC (or equivalent regimen) administered within 15 weeks after chemotherapy initiation, or seven to eight cycles of (F)AC-T within 28 weeks (recommended are 24). To control for co-morbidities as reason for suboptimal treatment, only women alive six months post-baseline were considered. (ii) The reference patient group for ET was defined as women with known HR+ or missing HR status. Due to limited study follow-up time ET treatment completion was approximated by at least three years of continued treatment, and only women alive at six months post-baseline were considered. (iii) The absolute cumulative initiation, and abandonment percentages of multimodality treatment (i.e., surgery and systemic therapy) were plotted over one year after baseline for each study population.
Data were analysed using Stata 15·0 (StataCorp LLC, College Station, Texas) and R Studio, version 3·6·1.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all data. The corresponding author has the final responsibility to submit for publication.
Results
Of 2313 women recruited into ABC-DO between 10 September 2014 and 31 December 2017, 1716 patients were non-metastatic (M0) at presentation (Figure 1). Of these women, 60% (1028/1716) presented with localised (T1-3N0-1M0) and 40% (688/1716) with locally advanced (T4N2-3M0) tumours (appendix p 5). The majority of women in South Africa and Namibia had HR+/HER− tumours (from 48% (147/309) in Namibian black to 69% (23/34) in South Africa mixed-race women). The socio-demographic profile is given in appendix p 4.
In the analysis of treatment data up to 15th July 2021, the median follow-up time was 5·2 years (interquartile range: 4·6 to 5·9). Of all, 16% (256/1716) died within one year after hospital presentation (data not shown). The majority of these women (71%, 181/256) had initiated treatment, mostly neoadjuvant chemotherapy (61%, 156/256) and/or surgery (31%, 80/256). Of the whole study population, 8% (137/1716) died before starting any treatment. For these, median time between hospital presentation and death was 10·1 months (interquartile range: 3·1 to 21·8).
In all, 68% (1163/1716) of women received surgery, ranging from 53% (79/150) in Zambia to 90% (82/91) in Namibian non-black women, 72% (1232/1716) initiated chemotherapy (> 70% in all population groups but Nigeria (49%, 143/292), 57% (973/1716) initiated ET (from 37% (109/292) in Nigeria to 77% (70/91) in Namibian non-black women), and 33% (569/1716) initiated radiotherapy (from 3% (9/292) in Nigeria to 66% (60/91) in Namibian non-black women, Table 1, Figure 2). Multimodality treatment (i.e. surgery and systemic therapy) with and without radiotherapy was initiated by 36% (370/1028) and 38% (386/1028) of women, respectively, with localised and 23% (156/688) and 24% (167/688) of women with locally advanced breast cancer but there were marked variations across patient groups (Table 2, appendix p 8 and 9). For both localised and locally-advanced tumours, initiation of multimodality treatment (with or without radiotherapy) was lowest in Nigeria (46% (58/127) and 38% (63/165), respectively) and highest in Namibian non-black women (89% (67/75) and 81% (13/16), respectively). Systemic therapy without surgery was received by 13% (135/1028) of women with localised and 34% (236/688) with locally advanced disease. No treatment was received by 8% (77/1028) of women with localised and 15% (104/688) with locally advanced tumours. Of all women initiating any treatment, 88% (1349/1535) - from 62% (131/212) in Nigeria to 99% (300/303) in black Namibian women - initiated it within three months of baseline (including 456 women starting treatment before baseline or on the day itself). The initiation, abandonment, and completion proportions of multimodality treatment (surgery and systemic therapy combined) of all women and by population group are displayed in Figure 3.
Table 1:
Descriptive statistics of breast cancer management for the treatment modalities of surgery, chemotherapy (CT) and endocrine therapy (ET) in women diagnosed with non-metastatic breast cancer with known TNM stage in the ABC-DO cohort
| Namibia black | Namibia non-black | Nigeria | SA black | SA mixed-race | Uganda | Zambia | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| No. study sample with non-metastatic breast cancer | 309 | 91 | 292 | 544 | 34 | 296 | 150 | 1716 | |
| Subset populating the denominator | N (column % among women who received the respective treatment) | ||||||||
| Breast cancer surgery performed | Whole study sample | 221/309 (71) | 82/91 (90) | 156/292 (53) | 415/544 (76) | 28/34 (82) | 182/296 (61) | 79/150 (53) | 1163/1716 (68) |
| Mastectomy performed | Received surgery | 205/221 (93) | 66/82 (80) | 80/156 (51) | 297/415 (71) | 18/28 (64) | 167/182 (92) | 76/79 (96) | 909/1163 (78) |
| Received consecutive radiotherapy | Received mastectomy | 160/205 (78) | 43/66 (65) | 7/80 (9) | 127/297 (43) | 12/18 (67) | 45/167 (27) | 32/76 (42) | 426/909 (47) |
