Abstract
Nephrectomy remains standard treatment for renal cell carcinoma (RCC). The Mayo Adhesive Probability (MAP) score is predictive of adherent perinephric fat and associated surgical complexity, and is determined by assessing perinephric fat and stranding. MAP has additionally predicted progression-free survival (PFS), though primarily reported in stage T1-T2 RCC. Here, we examine MAP’s ability to predict overall survival (OS) and PFS in T3-T4 RCC. From our prospectively maintained RCC database, patients that underwent radical nephrectomy (2009-2016) with available abdominal imaging (<90 days preop) and T3/T4 RCC underwent MAP scoring. Survival analyses were conducted with MAP scores as individual (0-5) and dichotomized (0-3 vs 4-5) using Kaplan-Meier method. Multivariable Cox proportional hazard regression models for PFS and OS were built with backward elimination. 141 patients were included. 134 (95%) and 7 (5%) had pT3 and pT4 disease, respectively. 46.1% of patients had an inferior vena cava thrombus. Mean MAP score was 3.22±1.52, with 75 (53%) patients having a score between 0-3 and 66 (47%) having a score of 4-5. Both male gender (p=0.006) and clear cell histology (p=0.012) were associated with increased MAP scores. On Kaplan-Meier and multivariable analysis, no significant associations were identified between MAP and PFS (HR=1.01, 95% CI 0.85-1.20, p=0.93) or OS (HR=1.01, 95% CI 0.84-1.21, p=0.917). In this cohort of patients with locally advanced RCC, high MAP scores were not predictive of worse PFS or OS.
Keywords: body composition, kidney cancer, mayo adhesive probability, renal cell carcinoma, survival
Introduction
In 2021, renal cell carcinoma (RCC) was responsible for nearly 14,000 deaths in the United States (1). Diagnosis of RCC has rapidly risen in recent decades, with a doubling in incidence since 1975 (1). Nephrectomy with curative intent remains the gold standard in RCC management; however, image-guided procedures, conservative treatment approaches, and active surveillance have gained popularity. Great interest persists in patient-specific preoperative risk stratification to inform management, rather than relying on postoperative information such as tumor pathology. Specifically, measurements on preoperative imaging may be informative and assist in preoperative prognostication to further guide clinical decision-making.
One radiographic feature that has demonstrated the ability to predict surgical risks and outcomes in RCC is the Mayo Adhesive Probability (MAP) score. MAP estimates the probability of encountering adherent perinephric fat (APF) (2) and has been associated with increased surgical complexity, operative time, and blood loss during partial nephrectomy (PN) (3). Moreover, Thiel et al. explored the association between MAP scores and progression-free survival (PFS). In their analysis, patients with high MAP scores (4–5) experienced inferior PFS (HR = 2.16, 95% CI 1.15–4.06, P = 0.017) following surgery for clinically localized RCC (4).
Accordingly, MAP score appears useful in clinically localized disease and is appealing given its quick and convenient measurement on routine preoperative imaging. However, little is known about its utility in locally advanced RCC. In the study by Thiel et al., 82% of patients had T1–T2 disease. As novel preoperative prognostic factors continue to emerge, understanding their value in all patient populations is necessary. To further elucidate the prognostic utility of MAP, we retrospectively analyzed the associations between preoperative MAP and both PFS and overall survival (OS) in patients with locally advanced nonmetastatic RCC.
Methods
Patient selection and data acquisition
Patients that underwent radical nephrectomy (RN) for RCC from 2009 to 2016 were identified in our institutional database. MAP scores were calculated for patients with available computerized tomography (CT) or magnetic resonance imaging (MRI) within 90 days before surgery, as previously described (2). MAP scores were acquired by two Medical Doctorate (MD) candidates pursuing urology residency training and familiar with renal imaging under the direct supervision of an attending urologic oncologist. Patients with T1–T2 disease were excluded. Patient characteristics included race, gender, age of surgery, Eastern Cooperative Oncology Group (ECOG) score, and BMI (<25 or ≥25). Clinical factors including presence of inferior vena cava (IVC) thrombus; laterality of kidney tumor; Fuhrman nuclear grade; presence of necrosis; pathologic N and T stage; stage, size, grade, and necrosis (SSIGN) score; University of California Los Angeles Integrated Staging System (UISS) score; systemic therapy history; corrected calcium; modified Glasgow prognostic score (mGPS); and histology (clear cell [ccRCC] or nonclear cell) were also obtained. All patients provided their informed consent in this study approved by the Institutional Review Board.
