Abstract
Objective
The prognostic factors for azacitidine in untreated acute myeloid leukemia (AML) patients ineligible for intensive therapy remain unknown. To identify prognostic factors for azacitidine monotherapy and assist clinicians in deciding whether to use azacitidine monotherapy or other therapies.
Methods
We retrospectively analyzed 27 patients with AML who were newly treated with azacitidine between 2013 and 2021 at our hospital. We evaluated potential predictors based on the overall survival (OS).
Results
A univariate analysis found that an Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2 and platelet count (Plt) <27,000/μL had a significant negative influence on the OS. A multivariate analysis confirmed that both factors had significant independent adverse effects on the OS. An ECOG PS ≥2 and Plt <27,000/μL were thus assigned 1 point each, and a clinical scoring system was created. Log-rank testing showed that the 0-point group (n=12) had a median OS of 680 days [95% confidence interval (CI) 220-898 days] and a 1-year OS rate of 80.8% (95% CI 42.3-94.9%), the 1-point group (n=11) had a median OS of 90 days (95% CI 62-345 days) and a 1-year OS rate of 18.2% (95% CI 2.9-44.2%), and the 2-point group (n=4) had a median OS of 82 days [95% CI 19-not applicable (NA) days] and a 1-year OS rate of 0% (95% CI NA-NA). The p value of 0.00008 indicated that this scoring was useful.
Conclusion
The ECOG PS and Plt can be used to predict the OS with azacitidine monotherapy in untreated AML patients ineligible for intensive therapy.
Keywords: acute myeloid leukemia, azacitidine, prognostic factors, overall survival
Introduction
Genetic mutations characteristic of acute myeloid leukemia (AML) are functionally classified into eight categories, one of which is DNA methylation (1). Mutations in the DNMT3A, IDH1, IDH2, and TET2 genes result in DNA methylation of CpG islands in the DNA promoter region, preventing transcription factors from binding and thereby decreasing the gene expression. Azacitidine induces gene expression by demethylating DNA, which prolongs the overall survival (OS) in AML patients (2). In clinical practice, some patients can survive for a long time with azacitidine, while others die early after azacitidine administration. However, the prognostic factors for azacitidine in AML remain unclear.
To decide whether to use azacitidine monotherapy or other therapies (e.g. venetoclax-based therapy or low-dose cytarabine therapy), prognostic factors for azacitidine monotherapy need to be identified. We therefore conducted a retrospective analysis of azacitidine monotherapy in untreated AML patients ineligible for intensive therapy at our hospital to identify factors that predict the OS with this therapy.
Materials and Methods
Using our medical records, we retrospectively analyzed AML patients ineligible for intensive therapy who were newly treated with azacitidine between 2013 and 2021 at our hospital. Patients with more than 20% blasts in the peripheral blood or bone marrow were diagnosed with AML based on the World Health Organization (WHO) classification. The observation period was from the date of treatment initiation to September 9, 2021. Patients with AML progressing from myeloproliferative neoplasms (MPNs), patients previously treated for myelodysplastic syndromes (MDS) or AML with anticancer agents, and patients who had received addition of venetoclax following its approval in Japan were excluded from the study (Fig. 1). AML was diagnosed by and bone marrow smear reports were prepared by hematologists certified by the Japanese Society of Hematology. Written informed consent for the administration of azacitidine was obtained for all patients prior to the start of treatment.
Figure 1.