| Breast conserving surgery | Received surgery | 16/221 (7) | 16/82 (20) | 76/156 (49) | 119/415 (29) | 10/28 (36) | 15/182 (8) | 3/79 (4) | 255/1163 (22) |
| Received consecutive radiotherapy | Received breast conserving surgery | 13/16 (81) | 15/16 (94) | 2/76 (3) | 67/119 (56) | 6/10 (60) | 4/15 (27) | 0/3 (0) | 107/255 (42) |
| Nodes dissected | Received surgery | ||||||||
| Not done | “ | 11/221 (5) | 0/82 (0) | 4/156 (3) | 6/415 (1) | 0/28 (0) | 23/182 (13) | 1/79 (1) | 45/1163 (4) |
| Sentinel or sampling | “ | 26/221 (12) | 15/82 (18) | 3/156 (2) | 0/415 (0) | 0/28 (0) | 0/182 (0) | 0/79 (0) | 44/1163 (4) |
| Complete axillary node dissection | “ | 40/221 (18) | 11/82 (13) | 6/156 (4) | 361/415 (87) | 26/28 (93) | 8/182 (4) | 4/79 (5) | 456/1163 (39) |
| unknown | “ | 144/221 (65) | 56/82 (68) | 143/156 (92) | 48/415 (12) | 2/28 (7) | 151/182 (83) | 74/79 (94) | 618/1163 (53) |
| Additional systemic therapyA | Received surgery | ||||||||
| Neoadjuvant | “ | 132/221 (60) | 45/82 (55) | 69/156 (44) | 157/415 (38) | 9/28 (32) | 23/182 (13) | 40/79 (51) | 475/1163 (41) |
| Adjuvant | “ | 143/221 (65) | 61/82 (74) | 76/156 (49) | 332/415 (80) | 25/28 (89) | 167/182 (92) | 47/79 (59) | 851/1163 (73) |
| No systemic therapy | “ | 6/221 (3) | 2/82 (2) | 35/156 (22) | 19/415 (5) | 1/28 (4) | 12/182 (7) | 9/79 (11) | 84/1163 (7) |
| Chemotherapy initiated | Whole study sample | 245/309 (79) | 65/91 (71) | 143/292 (49) | 415/544 (76) | 27/34 (79) | 228/296 (77) | 109/150 (73) | 1232/1716 (72) |
| Timing of chemotherapy | Initiated chemotherapy | ||||||||
| NeoadjuvantB | “ | 187/245 (76) | 44/65 (68) | 89/143 (62) | 226/415 (54) | 13/27 (48) | 75/228 (33) | 80/109 (73) | 714/1232 (58) |
| Adjuvant | “ | 58/245 (24) | 21/65 (32) | 54/143 (38) | 189/415 (46) | 14/27 (52) | 153/228 (67) | 29/109 (27) | 518/1232 (42) |
| Regimen C | Initiated chemotherapy | ||||||||
| AC/CMF | “ | 36/245 (15) | 16/65 (25) | 22/143 (15) | 75/415 (18) | 7/27 (26) | 8/228 (4) | 8/109 (7) | 172/1232 (14) |
| FAC | “ | 63/245 (26) | 6/65 (9) | 56/143 (39) | 31/415 (7) | 1/27 (4) | 44/228 (19) | 37/109 (34) | 238/1232 (19) |
| (F)AC-T | “ | 131/245 (543) | 34/65 (52) | 6/143 (4) | 253/415 (61) | 16/27 (59) | 6/228 (3) | 40/109 (37) | 486/1232 (39) |
| non-standard | “ | 6/245 (2) | 5/65 (8) | 7/143 (5) | 28/415 (7) | 1/27 (4) | 0/228 (0) | 2/109 (2) | 49/1232 (4) |
| unknown | “ | 9/245 (4) | 4/65 (6) | 52/143 (36) | 28/415 (7) | 2/27 (7) | 170/228 (75) | 22/109 (20) | 287/1232 (23) |
| Trastuzumab received | Whole study sample | 36/309 (12) | 9/91 (10) | 1/292 (0.3) | 1/544 (0.2) | 0/34 (0) | 0/296 (0) | 0/150 (0) | 47/1716 (3) |
| ET initiated | “ | 233/309 (75) | 70/91 (77) | 109/292 (37) | 309/544 (57) | 26/34 (77) | 161/296 (54) | 65/150 (43) | 973/1716 (57) |
| Timing | Initiated ET | ||||||||
| Neoadjuvant D | “ | 97/233 (42) | 15/70 (21) | 66/109 (61) | 42/309 (14) | 3/26 (12) | 56/161 (35) | 33/65 (51) | 312/973 (32) |
| Adjuvant | “ | 131/233 (56) | 55/70 (79) | 43/109 (39) | 267/309 (86) | 23/26 (88) | 95/161 (59) | 31/65 (48) | 645/973 (66) |
| Unknown | “ | 5/233 (2) | 0/70 (0) | 0/109 (0) | 0/309 (0) | 0/26 (0) | 10/161 (6) | 1/65 (2) | 16/973 (2) |
| Drug | Initiated ET | ||||||||
| Tamoxifen | “ | 196/233 (84) | 46/70 (66) | 107/109 (98) | 304/309 (98) | 25/26 (96) | 145/161 (90) | 60/65 (92) | 883/973 (91) |
| Aromatase inhibitor | “ | 5/233 (2) | 17/70 (24) | 0/109 (0) | 5/309 (2) | 1/26 (4) | 3/161 (2) | 0/65 (0) | 31/973 (3) |
| Tamoxifen -> Aromatase inhibitors | “ | 27/233 (12) | 7/70 (10) | 1/109 (1) | 0/309 (0) | 0/26 (0) | 11/161 (7) | 4/65 (6) | 50/973 (5) |
| Unable to determine | “ | 5/233 (2) | 0/70 (0) | 1/109 (1) | 0/309 (0) | 0/26 (0) | 2/161 (1) | 1/65 (2) | 9/973 (1) |
| Ovarian ablation | Whole study sample | 12/309 (4) | 2/91 (2) | 0/292 (0) | 0/544 (0) | 0/34 (0) | 0/296 (0) | 0/150 (0) | 14/1716 (1) |
Column percentage >100% due to 247 women receiving neoadjuvant and adjuvant systemic therapy;
Including 287 women not receiving surgery after initiated chemotherapy;
Information only available in subsample with medical CT records (N=975). Chemotherapy drugs contained in regimens: Cyclophosphamide (C), Doxorubicin (Adriamycin; A), Fluorouracil (F), Methotrexate (M) and Taxane (T). Amongst 50 patients with non-standard regimen: 41 patients received Taxane only, 2 Xeloda only, 5 Carboplatin and 1 Cyclophosphamide and Carboplatin;
Including 84 women not receiving surgery after initiated endocrine therapy
Figure 2:
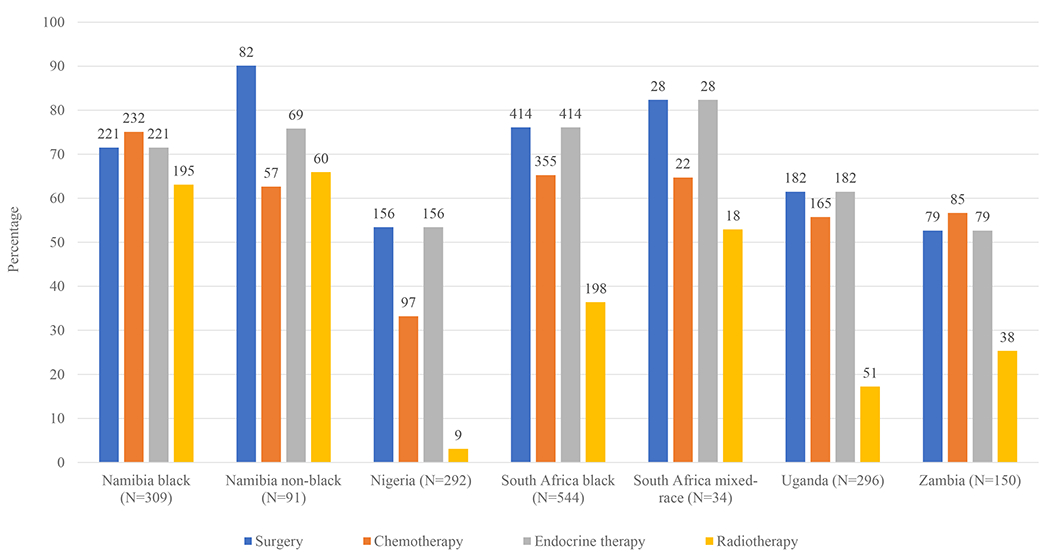
Treatment initiation among women with non-metastatic breast cancer and known TNM stage in the ABC-DO cohort, by population group. The bar height corresponds to the percentage of women who initiated treatment in each population group, bars are labelled with the number of women in the corresponding population group who initiated the respective treatment.