Data analysis
The primary objective of this study was to analyze the prognostic ability of MAP in patients with locally advanced, nonmetastatic RCC. The primary endpoints were PFS and OS.
For survival analyses, MAP scores were analyzed as individual scores (0–5) and dichotomized groups (0–3 vs. 4–5) using the Kaplan–Meier method. In addition, multivariable Cox proportional hazard regression models were built with backward elimination using an alpha level of removal of 0.1. All patient clinicopathologic and demographic features were included in the model. For both PFS and OS, two separate multivariable models were generated to include and exclude SSIGN score, which is only validated in patients with clear cell RCC (ccRCC). Additional subanalyses were conducted in patients with and without presence of IVC tumor thrombus. All statistical tests were two-sided with type I error set at 0.05. Statistical analysis was conducted using SAS Version 9.4 (Cary, NC, USA) and SAS macros developed by the Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute.
Results
A summary of patient demographics and clinicopathologic data is represented in Table 1. In total, 141 patients were included, of whom 134 (95%) had pT3 and 7 (5%) had pT4 disease. One hundred and seven (75.9%) patients had clear-cell histology and 65 (46.1%) patients had the presence of an IVC tumor thrombus. The final cohort was primarily male (n = 100, 71%) and white (n = 104; 74%). The median age was 63 years (IQR: 54–72) and median BMI was 28.5 kg/m2 (IQR: 24.6–32.6). In total, 47 (33.3%) patients received some form of systemic therapy, all of which were administered postoperatively. Mean MAP score was 3.22 ± 1.52, with 75 (53%) patients having a score between 0–3 and 66 (47%) having a score of 4–5. Both male gender (P = 0.006) and ccRCC histology (P = 0.012) were significantly associated with increased MAP scores, though pathologic staging, ECOG status, and various clinical scoring systems were not. Interestingly, low BMI patients appeared to have lower MAP scores, although the association between these two measures was not significant (P = 0.059).
Table 1:
Characteristics of study population subdivided by Mayo Adhesive Probability scores.
| MAP score, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | 0 (n = 14) | 1 (n = 3) | 2 (n = 22) | 3 (n = 36) | 4 (n = 31) | 5 (n = 35) | Total (n = 141) | P |
| Gender | ||||||||
| Male | 9 (64.3) | 1 (33.3) | 14 (63.6) | 19 (52.8) | 26 (83.9) | 31 (88.6) | 100 (70.9) | 0.006 |
| Female | 5 (35.7) | 2 (66.7) | 8 (36.4) | 17 (47.2) | 5 (16.1) | 4 (11.4) | 41 (29.1) | |
| Race | ||||||||
| White | 7 (50) | 1 (33.3) | 15 (68.2) | 28 (77.8) | 25 (80.6) | 28 (80) | 104 (73.8) | 0.12 |
| Non-white | 7 (50) | 2 (66.7) | 7 (31.8) | 8 (22.2) | 6 (19.4) | 7 (20) | 37 (26.2) | |
| IVC thrombus | 5 (35.7) | 0 (0) | 11 (50) | 17 (47.2) | 18 (58.1) | 14 (40) | 65 (46.1) | 0.343 |
