Abstract
The Miconia genus is traditionally used in folk medicine in Brazil and other tropical American countries and is represented by 282 species in this region. It is a multifaceted genus of medicinal plants widely used to treat rheumatoid arthritis (RA), pain, inflammatory diseases, and many more therapeutic applications. In the present study, we systematically identify and discuss the literature on in vivo and in vitro studies focusing on the therapeutic potentials and related molecular mechanisms of the Miconia genus. The review also assessed phytochemicals and their pharmacological properties and considered safety concerns related to the genus. Literature searches to identify studies on the Miconia genus were carried out through four main electronic databases, namely PubMed, Embase, Scopus, and Web of Science limited to Medical Subjects Headings (MeSH) and Descriptores en Ciencias de la Salud (DCS) (Health Sciences Descriptors) to identify studies published up to December 2022. The relevant information about the genus was gathered using the keywords ‘Miconia’, ‘biological activities’, ‘therapeutic mechanisms’, ‘animal model, ‘cell-line model’, ‘antinociceptive’, ‘hyperalgesia’, ‘anti-inflammatory’, and ‘inflammation’. The therapeutic potentials and mechanisms of action of 14 species from genus Miconia were examined in 18 in vitro studies and included their anti-inflammatory, anticancer, analgesic, antibacterial, cytotoxic, mutagenic, antioxidant, anti-leishmanial, antinociceptive, schistosomicidal, and anti-osteoarthritis potentials, and in eight in vivo studies, assessing their analgesic, antioxidant, antinociceptive, and anti-osteoarthritis activities. Some of the main related molecular mechanisms identified are the modulation of cytokines such as IL-1β, IL-6, and TNF-α, as well as the inhibition of inflammatory mediators and prostaglandin synthesis. The limited number of studies showed that commonly available species from the genus Miconia are safe for consumption. Miconia albicans Sw.Triana and Miconia rubiginosa (Bonpl.) DC was the most frequently used species and showed significant efficacy and potential for developing safe drugs to treat pain and inflammation.
Keywords: Miconia, Therapeutic mechanism, Animal model, Antioxidant, Analgesic, Anti-inflammatory
1. Introduction
The Miconia genus belongs to the Melastomataceae botanical family and comprises various flowering perennial arboreal medicinal shrubs widely distributed in tropical American countries [1]. The genus is distributed mainly in the Cerrado biome, a Brazilian savannah ecosystem in the Atlantic coastal forest of North-Eastern Brazil, occupying the fifth position in respect of species diversity, being represented by about 276 species, of which 121 are endemic [2,3]. Several Miconia fruits are edible and rich sources of phenolic compounds [4]. The Brazilian populations commonly use some species of Miconia for medicinal purposes to treat different diseases [5]. In traditional medicine, species of the Miconia genus, such as Miconia rubiginosa (Bonpl.) DC., and Miconia cinnamomifolia (DC.) Naudin treats pain, throat infections, colds, and fever [6]. Traditional healers use Miconia albicans (Sw.) Triana leaves to treat back pain and rheumatoid arthritis (RA), and its stem has significant antipyretic potential [7,8]. In Brazilian folk medicine, M. albicans has been given the popular names of ‘Canela-de-velho’ and ‘branda-fogo’ meaning “old man's ankle” or “heat reducer,” due to its purported ability to reduce joint pain in old people and the burning sensation of pain in the joints; however, the actual efficacy of many of these treatments has been little studied and/or reported.
Cunha et al. reviewed previous phytochemical investigations and reported 21 investigated species from Miconia, viz Miconia stenostachya DC., M. albicans, Miconia pepericarpa Mart. ex DC., Miconia sellowiana Naudin, Miconia fallax DC., M. rubiginosa, Miconia ligustroides (DC.) Naudin, Miconia ferruginata DC., Miconia langsdorffii Cogn., Miconia macrothyrsa Benth., Miconia affinis DC., Miconia lepidota DC., Miconia pilgeriana Ule., Miconia myriantha Benth., Miconia alypifolia Naudin., Miconia cannabina Markgr., Miconia cabucu Hoehne., Miconia willdenowii Klotzsch ex Naudin., Miconia prasina (Sw.) DC., Miconia ioneura Griseb., and Miconia trailii Cogn., containing no less than 79 phytochemicals including flavonoids, triterpenes, steroids, phenolic acids, quinones, tannins, and lignans [9]. The review further reported that flavonoids from the Miconia genus are mostly glycosylated with sugar units in the carbons 3 or 7, while others are aglycones such as quercetin, matteucinol, and kaempferol [9]. Among the pentacyclic triterpenes and derivatives isolated from the Miconia genus, the main ones are ursolic acid (UA), oleanolic acid (OA), α-amyrin, β-amyrin, α-amyrin acetate, β-amyrin acetate, arjunolic acid, sumaresinolic acid, 2-α-hydroxyursolic acid, and maslinic acid. In addition, constituents such as gallic acid, ellagic acid, primin, casuarictin, schizandriside, and several of their derivatives have also been reported to have been isolated from this genus.
Extracts, compounds, and their derivatives from Miconia species have been evaluated in vivo and in vitro studies regarding their biological and therapeutic potentials. The Miconia species showed various biochemical activities such as anti-inflammatory [10], anti-diabetic [11], anti-rheumatic [5], anti-mutagenic and anti-tumor [[12], [13], [14]], anti-microbial [[15], [16], [17], [18], [19]], schistosomicidal [20], antioxidant [[21], [22], [23], [24]], analgesic [10,25], anti-malarial [26,27], anti-nociceptive [21]; leishmanicidal [28], trypanocidal [24,26], insecticidal and fungicidal [29,30] activities. However, little is known about their safety, mechanism of action, and systemic toxicity in prolonged use, limiting their clinical use.
The pharmacological characteristics of the genus Miconia were also described by da Silva et al. in a recent review [31]. The authors also compiled the phytochemical reports in respect of Miconia species. They discovered 148 chemical compounds, including the primary constituents of the Miconia species, flavonoids, and phenolic acids. In this systematic review, we examine the phytochemical composition of the species of the Miconia genus, their safety, and potential health applications based on the identified in vivo and in vitro pharmacological studies, and in particular emphasize their related molecular mechanisms.
2. Materials and methods
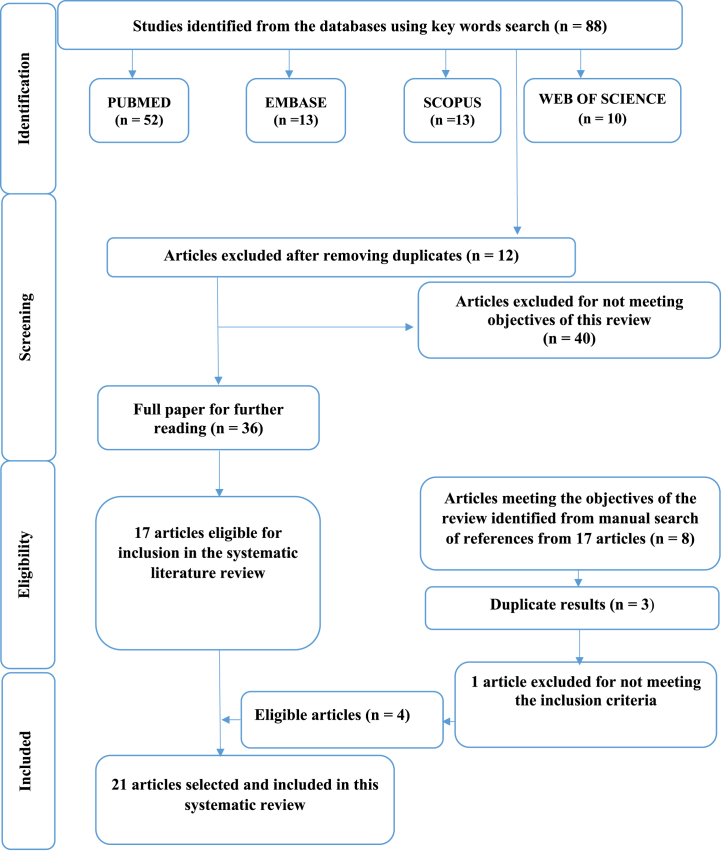
The current systematic review was designed and performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to detect current in vivo and in vitro studies linked to the therapeutic mechanisms of the plant species belonging to the Miconia genus [32] (Fig. 1).
Fig. 1.
Flow chart diagram of the selection process of eligible studies.
2.1. Search strategy
Literature searches were conducted using the four main electronic databases, namely PubMed, Embase, Scopus, and Web of Science, and limited to Medical Subjects Headings (MeSH) and Descriptores en Ciencias de la Salud (DCS) (Health Sciences Descriptors) to identify studies published to December 2022. Specific keywords such as ‘Miconia’, ‘biological activities’, ‘therapeutic mechanisms’, ‘animal model’, ‘cell-line model’, ‘antinociceptive’, ‘hyperalgesia’, ‘anti-inflammatory’, and ‘inflammation’ were used. Additionally, a manual search was done online and in Google Scholar searching both academic and preprint literature to detect any articles not found in the databases. The data were collected from online journals published in English, irrespective of the region and publication type.
2.2. Study selection
Initially, two authors (JSSQ and RQG) performed the literature search in the databases. Then the extracted titles, abstracts, and relevant full-text published articles were reviewed independently by three investigators (SRG, JSSQ, and GRG), with any disagreement being settled by consensus or, failing this, by a fifth author (LJQJ); the final selection of articles for systematic review was made in consultation with all co-authors. Only original research papers investigating the Miconia genus, its well-defined chemical composition, and its potential therapeutic mechanisms using in vivo and in vitro experimental models were included in this review. Review articles, meta-analyses, book chapters, conference proceedings, editorials/letters, patents, and case reports were excluded.
2.3. Data extraction
One of the authors (SRG) summarized data extraction from shortlisted studies individually. Table 1, Table 2 summarize the critical information from the selected studies: (a) extracts and natural molecules isolated from the plants' species belonging to the Miconia genus, (b) the animals/strains or cell lines used, (c) the doses/routes of administration, (d) the proposed mechanisms of action, (e) the first author's name and year of publication.
Table 1.
Description of the main characteristics of in vivo studies using the Miconia genus.
| Name of the plant | Animal/Strains | Dose/Route | Effects and molecular mechanism | References |
|---|---|---|---|---|
| Miconia rubiginosa (Bonpl.) DC. | Swiss mice and Wistar rats | 100, 200 and 300 mg/kg intraperitoneal | ↑central and peripheral analgesic; antinociceptive effect | [21] |
| Miconia albicans Sw. Triana | Swiss albino mice and Wistar rats | 200 mg/kg intraperitoneal | ↓PGE2α and PGF2α; ↑peripheral-mediated analgesic activity | [25] |
| M. albicans | Swiss mice and Wistar rats | 40 mg/kg intraperitoneal | ↓inflammatory mediators and inhibition of synthesis of prostaglandins and also the blockage of their receptor sites; ↑peripheral mediated analgesic effect | [10] |
| Miconia minutiflora (Bonpl.) DC. | Wistar rat and Swiss mice | 50, 100 and 200 mg/kg oral | reduced inflammatory activity by decrease in cell migration; ↓pro-inflammatory cytokines TNF-α and IL-1β; ↑central and peripheral analgesic effect | [33] |
| M. albicans | Swiss mice | 50 and 100 mg/kg oral | ↓leukocyte migration; ↓TNF-α, IL-1β, and IL-6; ↓nociceptive and hyperalgesic behaviors; ↓ipsilateral knee edema; ↑mobility and hindpaw grip strength | [36] |
| M. albicans | Swiss mice | 25, 50 and 100 mg/kg oral | ↓TNF-α and IL-1β; reduced inflammatory nociception and edema; exhibit antioxidant and anti-inflammatory properties | [44] |
| M. albicans and Curcuma longa Linn | Human knee's osteoarthritis | 1000 mg/kg oral | ↓score of WOMAC, VASP; ↑analgesic and exhibit anti-inflammatory effect on knee osteoarthritis | [39] |
| M. albicans | Swiss mice | 2.5 mg/kg on ear edema | ↓ear edema and MPO activity; revealed antioxidant and anti-inflammatory effects | [43] |
Table 2.
