Abstract
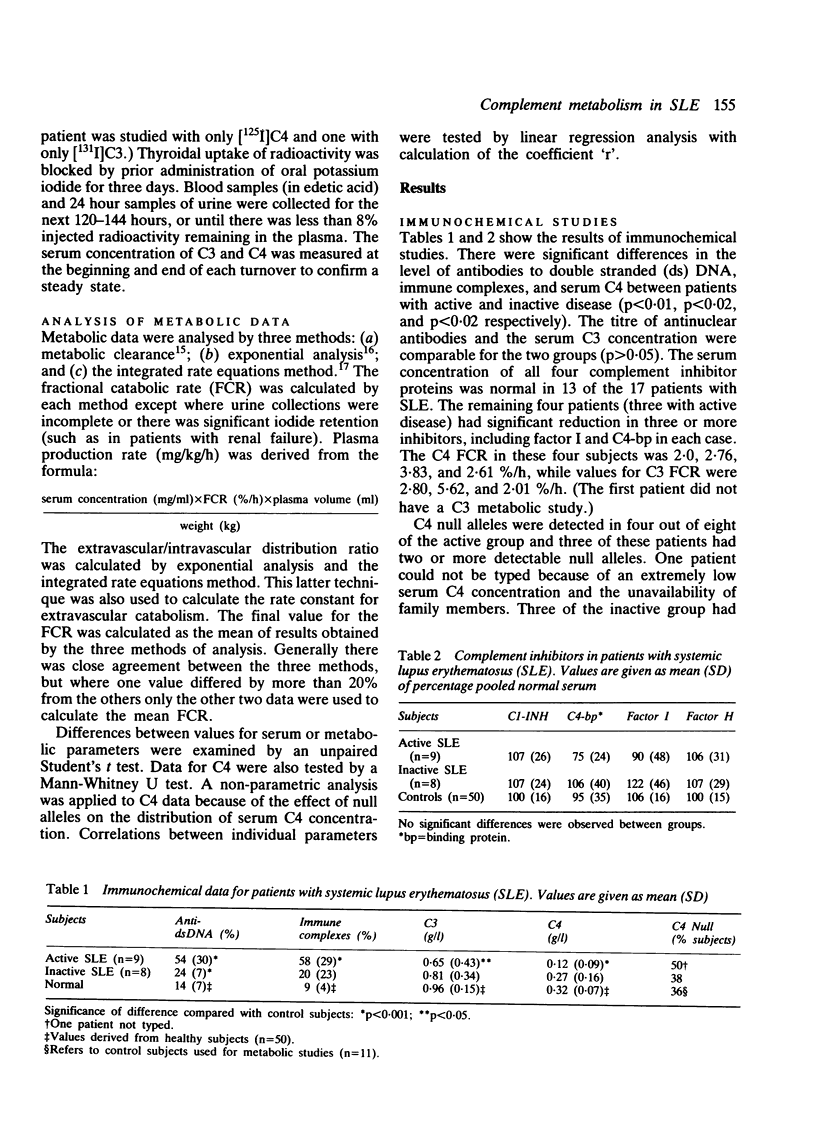
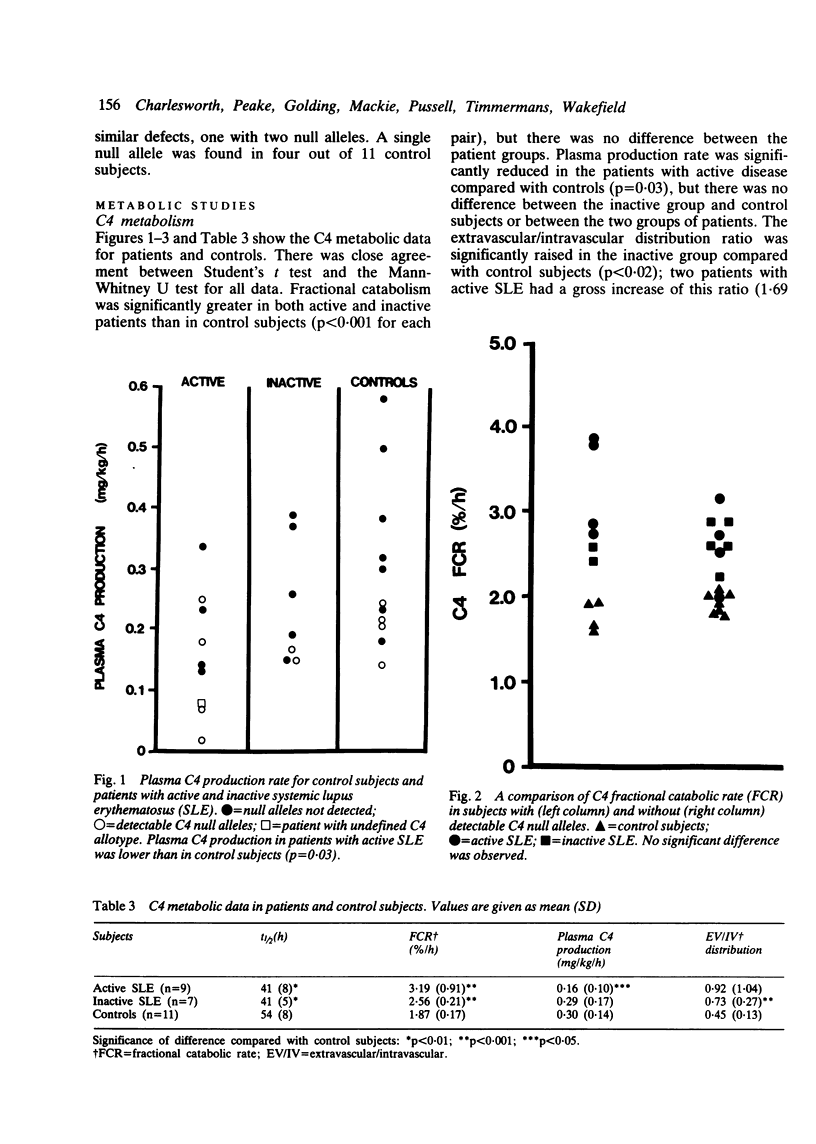
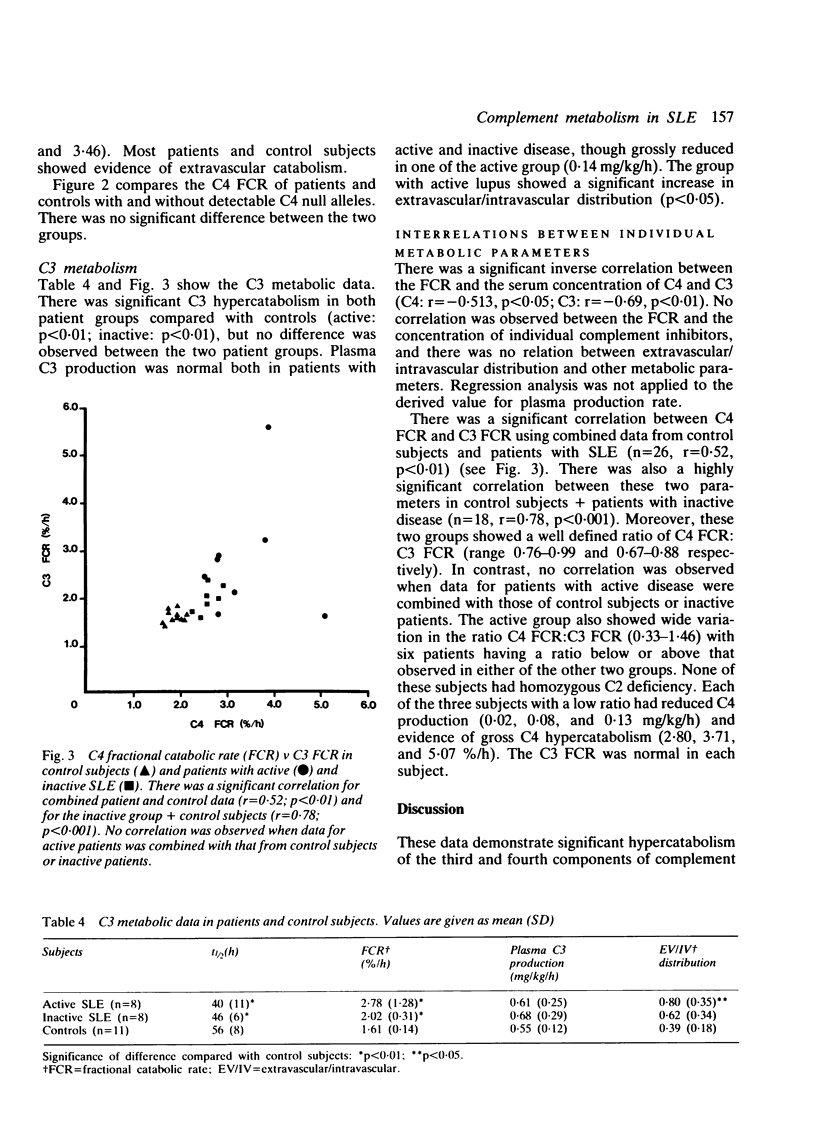
The metabolism of the complement proteins C3 and C4 was studied in patients with active and inactive systemic lupus erythematosus (SLE) using highly purified, functionally active preparations. Nine patients with active and eight with inactive SLE were examined and 11 control subjects. There was a significant difference in the level of double stranded DNA antibodies, immune complexes, and serum C4 between the patients with active and inactive disease. Seven of 16 patients had detectable C4 null alleles and four had low serum concentrations of complement inhibitors. Each subject received approximately 370 kBq [125I]C4 and 93 kBq [131I]C3. Both patient groups showed significant C4 hypercatabolism compared with control subjects, but there was no difference between patients with active and inactive disease. The fractional catabolic rate (FCR) of C4 was comparable in subjects with and without detectable C4 null alleles. C4 production rate was significantly lower in patients with active SLE than in control subjects. There was significant C3 hypercatabolism for both patient groups, but C3 production was normal. An inverse correlation was observed between serum concentration and