| ECOG | ||||||||
| ≥1 | 0 (0) | 1 (33.3) | 5 (22.7) | 14 (38.9) | 8 (25.8) | 5 (14.3) | 33 (23.4) | 0.052 |
| BMI* | 23.9 (20.1–29.0) | 30.0 (28.6–31.0) | 29.0 (24–35) | 26.0 (23.1–30.3) | 28.8 (26.2–32.9) | 29.9 (27–34.3) | 28.50 (24.6–32.6) | 0.059 |
| Age* | 54.2 (44.2–68.1) | 62.7 (51.2–77.7) | 59.4 (45.6–71.6) | 61.6 (51.5–71.3) | 66.9 (55.4–73.4) | 63.3 (54.5–73.4) | 62.7 (53.8–71.5) | 0.212 |
| Nephrectomy side | ||||||||
| Right | 8 (57.1) | 0 (0) | 12 (54.5) | 16 (44.4) | 17 (54.8) | 19 (54.3) | 72 (51.1) | 0.494 |
| Histology | ||||||||
| ccRCC | 6 (42.9) | 2 (66.7) | 14 (63.6) | 32 (88.9) | 24 (77.4) | 29 (82.9) | 107 (75.9) | 0.012 |
| non-ccRCC | 8 (57.1) | 1 (33.3) | 8 (36.4) | 4 (11.1) | 7 (22.6) | 6 (17.1) | 34 (24.1) | |
| pT stage | ||||||||
| T3 | 11 (78.6) | 3 (100) | 22 (100) | 35 (97.2) | 30 (96.8) | 33 (94.3) | 134 (95.0) | 0.077 |
| T4 | 3 (21.4) | 0 (0) | 0 (0) | 1 (2.8) | 1 (3.2) | 2 (5.7) | 7 (5.0) | |
| pN stage | ||||||||
| N1 | 2 (14.3) | 0 (0) | 2 (9.1) | 4 (11.1) | 5 (16.1) | 2 (6.3) | 15 (10.9) | 0.821 |
| Fuhrman nuclear grade | ||||||||
| 2 | 0 (0) | 2 (66.7) | 4 (18.2) | 3 (8.3) | 6 (19.4) | 5 (14.3) | 20 (14.2) | 0.246 |
| 3 | 8 (57.1) | 1 (33.3) | 11 (50) | 22 (61.1) | 15 (48.4) | 16 (45.7) | 73 (51.8) | |
| 4 | 6 (42.9) | 0 (0) | 7 (31.8) | 11 (30.6) | 10 (32.3) | 14 (40) | 48 (34.0) | |
| Necrosis | ||||||||
| Yes | 10 (71.4) | 2 (66.7) | 17 (77.3) | 24 (66.7) | 17 (54.8) | 24 (68.6) | 94 (66.7) | 0.659 |
| SSIGN score*** | ||||||||
| n (%) | 6 (5.6) | 2 (1.8) | 14 (13.08) | 32 (29.9) | 24 (22.4) | 29 (27.1) | 107 (100) | |
| Mean (std) | 7.3 (±1.8) | 3.5 (±2.1) | 5.1 (±1.6) | 6.1 (±1.7) | 6.3 (±1.8) | 6 (±1.6) | 6 (±1.76) | |
| UISS score** | 2.5 (±0.9) | 2 (±0.6) | 4 (±1.0) | 3 (±0.9) | 3.5 (±0.9) | 2 (± 1.0) | 2.94 (±0.93) | 0.57 |
| mGPS | ||||||||
| Low | 8 (66.7) | 2 (100) | 8 (38.1) | 13 (38.2) | 13 (44.8) | 12 (35.3) | 56 (42.4) | 0.211 |
| Intermediate | 2 (16.7) | 0 (0) | 4 (19) | 7 (20.6) | 9 (31) | 14 (41.2) | 36 (27.3) | |
| High | 2 (16.7) | 0 (0) | 9 (42.9) | 14 (41.2) | 7 (24.1) | 8 (23.5) | 40 (30.3) | |
| Missing | – | – | – | – | – | – | 9 (6.4%) | |
| Corrected calcium** | 9.8 (±0.6) | 9.8 (±0.6) | 9.9 (±0.7) | 9.8 (±0.8) | 9.7 (±0.6) | 9.6 (±0.4) | 9.74 (±0.65) | 0.447 |
| Received systemic therapy | 4 (28.6) | 0 (0) | 6 (27.3) | 12 (33.3) | 14 (45.2) | 11 (31.4) | 47 (33.3) | 0.547 |
| MAP score | ||||||||
| Mean** | – | – | – | – | – | – | 3.22 (±1.52) | |
| 0–3 | – | – | – | – | – | – | 75 (53.2) | |
| 4–5 | – | – | – | – | – | – | 66 (46.8) | |
MAP, Mayo Adhesive Probability; n, number; IVC, Inferior Vena Cava; ECOG, Eastern Cooperative Oncology Group; BMI, Body mass index; SSIGN, Stage, Size, Necrosis, Grade; ccRCC, Clear-cell renal cell carcinoma; UISS, UCLA Integrated Staging System; mGPS, modified Glasgow prognostic score. *Median (IQR), **Mean (standard deviation[std]), ***non-ccRCC not included (n = 34).