Description of the main characteristics of in vitro studies using Miconia species and its chemical composition.
| Plant Used |
Plant extract | Chemical composition | Therapeutic properties | Effects and Molecular Mechanism | References | ||
|---|---|---|---|---|---|---|---|
| Genus | Species | Plant part | |||||
| M. | rubiginosa | Aerial parts | Hexane, methylene chloride and ethanol extract | Triterpenes and sterols | Analgesic activity | ↑central and peripheral analgesic activity, antinociceptive effect; ↓prostaglandin synthesis | [21] |
| M. | albicans | Aerial parts | Methylene chloride extract | Ursolic acid and oleanolic acid | Analgesic and anti-inflammatory activity | ↑analgesic and anti-inflammatory activity; ↓ inflammatory mediators | [10] |
| Miconia | salicifolia (Bonpl. ex Naudin) | Leaves and bark | Ethanolic and aqueous extracts | N/A | Antibacterial activity | ethanol extract has strong antibacterial activity against Staphylococcus aureus and Escherichia coli | [64] |
| Miconia | ligustroides (DC.) Naudin and isolated triterpene acids | Aerial parts | Methylene chloride extract | Ursolic acid and oleanolic acid | Antimicrobial activity | M. ligustroides showed appreciable inhibition against Bacillus cereus; ursolic acid displayed efficient activity against B. cereus; oleanolic acid exhibited growth inhibitory activity against B. cereus and Streptococcus pneumoniae | [65] |
|
M. Miconia Miconia M. |
albicans, cabucu Hoehne, stenostachya DC., rubiginosa |
Aerial parts |
Methanol extract | Flavonoids and tannins | Mutagenicity and antimutagenicity of the extracts | ↑cytotoxic, antimutagenic activity; demonstrated the protective effects against DXR-induced DNA damage | [14] |
| Miconia | langsdorffii Cogn. | Aerial parts | Hydroalcoholic extract | Triterpenes | Antileishmanial activity | ↑in vitro antileishmanial activity against the promastigote forms of Leishmania amazonensis | [28] |
| M. | rubiginosa | Leaves | Aerial parts | Triterpenes, flavonoids and quinones | N/A | N/A | [5] |
| Miconia | willdenowii Klotzsch ex Naudin. | Leaves | Ethanol extract | Benzoquinone | Schistosomicidal activity | ↑ crude ethanolic extract of M. willdenowii showed the promising results, killing 65% of the Schistossoma mansoni worms. Primin as the active metabolite responsible for the observed schistosomicidal effect | [20] |
| M. | minutiflora | Leaves | Methanol extract | Ellagic acid, gallotannin and terpenes | Anti-inflammatory and antinociceptive | ↑antioxidant, anti-inflammatory, and antinociceptive activity induced by hydrolyzable tannins; ↓proinflammatory cytokines TNF and IL-1β, decrease edema in both phases of inflammation | [33] |
| M. | willdenowii | Leaves | Ethanol extract | 2-methoxy-6-pentyl-benzoquinone | Leishmanicidal and antimicrobial activities | inhibits promastigote forms of L. amazonensis; exhibited antimicrobial activity against pathogenic fungi, gram-positive and negative bacteria | [68] |
| Miconia | latecrenata Naudin. | Leaves | Aqueous extract | Tannins | Antioxidant, antibacterial, antimutagenic and antigenotoxic activities | ↑antioxidant property due to high total phenolic content; antibacterial activity to gram-positive and negative strains; and antimutagenic property by decreasing the ROS | [63] |
| Miconia | chamissois Naudin | Leaves | Hydroethanolic extract | Matteucinol | Cytotoxicity and anticancer potential | M. chamissois and matteucinol showed cytotoxicity and antitumor potential in glioblastoma cell lines | [77] |
| M. | albicans | Leaves | Ethanol extract | Quercetin and rutin | Antihyperalgesic and anti-inflammatory profile | ↓ levels of TNF-α and IL-1β in the joint; ↓ nociceptive and hyperalgesic behaviors | [36] |
| M. | albicans | Leaves | Ethanol extract | Polyphenols, leucoanthocyanins, tannins, steroids and saponins. | In vitro antioxidant activity | ↑ exhibited strong antioxidant profiles due to enhanced content of polyphenols | [44] |
| M. | latecrenata | Leaves | Organic extract | Phenolic compounds | Antibacterial activity | demonstrated the promising for inhibiting the growth of S. aureus and Pseudomonas aeruginosa | [69] |
| Miconia | burchellii Triana. | Leaves | Ethanol extract | triterpenes, flavonoids, steroids and pheophorbide | Cytotoxic activity | ↑ antiproliferative and demonstrated cytotoxic against leukemia cell lines | [62] |
| M. | albicans | Fruit | Methanol extract | Flavonoids, organic acids, tannins and triterpenes | In vitro antioxidant and antiproliferative properties | ↑antioxidant, ferrous chelating capacities; non-toxic to VERO cells, ensures cell viability, absence of antiproliferative effect against human tumor cell lines | [43] |
| Miconia | ferruginata DC. | Leaves, stem and flowers | Ethanol extract | flavonoids derivatives of quercetin, catechins, and phenolic acids | anticancer | ↑ anticancer; exhibits cytotoxicity against tumor cells of 4T1, A549, and MDA-MB-231 | [78] |
| M. | albicans | leaves | Hydroethanolic extract | N/A | anticancer | mixture of plant extracts exhibited cytotoxic potential; ↓ viability of human cancer cell lines | [79] |
| M. | chamissois | leaves | |||||
Abbreviations: Not applicable (N/A); prostaglandin E2α (PGE2α); prostaglandin F2α (PGF2α); tumor necrosis factor alpha (TNF-α); IL-1 beta (IL-1β); interleukin (IL); western Ontario and McMaster universities arthritis index (WOMAC); vasodilator stimulated phosphoprotein (VASP); reactive oxygen species (ROS); doxorubicin (DXR); myeloperoxidase (MPO).
3. Results and discussion
3.1. Search results
