Abstract
Background and Aim
Polypharmacy and sarcopenia are increasing public health problems worldwide. However, data on the prevalence, association, and prognostic significance of polypharmacy and sarcopenia in patients with liver cirrhosis are limited.
Methods
Polypharmacy and sarcopenia were assessed in 239 patients with liver cirrhosis. Polypharmacy was defined as the daily use of six or more medications, and sarcopenia was diagnosed based on muscle strength and mass evaluated on computed tomography. The association between polypharmacy and sarcopenia and their effects on mortality were analyzed using logistic regression and Cox proportional hazards models.
Results
Among the 239 patients, 52% were men, the median age was 68 years, and the number of medications used per patient was 6. Further, 53% and 29% patients had polypharmacy and sarcopenia, respectively. The number of medications used and the prevalence of sarcopenia increased with age. Patients with polypharmacy and sarcopenia had similar characteristics, such as older age, increased medication use, advanced liver disease, and decreased muscle strength and mass. After adjusting for confounders, polypharmacy was significantly associated with sarcopenia (odds ratio, 2.11; 95% confidence interval [CI], 1.07–4.17). During the median follow‐up of 2.2 years, 62 (26%) patients died. Polypharmacy (hazard ratio [HR], 1.83; 95% CI, 1.01–3.37) and sarcopenia (HR, 2.00; 95% CI, 1.12–3.50) independently predicted mortality. The prognostic significance of polypharmacy was more prominent in older adults than in younger adults (HR, 2.31; 95% CI, 1.01–5.67).
Conclusion
Polypharmacy and sarcopenia are interrelated and associated with poor prognosis in patients with cirrhosis. Further large, prospective, population‐based studies are required to validate these findings.
Keywords: cirrhosis, multimorbidity, older adults, polypharmacy, sarcopenia
Polypharmacy and sarcopenia are increasing public health problems worldwide, but the prevalence, association, and prognostic significance of polypharmacy and sarcopenia in patients with liver cirrhosis remain unclear. Our data indicate that polypharmacy and sarcopenia are common, interconnected, and associated with mortality in patients with cirrhosis.

Introduction
Polypharmacy and sarcopenia are serious public health problems worldwide, given the rapid increase in the older adult population. 1 , 2 , 3 Owing to the dramatic increase in life expectancy in the 20th century, the number of people aged ≥65 years is expected to increase from an estimated 524 million (8% of the world population) in 2010 to approximately 1.5 billion (16%) in 2050. 2 , 3 Japan is one of countries with the largest aged population in the world, with an average life expectancy of >83 years at birth. 3 , 4 In addition, 27% of the Japanese population was aged ≥65 years in 2016, and this is expected to reach 40% by 2060. 4 , 5 Reflecting the aging population, the number of older adults with liver cirrhosis has been increasing in Japan, with the average age of these patients increasing from 63.8 years in 2007 to 68.1 years in 2014. 6 , 7
Polypharmacy—the concurrent use of multiple medications—is common among older adults owing to the increased risk of age‐related complications, including chronic diseases, multimorbidity, and geriatric syndromes. 1 , 2 , 8 Aging‐related decreases in renal clearance, liver function, and skeletal muscle mass affect drug pharmacokinetics and pharmacodynamics. 1 , 8 , 9 Polypharmacy has recently attracted attention because it is associated with adverse drug reactions, drug–drug and drug–disease interactions, poor treatment adherence, decreased drug efficacy, falls, cognitive impairment, sarcopenia, impaired activities of daily living, increased hospitalization, and mortality. 1 , 2 , 8 In addition, patients with cirrhosis are at an increased risk of polypharmacy because of complications associated with cirrhosis. 10
Sarcopenia, a progressive and generalized skeletal muscle disorder characterized by loss of skeletal muscle mass and strength, is a well‐known geriatric syndrome. 1 Sarcopenia is common in not only older adults but also in patients with cancers and chronic diseases and is associated with adverse clinical outcomes, including falls, fractures, hospitalization, impaired activities of daily living, cognitive impairment, reduced quality of life, and mortality. 11 , 12 Substantial evidence shows that the prevalence of sarcopenia is higher in patients with cirrhosis than in the older adult population and in patients with other diseases such as heart failure, renal failure, chronic obstructive pulmonary disease, and cancer. 11 Therefore, patients with cirrhosis may be at increased risk of polypharmacy and sarcopenia due to age‐related physiological changes, multimorbidity, and impaired liver function. 6 , 10 , 13
Therefore, there is an urgent need to identify patients with cirrhosis at the risk of polypharmacy and sarcopenia, determine the prognostic significance of these factors, and provide appropriate pharmacotherapy to improve clinical outcomes. However, few studies have evaluated the prevalence, association, and prognostic significance of polypharmacy and sarcopenia, especially in older adults with cirrhosis. This study aimed to evaluate the characteristics of older adults with liver cirrhosis, prevalence and association of polypharmacy and sarcopenia, and the extent to which these factors affect the prognosis of these patients.