Table 2:
Breast cancer (BC) treatment initiation of surgery, systemic therapy (chemotherapy or endocrine therapy), and radiotherapy and their combinations in women diagnosed with non-metastatic breast cancer with known TNM stage information in the prospective African Breast Cancer-Disparities in Outcomes (ABC-DO) cohort, separately for women diagnosed with localised vs locally advanced tumours
| Type of treatment | Patient group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subset populating the denominator | Namibia black | Namibia non-black | Nigeria | South Africa black | South Africa mixed-race | Uganda | Zambia | All | |
| No. women | 309 | 91 | 292 | 544 | 34 | 296 | 150 | 1716 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| No. with localised BCA | Women with non-metastatic breast cancer | 178/309 (58) | 75/91 (82) | 127/292 (44) | 344/544 (63) | 26/34 (76) | 191/296 (65) | 87/150 (58) | 1028/1716 (60) |
| Received surgery, systemic therapy and radiotherapy | Women with localised BC | 111/178 (62) | 46/75 (61) | 6/127 (5) | 126/344 (37) | 14/26 (54) | 41/191 (21) | 26/87 (30) | 370/1028 (36) |
| Surgery, systemic therapy, no radiotherapy | “ | 30/178 (17) | 21/75 (28) | 52/127 (41) | 171/344 (48) | 9/26 (35) | 78/191 (41) | 25/87 (29) | 386/1028 (38) |
| Surgery (+/− radiotherapy) | “ | 3/178 (2) | 2/75 (3) | 25/127 (18) | 14/344 (4) | 1/26 (3.8) | 8/191 (4) | 7/87 (8) | 60/1028 (6) |
| Systemic therapy (+/− radiotherapy) | “ | 32/178 (18) | 6/75 (8) | 12/127 (9) | 20/344 (6) | 2/26 (8) | 47/191 (25) | 16/87 (18) | 135/1028 (13) |
| No treatment | “ | 2/178 (1) | 0/75 (0) | 32/127 (25) | 13/344 (4) | 0/26 (0) | 17/191 (9) | 13/87 (15) | 77/1028 (7) |
| No. locally advanced BCA | Women with non-metastatic breast cancer | 131/309 (42) | 16/91 (18) | 165/292 (57) | 200/ (37) | 8/34 (24) | 105/296 (35) | 63/150 (42) | 688/1716 (40) |
| Surgery, systemic therapy and radiotherapy | Women with locally advanced BC | 60/131 (46) | 11/16 (69) | 3/165 (2) | 65/200 (33) | 3/8 (38) | 8/105 (8) | 6/63 (10) | 156/688 (23) |
| Surgery, systemic therapy, no radiotherapy | “ | 14/131 (10) | 2/16 (13) | 60/165 (36) | 34/200 (17) | 1/8 (13) | 43/105 (41) | 13/63 (21) | 167/688 (24) |
| Surgery (+/− radiotherapy) | “ | 3/131 (2) | 0/16 (0) | 10/165 (6) | 6/200 (3) | 0/8 (0) | 4/105 (4) | 2/63 (3) | 25/688 (4) |
| systemic therapy (+/− radiotherapy) | “ | 50/131 (38) | 2/16 (13) | 44/165 (27) | 65/200 (33) | 4/8 (50) | 42/105 (40) | 29/63 (46) | 236/688 (34) |
| No treatment | “ | 4/131 (3) | 1/16 (6) | 48/165 (29) | 30/200 (15) | 0/8 (0) | 3/105 (3) | 13/63 (21) | 104/688 (15) |
| Any treatment initiated | Women with non-metastatic breast cancer | 303/309 (98) | 90/91 (99) | 212/292 (73) | 501/544 (92) | 34/34 (100) | 271/296 (92) | 124/150 (83) | 1535/1716 (89) |
| Initiated treatment within 3 months from baseline | Women with non-metastatic breast cancer who initiated treatment | 300/303 (99) | 87/90 (97) | 131/212 (62) | 467/501 (93) | 31/34 (91) | 235/271 (87) | 98/124 (79) | 1349/1535 (88) |
BC: breast cancer; CT: chemotherapy; ET: endocrine therapy; RT: radiotherapy; SA: South Africa; SX: surgery
Dichotomized variable derived from tumour size, nodes and metastases to assess correlation with the NCCN guidelines. Localized T1-3N0-1M0 vs locally advanced/inoperable tumours T4N2-3M0.
