Abstract
Chronic nausea and vomiting syndrome (CNVS), one of a functional gastroduodenal disorder, was identified in an 8-year-old girl and a 13-year-old boy who had complained of nausea for more than 4 months following coronavirus disease 2019 (COVID-19) due to normality of their head computed tomography and upper gastrointestinal tract images. The patients' symptoms responded quickly to acotiamide, a medication that is effective for treating functional dyspepsia (FD). Despite being a distinct illness from FD, CNVS is also a functional gastrointestinal disorder, and acotiamide may be just as effective for CNVS following COVID-19 as for FD.
Keywords: acotiamide, chronic nausea and vomiting syndrome, coronavirus disease 2019
Introduction
Chronic nausea and vomiting syndrome (CNVS) is a functional gastroduodenal disorder under the Rome IV criteria defined in cases with the following: 1) bothersome nausea occurring at least 1 day per week and/or ≥1 vomiting episodes per week; 2) self-induced vomiting, eating disorders, regurgitation, or rumination excluded; 3) no evidence of organic, systemic, or metabolic diseases likely to explain the symptoms on routine investigations; and 4) symptoms present for the past 3 months with an onset of at least 6 months prior (1).
Acotiamide is an acetylcholinesterase inhibitor reported to be effective in attenuating all symptoms of functional dyspepsia (FD) (2).
We herein report the first two cases in which acotiamide used according to the FD treatment protocol proved effective for CNVS that seem to be related to coronavirus disease 2019 (COVID-19).
Case Reports
Case 1
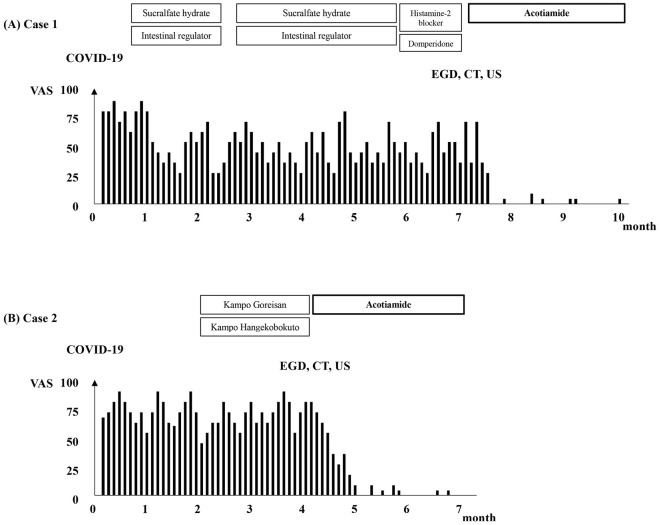
An eight-year-old girl with a complaint of pre-meal nausea lasting for more than six months was referred to our facility from a pediatric clinic. She had been in good health before contracting COVID-19, caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), 6 months ago. She had never received vaccination against SARS-CoV-2. Fig. 1A shows her clinical course.
Figure 1.
Clinical course of the two patients: (A) Case 1 and (B) Case 2. COVID-19: coronavirus disease 2019, EGD: esophagogastroduodenoscopy, CT: computed tomography, US: ultrasonography, VAS: visual analogue scale
Her symptoms began with a fever and cough, and after the fever disappeared after five days, she frequently experienced pre-meal nausea throughout the day. Her degree of nausea, assessed by a visual analog scale (VAS) on a 0-100 mm scale, ranged from 75 to 90. No gastrointestinal symptoms other than nausea, such as vomiting, abdominal pain, diarrhea, or abdominal discomfort, were observed.
At the referral pediatric clinic, she had been prescribed sucralfate hydrate and an intestinal regulator; however, her symptoms only improved slightly, eventually persisting thereafter. She was able to attend school but was unable to take classes in the classroom due to pre-meal nausea; however, her appetite was conserved, and she had no weight loss. Her height, body weight, and body mass index were 126.1 cm, 23.6 kg, and 14.8, respectively.
On her first visit to our hospital, a physical examination revealed no obvious abnormalities, and her vital signs were within the normal range. There were no obvious abnormalities in the results of general blood tests (Table 1), urine tests, stool culture tests, or abdominal radiographs. At this point, the causal relationship between pre-meal nausea and COVID-19 was unknown, and a histamine-2 (H2) blocker and domperidone (treatment for FD) were prescribed for her symptoms. However, despite a month of taking these medicines, her symptoms did not improve, and esophagogastroduodenoscopy (EGD), abdominal contrast-enhanced computed tomography (CT), abdominal ultrasonography (US), upper gastrointestinal fluoroscopy, and head CT were finally performed.
Table 1.