Patients and methods
Study design and participants
In total, 239 patients with cirrhosis treated at Gifu University Hospital (Gifu, Japan) between October 2011 and December 2020 were enrolled in this retrospective cohort study. The observation period was from the date of enrollment to the date of last visit, death, or December 31, 2020, whichever occurred first. The study protocol was reviewed and approved by the Institutional Review Board of Gifu University Graduate School of Medicine (approval number: 2021‐B190). This study was performed in accordance with the ethical standards of the Declaration of Helsinki 1964 and its later amendments.
Data collection
Patients were categorized into older adult (age ≥65 years) and younger adult groups (age <65 years). 3 , 4 , 5 Data on the number of medications used and demographic and clinical characteristics of patients within 1 month of sarcopenia assessment were collected from the prospective database using a standard template. Data on the following demographic and clinical characteristics were collected: age, sex, number of medications used, polypharmacy, height, weight, body mass index (BMI), grip strength, skeletal muscle mass, sarcopenia, etiology of cirrhosis, ascites, hepatic encephalopathy, Child–Pugh score, Model for End‐stage Liver Disease (MELD) score, and laboratory findings. Liver cirrhosis was diagnosed based on laboratory findings, clinical features (spider nevus, caput medusae, esophagogastric varices, and portosystemic shunt), and imaging and histological features. The inclusion criteria were age ≥20 years, cirrhosis of any etiology, and assessment of sarcopenia and polypharmacy. The exclusion criteria were a history of organ transplantation, hepatocellular carcinoma, active non‐hepatic malignancies, amino acid metabolism disorder, pregnancy, neurological or orthopedic diseases that affect grip strength measurement, and medical conditions such as severe sepsis, heart failure, respiratory failure, renal failure, and other acute life‐threatening diseases.
Polypharmacy
Data on medication use were recorded in electronic records by pharmacists who were not directly involved in patient care. Polypharmacy was assessed based on the number of oral medications used for at least 28 days within 1 month of sarcopenia assessment. Polypharmacy was defined as the daily use of six or more medications based on the criteria established by the Ministry of Health, Labour and Welfare. Polypharmacy is associated with an increased risk of adverse drug reactions in the older adult population in Japan. 14 , 15
Sarcopenia
Sarcopenia was diagnosed based on the revised sarcopenia criteria for liver disease (second edition) proposed by the Japanese Society of Hepatology, 16 including grip strength and skeletal muscle mass. Patients who met both criteria were diagnosed with sarcopenia. Grip strength was measured by a registered dietitian using a digital Smedley dynamometer (T.K.K.5101 GRIP‐D; Takei Scientific Instruments, Niigata, Japan), and the cutoff value for low grip strength was 28 kg for men and 18 kg for women. Skeletal muscle area was measured by estimating the cross‐sectional area of the abdominal skeletal muscles at the third lumbar vertebra using a computed tomography image analysis system (Synapse Vincent; Fujifilm, Tokyo, Japan). Skeletal muscle index (SMI) was calculated by dividing the skeletal muscle area by the square of the height, and the cutoff value for low SMI was 42 cm2/m2 for men and 38 cm2/m2 for women.
Statistical analysis
Continuous variables are presented as the median and interquartile range (IQR), and groups were compared using the Mann–Whitney U‐test or the Kruskal–Wallis test. The normality of the distribution of continuous variables was evaluated using the Shapiro–Wilk normality test. Categorical variables are presented as the number of patients and percentage, and groups were compared using the chi‐square test or Fisher's exact test. The association between polypharmacy and sarcopenia was assessed using the logistic regression model, and the results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). The prognostic significance of polypharmacy and sarcopenia was assessed using the Cox proportional hazards model, and the results are presented as hazard ratios (HRs) and 95% CIs. Overall survival (OS) was estimated using the Kaplan–Meier method, and the groups were compared using the log‐rank test. All tests were two sided, and the significance threshold was set at P <0.05. All analyses were performed using JMP software, version 9.0.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Among the 239 patients included, 125 (52%) were men, with a median age of 68 years, BMI of 22.7 kg/m2, Child–Pugh score of 7, and MELD score of 9. Altogether, 127 (53%) and 69 (29%) patients had polypharmacy and sarcopenia, respectively. The median number of medications used by each patient was 6 (IQR, 4–8, Table 1). The number of medications increased significantly with age (P < 0.037; Fig. 1a). Similarly, the prevalence of polypharmacy tended to increase with age (P = 0.153; Fig. 1b). The types of medication used are presented in Table S1. Seven patients who received beta‐blockers to prevent variceal bleeding were classified in the antihypertensive group.
Table 1.