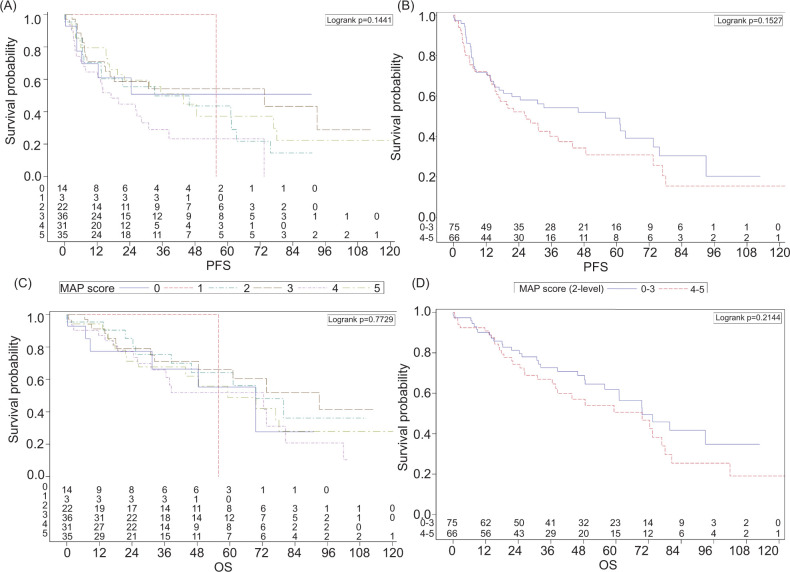
On Kaplan–Meier analysis, there were no significant associations between continuous or dichotomized MAP scores and PFS or OS (Figure 1). Similarly, multivariable Cox proportional hazard models (Table 2) demonstrated no significant associations both statistically and clinically between MAP and PFS (HR = 1.01, 95% CI 0.85–1.20, P = 0.93) or OS (HR = 1.01, 95% CI 0.84–1.21, P = 0.917). However, no receipt of systemic therapy was associated with better PFS (HR = 0.28, 95% CI 0.18–0.46, P < 0.001). The presence of IVC tumor thrombus was predictive of significantly worse OS (HR = 2.05, 95% CI 1.23–3.39, P = 0.006). These results were similar with and without the inclusion of SSIGN score.
Figure 1:
Kaplan–Meier curves for stage T3/T4 renal cell carcinoma patients (n = 141) displaying median progression-free survival (PFS) or overall survival (OS) with either individualized (0–5) or dichotomized (0–3 vs 4–5) Mayo Adhesive Probability (MAP) scores. (A) Median PFS with individualized MAP Scores. (B) Median PFS with dichotomized MAP scores. (C) Median PFS with individualized MAP Scores. (D) Median OS with dichotomized MAP scores.
Table 2:
Multivariable cox proportional hazard model for progression-free survival and overall survival.
| Covariate | N | Hazard ratio (95% CI) | P |
|---|---|---|---|
| Progression-free survival* | |||
| MAP score | 134 | 1.01 (0.85–1.20) | 0.926 |
| IVC tumor thrombus | |||
| Yes | 65 | 1.55 (0.98–2.47) | 0.061 |
| No | 76 | – | – |
| Received systemic therapy | |||
| Yes | 46 | – | – |
| No | 88 | 0.28 (0.18–0.46) | <0.001 |
| Corrected calcium | 134 | 1.38 (0.98–1.94) | 0.064 |
| Overall survival** | |||
| MAP score | 141 | 1.01 (0.84–1.21) | 0.917 |
| IVC tumor thrombus | |||
| Yes | 65 | 2.05 (1.23–3.39) | 0.006 |
| No | 76 | – | – |
MAP, Mayo Adhesive Probability; IVC, Inferior vena cava; ECOG, Eastern Cooperative Oncology Group; BMI, Body mass index. *Backward selection with an alpha level of removal of 0.1 was used. The following variables were removed from the model: Age at the Surgery, BMI, clear_cell, ECOG PS at presentation, Gender, Race, and Side of kidney. **Backward selection with an alpha level of removal of 0.1 was used. The following variables were removed from the model: Age at the Surgery, BMI, clear_cell, ECOG PS at presentation, Gender, Race, Ever received systemic therapy, and Side of Kidney.