FCR. There was a highly significant correlation between C4 FCR and C3 FCR for control subjects + patients with inactive disease but not for those with active SLE combined with either controls or the inactive group. We conclude that complement hypercatabolism occurs in SLE irrespective of disease activity and that accelerated turnover does not account completely for the low C4 concentration observed in patients with active disease. This low concentration also results from impaired plasma production, which could reflect a high incidence of C4 null alleles or (inhibitory) factors associated with pathological complement activation, or both. Low C4 production could affect generation of the C3 converting enzyme C4b, 2b and thus influence proceeding complement activation.
Full text
PDF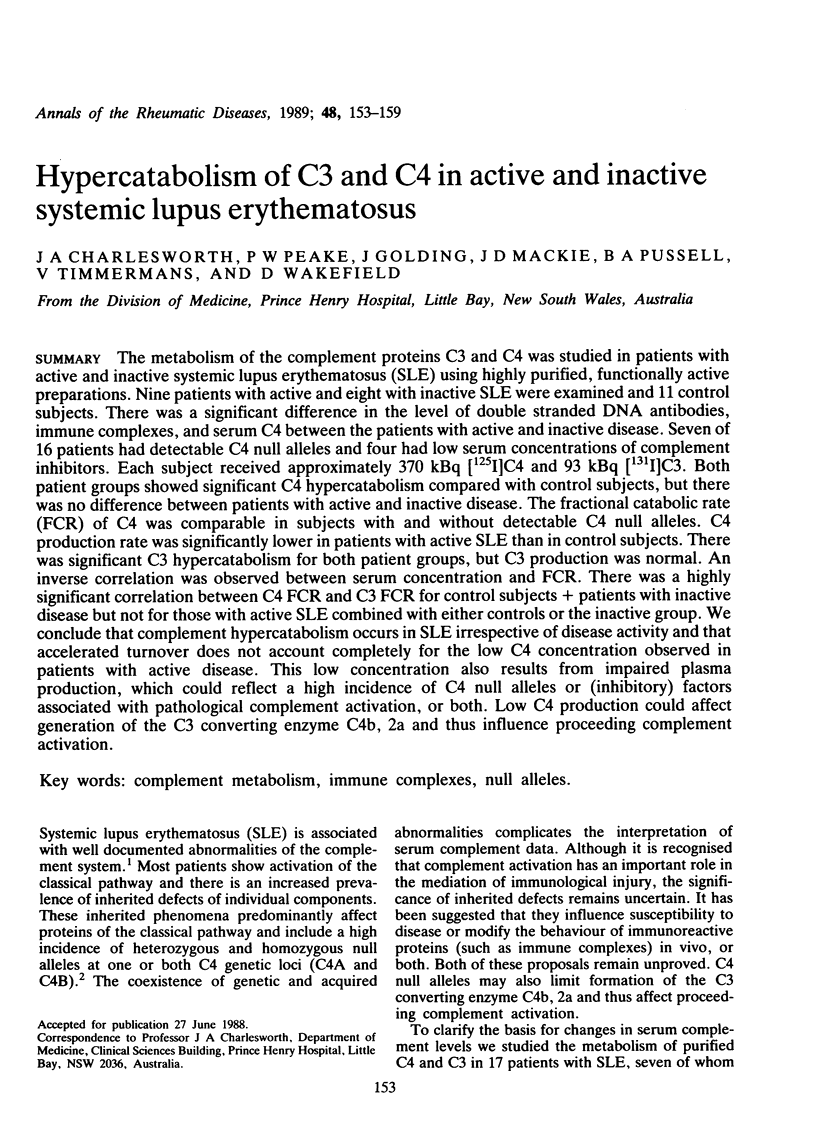



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Raum D., Alper C. A. Genetic polymorphism of human complement C4 and detection of heterozygotes. Nature. 1979 Nov 8;282(5735):205–207. doi: 10.1038/282205a0. [DOI] [PubMed] [Google Scholar]
- BERSON S. A., YALOW R. S. Distribution and metabolism of I131 labeled proteins in man. Fed Proc. 1957 Jul;16(2):suppl–18. [PubMed] [Google Scholar]