Baseline characteristics of patients with cirrhosis
| Total cohort | Younger adults | Older adults | P‐value* | |
|---|---|---|---|---|
| Characteristic | (n = 239) | (n = 91) | (n = 148) | |
| Age, years | 68 (58–76) | 55 (50–60) | 74 (70–79) | <0.001 |
| Men | 125 (52) | 53 (58) | 72 (49) | 0.182 |
| Number of medications used | 6 (4–8) | 5 (4–7) | 7 (3–9) | 0.069 |
| Polypharmacy | 127 (53) | 41 (45) | 86 (58) | 0.049 |
| Comorbidities | ||||
| Diabetes mellitus | 66 (28) | 23 (25) | 43 (29) | 0.555 |
| Hypertension | 151 (63) | 51 (56) | 100 (68) | 0.097 |
| Dyslipidemia | 6 (3) | 2 (2) | 4 (3) | 0.809 |
| Etiology of cirrhosis | ||||
| Hepatitis B virus | 17 (7) | 3 (3) | 14 (10) | 0.002 |
| Hepatitis C virus | 68 (29) | 19 (21) | 49 (33) | |
| Alcoholic | 60 (25) | 34 (37) | 26 (18) | |
| Others | 94 (39) | 35 (39) | 59 (40) | |
| Ascites | 113 (47) | 44 (48) | 69 (47) | 0.894 |
| Hepatic encephalopathy | 23 (10) | 7 (8) | 16 (11) | 0.503 |
| MELD score | 9 (7–12) | 10 (8–12) | 9 (7–12) | 0.155 |
| Child–Pugh score | 7 (5–9) | 7 (6–9) | 7 (5–9) | 0.525 |
| Albumin, g/dl | 3.2 (2.6–3.7) | 3.2 (2.5–3.5) | 3.2 (2.6–3.8) | 0.454 |
| Creatinine, mg/dl | 0.71 (0.60–0.90) | 0.65 (0.52–0.78) | 0.74 (0.63–0.93) | 0.002 |
| Sodium, mEq/L | 138 (136–140) | 138 (137–140) | 138 (136–140) | 0.401 |
| Total bilirubin, mg/dl | 1.2 (0.8–1.7) | 1.3 (0.9–1.9) | 1.1 (0.8–1.6) | 0.057 |
| International normalized ratio | 1.12 (1.03–1.26) | 1.18 (1.04–1.31) | 1.10 (1.02–1.23) | 0.008 |
| Body composition | ||||
| Height, cm | 158 (150–166) | 164 (158–171) | 155 (149–161) | <0.001 |
| Weight, kg | 58.3 (50.3–65.9) | 63.7 (53.9–74.6) | 56.0 (48.8–62.5) | <0.001 |
| Body mass index, kg/m2 | 22.7 (21.4–25.4) | 22.73 (21.8–26.3) | 22.7 (21.0–25.1) | 0.238 |
| Grip strength, kg | 20.9 (16.5–29.2) | 26.8 (19.7–35.5) | 18.6 (14.5–24.9) | <0.001 |
| Skeletal muscle index, cm2/m2 | 41.9 (36.2–49.1) | 44.9 (38.1–50.0) | 40.1 (35.7–47.8) | 0.011 |
| Sarcopenia | 69 (29) | 14 (15) | 55 (37) | <0.001 |
Note: Values are presented as numbers (percentages) or medians (interquartile ranges).
Abbreviation: MELD, Model for End‐Stage Liver Disease.
Clinical characteristics between the two groups were compared using the chi‐square test for categorical variables or the Mann–Whitney U‐test for continuous variables.
Figure 1.
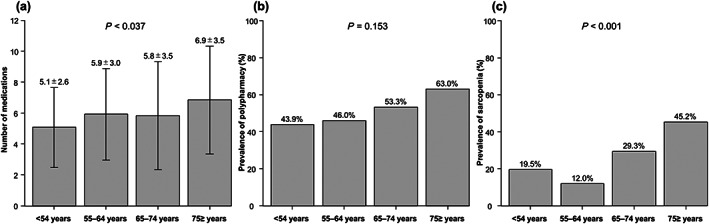
(a) Number of medications used, (b) prevalence of polypharmacy, and (c) prevalence of sarcopenia in 239 patients with cirrhosis in different age groups. Data are compared using the chi‐square test and Kruskal–Wallis test.
In total, 148 (62%) and 91 (28%) patients were categorized into the older and younger adult group, respectively (Table 1). Compared to the younger adult group, the older adult group had more patients with polypharmacy, high serum creatinine levels, and viral cirrhosis, but fewer patients with alcoholic cirrhosis. Height and weight were significantly lower in the older adult group than in the younger adult group; however, there was no significant intergroup difference in BMI. Grip strength and SMI were significantly lower in the older adult group than in the younger adult group, and sarcopenia was more predominant in the older adult group than in the younger adult group (37% vs 15%, P < 0.001; Table 1). The prevalence of sarcopenia increased with age (P < 0.001; Fig. 1c).