Figure 3:
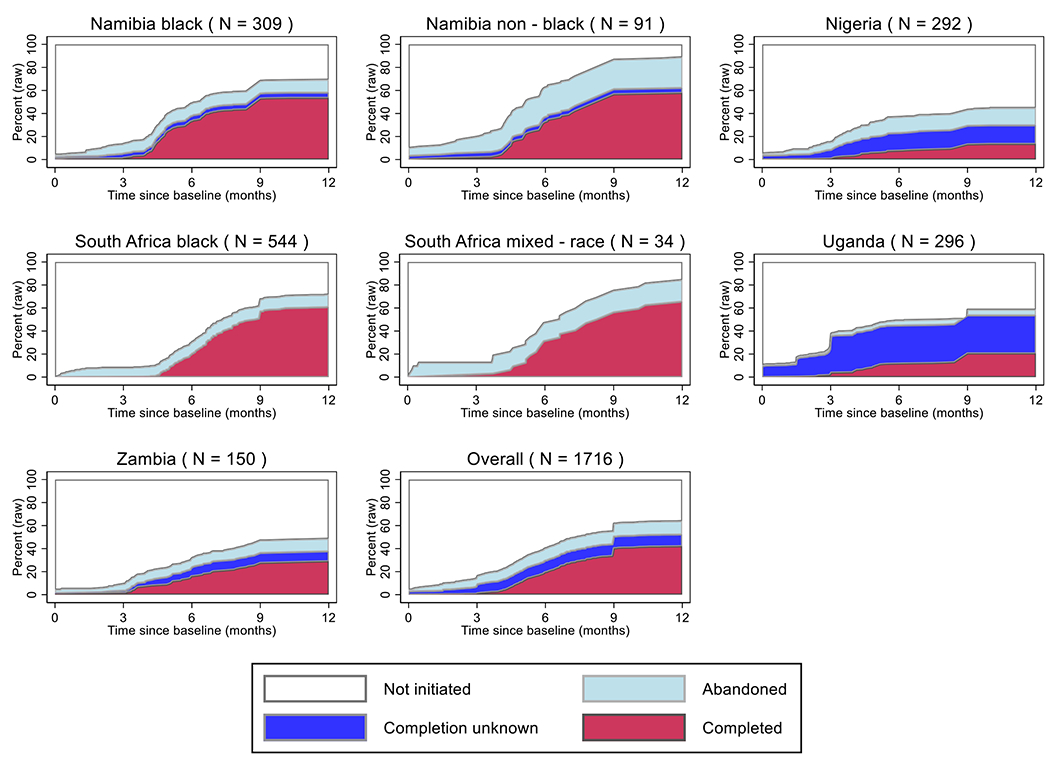
Cumulative percentage of multimodality treatment (surgery and systemic therapy) initiation, abandonment and completion in the African Breast Cancer-Disparities in Outcomes (ABC-DO) cohort for each study group and overall. Footnote: Percentages relate to the crude percentage amongst all women per site, unadjusted for death. Due to the x-axis restriction to one year post-baseline (i.e. presentation at the study hospital), endocrine therapy (ET) completion date was coded to 9 months post-baseline for all women who received at least 3 years of continued ET treatment. Date of treatment “Completed” was calculated as date of the 5th cycle of chemotherapy (CT; CT initiation date+5*mean inter-cycle duration), ET (9 months post-diagnosis), or surgery, whichever came last. Date of treatment “Abandoned” relates to the date of last completed CT cycle (CT initiation date+(number of cycles * mean inter-cycle duration)), ET initiation, or surgery, whichever came last. “Completion unknown” relates to the date of initiated treatment if systemic therapy completion was unknown due to missing medical records. Women who initiated only systemic therapy or surgery monotherapies are counted as “Not initiated”.
In total, 68% (1163/1716) of women underwent surgery and among these 1163 women, 78% (909/1163) received mastectomy (Table 1). Proportions of BCS were highest in Nigeria (49%, 76/156). Amongst all women receiving BCS, subsequent radiotherapy was initiated by 42% overall (107/255), but only two of these from Nigeria. In South Africa, the only setting with valid information on this procedure, complete axillary node dissection was performed in 87% (361/415) of black and 93% (26/28) of mixed-race women. Proportions differed little between localised and locally advanced tumours (appendix p 7).
Chemotherapy was mostly neoadjuvant (overall: 58%, 714/1232; from 33% (75/228) in Uganda to 76% (187/245) in black Namibian women, Table 1). The most common drug regimen was (F)AC-T (39%, 486/1232). ET was mostly Tamoxifen (91%, 883/973).
According to the NCCN guidelines, 95% (1530/1611, i.e. excluding 115 patients who died within six months after baseline) of patients should have received chemotherapy (Table 3). Of these, 66% (1013/1530) initiated treatment (neoadjuvant chemotherapy or surgery) within three months after baseline (exemplary treatment trajectories of 15 women per population group are displayed in appendix p 10). This percentage was over 60% in all population groups but Nigeria (39% (97/251), appendix p 8). Initiated chemotherapy was adequately completed by 28% (284/1013), marginally completed by 28% (97/1013) and abandoned by 20% (200/1013) of women. Completion was unknown in 15% (151/1013, of these 114 from Uganda) and 2% (19/1013) died within six months after chemotherapy initiation. Chemotherapy abandonment was particularly frequent in Nigeria (38%, 37/97), Zambia (29%, 25/85) and black women in Namibia (24%, 56/232). Of 80% (1375/1716) of women for whom ET was indicated (HR+ or HR unknown), 63% (920/1375) initiated treatment. ET duration was at least three years in 40% (367/920).