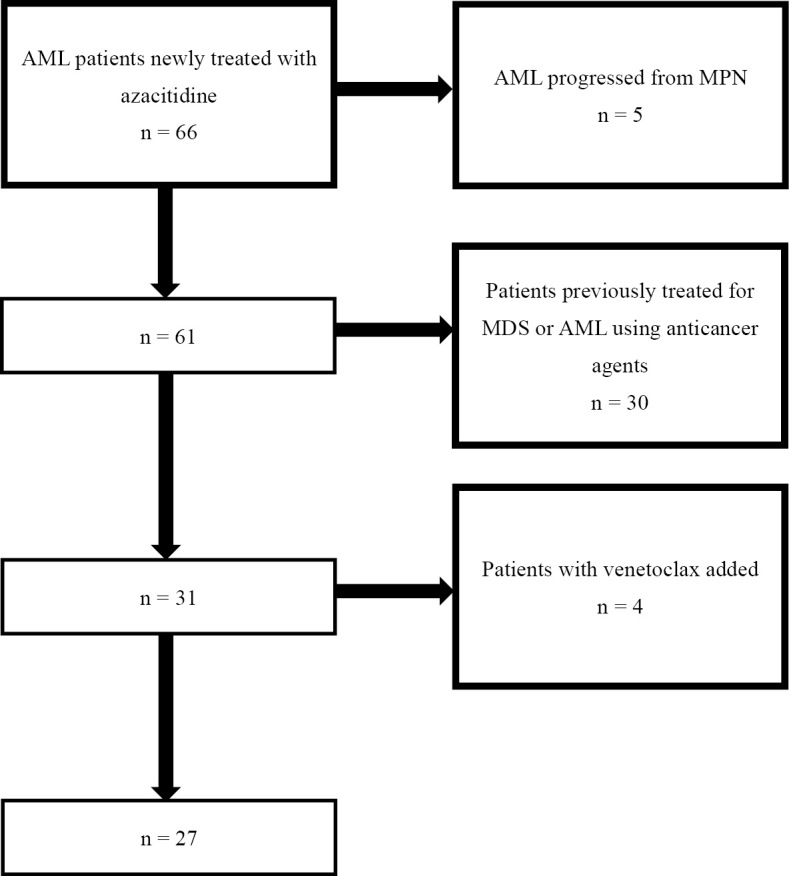
Flowchart for selection of patients. AML: acute myeloid leukemia, MPN: myeloproliferative disorders, MDS: myelodysplastic syndromes. Patients with AML progressing from MPN, patients previously treated for MDS or AML with anticancer agents, patients who had received addition of venetoclax following its approval in Japan were excluded from the study.
Azacitidine was planned to be administered at 75 mg/m2/day over 7 consecutive days every 28 days. Dose reductions were allowed at the discretion of each attending physician, taking into account the Eastern Cooperative Oncology Group (ECOG) performance status (PS), organ damage, comorbidities, and infections. Both subcutaneous and intravenous azacitidine administration methods were acceptable. Hematologic improvement (HI) was defined by the International Working Group response criteria (3). Only patients with Hb <11 g/dL or requiring red blood cell (RBC) transfusion, an absolute neutrophil count <1,000/μL, a and platelet count (Plt) <100,000/μL prior to initiation of azacitidine were considered eligible for an assessment of HI of the erythroid (HI-E), neutrophil (HI-N), and platelet (HI-P) lineages, respectively. The number of patients treated with best supportive care (BSC) or low-dose chemotherapy, i.e. low-dose cytarabine with or without aclarubicin, and their OS were also determined.
This study was approved by the ethics review committee of our hospital. Since this was a retrospective study, informed consent for the publication of this paper did not need to be obtained from individual patients.
Statistical analyses
We tabulated the following factors as potential predictors of treatment responsiveness. For blood tests, we examined the white blood cell count (WBC), neutrophil count (Neutro), hemoglobin (Hb), percentage of reticulocytes (Ret), red cell distribution width (RDW), Plt, lactate dehydrogenase (LDH), ferritin, C-reactive protein (CRP), and percentage of blasts in peripheral blood cells (PB blast). In the bone marrow examination, we investigated the cellularity, nucleated cell count (NCC), ratio of maturing myeloid cells to erythroid cells (M:E ratio), megakaryocyte count (MegK), percentage of blasts (BM blast), percentage of monocytes (BM mono), chromosome type, presence of an FLT3-ITD mutation, Wilms tumor 1 (WT1) mRNA quantification, and presence of an NPM1 mutation. Other items evaluated included the age, sex, ECOG PS, body mass index (BMI), azacitidine dose, WHO classification, French-American-British (FAB) classification, and National Comprehensive Cancer Network (NCCN) prognostic classification. If RBC transfusion had been received in the four weeks prior to the start of treatment, the laboratory values on the day of transfusion were adopted for Hb, RDW, and Ret. For the Plt, laboratory values on the day of transfusion were also used. In the case of Hb and Plt, if the values on the treatment day were lower than those on the transfusion day, the values on the treatment day were used.