Fig. 1 shows a PRISMA flow chart showing the process used in this systematic review's database search and articles assessment. According to the PRISMA statement, the systematic investigation included in vivo and in vitro studies relating to the therapeutic mechanisms of plant species belonging to the Miconia genus. Among the 88 potentially relevant articles from four databases (PubMed: 52, Embase: 13, Scopus: 13, Web of Science: 10), 12 were discarded for being duplicates and 40 were excluded after comprehensive screening for the following reasons: (a) studies unrelated to the objectives and aim of this systematic review; (b) studies established to be reviews, editorials, conference proceedings, and meta-analyses. 36 full-text articles were then evaluated independently, with 21 studies being identified that met the eligibility criteria of this review.
3.2. Phytochemical studies
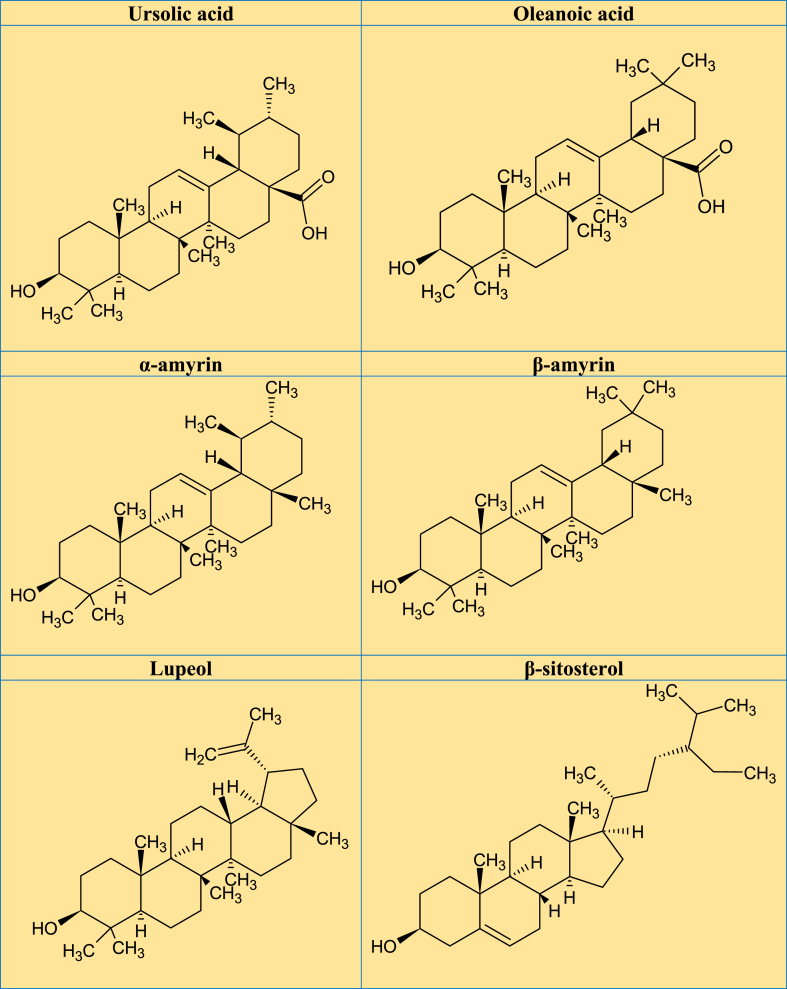
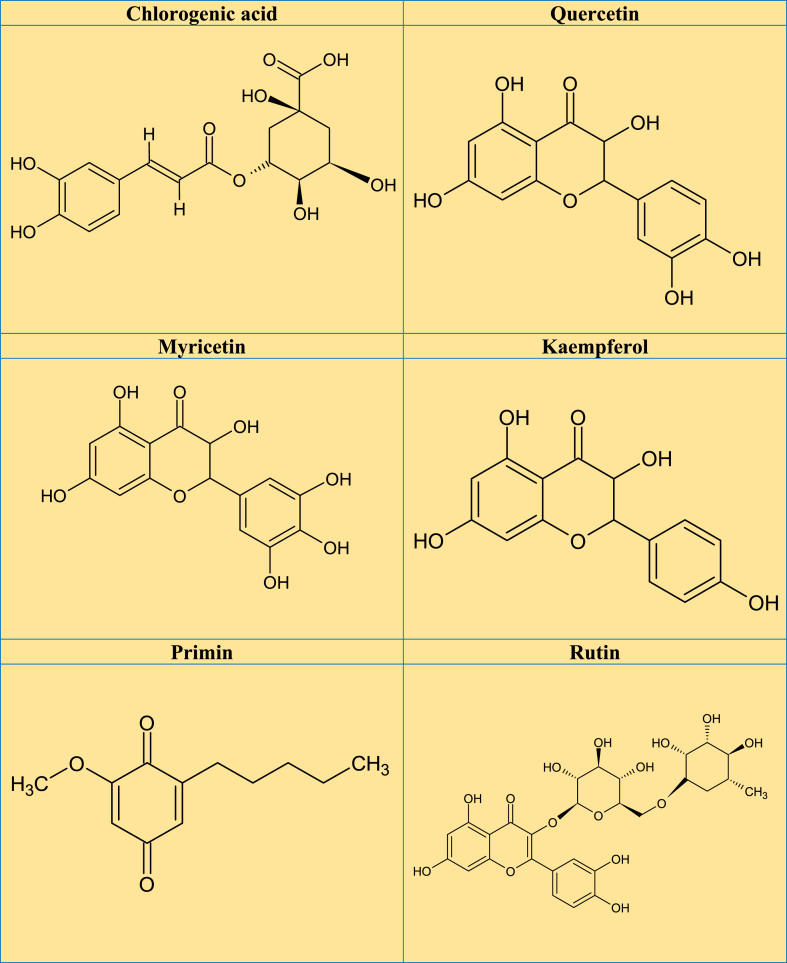
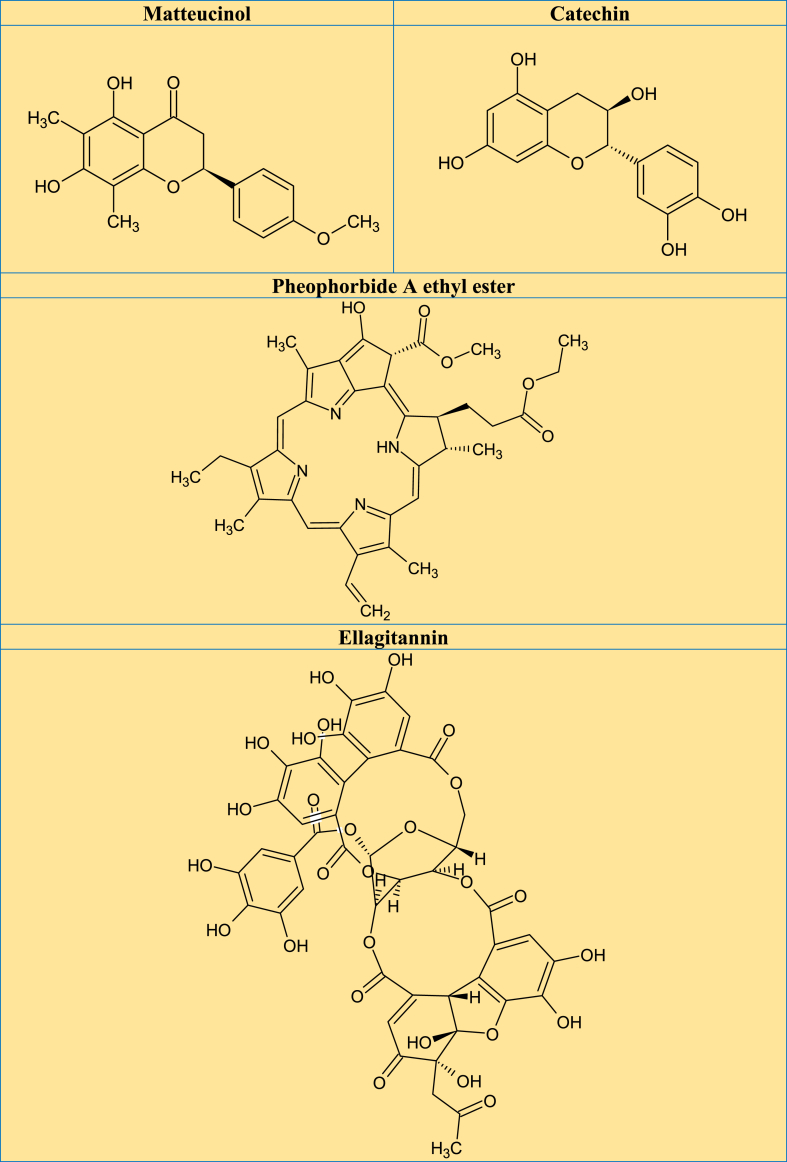
A previous review revealed that 28 glycosylated flavonoids and 10 aglycones (mostly quercetin, matteucinol and kaempferol) had been isolated and identified from plant species belonging to the Miconia genus [9]. The major pentacyclic triterpenes isolated from the Miconia genus include UA, OA, α-amyrin and, β-amyrin, and several bioactive derivatives. Other isolated phytochemicals include steroids and derivatives (β-sitosterol, stigmasterol, stigmast-4-ene-3,6-dione, campesterol, gallic acid, ellagic acid, and derivatives) [9]. Examination of the leaves from M. rubiginosa using high-speed counter-current chromatography showed the presence of phytochemicals including flavonoids, gallic acid, casuarictin, and schizandriside [5]. A study that examined the methanol extract of Miconia minutiflora (Bonpl.) DC., using UPLC-DAD-QTOF-MS/MS revealed the presence of ellagic acid, gallotannin, and terpenes [33]. UA and OA are the major secondary metabolites of triterpenes isolated from the plant species from Miconia, commonly as an isomeric mixture having a pentacyclic skeleton [34]. Other triterpenes reported are α-amyrin, β-amyrin, lupeol, maslinic acid, epibetulinic acid, and arjunolic acid [11]. Miconia plant species revealed the presence of some phytoconstituents such as flavonoid glycosides (quercetin, myricetin, catechin, and kaempferol), a phenolic acid (gallic acid) and bioflavonoids [35]. In addition, HPLC-DAD-ESI-MS/MS analysis of ethanol extract from the leaves of M. albicans identified 23 natural molecules, mostly glycoside flavonoids derived from quercetin, and rutin [36]. Phytochemical studies on this genus reported the presence of triterpenes [37], flavones [38], coumarins, and benzoquinones [12]. According to the studies reviewed, Fig. 3A-C depicted the potential phytoconstituents that have been found or extracted from various Miconia plant species that are associated with a range of biological functions.
Fig. 3.
A–C. Promising phytochemical structures identified/isolated from different Miconia plant species that are responsible for a variety of biological functions from the reviewed studies.
3.3. Evaluation of selected studies
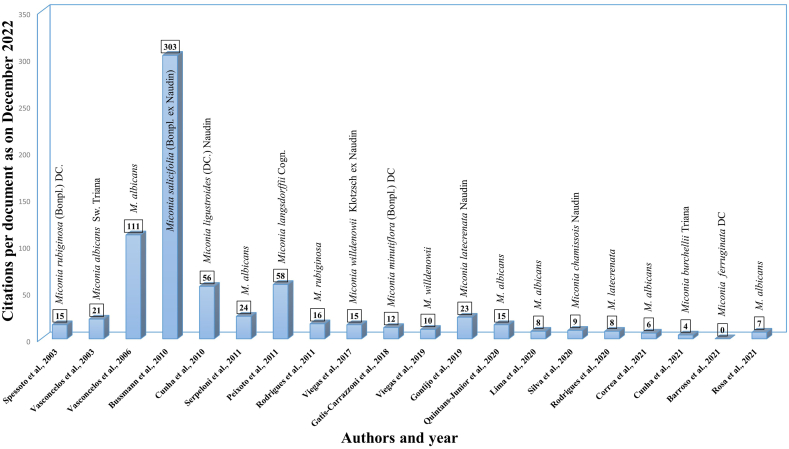
Our review found that the following 11 plant species from this genus have been reported as containing various promising bioactive phytochemicals in reports conducted in both in vitro and in vivo experiments: M. albicans, M. rubiginosa, Miconia salicifolia (Bonpl. ex Naudin) Naudin., M. ligustroides, M. stenostachya, M. cabucu, M. langsdorffii, M. willdenowii, M. minutiflora, Miconia latecrenata Naudin., and Miconia burchellii Triana. The following biological activities were evaluated in eight in vivo studies, including one human osteoarthritis clinical study by Gomes et al. [39]; antioxidant, antinociceptive, anti-inflammatory, anti-arthritic, and antinociceptive peripheral and central analgesic activity; while the in vitro studies investigated the extracts of plant species from Miconia for their antibacterial, anti-leishmanial, cytotoxic, mutagenic, schistosomicidal, anti-inflammatory, antioxidant, and anti-proliferative activities. The twenty-one articles reviewed here originated from Brazil (n = 20) and the USA (n = 1). Fig. 2 shows the number of publications with their citations and the year they were reported for each species of Miconia. Traditional populations and users of medicinal plants in the Northeast region of Brazil depend on folk medicine widely; most of these species are commonly found for sale in public markets [40]. Our results also show that plant species belonging to the Miconia genus that treat joint diseases (those with an inflammatory profile) are more popular with Brazilian folk medicine practitioners than medicinal plants used to treat other illnesses. This is because there is an increase in the elderly population and more people are getting these kinds of diseases [41,42].
Fig. 2.
A bar graph showing the number of articles, their citations, and the year they were published about the genus Miconia.
3.4. In vivo studies
Our literature search identified 8 in vivo studies relating to the biological activities of the extracts and isolated compounds from Miconia, the viz analgesic and antioxidant activity of M. rubiginosa [21], the antinociceptive and peripheral analgesic activity of M. albicans [25], the analgesic and anti-inflammatory activity of M. albicans [10], anti-arthritic and anti-inflammatory activity of M. albicans [36], antioxidant and anti-inflammatory activity of M. albicans [43], anti-inflammatory and antinociceptive of M. minutiflora [33], anti-inflammatory and antioxidative effect of M. albicans [44], and anti-osteoarthritis activity of M. albicans [39]. Most in vivo studies in our current review mainly focus on the analgesic and anti-inflammatory methodologies, which might be because plants of the Miconia genus are most commonly used to treat pain and inflammation in folk medicine [9].