Blood Test Results at the First Visit to Authors’ Hospital.
| Case 1 | Case 2 | Normal range | |||||
|---|---|---|---|---|---|---|---|
| WBC | 5,300 | 4,300 | /μL | 3,300-8,600 | |||
| Hb | 13.9 | 14.0 | g/dL | 11.6-14.8 | |||
| Plt | 246×103 | 273×103 | /μL | 158-348 | |||
| TP | 6.8 | 8.1 | g/dL | 6.6-8.1 | |||
| Alb | 4.2 | 5.0 | g/dL | 4.1-5.1 | |||
| T-Bil | 0.5 | 0.9 | mg/dL | 0.4-1.5 | |||
| D-Bil | 0.0 | 0.1 | mg/dL | 0.0-0.3 | |||
| AST | 27 | 20 | IU/L | 13-30 | |||
| ALT | 12 | 8 | IU/L | 7-23 | |||
| LDH | 231 | 187 | IU/L | 124-222 | |||
| ALP | 372 | 276 | IU/L | 38-113 | |||
| GGT | 7 | 15 | IU/L | 9-32 | |||
| BUN | 10.6 | 13.3 | mg/dL | 8.0-20.0 | |||
| Cre | 0.37 | 0.53 | mg/dL | 0.46-0.79 | |||
| Na | 140 | 140 | mEq/L | 138-145 | |||
| K | 4.2 | 4.0 | mEq/L | 3.6-4.8 | |||
| CRP | 0.02 | 0.01 | mg/dL | 0.00-0.14 | |||
| SAA | 3.1 | 0.6 | μg/mL | 0.0-8.0 | |||
| ESR | 3 | 3 | mm/h | 0-17 |
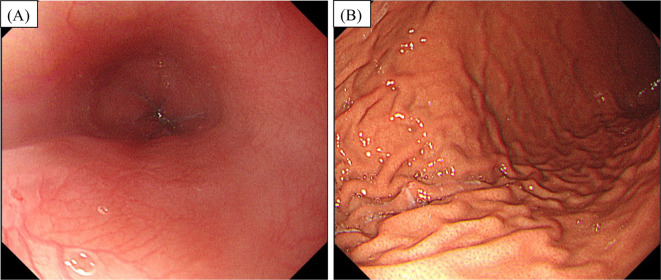
Helicobacter pylori infection was ruled out through invasive tests, and EGD (Fig. 2) revealed no obvious abnormalities. Likewise, neither CT nor ultrasound or fluoroscopy showed any abnormal findings to account for her symptoms. She was subsequently identified as having CNVS using the Rome IV criteria (3).
Figure 2.
Esophagogastroduodenoscopy revealed no remarkable abnormalities (A) in the esophagus or (B) the stomach.
Her symptoms vanished completely after a month and a half of taking acotiamide 100 mg three times a day before meals. After three months, the treatment was stopped without a relapse.
Case 2
A different pediatric clinic referred a 13-year-old boy who had been complaining of daytime nausea for more than 4 months to our facility. Before contracting COVID-19 caused by the SARS-CoV-2 Omicron variant four months earlier, he had been in good health. He had never been immunized against the SARS-CoV-2 virus.
The patient's clinical trajectory is depicted in Fig. 1B. His COVID-19 symptoms included a 5-day period of a fever and cough that quickly subsided, but nausea developed upon this resolution. His VAS score for his nausea ranged from 70 to 95. No other gastrointestinal symptoms, such as vomiting, abdominal pain, diarrhea, or discomfort in the abdomen, were noticed. On visiting the referring pediatric clinic two months after the nausea started, he was administered Kampo Goreisan and Kampo Hangekobokuto as medications, but his symptoms did not improve. Results from general blood tests (Table 1), urine tests, stool culture tests, and abdominal radiographs revealed no obvious abnormalities.
He was referred to our hospital two months after his initial visit to the pediatric referral clinic because his motion sickness symptoms did not improve. Despite his nausea, he had not lost any weight or his appetite. His height, body weight, and body mass index were 153.0 cm, 31.0 kg, and 13.2, respectively. No obvious abnormalities were found in his vital signs or during the physical examination. Our hospital's general blood tests, urine tests, stool culture tests, and abdominal radiographs also did not show any obvious abnormalities. We performed abdominal US, abdominal CT, and EGD; none of these tests found any anomalies. Through invasive and non-invasive testing (stool H. pylori antigen test), H. pylori infection was ruled out. He was finally identified as having CNVS using the Rome IV criteria (3).
Acotiamide was started at 100 mg three times a day orally before meals; as a result, his symptoms disappeared completely after a month, and treatment was discontinued after a total of 3 months, with no relapse occurring.