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Charlesworth J. A., Timmermans V., Golding J., Campbell L. V., Peake P. W., Pussell B. A., Wakefield D., Howard N. The complement system in type 1 (insulin-dependent) diabetes. Diabetologia. 1987 Jun;30(6):372–379. doi: 10.1007/BF00292537. [DOI] [PubMed] [Google Scholar]
- Gaither T. A., Alling D. W., Frank M. M. A new one-step method for the functional assay of the fourth component (C4) of human and guinea pig complement. J Immunol. 1974 Aug;113(2):574–583. [PubMed] [Google Scholar]
- Kaplan R. A., Curd J. G., Deheer D. H., Carson D. A., Pangburn M. K., Müller-Eberhard H. J., Vaughan J. H. Metabolism of C4 and factor B in rheumatoid arthritis. Relation to rheumatoid factor. Arthritis Rheum. 1980 Aug;23(8):911–920. doi: 10.1002/art.1780230806. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauff G., Alper C. A., Awdeh Z., Batchelor J. R., Bertrams J., Bruun-Petersen G., Dawkins R. L., Démant P., Edwards J., Grosse-Wilde H. Statement on the nomenclature of human C4 allotypes. Immunobiology. 1983 Mar;164(2):184–191. doi: 10.1016/s0171-2985(83)80009-6. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Nosslin B. Analysis of disappearance time-curves after single injection of labelled proteins. Ciba Found Symp. 1972;9:113–130. doi: 10.1002/9780470719923.ch7. [DOI] [PubMed] [Google Scholar]