On subanalysis of patients with the presence of IVC tumor thrombus (Table 3), MAP score continued to have no significant predictive value. Non-white race was associated with worse PFS and no receipt of systemic therapy was associated with improved PFS. For OS, non-white race was additionally associated with worse survival. Notably, only 14 of the patients in the thrombus cases were non-white.
Table 3:
Multivariable cox proportional hazard model for progression-free survival and overall survival in patients with the presence of an IVC tumor thrombus.
| Covariate | N | Hazard ratio (95% CI) | P |
|---|---|---|---|
| Progression-free survival* | |||
| MAP score | 64 | 0.92 (0.67–1.25) | 0.589 |
| Non-white race | 14 | 2.38 (1.07–5.33) | 0.034 |
| Received systemic therapy | |||
| Yes | 26 | – | – |
| No | 38 | 0.29 (0.14–0.57) | <0.001 |
| Age | 64 | 1.02 (1.00–1.05) | 0.082 |
| Corrected calcium | 64 | 1.56 (0.98–2.47) | 0.06 |
| Overall survival** | |||
| MAP score | 65 | 1.09 (0.83–1.44) | 0.539 |
| Non-white race | 14 | 2.31 (1.10–4.83) | 0.027 |
MAP, Mayo Adhesive Probability; IVC, Inferior vena cava; ECOG, Eastern Cooperative Oncology Group; BMI, Body mass index. *Backward selection with an alpha level of removal of 0.1 was used. The following variables were removed from the model: BMI, clear_cell, ECOG PS at presentation, Gender, and Side of kidney. **Backward selection with an alpha level of removal of 0.1 was used. The following variables were removed from the model: Age at the Surgery, BMI, Corrected Calcium, clear_cell, ECOG PS at presentation, Gender, Side of kidney, and systx_ever.
Discussion
Ultimately, a significant association of MAP with PFS and OS in patients with nonmetastatic T3–T4 RCC was not identified. These findings are important as our understandings of diagnostics, patient-specific prognostication, and the role of body composition in RCC continue to evolve. It is believed that the association between MAP and survival outcomes in RCC exists because perinephric fat thickness and stranding may serve as a proxy for visceral obesity and inflammation (4). Visceral adiposity and inflammation are interconnected, and each has additionally independently been identified as a risk factor for poorer RCC survival outcomes and more aggressive disease (5, 6).
It is unclear why our population of locally advanced RCC patients does not corroborate previous literature demonstrating the prognostic value of MAP scores. It is likely that, for locally advanced disease, extent of disease extension, Tumor, Node, Metastasis (TNM) stage, and other tumor-specific factors play a stronger role in determining survival, thus overpowering the effects of factors such as visceral obesity (7, 8). Moreover, patients with higher visceral obesity may be more likely to present with confounding factors harming survival, including advanced disease or cardiovascular comorbidities.
An important consideration in this cohort of patients with locally advanced disease is the utility of effective systemic therapy (i.e., immune-checkpoint inhibitors [ICI], tyrosine kinase inhibitors) in the neoadjuvant or adjuvant setting. While neoadjuvant therapy prior to nephrectomy has been reported as feasible (9–11), our patient cohort only received adjuvant systemic therapy since neoadjuvant systemic therapy outside of clinical trials is currently not routinely used for nonmetastatic RCC. Patients not receiving any systemic therapy actually experienced better survival, likely as a result of patient selection. Furthermore, in our cohort, there was no difference in receipt of adjuvant systemic therapy by MAP score. Though unable to be captured in this cohort, ICI-induced inflammatory response (12) could potentially be captured by radiographic features measured by MAP scoring given it may partially serve as a proxy for perinephric inflammation (4). Therefore, future studies are warranted in this patient population, specifically.