3.5. Analgesic and anti-inflammatory activity
The analgesic effect of M. rubiginosa extract was studied in rats and mice using the acetic acid-induced writhing and hot plate tests, classical experimental models of analgesic screening were widely used to validate this pharmacological property [21,45]. The extract (200 mg/kg/body wt.) showed a potent increase in the pain threshold and antinociceptive effect and inhibited acetic acid-induced writhing in mice. The presence of triterpenes (UA and OA, α-amyrin and β-amyrin) and sterols (lupeol and β-sitosterol) seems responsible for the central and peripheral analgesic and anti-inflammatory profiles. Previous studies have indicated that these triterpenes play a role in modulating pro-inflammatory mediators and mitigating the deleterious effects of inflammation and cell proliferation [46,47]. The results suggest that the favourable biological mechanism of action might be due to the inhibition of significant levels of prostaglandin, namely, PGE2α and PGF2α synthesis, the two critical prostanoids in the inflammatory cascade following the acetic acid injection, as well as through central inhibitory mechanisms. The synergistic action of natural molecules in the extract could have been accountable for the observed analgesic effect in biological activity studies [47]. Consequently, the analgesic activity study of the crude extracts of aerial parts of M. rubiginosa resulted in abdominal writhing inhibition and antinociceptive effects [21]. M. rubiginosa crude extracts due to the presence of anti-inflammatory phytochemicals might be involved in the peripheral analgesic activity [48]. Furthermore, the extracts of M. rubiginosa significantly reversed the acetic acid-induced writhing in mice. M. rubiginosa induced a significant increase in pain threshold throughout the experimental period, with a substantial increase in the percentage of protection. The presence of triterpenes and sterols in M. rubiginosa is the essential active principle responsible for the witnessed analgesic effect, possibly mediated by the modulation of prostaglandin synthesis, along with effects on central inhibitory mechanisms. Literature studies report that these compounds have analgesic and anti-inflammatory properties [49]. There is consistent evidence that lupeol and β-sitosterol mitigate tumor necrosis factor-α (TNF-α), vascular endothelial growth factor receptor −2 (VEGFR-2), and pro-inflammatory cytokines activity in cell proliferation, and the production of factors that drive the inflammatory process and, consequently, the pain process [46].
A similar study performed by Vasconcelos et al. using M. albicans crude extracts (200 mg/kg/body wt.) in Swiss albino mice and Wistar rats, did not exhibit any analgesic effect on the central nervous system (CNS); however, there were clear peripheral analgesic effects, and a reduction in pain behaviour, and anti-inflammatory activities [25]. Crude extract of the aerial parts of M. albicans has been shown to have analgesic effects due to the presence of triterpene acids and β-sitosterol [25]. β-sitosterol has been shown to have outstanding anti-inflammatory properties while reducing critical inflammatory mediators of the pain-related process [50].
The potential analgesic and anti-inflammatory activities of UA and OA, isolated from the aerial parts of M. albicans crude methylene chloride extract, were evaluated in a carrageenan-induced paw edema animal model. The compounds produced a significant anti-inflammatory effect and a prostaglandin synthesis inhibition. Thus, they showed a significant reduction in inflammatory pain, which seems to be confirmed by the amelioration of the increase in inflammatory mediators, such as the prostaglandins, induced by carrageenan and the subsequent inhibition of the inflammatory signaling pathways which is one of the main triggers for the pain profile of these animal models. The analgesic effect of the extract was mostly based on its peripheral mediated mechanism, which is compatible with the presence of the UA and OA compounds found [10]. UA and OA are inhibitors of key pro-inflammatory pathways in joint pain, such as the nuclear factor erythroid-2-related factor 2 (Nrf2) and nuclear factor-κB (NFκB) pathways [51,52].
Similarly, Lima et al. demonstrated that M. albicans extract possesses an effective treatment profile against RA in carrageenan-induced paw edema [44]. The study revealed that the extract reduced TNF-α, IL-1β, and consequently, the inflammatory nociception and edema caused by carrageenan injection. These results corroborated, at least in part, those reported by Vasconcelos et al. suggesting that M. albicans has potential therapeutic uses in chronic, difficult-to-treat conditions that are very disabling for patients, such as arthritis or other joint pain [10]. The chemical study of the extract produced a vast number of flavonoids with polyphenol structures, and the authors suggest that its bioactivity may be explicitly credited to the presence of the composition of flavonoids containing polyphenols structure, such as rutin and quercetin. Flavonoids are anti-inflammatory and are used to mitigate chronic inflammatory diseases [53,54]. Thus, these findings reinforce the use of these anti-inflammatory plants in Brazilian folk medicine to treat joint and related pain [44].
M. minutiflora leaf polyphenols rich-extract has shown anti-inflammatory activity and could reduce edema and the migration of leukocytes towards the site of inflammation, and was associated with suppressed concentrations of the pro-inflammatory cytokines such as TNF-α and IL-1β, while the antinociceptive actions involve central and peripheral mechanisms with the participation of α2-adrenergic receptors. The anti-inflammatory mechanisms of M. minutiflora might be related to the decrease in the level of several inflammatory and pro-inflammatory mediators in the edema tissue via the suppression of pro-inflammatory cytokines concentrations [33].
Thus, the major compounds in the studied extracts (especially terpenoids and flavonoids) are known to act directly by mitigating the production and levels of circulating pro-inflammatory cytokines, influencing pathways related to oxidative stress, which are suggested to be responsible for the anti-inflammatory activities of several medicinal plants [53,55]. These therapeutic points of view suggest using plant remedies to recommend these herb species used in traditional medicine to treat inflammation.
3.6. Anti-arthritic and anti-inflammatory activity
Studies have indicated that drugs that modulate or block the pro-inflammatory cytokine TNF-α and its related factors, including interleukins (IL)-1, IL-6, IL-8, and granulocyte macrophage-colony stimulating factor (GM-CSF) have been considered as one of the preferred treatments for the management of RA [[56], [57], [58]]. M. albicans ethanolic leaf extract (MAEE), in doses of 50 and 100 mg/kg/body wt., was assessed for its anti-hyperalgesic and anti-inflammatory profiles in a carrageenan-induced arthritis-like model. The results showed that MAEE significantly lessened leukocyte migration in the pleurisy model and suppressed TNF-α and IL-1β in pleural lavage. Moreover, in the Complete Freund's Adjuvant (CFA) mice model, a primary animal model that mimics the signs and symptoms of RA in humans, MAEE administration in rats resulted in a significant decrease in nociceptive pain and hyperalgesic actions in the rearing test and decreased mechanical hyperalgesia. Moreover, MAEE significantly improved mobility in the open-field test and increased hind paw grip strength without any apparent damage to the liver. MAEE drastically reduced the volume of CFA-induced ipsilateral knee edema, the solid anti-inflammatory potency could be related to its positive effect on IL-6 and TNF-α in the knee joint [36]. Moreover, a dried extract of M. albicans (DEMA) reduced the carrageenan-induced edema of the paw. It ameliorated the inflammatory reactions by downregulating TNF-α and IL-1β and reducing antioxidant parameters, consequently reducing inflammatory nociception, important factors in reducing the inflammatory cascade [44]. Similarly, the in vivo anti-inflammatory effect of M. albicans fruits methanol extract, which is rich in phenolic compounds, flavonoids, hydroxybenzoic acids, terpenoids, ellagitannins, chlorogenic acid, and fatty acids was assessed in croton oil-induced ear edema in mice and was reported to have potential health benefits [43].
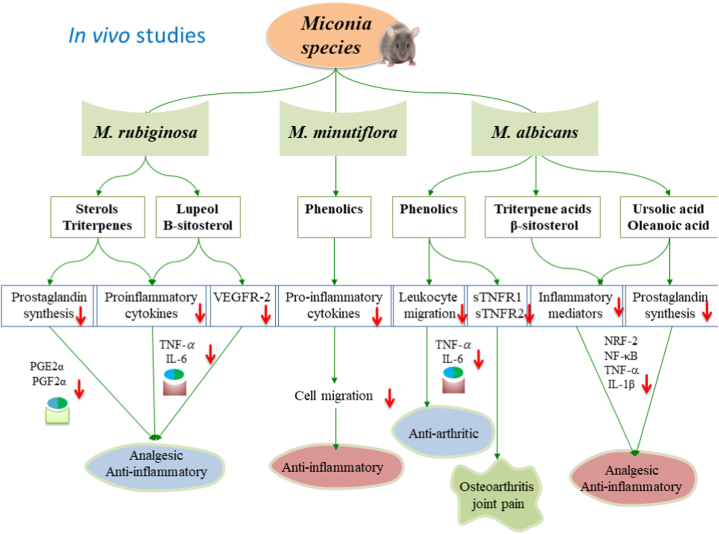
An important aspect in these recent studies with different extracts of M. albicans [36,43,44] is the possible ability of the extracts to reduce RA symptoms, mitigate pro-inflammatory pathways, and reduce oxidative stress by acting as a potential anti-RA agent, similar to RA blockers which reduce TNF-α, IL-1β and IL-6 levels [59,60]. Moreover, TNF-α and IL-6 are cytokines at high levels in the synovial fluid of RA patients. The reduction of these cytokines has been directly related to improvements in the general condition of patients, especially in respect of joint pain that produces the greatest disability in these persons [61]; thus the fact that the studied extracts of M. albicans seem to be able to act as TNF-α, IL-1β and IL-6 blockers are encouraging in respect of their potential use. Fig. 4 depicts the role of phytochemicals derived from the Miconia genus plant species in exhibiting their anti-inflammatory effect by modulating vital inflammatory mediators, which might be related to their antioxidative mechanism involving the normalization of oxidative stress-stimulated biomarkers.
Fig. 4.
The in vivo biological studies evaluated the therapeutic potentials of the Miconia species, such as their analgesic, anti-inflammatory activity, anti-arthritis, joint pain, and anti-osteoarthritis activities The extracts and secondary metabolites from Miconia species, such as phenolic compounds, hydroxybenzoic acids, flavonoids, terpenoids, triterpenes, sterols, ellagitannins independently or synergistically, might have contributed to strong antioxidant and anti-inflammatory activities through cytokine-mediated responses. Pro-inflammatory and anti-inflammatory cytokine production might have modulated various ILs-mediated cellular responses to the tested diseases, especially for treating osteoarthritis, and joint pain.
3.7. Osteoarthritis and joint pain
Interestingly, a clinical study conducted to assess the analgesic and immunomodulatory potential of M. albicans in knee osteoarthritis revealed its capability to lessen joint pain and inflammation and improve function [39]. This is probably the first clinical study using M. albicans for a common disease and provides further evidence for its use in traditional medicine. In the study, the oral administration of the extract at 1000 mg/day/body wt. for 30 days reduced the patients’ pain, decreased Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Visual Analogue Scale of Pain (VASP) scores, and knee joint effusion resulting in functional improvement. This clinical study demonstrated the analgesic and anti-inflammatory effect of M. albicans on knee osteoarthritis, correlating with changes in the expression of inflammatory mediators in the synovial fluid. Moreover, the treatment decreased the expression of resist in and soluble TNF-α receptors (soluble tumour necrosis factor receptor (sTNFR)1 and sTNFR2 and increased the expression of adiponectin and leptin. In this study, the authors demonstrated the analgesic efficacy of M. albicans and their possible mechanisms concerning pain modulation, reduced inflammation, and improved function in knee osteoarthritis. The study also corroborated the clinical safety of using this plant species and its therapeutic benefits [39].
Although this study lacked a more careful analysis of possible toxicity and did not evaluate the inflammatory mediators involved (blood levels of cytokines, inflammatory mediators common in osteoarthritis, among others) more systemically, it is the first study in humans to provide evidence that supports the popular use of M. albicans and shows that it can be effective for diseases such as osteoarthritis and RA. This is, therefore, a fundamental study and provides a reasonable basis for further controlled and randomized clinical trials.