Discussion
The present findings suggest that acotiamide may be effective for CNVS symptoms that develop after COVID-19. Several reports are available on gastrointestinal COVID-19 symptoms in both adults and children, although it was impossible to definitively determine whether or not the virus was responsible for the nausea in these cases (4). A list of studies demonstrating the association between COVID-19 and gastrointestinal symptoms with functional gastroduodenal disorders is provided in Table 2. A fever and respiratory symptoms are commonly present in patients with COVID-19; however, digestive symptoms, including anorexia, nausea, vomiting, and diarrhea, are also commonly reported.
Table 2.
A List of Papers Showing Associations between Coronavirus Disease 2019 and Gastrointestinal Symptoms with Functional Gastroduodenal Disorders.
| Reference | style | |
|---|---|---|
| 7 | Review | |
| 8 | Review | |
| 9 | A case-controll study | |
| 10 | Systematic review and meta-analysis | |
| 11 | Systematic review and meta-analysis | |
| 17 | Multicenter cohort study | |
| 18 | Meta-analysis | |
| 19 | Comment | |
| 20 | Cross sectional study | |
| 21 | Systematic review and meta-analysis | |
| 22 | A case-control study | |
| 23 | Review | |
| 24 | Retrospective controlled study | |
| 25 | Review | |
| 26 | Prospective control multinational study | |
| 27 | Prospective control study | |
| 28 | Prospective control multinational study | |
| 29 | Review |
The expression and distribution of angiotensin-converting enzyme 2 (ACE2) receptors in humans is a potential route of SARS-CoV-2 infection, as transmembrane ACE2 receptors are mainly located in the lung, intestine, esophagus and pancreas, heart, kidney, and liver (5). SARS-CoV-2 may enter into cells directly through the ACE2 receptor, which affects how the liver and digestive tract normally function (6). Following the resolution of COVID-19's acute respiratory symptoms, gastrointestinal symptoms appear frequently; indeed, 6 months after recovery, 10-25% of patients report persistent gastrointestinal symptoms (7). Although the pathophysiology underlying the long-lasting post-COVID gastrointestinal symptoms is unknown, post-infection irritable bowel syndrome offers a biologically tenable conceptual model (7).
Approximately 2-5 days after a positive sputum polymerase chain reaction (PCR) result, 36-53% of COVID-19 patients' fecal SARS-CoV-2 PCR showed a positive result (8). This fact may help explain why the female patient started feeling sick on day 5 after the onset of COVID-19. SARS-CoV-2 PCR testing in feces could not be performed in the present cases. In addition, functional gastroduodenal disorders develop after some infections, and COVID-19 causes post-COVID-19 functional gastrointestinal disorders (9). According to Benvari et al., SARS-CoV-2 RNA could be detected through children's gastrointestinal specimens for more than 70 days after the loss of detectable RNA in the respiratory tract (10). Patients with gastrointestinal symptoms had a higher percentage of fecal SARS-CoV-2 RNA than those without such symptoms according to Zhang et al. (11). We cannot rule out the possibility that the ongoing nausea in the current cases was brought on by COVID-19, but this is mere conjecture, since we were unable to measure SARS-CoV-2 RNA.
Acotiamide is an acetylcholinesterase inhibitor reported to be effective in the treatment of FD symptoms in adults (2,12). However, to our knowledge, data on the efficacy or safety of acotiamide in children are not available (13). In the Rome IV criteria, FD is defined in cases with one or more of four symptoms - postprandial fullness, early satiation, epigastric pain, and epigastric burning - that is unexplained after a routine clinical evaluation; however, the Asian consensus on FD does not restrict FD symptoms to the four specified in the Rome IV criteria (14). Although CNVS is different from FD, it is included among functional gastrointestinal disorders, so acotiamide might be as effective for CNVS as it is for FD.