Conclusions
This study is the first to examine MAP score in a locally advanced RCC cohort. No significant associations with survival outcomes were identified. Limitations of this study include its retrospective nature and relatively limited sample size. The data enhances our best use of MAP scores to the T1/T2 population. As we move forward in an age of precision medicine and patient-specific risk stratification, a comprehensive understanding of potential prognostic tools is crucial for decision-making and patient counseling.
Acknowledgements
None.
Footnotes
How to cite: Schmeusser B, et al. Mayo Adhesive Probability Score Does Not Have Prognostic Ability in Locally Advanced Renal Cell Carcinoma. J Kidney Cancer VHL. 2023; 10(1): 19–25.
Funding
We gratefully acknowledge support of the John Robinson Family Foundation, Christopher Churchill Foundation, and Cox Immunology Fund.
Declaration of Interest
All of the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Davidiuk AJ, Parker AS, Thomas CS, Leibovich BC, Castle EP, Heckman MG, et al. Mayo adhesive probability score: an accurate image-based scoring system to predict adherent perinephric fat in partial nephrectomy. Eur Urol. 2014;66(6):1165–71. 10.1016/j.eururo.2014.08.054 [DOI] [PubMed] [Google Scholar]
- 3.Yao Y, Xu Y, Gu L, Liu K, Li P, Xuan Y, et al. The Mayo adhesive probability score predicts longer dissection time during laparoscopic partial nephrectomy. J Endourol. 2020;34(5):594–9. 10.1089/end.2019.0687 [DOI] [PubMed] [Google Scholar]
- 4.Thiel DD, Davidiuk AJ, Meschia C, Serie D, Custer K, Petrou SP, et al. Mayo adhesive probability score is associated with localized renal cell carcinoma progression-free survival. Urology. 2016;89:54–60. 10.1016/j.urology.2015.10.034 [DOI] [PubMed] [Google Scholar]
- 5.Park YH, Lee JK, Kim KM, Kook HR, Lee H, Kim KB, et al. Visceral obesity in predicting oncologic outcomes of localized renal cell carcinoma. J Urol. 2014;192(4):1043–9. 10.1016/j.juro.2014.03.107 [DOI] [PubMed] [Google Scholar]
- 6.Hu X, Wang Y, Yang WX, Dou WC, Shao YX, Li X. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:6163–73. 10.2147/CMAR.S208839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol. 2018;36(12):1943–52. 10.1007/s00345-018-2309-4 [DOI] [PubMed] [Google Scholar]
- 8.Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. World J Urol. 2010;28(3):319–27. 10.1007/s00345-010-0540-8 [DOI] [PubMed] [Google Scholar]
- 9.Bilen MA, Jiang JF, Jansen CS, Brown JT, Harik LR, Sekhar A, et al. Neoadjuvant cabozantinib in an unresectable locally advanced renal cell carcinoma patient leads to downsizing of tumor enabling surgical resection: a case report. Front Oncol. 2020;10:622134. 10.3389/fonc.2020.622134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Master VA, Schmeusser BN, Osunkoya AO, Palacios AR, Midenberg E, Yantorni L, et al. Neoadjuvant nivolumab and ipilimumab for nonmetastatic renal cell carcinoma with tumor thrombus. J Immunother Precis Oncol. 2023;6(1):50–5. 10.36401/JIPO-22-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorin MA, Patel HD, Rowe SP, Hahn NM, Hammers HJ, Pons A, et al. Neoadjuvant nivolumab in patients with high-risk nonmetastatic renal cell carcinoma. Eur Urol Oncol. 2022;5(1):113–17. 10.1016/j.euo.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pignot G, Thiery-Vuillemin A, Walz J, Lang H, Bigot P, Werle P, et al. Nephrectomy after complete response to immune checkpoint inhibitors for metastatic renal cell carcinoma: a new surgical challenge? Eur Urol. 2020;77(6):761–3. 10.1016/j.eururo.2019.12.018 [DOI] [PubMed] [Google Scholar]