Table 3:
Guideline based systemic treatment initiation, abandonment, and completion in women with non-metastatic breast cancer and known TNM stage information in the prospective African Breast Cancer-Disparities in Outcomes cohort. To judge NCCN guideline adherence, chemotherapy completion and abandonment was calculated only for women having started treatment (including surgery before adjuvant chemotherapy) within 3 months after study initiation (i.e. baseline interview at presentation at the study hospital). Women who died within 6 months after baseline (BL) were excluded from the CT statistics as there might have been medical reasons not to initiate or complete treatment
| Patient group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subset populating the denominator | Namibia black | Namibia non-black | Nigeria | South Africa black | South Africa mixed-race | Uganda | Zambia | All | |
| No. study sample (women with non-metastatic breast cancer) | 309 | 91 | 292 | 544 | 34 | 296 | 150 | 1716 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Alive at 6 months post-baseline | Whole study sample | 298/309 (96) | 90/91 (99) | 256/292 (88) | 517/544 (95) | 32/34 (94) | 281/296 (95) | 137/150 (91) | 1611/1716 (94) |
| Chemotherapy indicated A | Women alive at 6 months | 292/298 (98) | 71/90 (79) | 251/256 (97) | 487/517 (94) | 29/32 (91) | 266/281 (95) | 134/137 (98) | 1530/1611 (95) |
| Treatment trajectory initiated within 3 months post-baseline: | Women alive at 6 months and chemotherapy indicated | 232/292 (79) | 57/71 (80) | 97/251 (39) | 355/487 (73) | 22/29 (76) | 165/266 (62) | 85/134 (63) | 1013/1530 (66) |
| Adequately completed B | Women alive at 6 months, chemotherapy indicated and treatment initiated | 97/232 (42) | 25/57 (44) | 9/97 (9) | 198/355 (56) | 14/22 (64) | 8/165 (5) | 8/85 (9) | 359/1013 (35) |
| Marginally completed C | “ | 74/232 (32) | 18/57 (32) | 21/97 (22) | 99/355 (28) | 6/22 (27) | 20/165 (12) | 46/85 (54) | 284/1013 (28) |
| Abandoned D | “ | 56/232 (24) | 11/57 (19) | 37/97 (38) | 54/355 (15) | 1/22 (5) | 16/165 (10) | 25/85 (29) | 200/1013 (29) |
| Died within 6 months after baseline | “ | 0/232 (0) | 0/57 (0) | 5/97 (5) | 4/355 (1) | 1/22 (5) | 7/165 (4) | 2/85 (2) | 19/1013 (2) |
| completion unknown | “ | 5/232 (2) | 3/57 (5) | 25/97 (268) | 0/355 (0) | 0/22 (0) | 114/165 (69) | 4/85 (5) | 151/1013 (15) |
| Endocrine therapy (ET) indicated E | Women alive at 6 months post-baseline | 224/298 (75) | 72/90 (80) | 246/256 (95) | 412/517 (80) | 30/32 (94) | 269/281 (96) | 122/137 (89) | 1375/1611 (80) |
| ET initiated | Women alive at 6 months and ET indicated | 213/224 (95) | 69/72 (96) | 98/246 (40) | 297/412 (72) | 26/30 (87) | 155/269 (58) | 62/122 (51) | 920/1375 (63) |
| ≥ 3 years continued | Women alive at 6 months, ET indicated and treatment initiated | 112/213 (53) | 37/69 (54) | 30/98 (31) | 104/297 (35) | 8/26 (31) | 53/155 (34) | 23/62 (37) | 367/920 (40) |
| <3 years continued | “ | 31/213 (15) | 17/69 (25) | 10/98 (10) | 106/297 (36) | 11/26 (42) | 32/155 (21) | 8/62 (13) | 215/920 (23) |
| Died within 3 years after initiation | “ | 42/213 (20) | 3/69 (4) | 23/98 (23) | 59/297 (20) | 3/26 (12) | 38/155 (25) | 9/62 (15) | 177/920 (19) |
| No information on duration | “ | 28/213 (13) | 12/69 (17) | 35/98 (36) | 28/297 (9) | 4/26 (15) | 32/155 (21) | 22/62 (35) | 161/920 (18) |
| Hormone receptor (HR) positive F | Whole study sample | 223/309 (72) | 71/91 (78) | 23/292 (8) | 428/544 (79) | 31/34 (91) | 25/296 (8) | 27/150 (18) | 828/1716 (48) |
| ET initiated | Women with HR+ tumours | 213/223 (96) | 68/71 (96) | 15/23 (65) | 300/428 (70) | 26/31 (84) | 15/25 (60) | 21/27 (78) | 658/828 (79) |
| Chemotherapy initiated | “ | 172/223 (77) | 47/71 (66) | 21/23 (91) | 315/428 (74) | 24/31 (77) | 23/25 (92) | 23/27 (85) | 625/828 (76) |
| HR negative F | Whole study sample | 79/309 (26) | 19/91 (21) | 13/292 (4) | 114/544 (21) | 3/34 (9) | 13/296 (4) | 16/150 (11) | 257/1716 (15) |
| ET initiated | Women with HR− tumours | 17/79 (22) | 1/19 (5) | 6/13 (46) | 9/114 (8) | n.r.G | 7/13 (54) | 3/16 (19) | 43/257 (17) |
| Chemotherapy initiated | “ | 68/79 (86) | 17/19 (89) | 12/13 (92) | 99/114 (87) | n.r. G | 11/13 (85) | 12/16 (75) | 222/257 (86) |
| HR status unknown F | Whole study sample | 7/309 (2) | 1/91 (1) | 256/292 (88) | 2/544 (0.4) | 0/34 (0) | 258/296 (87) | 107/150 (71) | 631/1716 (37) |
| ET given if HR NK | Women with unknown HR status | n.r. G | n.r. G | 88/256 (34) | n.r. G | n.r. G | 140/258 (54) | 41/107 (38) | 273/631 (43) |
| Chemotherapy given if HR NK | “ | n.r. G | n.r. G | 110/256 (43) | n.r. G | n.r. G | 194/258 (75) | 74/256 (69) | 385/631 (61) |
| HER2 positive F | Whole study sample | 99/309 (32) | 18/91 (20) | 4/292 (1) | 119/544 (22) | 7/34 (21) | 6/296 (2) | 6/150 (4) | 259/1716 (15) |
| Trastuzumab received if HER2+ | Women with HER2+ tumours | 33/99 (33) | 9/18 (50) | n.r. G | 0/119 (0) | n.r. G | n.r. G | n.r. G | 42/259 (16) |
| BL: Baseline, i.e. first presentation at ABC-DO study hospital; ET: Endocrine therapy; HR: Hormone receptors; | |||||||||
Defined at least one of the following: HER+, HR−, N+(≥2 nodes affected) , ≥T2 , G3 , young age (<35);
Over 85% chemotherapy cycles completed timely: Defined as at least 5 cycles of FAC, or equivalent regimen, within ≤15 weeks or seven to eight cycles (F)AC-T within 28 weeks;
Defined as 4*AC within 12 weeks or at least 5 cycles of FAC, (F)AC-T, or equivalent regimen, within 15-20 weeks;
Including 24 patients with non-standard regimen (Taxane alone, carboplatin or monotherapy of A,C or F);
defined as women with known HR+ disease or missing HR status;
Hormone receptor positive: Oestrogen and/or progesterone positive; hormone receptors negative: Oestrogen and progesterone negative. IHC testing was only routinely done in Namibia and South Africa. Information on IHC determined oestrogen (ER), progesterone (PR) and human epidermal growth factor 2 receptors (HER2) were extracted, the latter with florescent in-situ hybridization (FISH) for equivocal results. ER and PR were considered positive with >1% staining, and HER2+ if FISH was positive or the HER2 score was 3;
not reported: within column n ≤ 10
Guideline-compliant, 80% (658/828) received ET (from 60% (15/25) in Uganda to 96% (68/71) in Namibian non-black women). Women with unknown HR/HER2 status, a scenario not considered in the NCCN Harmonized Guidelines™, received ET and chemotherapy in 43% (273/631) and 61% (385/631) of cases, respectively. Targeted therapy (trastuzumab) was received only in Namibia by 50% (9/18) of non-black and 33% (33/99) of black women with HER2 positive tumours.