Polypharmacy and sarcopenia in patients with cirrhosis
The prevalence of sarcopenia was significantly higher in patients with polypharmacy than those without polypharmacy (36% vs 21%; P = 0.010). Similarly, the prevalence of polypharmacy was higher in patients with sarcopenia than those without sarcopenia (67% vs 48%; P = 0.010). The patients with polypharmacy and those with sarcopenia had similar characteristics, such as older age, increased medication use, advanced liver disease, and lower grip strength and SMI (Table 2).
Table 2.
Clinical characteristics of patients with cirrhosis, sarcopenia, and polypharmacy
| No polypharmacy | Polypharmacy | P‐value* | No sarcopenia | Sarcopenia | P‐value* | |
|---|---|---|---|---|---|---|
| Characteristic | (n = 112) | (n = 127) | (n = 170) | (n = 69) | ||
| Age, years | 67 (57–74) | 71 (60–77) | 0.010 | 66 (57–74) | 74 (68–78) | <0.001 |
| Men | 66 (59) | 59 (47) | 0.069 | 88 (52) | 37 (54) | 0.886 |
| Number of medications used | 3 (2–4) | 8 (7–10) | <0.001 | 5 (3–8) | 8 (5–10) | <0.001 |
| Polypharmacy | 0 (0) | 127 (100) | <0.001 | 81 (48) | 46 (67) | 0.010 |
| Etiology of cirrhosis | ||||||
| Hepatitis B virus | 7 (6) | 10 (8) | 0.788 | 13 (8) | 4 (6) | 0.750 |
| Hepatitis C virus | 29 (26) | 39 (31) | 50 (29) | 18 (26) | ||
| Alcoholic | 30 (27) | 30 (24) | 44 (26) | 16 (23) | ||
| Others | 46 (41) | 48 (38) | 63 (37) | 31 (45) | ||
| Ascites | 26 (23) | 87 (69) | <0.001 | 71 (42) | 42 (61) | 0.010 |
| Hepatic encephalopathy | 2 (2) | 21 (17) | <0.001 | 13 (8) | 10 (15) | 0.144 |
| MELD score | 8 (7–10) | 10 (8–13) | <0.001 | 9 (7–12) | 9 (7–12) | 0.897 |
| Child–Pugh score | 6 (5–8) | 8 (7–10) | <0.001 | 7 (5–9) | 8 (6–10) | 0.001 |
| Albumin, g/dl | 3.5 (3.1–3.9) | 2.9 (2.3–3.3) | <0.001 | 3.3 (2.9–3.7) | 2.6 (2.2–3.6) | 0.001 |
| Creatinine, mg/dl | 0.70 (0.58–0.83) | 0.73 (0.60–0.96) | 0.083 | 0.70 (0.59–0.88) | 0.75 (0.63–0.92) | 0.077 |
| Sodium, mEq/L | 139 (138–140) | 137 (135–139) | <0.001 | 138 (137–140) | 138 (135–140) | 0.022 |
| Total bilirubin, mg/dl | 1.1 (0.8–1.6) | 1.3 (0.9–1.7) | 0.106 | 1.2 (0.9–1.7) | 1.1 (0.8–1.6) | 0.571 |
| International normalized ratio | 1.07 (1.02–1.19) | 1.16 (1.05–1.31) | <0.001 | 1.12 (1.04–1.25) | 1.15 (1.01–1.29) | 0.877 |
| Body composition | ||||||
| Height, cm | 160 (152–166) | 158 (150–165) | 0.150 | 160 (152–166) | 155 (149–162) | 0.039 |
| Weight, kg | 60.0 (51.6–66.4) | 56.6 (49.4–65.3) | 0.130 | 61.2 (52.3–68.4) | 53.5 (47.7–58.3) | <0.001 |
| Body mass index, kg/m2 | 22.9 (21.0–25.5) | 22.4 (21.4–25.1) | 0.713 | 23.6 (22.0–25.9) | 21.7 (19.4–23.1) | <0.001 |
| Grip strength, kg | 25.1 (18.6–34.1) | 18.5 (14.8–24.9) | <0.001 | 24.5 (18.5–32.5) | 16.5 (14.0–19.8) | <0.001 |
| Skeletal muscle index, cm2/m2 | 43.6 (37.9–49.7) | 40.5 (35.4–48.0) | 0.012 | 46.0 (41.4–51.3) | 34.9 (32.0–37.1) | <0.001 |
| Sarcopenia | 23 (21) | 46 (36) | 0.010 | 0 (0) | 69 (100) | <0.001 |
Note: Values are presented as numbers (percentages) or medians (interquartile ranges).
Abbreviation: MELD, Model for End‐Stage Liver Disease.
Clinical characteristics between the two groups were compared using the chi‐square test for categorical variables or the Mann–Whitney U‐test for continuous variables.