For continuous variables, a receiver operating characteristic (ROC) curve analysis was performed to determine the cut-off value, with the dependent variable being the survival at one year. The cut-off was determined based on the maximum value of the Youden index for the ROC curve. The Kaplan-Meier method was used for the survival analysis, and log-rank testing was used for univariate analyses between groups. A multivariate Cox proportional hazards model was used to identify independent risk factors for the OS. Cases with missing data were excluded from the analysis of relevant variables. Values of p<0.05 were considered statistically significant.
Prognostic tools for intensive chemotherapy in elderly AML patients, such as the Wheatley index (4), have been used in clinical practice. In this study, we investigated whether or not the Wheatley index could be used to predict the prognosis of azacitidine therapy in elderly AML patients who were not eligible for intensive chemotherapy. In more detail, risk classification was performed according to the Wheatley index, and log-rank testing was implemented for each risk classification.
All statistical analyses were performed with EZR (5) (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Sixty-six patients were enrolled in the study from May 2013 to September 2020. Of these, 39 patients were excluded, and the remaining 27 were included (Fig. 1). The median age was 75 (range, 66-91) years old, 13 (48%) were men, and 20 (74%) had an ECOG PS of 0 or 1. There was 1 patient (4%) with disseminated intravascular coagulation syndrome (6) at the start of treatment. Twenty patients (74%) had a WHO diagnosis of AML with myelodysplasia-related changes, and 10 (39%) had an FAB diagnosis of MDS-refractory anemia with excess blasts in transformation. Eleven patients (39%) had a complex karyotype, and 12 patients (44%) were classified as having a poor prognosis according to the NCCN risk group. Only 5 patients were analyzed for the FLT3-ITD mutation, of whom 1 (4%) was mutation-positive (Table 1). For the total cohort, the median observation period was 164 [interquartile range (IQR) 81.5-419.5] days, and the median survival was 220 [95% confidence interval (CI) 89-621] days. The median number of azacitidine courses was 2 (IQR 1-13.5), and the median dose of azacytidine was 89.4% (IQR 78.7-94.1%). Twelve patients completed up to two courses of the treatment, and the reasons included patient preference due to adverse events in six cases, death in three cases (one with cerebral hemorrhaging and two with infections), infection in two cases, and exacerbation of chronic heart failure in one case. The dose of azacitidine was reduced below 80% in 6 patients, including 4 with a poor general condition (ECOG PS ≥2), 1 with infection, and 1 with severe pancytopenia. There were 23 deaths, and the causes of death were cerebral hemorrhaging in 2 cases, upper gastrointestinal hemorrhaging in 1 case, progression of AML in 7 cases, pneumonia in 4 cases, sepsis in 3 cases, liver failure in 1 case, arrhythmia in 1 case, and death from unknown cause in 4 cases. There were 26, 18, and 22 subjects who were eligible for HI-E, HI-N, and HI-P assessments, respectively. HI-E, HI-N, and HI-P were observed in 23.1% (n=6), 11.1% (n=2), and 27.3% (n=6) of subjects, respectively. There were 11 cases in which peripheral blood WT1 mRNA was measured prior to the treatment and then re-measured at least once after the start of treatment. Of those patients, 7 (64%) had decreased levels of peripheral blood WT1 mRNA. During the same period, there were 19 patients treated with BSC due to infection or patient preference and 7 patients treated with low-dose chemotherapy. The median survival durations were 52 (95% CI 24-238) days and 32 (95% CI 5-301) days, which was significantly shorter than in the azacitidine group (p value=0.009).