Discussion
Our ABC-DO study findings present a detailed profile of breast cancer treatment across SSA hospital settings. The results provide comprehensive benchmark estimates to inform the third pillar of the GBCI, and quantify the needs to enhance treatment quality and reduce treatment abandonment in SSA. In particular, the results highlight the unacceptably high proportions of patients with localised tumours lacking surgical tumour resection and abandoned or not initiated guideline-concordant systemic therapy leading to impaired survival chances of these women with potentially curable disease in SSA. Our data also emphasize the lack of, in the majority of settings, necessary infrastructure for guideline-compliant treatment - notably concerning the availability of radiotherapy, IHC and diagnostic imaging - highlighting the needs for overall health systems improvements, and government investment in assuring equitable access to quality cancer care.
Breast cancer treatment requires multimodality treatment (surgery, systemic therapy, and often radiotherapy) which was initiated by only ~70% of women with localised and ~50% of women with locally advanced disease in ABC-DO. Further, treatment abandonment was common, particularly in Nigeria and Namibia. These observed treatment initiation and rates can be compared to those reported in three recent publications from SSA. The first report analysing population-based cancer retrospective registry data from 11 countries of the African Cancer Registry Network (AFRCN), found an overall treatment initiation percentage of surgery and systemic therapy of 45% amongst women with non-metastatic disease.14 This lower percentage in AFCRN could be due to the earlier time-point (2009-2015) and nature of data collection (population-based, retrospective). A higher initiation percentage than in ABC-DO (57%) was reported in a smaller patient sample from a cancer center in Rwanda in 2012-13.15 Chemotherapy completion in this study was 74% (at least four cycles) compared to ~35% for adequate and ~30% marginal completion in ABC-DO. The third study, conducted by the African Research Group of Oncology (ARGO) in Nigeria, reported separate percentages for surgery, chemotherapy, and ET and combined women with non-metastatic and metastatic disease.16 The proportion of women undergoing surgery (50%) in ARGO was similar to Nigerian ABC-DO patients’, but chemotherapy initiation was higher (70% vs 39%) whereas abandonment was ~40% in both studies. Some heterogeneity between studies was likely due to more specialized cancer centres in ARGO.
Nearly one-third of patients did not undergo surgery. Women with locally advanced large tumour masses (~40% of patients with ≥5 cm) may not respond to (incomplete) neoadjuvant therapy and develop metastasis. In this case, not proceeding to a futile surgical resection would be guideline-concordant. Contrariwise, metastatic spread could have already occurred during the prolonged delay to treatment initiation. As we measured TNM stage only once at baseline (possibly under-staged due to the lack of diagnostic imaging (appendix p 6)), we unfortunately could not assess disease progression. Another reason for not receiving surgery, conveyed to us by the local investigators in Namibia, has been the old age, and comorbidity. The respective women (n=11) were treated with Tamoxifen and/or radiotherapy. However, approximately 20% of women with localised tumours did not receive surgery. The precise reasons were not documented but they will include costs, reliance on traditional medicine, fear of mastectomy and psychological distress.6, 17
Among women receiving surgery, the dominant type in Namibia, Uganda and Zambia was mastectomy, the guideline recommendation in the absence of radiotherapy facilities and/or for large tumour masses.9 The proportion of women undergoing BCS, the recommendation for localised or small tumours if radiation is provided, was highest in Nigeria but worryingly only two of these patients initiated radiotherapy. In contrast, almost all Namibian patients receiving BCS initiated consecutive radiotherapy.
Almost all women in ABC-DO (~95%) would have needed chemotherapy, as is indicated for advanced or aggressive tumours, independently of their HR/HER2 status. Nevertheless, only ~65% of these patients initiated timely treatment and of those, only one third adequately completed it. In low income countries without universal health coverage (in ABC-DO Nigeria, Uganda, and Zambia) treatment is often aborted due to financial barriers.6, 18, 19 In addition, long travel times and lack of transport is a known barrier to healthcare access in ABC-DO.20 Side effects from chemotherapy may have also contributed to the high abandonment rates, especially in HIV+ women, but their impact could not be evaluated due to incomplete data.