Association between polypharmacy and sarcopenia
The unadjusted OR for polypharmacy in sarcopenia was 2.20 (95% CI, 1.23–3.94; P = 0.008) (Table 3). After adjusting for age, sex, BMI, etiology of cirrhosis, and MELD score, polypharmacy remained significantly associated with sarcopenia (OR, 2.11; 95% CI, 1.07–4.17; P = 0.032). This association was more pronounced in the older adult group (OR, 2.62; 95% CI, 1.14–6.02; P = 0.023) than in the younger adult group (OR, 1.56; 95% CI, 0.40–6.01; P = 0.522).
Table 3.
Association between sarcopenia and polypharmacy
| Total cohort | Younger adults | Older adults | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Polypharmacy unadjusted | 2.20 (1.23–3.94) | 0.008 | 1.78 (0.56–5.62) | 0.327 | 2.10 (1.04–4.23) | 0.039 |
| Polypharmacy adjusted for age | 1.93 (1.06–3.51) | 0.032 | 1.79 (0.56–5.66) | 0.916 | 1.97 (0.97–4.01) | 0.061 |
| Polypharmacy adjusted for age and sex | 2.01 (1.10–3.70) | 0.024 | 1.91 (0.59–6.12) | 0.279 | 2.03 (0.99–4.15) | 0.053 |
| Polypharmacy adjusted for age, sex, and BMI | 2.02 (1.06–3.84) | 0.032 | 2.17 (0.63–7.50) | 0.221 | 1.95 (0.91–4.15) | 0.085 |
| Polypharmacy adjusted for age, sex, BMI, and etiology | 2.10 (1.09–4.04) | 0.026 | 2.12 (0.60–7.56) | 0.245 | 2.23 (1.01–4.90) | 0.046 |
| Polypharmacy adjusted for age, sex, BMI, etiology, and MELD score | 2.11 (1.07–4.17) | 0.032 | 1.56 (0.40–6.01) | 0.522 | 2.62 (1.14–6.02) | 0.023 |
Abbreviations: BMI, body mass index; CI, confidence interval; MELD, Model for End‐Stage Liver Disease; OR, odds ratio.
Overall survival
During a median follow‐up of 2.2 years (IQR, 0.9–4.0), 62 (26%) patients died. None of the patients underwent liver transplantation during follow‐up. OS was not significantly different between the groups (P = 0.324, Fig. 2a), with an HR of 1.30 (95% CI, 0.77–2.20; P = 0.325). The causes of death are listed in Table S2.
Figure 2.
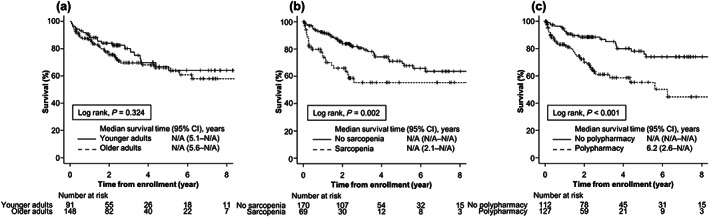
Overall survival in (a) older and younger adults, (b) patients with and without sarcopenia, and (c) patients with and without polypharmacy. Overall survival is estimated using the Kaplan–Meier method and compared between groups using the log‐rank test.
OS was lower in patients with sarcopenia than those without sarcopenia (P = 0.002; Fig. 2(b)), with an HR of 2.20 (95% CI, 1.32–3.67; P = 0.003). Similarly, OS was lower in patients with polypharmacy than in those without polypharmacy (P < 0.001; Fig. 2c), with an HR of 2.75 (95% CI, 1.60–4.71; P < 0.001).
In subgroup analysis, sarcopenia was significantly associated with poor prognosis in the younger adult group (P = 0.026; Fig. S1a), with an HR of 2.81 (95% CI, 1.09–7.28; P = 0.033), and in the older adult group (P = 0.045; Fig. S1b), with an HR of 1.87 (95% CI, 1.01–3.49; P = 0.048). However, polypharmacy was significantly associated with poor prognosis in the older adult group (P < 0.001; Fig. S2b), with an HR of 3.80 (95% CI, 1.79–8.05; P < 0.001), but not in the younger adult group (P = 0.265; Fig. S2a), with an HR of 1.63 (95% CI, 0.68–3.90; P = 0.269).
Prognostic significance of polypharmacy and sarcopenia in patients with cirrhosis
The prognostic factors of patients with cirrhosis evaluated by multivariate analysis are listed in Table 4. After adjusting for confounders, sarcopenia (HR, 2.00; 95% CI, 1.12–3.50; P = 0.020), polypharmacy (HR, 1.83; 95% CI, 1.01–3.37; P = 0.045), and MELD score (HR, 1.28; 95% CI, 1.19–1.38; P < 0.001) were significantly associated with mortality.
Table 4.