Table 1.
Baseline Patient Characteristics (n=27).
| Number or median | Percentage or range | |
|---|---|---|
| Age (years) | 75 | 66-91 |
| Sex | ||
| Male | 13 | 48 |
| Female | 14 | 52 |
| ECOG PS | ||
| 0 or 1 | 20 | 74 |
| ≥2 | 7 | 26 |
| WHO diagnosis | ||
| AML-MRC | 20 | 74 |
| t-AML | 3 | 11 |
| AML-NOS | 2 | 8 |
| AML with t(8;21)(q22;q22) | 1 | 4 |
| AML with inv(16)(p13.1q22) | 1 | 4 |
| FAB diagnosis | ||
| AML | 17 | 63 |
| MDS RAEB-t | 10 | 37 |
| Karyotype | ||
| Complex | 11 | 41 |
| Other | 15 | 56 |
| Unknown | 1 | 4 |
| FLT3-ITD mutation | ||
| Yes | 1 | 4 |
| No | 4 | 15 |
| Unknown | 22 | 81 |
| NCCN risk group | ||
| Favorable | 3 | 11 |
| Intermediate | 11 | 41 |
| Poor | 12 | 44 |
| Unknown | 1 | 4 |
ECOG PS: Eastern Cooperative Oncology Group performance status, WHO: World Health Organization, AML: acute myeloid leukemia, AML-MRC: acute myeloid leukemia with myelodysplasia-related changes, t-AML: therapy-related acute myeloid leukemia, AML-NOS: acute myeloid leukemia not otherwise specified, FAB: French-American-British, MDS RAEB-t: myelodysplastic syndromes refractory anemia with excess blasts in transformation, NCCN: National Comprehensive Cancer Network
A univariate analysis was performed using the age, sex, ECOG PS, diagnosis, prognostic classification, and laboratory values. The age, sex, karyotype, FLT3-ITD mutation, NCCN risk group, WT1 mRNA, BMI, WBC, PB blast, Hb, Ret, LDH, ferritin, CRP, cellularity, NCC, M:E ratio, BM blast, and BM mono did not significantly affect the OS. However, an ECOG PS ≥2, FAB diagnosis of AML, azacitidine dose <89.46%, RDW ≥14.5%, Plt <27,000/μL, and MegK <19/μL all showed significant negative impacts on the OS (p<0.05 each) (Table 2).
Table 2.
Univariable Analysis for Overall Survival.
| Category (units) | Median (IQR) | Criterion | N | Median survival time (95% CI) | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 75 (75-85) | ||||||||||
| <81 | 12 | 224 (79-621) | 0.8 | ||||||||
| ≥81 | 15 | 220 (62-680) | |||||||||
| Sex | Male | 13 | 164 (73-345) | 0.1 | |||||||
| Female | 14 | 411 (75-680) | |||||||||
| ECOG PS | 0-1 | 20 | 429 (164-680) | 0.00001 | |||||||
| ≥2 | 7 | 79 (19-89) | |||||||||
| FAB diagnosis | AML | 17 | 90 (73-429) | 0.043 | |||||||
| MDS RAEB-t | 10 | 393 (130-898) | |||||||||
| Karyotype | Complex | 11 | 90 (75-224) | 0.212 | |||||||
| Other | 15 | 621 (62-680) | |||||||||
| FLT3-ITD mutation | Yes | 1 | 62 (NA-NA) | 0.515 | |||||||
| No | 4 | 84 (19-NA) | |||||||||
| NCCN risk group | Favorable | 3 | NA | 0.105 | |||||||
| Intermediate | 10 | 621 (14-699) | |||||||||
| poor | 13 | 90 (73-224) | |||||||||
| WT1 mRNA (copy/μgRNA) | 58,000 (27,250-122,500) | <47,000 | 3 | 130 (90-NA) | 0.456 | ||||||
| ≥47,000 | 5 | 75 (62-NA) | |||||||||
| BMI (kg/m2) | 22.37 (19.14-23.78) | <21.53 | 11 | 90 (62-345) | 0.408 | ||||||
| ≥21.53 | 16 | 429 (90-680) | |||||||||
| Azacitidine dose (%) | 89.41 (78.67-94.06) | <89.46 | 14 | 90 (73-345) | 0.013 | ||||||
| ≥89.46 | 13 | 429 (90-699) | |||||||||