Systemic and targeted therapy for breast cancer is tailored to the tumour’s receptor status: HR+ tumours are treated with ET, whereas HR−, and to a lesser extent HR+ tumours require chemotherapy, and HER2+ tumours respond to additional targeted therapy. Therefore, knowledge of receptor status is a minimum requirement for treatment planning even in the NCCN Harmonized Guidelines™.9 As in many countries in SSA, the pathology infrastructure in Nigeria, Uganda, and Zambia is poor and IHC testing needs to be arranged personally and paid out-of-pocket (appendix p 3).21, 22 As a result, for up to 90% of women with unknown receptor status in these countries, evidence based systemic treatment decisions could not be provided. However, in Namibia and South Africa, where IHC testing is standard, almost all women with HR+ disease started ET, with the exception of South African black women among whom 30% did not initiate ET, despite its substantial survival benefits and, compared to chemotherapy, fewer side effects.23, 24 Another indication of limited resources are prolonged times to treatment initiation that should, as a critical cut-off for improved survival and adopted by the GBCI, not exceed three months, but were longer than this in over 40% of treated patients in Nigeria, Uganda, and Zambia.5, 25 Moreover, ~10% of patients with non-metastatic breast cancer did not initiate any treatment. Determinants of limited treatment access in ABC-DO have been described previously.6
A unique strength of the ABC-DO study was the mHealth-based prospective follow-up design which allowed assessment of timeliness, composition and completeness of breast cancer therapy – and reduced missing data and losses to follow-up, both common problems in cohort studies in SSA.11, 26 As a limitation, the chemotherapy completion could not account for the dose-intensity as documentation of actual dose received was not precise (only included the regimen in some hospitals) and exact timeliness was not always documented. Nevertheless, we were able to extract the planned regimen, total number of cycles and start- and end date of chemotherapy. Based on this information we accounted for different treatment schedules and treatment quality in stratifying treatment completion over chemotherapy regimen. In addition, we introduced a category of marginal treatment completion, to capture even the slightest treatment benefits in settings where various issues may hinder planned optimal treatment schedules. Several medical records were missing and for some patients results relied entirely on limited self-reported data (only treatment yes/no, side-effects and reasons for treatment abandonment), resulting in difficulties to judge treatment completion in Uganda. However, the concordance of medical records and the self-reported treatment data at six months post-baseline was rather high.27 For example: 86% (460/533) of chemotherapy and 83% (357/428) of surgery medical treatment records lying within six months post-baseline had corresponding self-reported data. Finally, the results cannot be generalised to all patients in the respective countries. In Namibia, patients who were referred to and reached Windhoek Central Hospital, but many patients will not reach a specialized oncology centre. The catchment population in the Nigerian hospitals, FMC Owerri and Aba, were local and most often the patients primary care contact (appendix p 3).28
Effective interventions are needed to improve breast cancer survival.2 In addition to programs to achieve earlier diagnosis, such a shift will only deliver the hoped-for survival gains if they are accompanied by comprehensive treatment. For the latter, professionals, diagnostic and therapeutic equipment, including pathology infrastructure, are needed at the health system level, and support in terms of finance, transport, accommodation, and education is needed at the patient level.
Supplementary Material
Research in context.
Evidence before this study : We searched PubMed and Google scholar using the search terms “breast cancer” in combination with “SSA” or “sub-Saharan Africa”, or each of its individually named countries, and at least one of “treatment”, “management”, “resources”, “care”, and “abandonment” from database inception up to November 1, 2021 with no language restrictions. Our review revealed only 12 studies that published unbiased basic percentages of multimodality breast cancer treatment initiation (surgery and systemic therapy), of which seven reported also on completeness of treatment. Of these, five analysed data from earlier than 2010. The two others were hospital-based studies from Nigeria and Rwanda. While crude treatment percentages were comparable, treatment completion was defined by only four cycles of chemotherapy in the Rwandan study and the Nigerian study failed to report on combined therapies. Also, both publications were single-country analysis and thus not suitable to detect regional differences within sub-Saharan Africa.
Added value of this study: In 2021 the WHO launched the Global Breast Cancer Initiative (GBCI) to improve breast cancer survival worldwide. One of its strategic pillars is to improve breast cancer management, specifically to promote multimodality treatment and reduce treatment abandonment. Whilst it is broadly known that these areas need improving in sub-Saharan Africa, there is a lack of detailed high quality data on breast cancer management and the challenges to follow resource-stratified treatment guidelines i.e. the NCCN Harmonized Guidelines for SSA. Herein, we provide this necessary data on treatment initiation, guideline adherence and completion, which were collected amongst female breast cancer patients in five countries from the African Breast Cancer - Disparities in Outcomes (ABC-DO) study, the first multi-centric prospective breast cancer cohort in SSA. We found large between-country differences in initiation of multimodality breast cancer treatment. In women presenting with localised tumours (T1-3N0-1M0) only 50% in Nigeria against more than 90% in South Africa and Namibia initiated multimodality treatment whereby lack of surgical tumour removal was a major problem. Our results further emphasize the need to overcome treatment abandonment; only one third of women requiring chemotherapy and initiating timely treatment, adequately completed (i.e. >85%) of the scheduled treatment cycles. This is particularly worrying, as marginally completed or abandoned chemotherapy will leave the patient with only minimal or without survival benefit, respectively, but not without its side effects. Further, in the participating hospitals in Nigeria, Zambia and Uganda, immunohistochemistry is not routinely available, thus adherence to breast cancer management guidelines is impossible, and radiotherapy facilities are few and overstretched, substantially hampering treatment quality.
Implications of all the available evidence: We found an unacceptably high proportion of early stage patients lacking surgical tumour removal and remaining without initiated or abandoning systemic therapy. Alongside early detection efforts to shift stage at presentation towards better prognosis tumours in SSA, in this region with the lowest breast cancer survival world, timely high-quality treatment needs to be assured to fulfil the ultimate goal of improving survival. Our results also highlight the need to improve basic oncology infrastructure in SSA, such as radiotherapy and pathology services, to enable guideline-concordant cancer treatment and also the need to clearly understand concepts and values of the affected women and health workers in their societies to assure their understanding of the disease and treatment options.
Acknowledgements
The ABC-DO study was funded by NIH (1R01CA244559) and Susan G Komen (IIR 13264158 to Valerie McCormack and Isabel dos-Santos-Silva, GSP18IARC001 and GSP19IARC001, and as part of “Implementing breast cancer care efficiency in Zambia through specialized health provider training and mhealth evaluation of patient outcomes” for the Zambian site to Groesbeck Parham) and by the International Agency for Research on Cancer. Moses Galukande is a THRiVE-2 fellow (supported by DELTA African initiative DEL-15-011). Leeya Pinder is supported by the University of Washington T32 Fellowship (5T32CA009515-34). We acknowledge the contribution of all patients who participated in ABC-DO and the original PI at Aba, our friend and colleague Dr Charles Adisa. We would like to thank the research assistants and nurses who interviewed women in the ABC-DO cohort.