Prognostic value of sarcopenia and polypharmacy in multivariate analyses*
| Total cohort | Younger adults | Older adults | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Sarcopenia | 2.00 (1.12–3.50) | 0.020 | 2.57 (0.82–7.44) | 0.102 | 1.71 (0.83–3.51) | 0.148 |
| Polypharmacy | 1.83 (1.01–3.37) | 0.045 | 1.47 (0.54–4.01) | 0.444 | 2.31 (1.01–5.67) | 0.046 |
| MELD score | 1.28 (1.19–1.38) | <0.001 | 1.30 (1.15–1.47) | <0.001 | 1.26 (1.14–1.40) | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; MELD, Model for End‐Stage Liver Disease.
Adjusted for age, sex, body mass index, and etiology.
In younger adults, MELD score was significantly associated with mortality (HR, 1.30; 95% CI, 1.15–1.47; P < 0.001). In older adults, polypharmacy (HR, 2.31; 95% CI, 1.01–5.67; P = 0.046) and MELD score (HR, 1.26; 95% CI, 1.14–1.40; P < 0.001), but not sarcopenia (HR, 1.71; 95% CI, 0.83–3.51; P = 0.148), were significantly associated with mortality. In addition, the impact of polypharmacy and sarcopenia on mortality was compared in two groups, young and old. The results showed that polypharmacy (HR, 2.53; 95% CI, 1.48–4.47) and sarcopenia (HR, 1.92; 95% CI, 1.11–3.25) were independently associated mortality.
Discussion
This study, to our knowledge, is the first to evaluate the significance of polypharmacy and sarcopenia in patients with cirrhosis. First, the prevalence of polypharmacy and sarcopenia was high in these patients and increased with age. Second, patients with polypharmacy and sarcopenia had similar characteristics, such as older age, increased medication use, advanced liver disease, and decreased skeletal muscle mass and strength. Third, there was a strong association between polypharmacy and sarcopenia, especially in the older adult group. Fourth, these two factors adversely affected prognosis; more importantly, the prognostic significance of polypharmacy was more pronounced in older adults than in younger adults. It is difficult to distinguish between appropriate polypharmacy, which has health benefits, and inappropriate polypharmacy, in which multiple medications are no longer required. 2 However, our results suggest that polypharmacy and sarcopenia are important predictors of prognosis and that mitigating the impact of polypharmacy on sarcopenia through the appropriate use of medications improves survival, especially in older adults with cirrhosis.
Polypharmacy increases the likelihood of adverse events, including drug–drug interactions, poor medication adherence, falls, cognitive impairment, and death. 1 , 15 A nationwide survey in Japan showed that the prevalence of polypharmacy was 28% and 41% in the population aged 65–74 and ≥75 years, respectively. 15 In our cohort, the prevalence of polypharmacy was higher in these two groups, corresponding to 53.3% and 63%, respectively, demonstrating that polypharmacy is common in patients with cirrhosis. Moreover, 53% of patients with cirrhosis had polypharmacy, consistent with the rate reported in a cross‐sectional study of patients with cirrhosis (56%). 10 Aging commonly involves morphological and functional changes in the liver, including decreased liver volume, reduced blood flow, and impaired metabolic and detoxification functions. 1 , 8 , 9 Moreover, patients with cirrhosis are at an increased risk of altered drug pharmacokinetics because of pathophysiological changes such as decreased metabolic capacity, lower albumin synthesis, and portosystemic shunt. 6 , 10 , 13 Recent evidence shows that 28% and 22% patients with cirrhosis have adverse drug reactions and drug–drug interactions, respectively. 13 Thus, these patients are more prone to adverse reactions caused by polypharmacy than those without cirrhosis, and more attention should be paid to optimizing the number of medications to reduce the risk of adverse events in patients with cirrhosis. 10
Polypharmacy and sarcopenia are common geriatric syndromes; however, their interaction is complex. 1 The present study found that patients with polypharmacy had a twofold higher OR for sarcopenia, supporting the findings of previous studies. 1 , 17 Several mechanisms may underlie the association between polypharmacy and sarcopenia. Medications that cause side effects, including dysphagia and gastrointestinal symptoms, can lead to poor nutritional status and sarcopenia. 1 , 18 In addition, medications for liver disease, such as loop diuretics, proton pump inhibitors, and glucocorticoids, can have detrimental effects on skeletal muscle. 1 , 19 , 20 , 21 , 22 , 23 In the present study, patients with polypharmacy used loop diuretics, proton pump inhibitors, and glucocorticoids more frequently than those without polypharmacy. In addition, several medications (e.g., statins and targeted anticancer agents) are known to contribute to the pathogenesis of sarcopenia through mitochondrial dysfunction, activation of the transcription factor NF‐kappa B, and increases in reactive oxygen species and inflammatory cytokines. 1 The insights gained from this study may help determine the extent to which these medications contribute to sarcopenia in cirrhosis.