| WBC (/μL) | 3,400 (1,850-7,600) | <5,600 | 17 | 224 (84-638) | 0.202 | ||||||
| ≥5,600 | 10 | 130 (14-NA) | |||||||||
| Neutro (/μL) | 930 (585.5-1,938) | <1,727 | 18 | 393 (84-638) | 0.597 | ||||||
| ≥1,727 | 9 | 130 (14-345) | |||||||||
| PB blast (%) | 10 (3.25-38.75) | <3 | 6 | 422.5 (19-NA) | 0.639 | ||||||
| ≥3 | 21 | 220 (84-429) | |||||||||
| Hb (g/dL) | 7.9 (6.35-9.2) | <7.8 | 12 | 147 (73-224) | 0.144 | ||||||
| ≥7.8 | 15 | 429 (75-699) | |||||||||
| RDW (%) | 15.95 (14.55-17.575) | <14.5 | 6 | 668.5 (89-NA) | 0.031 | ||||||
| ≥14.5 | 20 | 220 (75-393) | |||||||||
| Ret (%) | 2.4 (0.9-4.65) | <2 | 10 | 90 (14-NA) | 0.326 | ||||||
| ≥2 | 17 | 393 (89-638) | |||||||||
| Plt (/μL) | 27,000 (18,000-65,500) | <27,000 | 12 | 90 (19-345) | 0.006 | ||||||
| ≥27,000 | 15 | 621 (84-699) | |||||||||
| LDH (U/L) | 279 (225-458) | <279 | 13 | 429 (75-699) | 0.099 | ||||||
| ≥279 | 14 | 164 (79-393) | |||||||||
| Ferritin (ng/mL) | 549 (302.45-815) | <560 | 10 | 284.5 (62-638) | 0.599 | ||||||
| ≥560 | 10 | 130 (19-NA) | |||||||||
| CRP (mg/dL) | 0.52 (0.19-1.95) | <0.35 | 10 | 491.5 (14-898) | 0.093 | ||||||
| ≥0.35 | 17 | 175 (79-429) | |||||||||
| Bone marrow cellularity | Hypercellular | 5 | 429 (626-NA) | 0.58 | |||||||
| Normocellular | 10 | 130 (14-638) | |||||||||
| Hypocellular | 8 | 422.5 (73-898) | |||||||||
| NCC (104/μL) | 5.6 (3.1-14.5) | <16.8 | 18 | 393 (89-638) | 0.301 | ||||||
| ≥16.8 | 5 | 84 (14-NA) | |||||||||
| M:E ratio | 1.32 (0.7-3.13) | <0.88 | 8 | 411 (62-NA) | 0.717 | ||||||
| ≥0.88 | 16 | 224 (89-680) | |||||||||
| MegK (/μL) | 13 (0-50) | <19 | 12 | 638 (89-898) | 0.019 | ||||||
| ≥19 | 11 | 90 (62-429) | |||||||||
| BM blast (%) | 39.4 (25.6-50.6) | <40.4 | 13 | 393 (220-680) | 0.07 | ||||||
| ≥40.4 | 11 | 84 (62-638) | |||||||||
| BM mono (%) | 2.9 (0.6-4.55) | <1.6 | 8 | 621 (14-699) | 0.553 | ||||||
| ≥1.6 | 16 | 224 (79-429) |
IQR: interquartile range, N: number, CI: confidence interval, ECOG PS: Eastern Cooperative Oncology Group performance status, FAB: French-American-British, AML: acute myeloid leukemia, MDS RAEB-t: myelodysplastic syndromes refractory anemia with excess blasts in transformation, NA: not applicable, NCCN: National Comprehensive Cancer Network, WT1: Wilms tumor 1, BMI: body mass index, WBC: white blood cell count, PB blast: percentage of blasts in white blood cells, Hb: hemoglobin, RDW: red cell distribution width, Ret: percentage of reticulocytes, Plt: platelet count, LDH: lactate dehydrogenase, CRP: C-reactive protein, NCC: nucleated cell count in bone marrow, M:E ratio: ratio of maturing myeloid cells to erythroid cells in bone marrow, MegK: megakaryocyte count in bone marrow, BM blast: percentage of blasts in bone marrow, BM mono: percentage of monocytes in bone marrow
For continuous variables, the criterion was determined based on the maximum value of the Youden index for the receiver operating characteristic curve with the dependent variable being survival at 1 year. Univariate analyses were conducted with log-rank testing.