In summary, eight in vivo studies of the biological efficacy of the various extracts and their natural molecules from the Miconia genus were found to have potential health benefits, including analgesic, antioxidant, antinociceptive, and anti-osteoarthritis activities. Most importantly, M. albicans fruit extract has higher concentrations of flavonoids (quercetin, myricetin, kaempferol), terpenoids, and fatty acids such as palmitic, stearic, arachidic, behenic, elaidic, oleic, eicosenoic, and linoleic acids, as well as others, which might have contributed to the strong antioxidant and anti-inflammatory activities [43]. But there is still a lack of scientific evidence about some clinical aspects (molecular information, acute and chronic toxicity, effectiveness, etc.) and the possible effects of long-term use of pharmaceutical preparations that contain extracts from Miconia genus plants or their isolated compounds. This is an area that needs to be looked into more.
3.8. In vitro studies
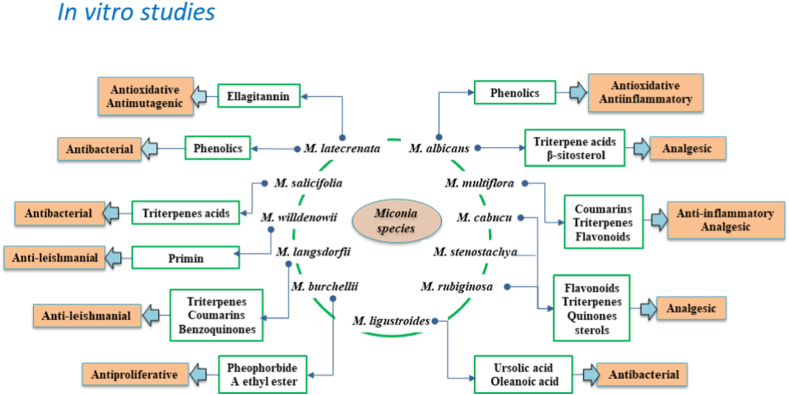
In our systematic search, we identified 18 in vitro studies of the pharmacological activities of the plant species from the Miconia genus with enriched bioactives (Fig. 5), viz., the antioxidant property of M. albicans [43], the cytotoxic activity of M. burchellii [62], the antioxidative effect of M. albicans [44], a chemo profile study of M. albicans [36], the antioxidant, the antibacterial and antimutagenic activity of M. latecrenata [63], the phytochemistry, anti-inflammatory and antinociceptive properties of M. minutiflora [33], the schistosomicidal activity of M. willdenowii [20], the phytochemistry of M. rubiginosa [5], the anti-leishmanial activity of M. langsdorffii [28], the cytotoxic and mutagenic activity of M. albicans, M. cabucu, and M. stenostachya [14], the antibacterial activity of M. salicifolia [64], the antibacterial activity of M. ligustroides [65], and the phytochemical and antioxidant activity of M. rubiginosa [21].
Fig. 5.
In vitro pharmacological studies of Miconia species showed several crucial health benefits, mainly focused on anti-inflammatory, antioxidant, analgesic, antibacterial, anti-leishmanial, antinociceptive, schistosomicidal, anti-osteoarthritis, cytotoxic, and mutagenic activities. Phenolic compounds, hydroxybenzoic acids, flavonoids, terpenoids, ellagitannins, chlorogenic acids, fatty acids, and many more, might have contributed to the aforementioned therapeutic potentials of Miconia species. Evidence indicates that the extracts and secondary metabolites from Miconia species are also safe for consumption and could be explored further for managing diseases.
3.9. Antibacterial and antifungal activities
A study conducted by Bussmann et al. reported that various solvent extracts prepared from 51 Peruvian medicinal plant species had antibiotic and inhibitory activity regarding microbial growth [64]. The extracts inhibited Escherichia coli and Staphylococcus aureus. The ethanolic extracts exhibited higher inhibitory potential than the aqueous extracts against tested microorganisms. M. salicifolia, had the lowest MIC values, indicating its antibacterial activity. Several triterpene acids have great potential as antimicrobial compounds in treating infectious diseases [66]. UA and OA from M. ligustroides have anti-microbial activity against some multi-resistant bacteria but not against many others [65,67]. A synergetic effect was noted when different concentrations of various UA derivatives were evaluated to inhibit the growth of Bacillus cereus, Vibrio cholerae, Salmonella choleraesuis, Klebsiella pneumoniae, and Streptococcus pneumonia, and was found to have an antimicrobial effect against some of the microbes. The antimicrobial activity of several species from Miconia against various microorganisms has already been reported in the literature [15]. The leaves of M. latecrenata, which contain copious amounts of ellagitannin, presented higher antibacterial effects against some gram-positive and negative strains. On the other hand, the phenolic-rich M. latecrenata show antimicrobial activity against antibiotic-resistant bacteria, which cause some significant infections to human health [63]. The M. willdenowii leaves collected from the Brazilian Atlantic forest have antibacterial properties, and evidence would suggest that primin, the component with the highest concentration, is the main bioactive metabolite in the plant. Additionally, the findings reveal for the first time the extract's potent antibacterial and antifungal effects on S. aureus, Candida krusei, and Candida glabrata [68]. The phenolic compounds extracted from M. latecrenata leaves acquired from the Brazilian Atlantic forest showed the greatest potential for preventing the growth of S. aureus and Pseudomonas aeruginosa. Following bio-guided fractionation of the extract, the fraction demonstrated synergism with ampicillin and tetracycline for S. aureus and P. aeruginosa, respectively. These results imply that M. latecrenata leaf extracts and fractions may be employed as therapeutic antibacterial agents [69].
3.10. Anti-leishmanial and schistosomicidal activities
The extract was prepared from the aerial parts of M. langsdorffii and was evaluated for their potential against promastigotes, mainly Leishmania amazonensis, the major parasite responsible for leishmaniasis in humans [70]. Bioassay-guided fractionation of this extract revealed the presence of an extensive concentration of triterpenes. The compounds, UA and OA were observed to be the primary compounds in the plant extracts. Among the UA-derived substances, the C-28 methyl ester derivative exhibited the best activity [70]. The study results showed that UA and OA are highly potent compounds of antileishmanial action and can be effective agents against leishmania in the clinical location. An acute toxicity study of the molecules found they were safe even at high concentrations, but further studies using animal models are warranted to screen these compounds for developing new antiprotozoal agents [28].
The crude ethanolic extract from M. willdenowii was assessed for its schistosomicidal activity [20]. The extract showed greater schistosomicidal activity against S. mansoni worms than praziquantel. The ethanolic extract was further subjected to fractionation to identify its active lead molecule(s), with the hexane sub-fraction having considerably greater schistosomicidal activity against the adult worms. Moreover, chromatographic isolation of this active sub-fraction led to the isolation of 2-methoxy-6-pentyl-benzoquinone, also known as primin. This activity may be attributed to this significant bioactive metabolite. The authors reported that M. willdenowii extracts containing its active lead molecule primin showed important antischistosomal activity. Thus, these substances could be novel candidates for the treatment and management of microbes and could also act as a less toxic natural remedy against schistosomiasis [20]. The leishmanicidal activity of primin-containing ethanolic extract of M. willdenowii was also reported by Viegas et al. which demonstrated inhibition of 99.7% of the promastigote forms of L. amazonensis at a concentration of 80 μg/mL [68].
3.11. Cytotoxic and mutagenic effects
Knowledge of plant genotoxicity and potential mutagenic effects is necessary to develop plant-based phytochemical products and drugs. Extracts of plant species from Miconia were prepared and assessed for their cytotoxicity, mutagenicity, and protective effects on Chinese hamster lung fibroblast cell cultures (V79). The cytotoxicity study indicated a remarkable decline in cell viability at higher concentrations of plant extracts from Miconia, suggesting that the mixtures of polyphenols present in these extracts could contribute to the dose-dependent cytotoxicity potential of this plant and should be further validated by the pharmaceutical industry due to its ever-growing number of nutraceutical properties [71].
The plant extracts found to be the most active as pro- or anti-oxidants revealed the presence of a copious amount of phenolic compounds. Hence, the reports of the study suggest that the plant extracts prepared from the Miconia genus containing large amounts of phenolics are responsible for the antioxidant activity in vitro due to their free radical scavenging properties. Phenolic components in fruits, vegetables, and medicinal herbs have been proposed to be active secondary metabolites and have antioxidant and anticancer properties [72]. The interactions and potential synergistic properties of various plant extract polyphenols and their beneficial effects were reported in the study by Feinstein et al. including regarding doxorubicin (XDR)-induced damage [73]. In addition to XDR, the DNA-damaging potential of adriamycin has been reported [73]. Adriamycin is a substance that causes excessive production of free radicals, resulting in the generation of oxidative injuries to DNA and the production of oxidative stress-mediated lipid peroxidation [74]. The amelioration of DNA impairment reported in the study suggests that Miconia extracts containing phenolics may have protective effects on XDR-induced DNA damage by neutralizing the free radicals-mediated inflammatory reactions. Polyphenols have been shown as worthy quenchers of circulating free radicals, and therefore they inhibit DNA damage and act as vital antioxidant molecules [75]. The most outstanding feature of the present study is the indication of a therapeutic role of Miconia extracts in the recovery of XDR-induced DNA damage by enhancing DNA-repair efficiency in the damaged cells, which has been attributed to the presence of high levels of bioactive polyphenols [14]. Therefore, this study suggests Miconia species rich in polyphenols have anti-oxidant effects and high anti-mutagenic activity.
3.12. Anti-inflammatory and antioxidant properties
This study summarizes the recent investigations into the anti-inflammatory effect of M. albicans fruit extract (MAFRE). The chemical profile showed a high concentration of phenolic compounds, flavonoids, and fatty acids, that benefit the counter-inflammatory response with less toxicity on VERO cells. Flavonoids are the predominant substances in MAFRE and it has been shown that they are natural immunomodulators of pro and anti-inflammatory molecules [53]. Nine fatty acids have been found in MAFRE, in addition to linoleic acid, which is one of its major constituents. The phytochemical contents of M. albicans fruit have been previously investigated and shown to contain untapped resources of phytochemicals with effective pharmacological actions beneficial for pharmaceutical and nutritional purposes [43]. M. albicans extract exhibited potent antioxidant activity, probably due to the high concentration of flavonoids, tannins, saponins, leucoanthocyanins, and, steroids. Significant levels of total phenolic (551.3 mg gallic acid equivalent/g of dried extract) and flavonoid contents (367.19 mg catechin equivalent/g of dried extract) have been identified. A study using HPLC-PDA revealed the presence of rutin and quercetin as two major flavonoids in the extract which act strongly to inhibit levels of nitric oxide, the intracellular reactive oxygen species (ROS) pathway, and pro-inflammatory cytokines, thus reducing, for example, the levels of TNF-α and IL-6, whilst also mitigating the oxidative imbalance common in the inflammatory process and tissue injury [76]. Regarding the antioxidant activity of this standardized extract, it has been shown that the anti-inflammatory phytochemicals rutin and quercetin present in M. albicans appears to exhibit profound activity in modulating the damaging effects of reactive oxygen species [44].