Polypharmacy and sarcopenia worsen prognosis in older adults 1 ; however, there is limited evidence on the prognostic significance of these factors in patients with cirrhosis. In our study, polypharmacy and sarcopenia independently predicted mortality. Furthermore, the prognostic significance of polypharmacy was stronger in older adults than in younger adults. This result might be attributed to the higher incidence of adverse drug reactions, geriatric syndromes, functional and cognitive impairments, and multimorbidity in the former group. 13 , 24 Therefore, assessment of polypharmacy may be useful to stratify the risk of mortality in patients with cirrhosis. 9 However, the ideal timing and frequency of polypharmacy assessment and effect of polypharmacy on the health outcomes of these patients need to be investigated. 25 , 26 , 27
This study has two positive points. First, the number of participants (N = 239) and long follow‐up period (minimum 2 years) may allow us to estimate adjusted ORs for sarcopenia and HRs for death. It is well known that as the age increases, the number of comorbidities and therefore treatment (drugs) increases. The findings of our study need validation in large population‐based prospective studies. Second, the analyses were adjusted for important confounders in the association between polypharmacy and sarcopenia or mortality.
This study has limitations. First, the observational design does not allow for the identification of adverse drug reactions, drug–drug interactions, or drug–disease interactions, which may affect the interpretation of the results. In addition, the findings may be influenced by residual confounding and clinically relevant factors such as daily physical activity, comorbidities, cognitive impairment, and malnutrition. 17 , 18 Second, polypharmacy and sarcopenia were assessed at a single time point, preventing the evaluation of changes over time. Third, data on nonprescribed medications, such as dietary supplements, over‐the‐counter medications, and traditional and complementary medicines that may cause adverse events or interactions with prescribed medications, 2 were not collected and analyzed. Fourth, the definition of polypharmacy and diagnostic criteria for sarcopenia vary widely. 1 , 28 Considering that the diagnoses of polypharmacy and sarcopenia were based on Japanese guidelines, 14 , 15 , 16 our results might not be extrapolated to other cohorts in different regions and clinical settings. Thus, these findings should be interpreted with caution and validated in a large external cohort. However, we believe that this study serves as a basis for future research.
Conclusion
In conclusion, our results provide convincing evidence that polypharmacy and sarcopenia are common, interconnected, and useful in predicting mortality in patients with cirrhosis. This study underscores the importance of avoiding inappropriate polypharmacy, especially for patients with cirrhosis, and the need to implement other strategies to ensure appropriate pharmacotherapy and prevent or delay sarcopenia and related adverse events.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Supporting information
Figure S1. Overall survival of patients with and without sarcopenia in the (a) younger adult group and (b) older adult group. Overall survival is estimated using the Kaplan–Meier method and compared between groups using the log‐rank test.
Figure S2. Overall survival of patients with and without polypharmacy in the (a) younger adult group and (b) older adult group. Overall survival is estimated using the Kaplan–Meier method and compared between groups using the log‐rank test.
Table S1. Types of medications used by patients†
Table S2. Causes of death in our cohort
Author contribution: All authors contributed to the conception and design of this study. Data collection and analysis were performed by Tatsunori Hanai, Kayoko Nishimura, Takao Miwa, Toshihide Maeda, Kenji Imai, Atsushi Suetsugu, and Koji Takai. The first draft of the manuscript was written by Tatsunori Hanai, and all authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript.
Declaration of conflict of interest: The authors declare no conflict of interest.
Financial support: This work was supported by a Grant‐in‐Aid for Research from the Japan Agency for Medical Research and Development (JP22fk0210113).
Data availability statement
The data presented in this study are available upon request from the corresponding author.
References
- 1. Pana A, Sourtzi P, Kalokairinou A, Velonaki VS. Sarcopenia and polypharmacy among older adults: a scoping review of the literature. Arch. Gerontol. Geriatr. 2022; 98: 104520. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Medication safety in polypharmacy: technical report. World Health Organization/Utah Humanities Council/Saudi Dental Society/2019.11. Available at: https://apps.who.int/iris/handle/10665/325454.
- 3. National Institute on Aging, World Health Organization . Global Health and Aging. NIH Publication no. 11–7737. 2011. Available at: https://www.who.int/ageing/publications/global_health.pdf.