Footnotes
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Conflict of interest
All authors have no conflict of interests to disclose.
Data sharing statement
The protocol of ABC-DO has been published. Data collected for the study will be made available upon reasonable request to the principle investigators of ABC-DO Valerie McCormack and Isabel dos Santos Silva. Reasonable access criteria include research proposals for stand-alone analysis within the scope of the study. Such proposals will be evaluated by the PIs and supported if deemed relevant. Proposals from researchers and doctoral students from LMICs are encouraged. Relevant data will be shared with a signed data access agreement.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2.McCormack V, McKenzie F, Foerster M, et al. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. The Lancet Global health 2020; 8: e1203–e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joko-Fru W, Miranda-Filho A, Soerjomataram I, et al. Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: A population-based registry study. International journal of cancer 2020; 146: 1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2021. Atlanta: American Cancer Society; 2021 [Google Scholar]
- 5.Anderson BO, Ilbawi AM, Fidarova E, et al. The Global Breast Cancer Initiative: a strategic collaboration to strengthen health care for non-communicable diseases. The Lancet Oncology 2021; 22: 578–581. [DOI] [PubMed] [Google Scholar]
- 6.Foerster M, Anderson BO, McKenzie F, et al. Inequities in breast cancer treatment in sub-Saharan Africa: findings from a prospective multi-country observational study. Breast Cancer Research 2019; 21: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonadonna G, Valagussa P, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer—the results of 20 years of follow-up. New England Journal of Medicine 1995; 332: 901–906. [DOI] [PubMed] [Google Scholar]
- 8.Brandão M, Guisseve A, Bata G, et al. Breast cancer subtypes: implications for the treatment and survival of patients in Africa—a prospective cohort study from Mozambique. ESMO open 2020; 5: e000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN Harmonized Guidelines for Sub-Saharan Africa. 2019.
- 10.McKenzie F, Zietsman A, Galukande M, et al. African Breast Cancer—Disparities in Outcomes (ABC-DO): protocol of a multicountry mobile health prospective study of breast cancer survival in sub-Saharan Africa. BMJ open 2016; 6: e011390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foerster M, Anele A, Adisa C, et al. Few Losses to Follow-up in a Sub-Saharan African Cancer Cohort via Active Mobile Health Follow-up: The African Breast Cancer—Disparities in Outcomes Study. American journal of epidemiology 2020; 189: 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cubasch H, Ruff P, Joffe M, et al. South African breast cancer and HIV outcomes study: methods and baseline assessment. Journal of global oncology 2017; 3: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: a cancer journal for clinicians 2017; 67: 93–99. [DOI] [PubMed] [Google Scholar]
- 14.Joko-Fru WY, Griesel M, Mezger NCS, et al. Breast Cancer Diagnostics, Therapy, and Outcomes in Sub-Saharan Africa: A Population-Based Registry Study. Journal of the National Comprehensive Cancer Network 2021; 1: 1–11. [DOI] [PubMed] [Google Scholar]
- 15.O’Neil DS, Keating NL, Dusengimana JMV, et al. Quality of breast cancer treatment at a rural cancer center in Rwanda. Journal of global oncology 2017; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olasehinde O, Alatise O, Omisore A, et al. Contemporary management of breast cancer in Nigeria: Insights from an institutional database. International Journal of Cancer 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martei YM, Vanderpuye V and Jones BA. Fear of mastectomy associated with delayed breast cancer presentation among Ghanaian women. The oncologist 2018; 23: 1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaidi AA, Ansari TZ and Khan A. The financial burden of cancer: estimates from patients undergoing cancer care in a tertiary care hospital. International journal for equity in health 2012; 11: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian S, Gakunga R, Jones M, et al. Financial barriers related to breast cancer screening and treatment: A cross-sectional survey of women in Kenya. Journal of Cancer Policy 2019; 22: 100206. [Google Scholar]
- 20.Togawa K, Anderson BO, Foerster M, et al. Geospatial barriers to healthcare access for breast cancer diagnosis in sub-Saharan African settings: The African Breast Cancer—Disparities in Outcomes Cohort Study. International journal of cancer 2021; 148: 2212–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martei YM, Pace LE, Brock JE, et al. Breast cancer in low-and middle-income countries: why we need pathology capability to solve this challenge. Clinics in laboratory medicine 2018; 38: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegenhorn H-V, Frie KG, Ekanem I-O, et al. Breast cancer pathology services in sub-Saharan Africa: a survey within population-based cancer registries. BMC Health Services Research 2020; 20: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor–positive tumors. N Engl J Med 1989; 320: 479–484. [DOI] [PubMed] [Google Scholar]
- 24.Group EBCTC. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. The lancet 2011; 378: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards M, Westcombe A, Love S, et al. Influence of delay on survival in patients with breast cancer: a systematic review. The Lancet 1999; 353: 1119–1126. [DOI] [PubMed] [Google Scholar]
- 26.Cubasch H, Dickens C, Joffe M, et al. Breast cancer survival in Soweto, Johannesburg, South Africa: a receptor-defined cohort of women diagnosed from 2009 to 11. Cancer epidemiology 2018; 52: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foerster M, Anderson B, McKenzie F, et al. Inequities in breast cancer treatment in sub-Saharan Africa: findings from a prospective multi-country observational study. Breast Cancer Research 2019: submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foerster M, McKenzie F, Zietsman A, et al. Dissecting the journey to breast cancer diagnosis in sub-Saharan Africa: Findings from the multicountry ABC-DO cohort study. International journal of cancer 2021; 148: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protocol of ABC-DO has been published. Data collected for the study will be made available upon reasonable request to the principle investigators of ABC-DO Valerie McCormack and Isabel dos Santos Silva. Reasonable access criteria include research proposals for stand-alone analysis within the scope of the study. Such proposals will be evaluated by the PIs and supported if deemed relevant. Proposals from researchers and doctoral students from LMICs are encouraged. Relevant data will be shared with a signed data access agreement.