The primary pharmacological activities of M. latecrenata support its therapeutic potential concerning ROS/reactive nitrogen species (RNS) related anti-inflammatory disorders. Furthermore, the phenolic compounds from M. latecrenata significantly contribute to minimizing or inhibiting biological macromolecule damage, especially to DNA molecules. Phytochemical analysis of M. latecrenata revealed a high total phenolic content, especially ellagitannins, demonstrating a potential pharmacological activity. In addition, the extract's high antioxidant, antibacterial, and antimutagenic activities were observed in different tests. Therefore, this ellagitannin-rich extract can accelerate and reduce costs in the search for new therapeutic agents [63].
3.13. Anticancer and antiproliferative activity
The in vitro anticancer potential of Miconia chamissois Naudin for treating glioblastomas was examined in the study by Silva et al. [77]. The hydroethanolic extract of M. chamissois and its chloroform partition were tested for cytotoxicity in glioblastoma cell lines. A single molecule, matteucinol, was identified in the fraction. In the adult glioblastoma cell lines, matteucinol induced intrinsic apoptosis, which induced cell death [77]. Additionally, matteucinol markedly decreased the tumour cells' invasion, migration, and clonogenicity. In an in vitro study, M. burchellii leaves were reported to have antiproliferative activity with the ethyl acetate fraction showing potent cytotoxic activity against four of the five tumor cell lines tested. The cytotoxic activity was attributed to the strong presence of pheophorbide A ethyl ester in the respective fraction, which is projected to be effective against leukemia cell lines. The investigation showed that neither the fractions nor the compounds from M. burchellii contributed significantly to the antiproliferative potential [62]. Additionally, M. ferruginata, a native Brazilian plant from the Cerrado biome known as “pixirica” or “babatena,” which is rich in flavonoid derivatives from quercetin, catechins, and phenolic acids, showed potential cytotoxicity against tumor cells of 4T1, A549, and MDA-MB-231 in association with minimal cell toxicity against fibroblasts and should be taken into consideration for further research against the treatment of cancer [78]. Hydroethanolic extracts from the leaves of M. albicans and M. chamissois; reduced the viability of human cancer cell lines. They exhibited cytotoxic potential, leading to the discovery of novel chemotherapeutic agents for cervical cancer [79].
To summarize, the in vitro studies reported that extracts from the Miconia genus and isolated compounds had anti-proliferative, anticancer, analgesic, antibacterial, cytotoxic, mutagenic, anti-leishmanial, antinociceptive, schistosomicidal and anti-osteoarthritic properties. The presence of phenolic compounds, hydroxybenzoic acids, flavonoids, terpenoids, ellagitannins and chlorogenic acids, and fatty acids such as palmitic, stearic, arachidic, behenic, elaidic, oleic, eicosenoic, linoleic acids in the Miconia genus may have contributed to the strong antioxidant, anti-cancer, and anti-inflammatory activities [43].
4. Safety of Miconia
Several in vivo experiments indicate that extracts from certain Miconia species and their isolated phytochemicals are safe for medical use, at least in the doses and routes assessed in our survey. However, there is a lack of studies demonstrating safety in controlled clinical trials. The consumption of Miconia extracts and biomolecules from the aerial parts of the plants has not been reported to cause any undesirable outcome. M. albicans methanol fruit extract has strong antioxidant and anti-inflammatory properties and contains phenolic compounds, flavonoids, terpenoids, ellagitannins, and chlorogenic acid, and is potentially non-toxic to VERO cells, with 95% cell viability [43]. Moreover, no untoward outcomes were reported in a recently published clinical trial in patients with knee osteoarthritis. Patients received M. albicans extracts (1000 mg/day/body wt. orally for 30 days) [39]. An acute toxicity study of M. minutiflora leaf methanolic extract (2000 mg/kg/body wt., orally) in experimental models in vivo showed no significant changes in weight, or food and water intake, and did not produce any deaths or treatment-linked adverse reactions [33].
In general, in vitro studies reveal the antioxidant potential of plants from the Miconia species [43,44,63]. The n-butanol fraction and the isolated flavonoids from the methanolic extract of M. albicans leaves had significant antioxidant activities with a scavenging capacity against 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) [24]. The strong occurrence of phytochemicals, such as steroids, triterpenes, alkaloids, anthraquinones, glycosides, flavonoids, leucoanthocyanins, tannins, and saponins present in Miconia species, are known to have significant free radical scavenging capacity.
Therefore, the in vitro and in vivo studies suggested that the consumption of a limited number of the species from the Miconia genus is safe and can be used for clinical and therapeutic purposes; this is supported by the fact that it has been used in folk medicines around the globe for a long period without any adverse outcomes being reported.
5. Conclusion
Miconia species is of growing public interest and considered one of the largest genera of angiosperms belonging to the Melastomataceae family, with 1050 species distributed in the South American continent, with more than 282 species in Brazil alone, of which 121 are endemic [80]. Although it occupies a large area, has a widespread distribution, and is associated with several traditional uses, studies of its phytochemical and biological activity are scarce. Here, we reviewed all current studies of the Miconia genus and its phytochemical and pharmacological properties to provide an up-to-date understanding of its therapeutic potential. In vitro pharmacological studies were mainly focused on its anti-inflammatory, analgesic, antibacterial, cytotoxic, mutagenic, antioxidant, anti-leishmanial, antinociceptive, schistosomicidal, and anti-osteoarthritic properties. The in vivo biological studies mostly evaluated its therapeutic potential regarding its analgesic, antioxidant, antinociceptive, and anti-osteoarthritic properties. The identification and authentications of phenolic compounds; hydroxybenzoic acids; flavonoids; terpenoids; ellagitannins; chlorogenic acid; fatty acids such as palmitic, stearic, arachidic, behenic, elaidic, oleic, eicosenoic, and linoleic acids, among many others, has sparked increasing interest in these species as a strong antioxidant and anti-inflammatory agents. In addition, analysis of the phytochemical composition of this species yielded eight bioactive phytomolecules: pheophorbide an ethyl ester, kaempferol, kaempferol-3-O-β-glucopyranoside, kaempferol-3-O-β-galactopyranoside, OA, UA, lupeol, and β-sitosterol that added therapeutic value to each plant belonging to the Miconia species. Phytochemical and pharmacological studies focused on the aerial parts belonging to M. albicans, M. rubiginosa, M. salicifolia, M. ligustroides, M. stenostachya, M. cabucu, M. langsdorffii, M. willdenowii, M. minutiflora, M. latecrenata, and M. burchellii. There is sufficient in vitro and in vivo evidence to suggest that the extracts and natural molecules from Miconia are safe for consumption for clinical and therapeutic purposes, which is supported by the fact that no adverse outcomes have been reported regarding their traditional use. Given the variety of their effects and potential health benefits, future investigations are warranted, particularly studies that examine the most effective delivery mechanisms and more randomized studies of more species and controlled clinical trials to further examine their potential beneficial effects.
Ethics approval
Not applicable.
Availability of data and materials
Not applicable to this article.
Consent to participate
Not applicable.
Consent for publication
All the authors have approved the manuscript for publication.
Author contribution statement
SRG, LJQJ, RQG, and JSSQ: Conceived and designed the study; SRG, GRG, SAC, LJQJ, and RQG: Analyzed and interpreted the data; SRG, GRG, PJA, and LJQJ: Wrote the original draft; VEH, LJQJ, and RQG: Reviewed and edited the final draft; GH and YL: Performed the graphical abstract, figures 4 and 5 using image editing software. All authors have read and agreed to the published version of the manuscript.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to acknowledge the support of the Brazilian National Council for Scientific and Technological Development (CNPq), the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES), and the Foundation for Research Support of the State of Sergipe (FAPITEC), and the Federal University of Sergipe, São Cristóvão, SE, Brazil. SRG is a doctoral student in the Postgraduate Program of Health Sciences (PPGCS) under Prof. Dr. LJQJr of the Federal University of Sergipe.
References
- 1.Leitao F., Leitao S.G., Fonseca-Kruel V.S., Silva I.M., Martins K. Medicinal plants traded in the open-air markets in the State of Rio de Janeiro, Brazil: an overview on their botanical diversity and toxicological potential. Rev. Bras. Farmacogn. 2014;24:225–247. [Google Scholar]
- 2.Reis C.D., Bieras A.C., Sajo M.G. Anatomia foliar de Melastomataceae do cerrado do Estado de Sao Paulo. Rev. Bras. Botânica. 2005;28:451–466. [Google Scholar]
- 3.Pessoa M.S., Vleeschouwer K.M., Talora D.C., Rocha L., Amorim A.M.A. Reproductive phenology of Miconia mirabilis (melastomataceae) within three distinct physiognomies of Atlantic forest, Bahia, Brazil. Biota Neotropica. 2012;12:49–56. [Google Scholar]
- 4.Vieira F.A., de Carvalho D. Maturation and morphometrics of the fruits of Miconia albicans (Swartz) triana (melastomataceae) in a remnant of montane seasonal semideciduous forest in Lavras, MG. Rev. Árvore. 2009;33:1015–1023. [Google Scholar]
- 5.Rodrigues J., Rinaldo D., da Silva M.A., dos Santos L.C., Vilegas W. Secondary metabolites of Miconia rubiginosa. J. Med. Food. 2011;14:834–839. doi: 10.1089/jmf.2010.0157. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues V.E.G., Carvalho D.A.D. Levantamento etnobotânico de plantas medicinais No domíniodo cerrado Na Região do Alto Rio Grande-Minas Gerais. Ciênc. Agrotec. 2001;23:102–123. [Google Scholar]