- Pereira Da Silva J. A., Elkon K. B., Hughes G. R., Dyck R. F., Pepys M. B. C-reactive protein levels in systemic lupus erythematosus: a classification criterion? Arthritis Rheum. 1980 Jun;23(6):770–771. doi: 10.1002/art.1780230609. [DOI] [PubMed] [Google Scholar]
- Russo M. de C., Okamoto T., Holland R. Treatment of inflamed pulp in deciduous teeth. Histological study in dog. Bull Tokyo Dent Coll. 1972 Feb;13(1):9–20. [PubMed] [Google Scholar]
- Rynes R. I. Inherited complement deficiency states and SLE. Clin Rheum Dis. 1982 Apr;8(1):29–47. [PubMed] [Google Scholar]
- Schutte M., DiCamelli R., Murphy P., Sadove M., Gewurz H. Effects of anesthesia, surgery and inflammation upon host defense mechanisms. I. Effects upon the complement system. Int Arch Allergy Appl Immunol. 1975;48(5):706–720. doi: 10.1159/000231358. [DOI] [PubMed] [Google Scholar]
- Uko G., Christiansen F. T., Dawkins R. L., McCann V. J. Reference ranges for serum C4 concentrations in subjects with and without C4 null alleles. J Clin Pathol. 1986 May;39(5):573–576. doi: 10.1136/jcp.39.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Schur P. H., Ruddy S. Relative importance of C3b inactivator and beta 1H globulin in the modulation of the properdin amplification loop in systemic lupus erythematosus. Clin Exp Immunol. 1979 Jun;36(3):408–414. [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]