- 4. Nomura S, Sakamoto H, Glenn S et al. Population health and regional variations of disease burden in Japan, 1990–2015: a systematic subnational analysis for the Global Burden of Disease Study 2015. Lancet. 2017; 390: 1521–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikeda N, Saito E, Kondo N et al. What has made the population of Japan healthy? Lancet. 2011; 378: 1094–105. [DOI] [PubMed] [Google Scholar]
- 6. Kamimura K, Sakamaki A, Kamimura H et al. Considerations of elderly factors to manage the complication of liver cirrhosis in elderly patients. World J. Gastroenterol. 2019; 25: 1817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Enomoto H, Ueno Y, Hiasa Y et al. Transition in the etiology of liver cirrhosis in Japan: a nationwide survey. J. Gastroenterol. 2020; 55: 353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Milton JC, Hill‐Smith I, Jackson SH. Prescribing for older people. BMJ. 2008; 336: 606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet. 2007; 370: 185–91. [DOI] [PubMed] [Google Scholar]
- 10. Elzouki AN, Zahid M, Akbar RA et al. Polypharmacy and drug interactions amongst cirrhotic patients discharged from a tertiary center: results from a national quality improvement audit. Arab. J. Gastroenterol. 2020; 21: 216–8. [DOI] [PubMed] [Google Scholar]
- 11. Dasarathy S. Consilience in sarcopenia of cirrhosis. J. Cachexia. Sarcopenia Muscle. 2012; 3: 225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanai T, Nishimura K, Miwa T et al. Usefulness of nutritional therapy recommended in the Japanese Society of Gastroenterology/Japan Society of Hepatology evidence‐based clinical practice guidelines for liver cirrhosis 2020. J. Gastroenterol. 2021; 56: 928–37. [DOI] [PubMed] [Google Scholar]
- 13. Franz CC, Egger S, Born C, Rätz Bravo AE, Krähenbühl S. Potential drug–drug interactions and adverse drug reactions in patients with liver cirrhosis. Eur. J. Clin. Pharmacol. 2012; 68: 179–88. [DOI] [PubMed] [Google Scholar]
- 14. Kojima T, Akishita M, Kameyama Y et al. High risk of adverse drug reactions in elderly patients taking six or more drugs: analysis of inpatient database. Geriatr. Gerontol. Int. 2012; 12: 761–2. [DOI] [PubMed] [Google Scholar]
- 15. Ministry of Health, Labour and Welfare . Guidelines for the appropriate use of medicines for the elderly; 2018 (in Japanese). Available at: https://www.mhlw.go.jp/content/11121000/kourei-tekisei_web.pdf.
- 16. Assessment Criteria for Sarcopenia in Liver Disease Proposed by the Japan Society of Hepatology (JSH), second edn. Tokyo, Japan: The Japan Society of Hepatology. 2021. Available at: https://www.jsh.or.jp/lib/files/english/guidelines_en/sarcopenia.pdf. [Google Scholar]
- 17. König M, Spira D, Demuth I, Steinhagen‐Thiessen E, Norman K. Polypharmacy as a risk factor for clinically relevant sarcopenia: results from the Berlin aging Study II. J. Gerontol. A Biol. Sci. Med. Sci. 2017; 73: 117–22. [DOI] [PubMed] [Google Scholar]
- 18. Jokanovic N, Tan EC, Dooley MJ, Kirkpatrick CM, Bell JS. Prevalence and factors associated with polypharmacy in long‐term care facilities: a systematic review. J. Am. Med. Dir. Assoc. 2015; 16: e1–12. [DOI] [PubMed] [Google Scholar]
- 19. Musarò A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF‐1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA‐2 and NF‐ATc1. Nature. 1999; 400: 581–5. [DOI] [PubMed] [Google Scholar]
- 20. Kim HJ, Cha JY, Seok JW et al. Dexras1 links glucocorticoids to insulin‐like growth factor‐1 signaling in adipogenesis. Sci. Rep. 2016; 6: 28648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maggio M, Lauretani F, De Vita F et al. Relationship between use of proton pump inhibitors and IGF system in older subjects. J. Nutr. Health Aging. 2014; 18: 420–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandai S, Furukawa S, Kodaka M et al. Loop diuretics affect skeletal myoblast differentiation and exercise‐induced muscle hypertrophy. Sci. Rep. 2017; 7: 46369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanai T, Shiraki M, Miwa T et al. Effect of loop diuretics on skeletal muscle depletion in patients with liver cirrhosis. Hepatol. Res. 2019; 49: 82–95. [DOI] [PubMed] [Google Scholar]
- 24. Hilmer SN, Gnjidic D. The effects of polypharmacy in older adults. Clin. Pharmacol. Ther. 2009; 85: 86–8. [DOI] [PubMed] [Google Scholar]
- 25. O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015; 44: 213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel . American Geriatrics Society 2019 Updated AGS beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2019: 67: 674–94. [DOI] [PubMed] [Google Scholar]
- 27. Scott IA, Hilmer SN, Reeve E et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern. Med. 2015; 175: 827–34. [DOI] [PubMed] [Google Scholar]
- 28. Masnoon N, Shakib S, Kalisch‐Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017; 17: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overall survival of patients with and without sarcopenia in the (a) younger adult group and (b) older adult group. Overall survival is estimated using the Kaplan–Meier method and compared between groups using the log‐rank test.
Figure S2. Overall survival of patients with and without polypharmacy in the (a) younger adult group and (b) older adult group. Overall survival is estimated using the Kaplan–Meier method and compared between groups using the log‐rank test.
Table S1. Types of medications used by patients†
Table S2. Causes of death in our cohort
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.