- 7.de Albuquerque U.P., Monteiro J.M., Ramos M.A., de Amorim E.L.C. Medicinal and magic plants from a public market in north-eastern Brazil. J. Ethnopharmacol. 2007;110:76–91. doi: 10.1016/j.jep.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro R.V., Bieski I.G.C., Balogun S.O., Martins D.T.O. Ethnobotanical study of medicinal plants used by ribeirinhos in the north Araguaia microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017;205:69–102. doi: 10.1016/j.jep.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Cunha G.O.S., Cruz D.C., Menezes A.C.S. An overview of Miconia genus: chemical constituents and biological activities. Pharmacogn. Rev. 2019;13:77–88. [Google Scholar]
- 10.Vasconcelos M.A.L., Royo V.A., Ferreira D.S., Crotti A.E.M., e Silva M.L.A., Carvalho J.C.T., Bastos J.K., Cunha W.R. In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae) Z. Naturforsch., C: J. Biosci. 2006;61:477–482. doi: 10.1515/znc-2006-7-803. [DOI] [PubMed] [Google Scholar]
- 11.Lima R.C.L., Kongstad K.T., Kato L., Silva M.J., Franzyk H., Staerk D. High-resolution PTP1B inhibition profiling combined with HPLC-HRMS-SPE-NMR for identification of PTP1B inhibitors from Miconia albicans. Molecules. 2018;23:1–13. doi: 10.3390/molecules23071755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunatilaka A.A., Berger J.M., Evans R., Miller J.S., Wisse J.H., Neddermann K.M., Bursuker I., Kingston D.G. Isolation, synthesis, and structure-activity relationships of bioactive benzoquinones from Miconia lepidota from the Suriname rainforest. J. Nat. Prod. 2001;64:2–5. doi: 10.1021/np000219r. [DOI] [PubMed] [Google Scholar]
- 13.Cunha W.R., Silva M.L.A., dos Santos F.M., Montenegro I.M., Oliveira A.R.A., Tavares H.R., Santos H.S.L.D., Bizario J.C.D.S. In vitro inhibition of tumor cell growth by Miconia fallax. Pharm. Biol. 2008;46:292–294. [Google Scholar]
- 14.Serpeloni J.M., Barcelos G.R.M., Mori M.P., Yanagui K., Vilegas W., Varanda E.A., Colus I.M.S. Cytotoxic and mutagenic evaluation of extracts from plant species of the Miconia genus and their influence on doxorubicin-induced mutagenicity: an in vitro analysis. Exp. Toxicol. Pathol. 2011;63:499–504. doi: 10.1016/j.etp.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Celotto A.C., Nazario D.Z., Spessoto M.A., Martins C.H.G., Cunha W.R. Evaluation of the in vitro antimicrobial activity of crude extracts of three Miconia species. Braz. J. Microbiol. 2003;34:339–340. [Google Scholar]
- 16.Cunha L.C.S., Andrade E.S.M.L., Furtado N.A.J.C., Vinholis A.H.C., Martins C.H.G., Filho A.D.S., Cunha W.R. Antibacterial activity of triterpene acids and semi-synthetic derivatives against oral pathogens. Z. Naturforsch., C: J. Biosci. 2007;62:668–672. doi: 10.1515/znc-2007-9-1007. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues J., Michelin D.C., Rinaldo D., Zocolo G.J., Santos L.C., Vilegas W., Salgado H.R.N. Antimicrobial activity of Miconia species (melastomataceae) J. Med. Food. 2008;11:120–126. doi: 10.1089/jmf.2007.557. [DOI] [PubMed] [Google Scholar]
- 18.Queiroz G.M., de Souza M.G.M., de Carvalho T.C., Casemiro L.A., Cunha W.R., Martins C.H.G. Absence of the antibacterial activity of the crude extracts and compounds isolated from M. rubiginosa against extended-spectrum β-lactamase producing enterobacteria. J. Pharm. Negat. Results. 2011;2:1–7. [Google Scholar]
- 19.Tracanna M.I., Amani S.M., Romano E., Raschi A.B., Molina L.R.H., Piro O.E., Castellano E.E., Benavente A.M. Crystal structure, spectroscopic properties and antimicrobial activity of 4H (1-Benzopyran-4-one, 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-6, 8-dimethyl from Miconia ioneura Griseb. Mol. Med. Chem. 2010;21:94–104. [Google Scholar]
- 20.Viegas F.P.D., de Castro A.T., Castro A.P., Siqueira I., Rosa W., Espuri P.F., Coelho L.F.L., Marques M.J., Soares M.G. In vitro schistosomicidal activity of the crude extract, fractions and Primin, the major active benzoquinone constituent from the leaves of Miconia willdenowii (Melastomaceae) S. Afr. J. Bot. 2017;111:365–370. [Google Scholar]
- 21.Spessoto M.A., Ferreira D.S., Crotti A.E.M., Silva M.L.A., Cunha W.R. Evaluation of the analgesic activity of extracts of Miconia rubiginosa (Melastomataceae) Phytomedicine. 2003;10:606–609. doi: 10.1078/094471103322331629. [DOI] [PubMed] [Google Scholar]
- 22.Mancini E., DeMartino L., Belisario M.A., De Feo V. Flavonoids of Miconia alypifolia and their antioxidant activity. Pharmacologyonline. 2008;2:452–460. [Google Scholar]
- 23.Mosquera O.M., Correra Y.M., Niño J. Antioxidant activity of plants from Colombian flora. Rev, Bras. Farmacogn. 2009;19:382–387. [Google Scholar]
- 24.Pieroni L.G., de Rezende F.M., Ximenes V.F., Dokkedal A.L. Antioxidant activity and total phenols from the methanolic extract of Miconia albicans (Sw.) Triana leaves. Molecules. 2011;16:9439–9450. doi: 10.3390/molecules16119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasconcelos M.A.L., Ferreira D.S., e Silva M.L.A., Veneziani R.C.S., Cunha W.R. Analgesic effects of crude extracts of Miconia albicans (Melastomataceae) Boll. Chim. Farm. 2003;142:333–335. [PubMed] [Google Scholar]
- 26.Cunha W.R., Martins C., Ferreira D.S., Crotti A.E.M., Lopes N.P., Albuquerque S. In vitro trypanocidal activity of triterpenes from Miconia species. Planta Med. 2003;69:470–472. doi: 10.1055/s-2003-39719. [DOI] [PubMed] [Google Scholar]
- 27.Lima R.B.S., Rocha E.S.L.F., Melo M.R.S., Costa J.S., Picanco N.S., Lima E.S., Vasconcellos M.C., Boleti A.P.A., Santos J.M.P., Amorim R.C.N., Chaves F.C.M., Coutinho J.P., Tadei W.P., Kretti A.U., Pohlit A.M. In vitro and in vivo anti-malarial activity of plants from the Brazilian Amazon. Malar. J. 2015;14:508. doi: 10.1186/s12936-015-0999-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peixoto J.A., e Silva M.L.A., Crotti A.E.M., Veneziani R.C.S., Gimenez V.M.M., Januario A.H., Groppo M., Magalhales L.G., Santos F.F.D., Albuquerque S., Filho A.A.S., Cunha W.R. Antileishmanial activity of the hydroalcoholic extract of Miconia langsdorffii, isolated compounds, and semi-synthetic derivatives. Molecules. 2011;16:1825–1833. doi: 10.3390/molecules16021825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guldbrandsen N., De Mieri M., Gupta M., Seiser T., Wiebe C., Dickhaut J., Reingruber R., Sorgenfrei O., Hamburger M. Screening of Panamanian plant extracts for pesticidal properties and HPLC-based identification of active compounds. Sci. Pharm. 2015;83:353–367. doi: 10.3797/scipharm.1410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunha G.O.S., Matos A.P., Bernardo A.R., Menezes A.C.S., Burger M.C.D.M., Vieira P.C., Forim M.R., Fernandes J.B., Silva M.F.G.F. Chemical constituents and insecticidal activity of the Miconia ferruginata. Quím. Nova. 2017;40:1158–1163. [Google Scholar]
- 31.da Silva V.B., Almeida-Bezerra J.W., Costa A.R., Morais-Braga M.F.B., de Oliveira M.G., Pinheiro A.A.V., Sampaio R.S.L., Castro J.W.G., dos Santos M.A.F., Ulisses V.R.D.A., Pereira M.E.S., de Souza D.L., de Alcântara B.M., Generino M.E.M., Silva J.T.C., Filho A.M., da Silva S.B., Moon M., Kim B., da Costa J.G.M. The genus Miconia Ruiz & Pav. (Melastomataceae): ethnomedicinal uses, pharmacology, and phytochemistry. Molecules. 2022;27:4132. doi: 10.3390/molecules27134132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group 2009, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 33.Gatis-Carrazzoni A.S.S.G., Mota F.V.B., Leite T.C.C., de Oliveira T.B., da Silva S.C., Bastos I.V.A., de Souza Maia M.B., Pereira P.S., Neto P.P.M., de Oliveira Chagas E.C., Silva T.M.S., do Nascimento M.S., da Silva T.G. Anti-inflammatory and antinociceptive activities of the leaf methanol extract of Miconia minutiflora (Bonpl.) DC. and characterization of compounds by UPLC-DAD-QTOF-MS/MS. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018;392:55–68. doi: 10.1007/s00210-018-1561-x. [DOI] [PubMed] [Google Scholar]
- 34.Cunha W.R., Crevelin E.J., Arantes G.M., Crotti A.E.M., e Silva M.L.A., Furtado N.A.J.C., Albuquerque S., Ferreira D.D.S. A study of the trypanocidal activity of triterpene acids isolated from Miconia species. Phytother Res. 2006;20:474–478. doi: 10.1002/ptr.1881. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues J., Rinaldo D., dos Santos L.C., Vilegas W. An unusual C6-C6" linked flavonoid from Miconia cabucu (Melastomataceae) Phytochemistry. 2007;68:1781–1784. doi: 10.1016/j.phytochem.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Quintans-Júnior L.J., Gandhi S.R., Passos F.R.S., Heimfarth L., Pereira E.W.M., Monteiro B.S., Santos K.S.D., Duarte M.C., Abreu L.S., Nascimento Y.M., Tavares J.F., Silva M.S., Menezes I.R.A., Coutinho H.D.M., Lima A.A.N., Zengin G., Quintans J.S.S. Dereplication and quantification of the ethanol extract of Miconia albicans (Melastomaceae) by HPLC-DAD-ESI-/MS/MS, and assessment of its anti-hyperalgesic and anti-inflammatory profiles in mice arthritis-like model: evidence for involvement of TNF-α, IL-1β and IL-6. J. Ethnopharmacol. 2020;258:1–10. doi: 10.1016/j.jep.2020.112938. [DOI] [PubMed] [Google Scholar]
- 37.Tarawneh A.H., León F., Ibrahim M.A., Pettaway S., McCurdy C.R., Cutler S.J. Flavanones from Miconia prasina. Phytochem. Lett. 2014;7:130–132. doi: 10.1016/j.phytol.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z., ElSohly H.N., Li X.C., Khan S.I., Broedel S.E., Raulli R.E., Cihlar R.L., Walker L.A. Flavanone glycosides from Miconia trailii. J. Nat. Prod. 2003;66:39–41. doi: 10.1021/np020429z. [DOI] [PubMed] [Google Scholar]
- 39.Gomes T.P.O., Souza J.I.N., Somerlate L.C., Mendonca V.A., Lima N.M., Carli G.P., Castro S.B.R., Andrade T.J.A.S., Dias J.V.L., Oliveira M.A.L., Alves C.C.S., Carli A.P. Miconia albicans and Curcuma longa herbal medicines positively modulate joint pain, function and inflammation in patients with osteoarthritis: a clinical study. Inflammopharmacology. 2021;29:377–391. doi: 10.1007/s10787-020-00781-9. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira E.C., de Lucena R.F.P., Bussmann R.W., Zambrana N.Y.P., Cruz D.D. Temporal assessment of the medicinal plants trade in public markets of the state of Paraíba, northeastern Brazil. J. Ethnobiol. Ethnomed. 2021;17:1–24. doi: 10.1186/s13002-021-00496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moura M.D.G., Lopes L.C., Biavatti M.W., Busse J.W., Wang L., Kennedy S.A., Bhatnaga N., Bergamaschi C.C. Brazilian oral herbal medication for osteoarthritis: a systematic review protocol. Syst. Rev. 2016;5:1–7. doi: 10.1186/s13643-016-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zank S., Hanazaki N. The coexistence of traditional medicine and biomedicine: a study with local health experts in two Brazilian regions. PLoS One. 2017;12:1–17. doi: 10.1371/journal.pone.0174731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrêa J.G.S., Bianchin M., Lopes A.P., Silva E., Ames F.Q., Pomini A.M., Carpes S.T., Rinaldi J.D.C., Melo R.C., Kioshima E., Bersani-Amado C.A., Pilau E.J., Carvalho J.E.D., Ruiz A.L.T.G., Visentainer J.V., Santin S.M.D.O. Chemical profile, antioxidant and anti-inflammatory properties of Miconia albicans (Sw.) Triana (Melastomataceae) fruits extract. J. Ethnopharmacol. 2021;273:1–14. doi: 10.1016/j.jep.2021.113979. [DOI] [PubMed] [Google Scholar]