A multivariate analysis was performed using the Cox proportional hazards model. The number of events (deaths) was small (n=23), so only 2 independent variables could be included to avoid overfitting. The attending physician adjusted the azacitidine dose based on the ECOG PS of the patient, and the ECOG PS and azacitidine dose were considered to be strongly correlated. We therefore adopted ECOG PS ≥2, which had a lower p value in the univariate analysis; Plt and MegK also showed a strong correlation, so we adopted Plt <27,000/μL, which displayed a lower p value in the univariate analysis. The FAB diagnosis of AML and RDW ≥14.5 showed larger p values than ECOG PS ≥2 or Plt <27,000/μL. Therefore, a multivariate analysis was performed with ECOG PS ≥2 and Plt <27,000/μL as independent variables, and it was confirmed that each exerted a significant independent adverse effect on the OS (Table 3) [Plt <27,000/μL: hazard ratio (HR) 2.758, 95% CI 1.009-7.539, p=0.048; ECOG PS ≥2: HR 9.609, 95% CI 2.324-39.73, p=0.002].
Table 3.
Multivariable Analysis for OS.
| Categories | Hazard ratio | 95%CI | p value | Prognostic score | ||||
|---|---|---|---|---|---|---|---|---|
| Plt <27,000/μL | 2.758 | 1.009-7.539 | 0.048 | 1 | ||||
| ECOG PS ≥2 | 9.609 | 2.324-39.73 | 0.002 | 1 |
CI: confidence interval, ECOG PS: Eastern Cooperative Oncology Group performance status
The multivariable analysis was conducted with multivariate Cox proportional hazards model.
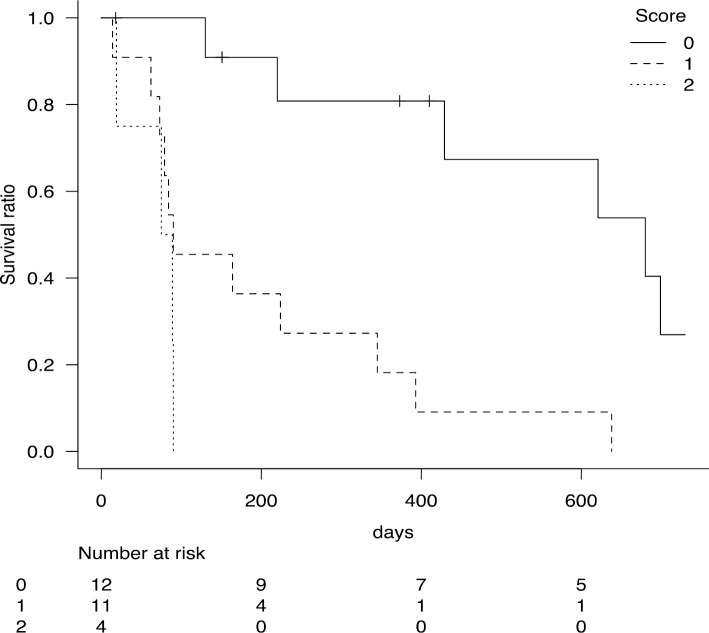
ECOG PS ≥2 and Plt <27,000/μL were assigned a value of 1 point each, and a clinical scoring system was created. Log-rank testing was performed for three point groups of 0, 1, and 2. The results showed that the 0-point group (n=12) had a median OS of 680 (95% CI 220-898) days and a 1-year OS rate of 80.8% (95% CI 42.3-94.9%), the 1-point group (n=11) had a median OS of 90 (95% CI 62-345) days and a 1-year OS rate of 18.2% (95% CI 2.9-44.2%), and the 2-point group (n=4) had a median OS of 82 [95% CI 19-not applicable (NA)] days and a 1-year OS rate of 0% (95% CI NA-NA). The p value of 0.00008 indicated that this scoring was useful (Fig. 2, Table 4). A prognostic analysis of the number of courses of azacitidine in the 0-point group showed a trend toward a longer OS for those with more than 14 courses of azacitidine than in those with fewer than 14 courses, but the difference was not significant.