To our knowledge, this is the first case report demonstrating that acotiamide may be effective for CNVS symptoms after COVID-19. Direct evidence that nausea was caused by COVID-19 could not be presented, but the rapid disappearance of nausea after acotiamide administration is a compelling piece of evidence. Andrews et al. (15) suggested that the combination of a 5-HT3 receptor antagonist and a neurokinin receptor antagonist, which would inhibit the activation/sensitization of vagal afferents and nucleus of the solitary tract/area postrema, might be effective for relieving nausea and vomiting in patients with COVID-19. Kow et al. (16) suggested that prochlorperazine might also be effective for relieving nausea and vomiting in patients with COVID-19, based on the pathophysiology of nausea and vomiting in patients with COVID-19. At present, the pathophysiology of vomiting and nausea in COVID-19 is remains poorly elucidated, so more studies on CNVS after COVID-19 and its treatment are needed.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Aziz I, Palsson OS, Whitehead WE, Sperber AD, Simrén M, Törnblom H. Epidemiology, clinical characteristics, and associations for Rome IV functional nausea and vomiting disorders in adults. Clin Gastroenterol Hepatol 17: 878-886, 2019. [DOI] [PubMed] [Google Scholar]
- 2. Porika SK, Veligandla KC, Muni SK, Acharya S, Mehta SC, Sharma AD. Real-world, non-interventional, observational study to evaluate effectiveness and tolerability of acotiamide hydrochloride hydrate in treatment of functional dyspepsia. Adv Ther 35: 1884-1893, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology 150: 1257-1261, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Pegoraro F, Trapani S, Indolfi G. Gastrointestinal, hepatic and pancreatic manifestations of COVID-19 in children. Clin Res Hepatol Gastroenterol 46: 101818, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen TH, Hsu MT, Lee MY, Chou CK. Gastrointestinal involvement in SARS-CoV-2 infection. Viruses 14: 1188, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lei HY, Ding YH, Nie K, et al. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed Pharmacother 133: 111064, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freedberg DE, Chang L. Gastrointestinal symptoms in COVID-19: the long and the short of it. Curr Opin Gastroenterol 38: 555-561, 2022. [DOI] [PubMed] [Google Scholar]
- 8. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther 51: 843-851, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghoshal UC, Ghoshal U, Rahman MM, et al. Post-infection functional gastrointestinal disorders following coronavirus disease-19: a case-control study. J Gastroenterol Hepatol 37: 489-498, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benvari S, Mahmoudi S, Mohammadi M. Gastrointestinal viral shedding in children with SARS-CoV-2: a systematic review and meta-analysis. World J Pediatr 18: 582-588, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Cen M, Hu M, et al. Prevalence and persistent shedding of fecal SARS-CoV-2 RNA in patients with COVID-19 infection: a systematic review and meta-analysis. Clin Transl Gastroenterol 12: e00343, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miwa H, Nagahara A, Asakawa A, et al. Evidence-based clinical practice guidelines for functional dyspepsia 2021. J Gastroenterol 57: 47-61, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manini ML, Camilleri M. How does one choose the appropriate pharmacotherapy for pediatric patients with functional dyspepsia? Expert Opin Pharmacother 20: 1921-1924, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miwa H, Ghoshal UC, Fock KM, et al. Asian consensus report on functional dyspepsia. J Gastroenterol Hepatol 27: 626-641, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Andrews PLR, Cai W, Rudd JA, et al. COVID-19, nausea, and vomiting. J Gastroenterol Hepatol 36: 646-656, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kow CS, Hasan SS. Prochlorperazine for nausea and vomiting accompanied COVID-19. J Gastroenterol Hepatol 36: 524-525, 2021. [DOI] [PubMed] [Google Scholar]
- 17. Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology 159: 765-767.e762, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung TYM, Chan AYL, Chan EW, et al. Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg Microbes Infect 9: 2190-2199, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158: 1831-1833.e1833, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol 92: 833-840, 2020. [DOI] [PubMed] [Google Scholar]
- 21. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 159: 81-95, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology 159: 373-375.e372, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmulson M, Ghoshal UC, Barbara G. Managing the inevitable surge of post-COVID-19 functional gastrointestinal disorders. Am J Gastroenterol 116: 4-7, 2021. [DOI] [PubMed] [Google Scholar]
- 24. Blackett JW, Li J, Jodorkovsky D, Freedberg DE. Prevalence and risk factors for gastrointestinal symptoms after recovery from COVID-19. Neurogastroenterol Motil 34: e14251, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blackett JW, Wainberg M, Elkind MSV, Freedberg DE. Potential long coronavirus disease 2019 gastrointestinal symptoms 6 months after coronavirus infection are associated with mental health symptoms. Gastroenterology 162: 648-650.e642, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernández-de-Las-Peñas C, Martín-Guerrero J, Navarro-Pardo E, Torres-Macho J, Canto-Diez MG, Pellicer-Valero O. Gastrointestinal symptoms at the acute COVID-19 phase are risk factors for developing gastrointestinal post-COVID symptoms: a multicenter study. Intern Emerg Med 17: 583-586, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qian Q, Fan L, Liu W, et al. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis 73: 361-366, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marasco G, Cremon C, Barbaro MR, et al. Prevalence of gastrointestinal symptoms in severe acute respiratory syndrome coronavirus 2 infection: results of the prospective controlled multinational GI-COVID-19 study. Am J Gastroenterol 117: 147-157, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golla R, Vuyyuru SK, Kante B, Kedia S, Ahuja V. Disorders of gut-brain interaction in post-acute COVID-19 syndrome. Postgrad Med J. Forthcoming. [DOI] [PubMed] [Google Scholar]