- 44.Lima T.C., Matos S.S., Carvalho T.F., Silveira-Filho A.J., Couto L.P.S.M., Quintans-Junior L.J., Quintans J.S.S., Silva A.M.O., Heimfarth L., Passos F.R.S., Gandhi S.R., Lima B., Silva F.A. Evidence for the involvement of IL-1β and TNF-α in anti-inflammatory effect and antioxidative stress profile of the standardized dried extract from Miconia albicans Sw. (Triana) leaves (Melastomataceae) J. Ethnopharmacol. 2020;259:1–14. doi: 10.1016/j.jep.2020.112908. [DOI] [PubMed] [Google Scholar]
- 45.Bars D.L., Gozariu M., Cadden S.W. Animal models of nociception. Pharmacol. Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 46.Kangsamaksin T., Chaithongyot S., Wootthichairangsan C., Hanchaina R., Tangshewinsirikul C., Svasti J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS One. 2017;12:1–16. doi: 10.1371/journal.pone.0189628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandeep S.G. Triterpenoids: structural diversity, biosynthetic pathway, and bioactivity. Stud. Nat. Prod. Chem. 2020;67:411–461. [Google Scholar]
- 48.Diaz A.M., Abad M.J., Fernandez L., Recuero C., Villaescusa L., Silvan A.M., Bermejo P. In vitro anti-inflammatory activity of iridoids and triterpenoids compounds isolated from Phillyrea latifolia. Biol. Pharm. Bull. 2000;23:1307–1313. doi: 10.1248/bpb.23.1307. [DOI] [PubMed] [Google Scholar]
- 49.Geetha T., Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J. Ethnopharmacol. 2001;76:77–80. doi: 10.1016/s0378-8741(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 50.Liu R., Hao D., Xu W., Li J., Li X., Shen D., Sheng K., Zhao L., Xu W., Gao Z., Zhao X., Liu Q., Zhang Y. β-sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 2019;57:161–168. doi: 10.1080/13880209.2019.1577461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J., Cha Y.N., Surh Y.J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Li W., Guo Y., Zhang C., Wu R., Yang A.Y., Gaspar J., Kong A.T. Dietary phytochemicals and cancer chemoprevention: a perspective on oxidative stress, inflammation, and epigenetics. Chem. Res. Toxicol. 2016;29:2071–2095. doi: 10.1021/acs.chemrestox.6b00413. [DOI] [PubMed] [Google Scholar]
- 53.Gandhi G.R., Neta M.T.S.L., Sathiyabama R.G., Quintans J.S.S., Silva A.M.O.E., Araujo A.A.S., Narain N., Junior L.J.Q., Gurgel R.Q. Flavonoids as Th1/Th2 cytokines immunomodulators: a systematic review of studies on animal models. Phytomedicine. 2018;44:74–84. doi: 10.1016/j.phymed.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 54.Carvalho M.T.B., Araújo-Filho H.G., Barreto A.S., Quintans-Junior L.J., Quintans J.S.S., Baretto R.S.S. Wound healing properties of flavonoids: a systematic review highlighting the mechanisms of action. Phytomedicine. 2021;90 doi: 10.1016/j.phymed.2021.153636. [DOI] [PubMed] [Google Scholar]
- 55.Quintans J.S.S., Shanmugam S., Heimfarth L., Araujo A.A.S., Almeida J.R.G.S., Picot L., Quintans-Junior L.J. Monoterpenes modulating cytokines-A review. Food Chem. Toxicol. 2019;123:233–257. doi: 10.1016/j.fct.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 56.Brennan F.M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF-alpha; antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;334:244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 57.Brennan F.M., Maini R.N., Feldmann M. TNF alpha-a pivotal role in rheumatoid arthritis? Br. J. Rheumatol. 1992;31:293–298. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- 58.Ma X., Xu S. TNF inhibitor therapy for rheumatoid arthritis (Review) Biomed. Rep. 2013;1:177–184. doi: 10.3892/br.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zangerle P.F., De Groote D., Lopez M., Meuleman R.J., Vrindts Y., Fauchet F., Dehart I., Jadoul M., Radoux D., Franchimont P. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood: II. Application to Rheumatoid Arthritis and osteoarthritis. Cytokine. 1992;4:568–575. doi: 10.1016/1043-4666(92)90021-i. [DOI] [PubMed] [Google Scholar]
- 60.Tissi L., Puliti M., Barluzzi R., Orefici G., Hunolstein C.V., Bistoni F. Role of tumor necrosis factor-alpha, interleukin-1beta, and interleukin-6 in a mouse model of group B streptococcal arthritis. Infect. Immun. 1999;67:4545–4550. doi: 10.1128/iai.67.9.4545-4550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei S.T., Sun Y.H., Zong S.H., Xiang Y.B. Serum levels of IL-6 and TNF-α may correlate with activity and severity of Rheumatoid Arthritis. Med. Sci. Monit. 2015;21:4030–4038. doi: 10.12659/MSM.895116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cunha G.O.S., da Silva D.M., dos Santos M.L., Filho M.O.M., O Pessoa C., Guimaraes C.J., Silva M.F.S., Menezes A.C.S. Chemical constituents and cytotoxic activity of Miconia burchellii Triana (Melastomataceae) leaves. S. Afr. J. Bot. 2021;137:345–350. [Google Scholar]
- 63.Gontijo D.C., Gontijo P.C., Brandão G.C., Diaz M.A.N., Oliveira A.B.D., Fietto L.G., Leite J.P.V. Antioxidant study indicative of antibacterial and antimutagenic activities of an ellagitannin-rich aqueous extract from the leaves of Miconia latecrenata. J. Ethnopharmacol. 2019;236:114–123. doi: 10.1016/j.jep.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Bussmann R.W., Malca-García G., Glenn A., Sharon D., Chait G., Diaz D., Pourmand K., Jonat B., Somogy S., Guardado G., Aguirre C., Chan R., Meyer K., Kuhlman A., Townesmith A., Effio-Carbajal J., Frias-Fernandez F., Benito M. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010;132:101–108. doi: 10.1016/j.jep.2010.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunha W.R., de Matos G.X., Souza M.G.M., Tozatti M.G., Silva M.L.A., Martins C.H.G., Silva R.D., Filho A.A.D.S. Evaluation of the antibacterial activity of the methylene chloride extract of Miconia ligustroides, isolated triterpene acids, and ursolic acid derivatives. Pharm. Biol. 2010;48:166–169. doi: 10.3109/13880200903062648. [DOI] [PubMed] [Google Scholar]
- 66.Horiuchi K., Shiota S., Hatano T., Yoshida T., Kuroda T., Tsuchiya T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE) Biol. Pharm. Bull. 2007;30:1147–1149. doi: 10.1248/bpb.30.1147. [DOI] [PubMed] [Google Scholar]
- 67.Liu J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 68.Viegas F.P.D., Espuri P.F., Oliver J.C., Silva N.C., Dias A.L.T., Marques M.J., Soares M.G. Leishmanicidal and antimicrobial activity of primin and primin-containing extracts from Miconia willdenowii. Fitoterapia. 2019;138 doi: 10.1016/j.fitote.2019.104297. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues L.A., Almeida A.D.C., Gontijo D.C., Salustiano I.V., Almeida A.A., Brandão G.C., Ribon A.O.B., Leite J.P.V. Antibacterial screening of plants from the Brazilian Atlantic Forest led to the identification of active compounds in Miconia latecrenata (DC.) Naudin. Nat. Prod. Res. 2020;35:5904–5908. doi: 10.1080/14786419.2020.1802271. [DOI] [PubMed] [Google Scholar]
- 70.Andrade S.F., Da Silva Filho A.A., Resende D.O., e Silva M.L.A., Cunha W.R., Nanayakkara N.P.D., Bastos J.K. Antileishmanial, antimalarial and antimicrobial activities of the extract and isolated compounds from Austroplenckia populnea (Celastraceae) Z. Naturforsch., C: J. Biosci. 2008;63:7–8. doi: 10.1515/znc-2008-7-805. [DOI] [PubMed] [Google Scholar]
- 71.Halliwell B. Flavonoids: a re-run of the carotenoids story? Novartis Found. Symp. 2007;282:93–101. doi: 10.1002/9780470319444.ch7. [DOI] [PubMed] [Google Scholar]
- 72.Liu R.H. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J. Nutr. 2004;134:3479–3485. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 73.Feinstein E., Canaani E., Weiner L.M. Dependence of nucleic acid degradation on in situ free-radical production by Adriamycin. Biochemistry. 1993;32:13156–13161. doi: 10.1021/bi00211a026. [DOI] [PubMed] [Google Scholar]
- 74.Quiles J.L., Huertas J.R., Battino M., Mataix J., Ramirez-Torotosa M.C. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;180:79–95. doi: 10.1016/s0300-483x(02)00383-9. [DOI] [PubMed] [Google Scholar]
- 75.Galati G., O'Brien P.J. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 76.Shanmugasundaram D., Roza J.M. Assessment of anti-inflammatory and antioxidant activity of quercetin-rutin blend (SophorOx™) - an in vitro cell-based assay. J. Compl. Integr. Med. 2022;18:2022. doi: 10.1515/jcim-2021-0568. [DOI] [PubMed] [Google Scholar]
- 77.Silva A.G., Silva V.A.O., Oliveira R.J.S., de Rezende A.R., Chagas R.C.R., Pimenta L.P.S., Romão W., Santos H.B., Thomé R.G., Reis R.M., Ribeiro R.I.M.A. Matteucinol, isolated from Miconia chamissois, induces apoptosis in human glioblastoma lines via the intrinsic pathway and inhibits angiogenesis and tumor growth in vivo. Invest. New Drugs. 2020;38:1044–1055. doi: 10.1007/s10637-019-00878-1. [DOI] [PubMed] [Google Scholar]
- 78.Barroso P.R., Gregório L.E., Kato K.C., Campos F.F., Oliveira F.D., Leite E.A., De Melo G.E.B.A., Martins H.R. Biological activity and chemical composition of the ethanolic extracts of Miconia ferruginata DC. Braz. J. Dev. 2021;7:37798–37819. [Google Scholar]
- 79.Rosa M.N., e Silva L.R.V., Longato G.B., Evangelista A.F., Gomes I.N.F., Alves A.L.V., de Oliveira B.G., Pinto F.E., Romão W., de Rezende A.R., Araújo A.A.C., Oliveira L.S.F.M., Souza A.A.M., Oliveira S.C., Ribeiro R.I.M.A., Silva V.A.O., Reis R.M. Bioprospecting of natural compounds from Brazilian cerrado biome plants in human cervical cancer cell lines. Int. J. Mol. Sci. 2021;22:3383. doi: 10.3390/ijms22073383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldenberg R., Penneys D.S., Almeda F., Judd W.S., Michelangeli A. Phylogeny of Miconia (Melastomataceae): patterns of stamen diversification in a megadiverse neotropical genus. Int. J. Plant Sci. 2008;169:963–979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable to this article.
Data will be made available on request.