Figure 2.
Overall survival classified according to the scoring system. ECOG PS ≥2 and Plt <27,000 /μL were given one point each, and a clinical scoring system was created. The Kaplan-Meier method was used for survival analysis on the three groups based on the scores.
Table 4.
Overall Survival Classified According to the Scoring System.
| Score | N | Median OS (95%CI) | 1-year OS rate (95%CI) | p value | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 680 days (220-898 days) | 80.8% (42.3-94.9%) | 0.00008 | ||||
| 1 | 11 | 90 days (62-345 days) | 18.2% (2.9-44.2%) | |||||
| 2 | 4 | 82 days (19 days-NA) | 0% (NA-NA) |
N: number, OS: overall survival, CI: confidence interval, NA: not applicable
ECOG PS ≥2 and Plt <27,000/μL were given one point each, and a clinical scoring system was created. Log-rank testing was performed on the three groups based on the scores.
According to the Wheatley index, there were 0 patients in the good-risk group, 7 in the intermediate-risk group, and 20 in the poor-risk group. With log-rank testing, the intermediate-risk group had a median OS of 638 (95% CI 130-NA) days, and the poor-risk group had a median OS of 90 (95% CI 75-393) days. However, the p value of 0.146 indicated that this scoring approach was not useful in this circumstance.
Discussion
The scoring system in this study allows for the prognostic classification of untreated AML patients who are ineligible for intensive therapy with azacitidine monotherapy, a feat that had been unable to be achieved with the Wheatley index. The prognostic factors were Plt and ECOG PS. A high ECOG PS can easily be imagined to be associated with a poor prognosis, so we examined why the Plt might be associated with the prognosis. Among the 19 cases in which the cause of death was able to be determined, bleeding contributed to death in 3 cases. Many of the 16 patients who died of causes other than hemorrhaging and the 4 patients whose cause of death could not be determined had complications of disseminated intravascular coagulation syndrome or thrombocytopenia immediately prior to death; however, a detailed examination was not requested, so it is possible that hemorrhaging was indeed the true cause of death but had not been diagnosed. In other words, the actual number of hemorrhagic complications may have been much higher than initially determined. Previous reports have suggested that MDS with a low Plt is associated with increased mortality due to hemorrhagic complications, and our findings were consistent with this (7,8).
Patients exhibiting evidence of other clinically significant uncontrolled systemic infections requiring therapy (viral, bacterial or fungal) were not included in the azacitidine and venetoclax therapy clinical trial (9). In the trial, grade ≥3 neutropenia and infection occurred in more than 50% of patients (9), suggesting that azacitidine and venetoclax therapy is associated with a high risk of exacerbating infection in patients with coexisting infections. In patients with infection and a Plt ≥27,000/μL or ECOG PS <2, azacitidine monotherapy may be the better choice, as it is safer and results in a longer OS. However, patients in the 1- and 2-point groups who show a poor prognosis according to this scoring system may need to be treated with venetoclax-based therapy or other methods instead of azacitidine monotherapy. When venetoclax is selected for patients in the 1- and 2-point groups, it should be noted that venetoclax-based therapy causes thrombocytopenia and requires frequent blood tests as well as the preparation of platelet products to avoid severe hemorrhagic complication.
Surprisingly, age had no marked effect on the prognosis. This was because the two youngest patients died early after starting azacitidine, as they had received chemotherapy and radiation for other malignancies immediately before being diagnosed with AML. Although no significant difference was found, the poor NCCN prognosis group and patients with Hb <7.8 g/dL tended to show a worse prognosis than others. Poor-risk cytogenetics and RBC transfusion dependency have been reported as predictors of response to azacitidine for high-risk MDS (10), and our findings were consistent with these reports. LDH ≥279 U/L and CRP ≥0.35 mg/dL also tended to be associated with a poor prognosis, with a high LDH level indicating AML disease activity and a high CRP level indicating concomitant infection. These factors may have influenced the prognosis.
Of note, high RDW had a significantly adverse prognostic effect in the univariate analysis. RDW is reportedly associated with the prognosis in coronary artery disease and hematological diseases other than AML (11,12). Recently, it was reported that RDW is correlated with increased values of inflammatory markers, such as the erythrocyte sedimentation rate, interleukin-6, and CRP, as well as malnutrition (13,14). The present results suggest that RDW reflects a worsening of the general condition and may also affect the prognosis of AML.
Several limitations to this study should be acknowledged. First, subject enrolment was not prospective and may have been influenced by selection bias. In our hospital, there were no clear criteria for whether to use azacitidine or low-dose chemotherapy, and the decision was left to each attending physician. Second, the number of patients was small, which limited the number of independent variables that could be included in the multivariate analysis. More patients need to be studied to verify the utility of RDW. Third, we did not validate the scoring of this study in an independent patient group. Fourth, in Japan, few genetic tests are covered by insurance, so we were unable to search for all of the genetic abnormalities listed in the NCCN prognostic classification. The NCCN prognostic classification may thus have been inaccurate.
Conclusion
Plt and ECOG PS can be used to predict the OS with azacitidine monotherapy in untreated AML patients ineligible for intensive therapy. Patients with Plt <27,000/μL or ECOG PS ≥2 may need to be treated with something other than azacitidine monotherapy. In the future, more patients need to be studied to validate the usefulness of RDW and other categories.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We greatly appreciate the assistance of the clinical laboratory department and pharmacy department in this research.
References
- 1. Ley TJ, Miller C, Ding L, et al. ; Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368: 2059-2074, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126: 291-299, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 96: 3671-3674, 2000. [PubMed] [Google Scholar]
- 4. Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol 145: 598-605, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asakura H, Takahashi H, Uchiyama T, et al. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J 14: 42, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kantarjian H, Giles F, List A, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer 109: 1705-1714, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Li W, Morrone K, Kambhampati S, Will B, Steidl U, Verma A. Thrombocytopenia in MDS: epidemiology, mechanisms, clinical consequences and novel therapeutic strategies. Leukemia 30: 536-544, 2016. [DOI] [PubMed] [Google Scholar]
- 9. DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383: 617-629, 2020. [DOI] [PubMed] [Google Scholar]
- 10. Itzykson R, Thépot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 117: 403-411, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Bujak K, Wasilewski J, Osadnik T, et al. The prognostic role of red blood cell distribution width in coronary artery disease: a review of the pathophysiology. Dis Markers 2015: 824624, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int 18: 61, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 133: 628-632, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Periša V, Zibar L, Sinčić-Petričević J, Knezović A, Periša I, Barbić J. Red blood cell distribution width as a simple negative prognostic factor in patients with diffuse large B-cell lymphoma: a retrospective study. Croat Med J 56: 334-343, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]