Abstract
Background: Type 2 diabetes
Type 2 diabetes mellitus (T2DM) is a strong risk factor for peripheral artery disease (PAD) and PAD diagnosis in T2DM may indicate coexisting coronary artery disease as well. Postexercise ankle brachial index (ABI) and transcutaneous partial pressure of oxygen (TcPO2) have not been evaluated for PAD diagnosis among Indian T2DM patients. This study aimed to evaluate the performance of resting + postexercise(R + PE) ABI and R + PE-TcPO2 for PAD diagnosis among T2DM patients at increased PAD risk, using colour duplex ultrasound (CDU) as reference standard.
Methods
This prospectively conducted diagnostic accuracy study involved T2DM patients at increased PAD risk. R-ABI≤0.9 or PE-ABI decline >20% from resting value in those with R-ABI between 0.91 and 1.4, R–TcPO2 <30 mm Hg or PE decline of TcPO2 to <30 mm Hg in those with R–TcPO2 ≥30 mm Hg, CDU showing >50% stenosis or complete occlusion of lower extremity arteries constituted PAD.
Results
Among 168 patients enrolled, R + PE-ABI diagnosed PAD in 19(11.3%), R + PE-TcPO2 in 61 (36.3%) and 17 (≈10%) had PAD finally confirmed by CDU. Sensitivity, specificity, PPV and NPV of R + PE-ABI for PAD diagnosis were 82.3%, 96.7%, 73.7% and 98% and that of R + PE-TcPO2 were 76.5%, 68.2%, 21.3% and 96.2%, respectively. PE-ABI increased the sensitivity of ABI by ≈ 18% and had 100% PPV for PAD. When both ABI and TcPO2 (R + PE tests) were normal, PAD could be safely excluded in 88% of patients.
Conclusion
PE-ABI should be routinely employed and TcPO2(R/PE) is unreliable as a stand-alone test for PAD detection among moderate to high risk T2DM patients.
Keywords: Type 2 diabetes, Peripheral artery disease, Ankle brachial index, Transcutaneous partial pressure of oxygen, Post-exercise test
Introduction
Peripheral artery disease (PAD) most often results from atherosclerotic narrowing or occlusion of iliac and other lower extremity arteries. Apart from giving rise to troublesome intermittent claudication, functional disability, foot ulcers/gangrene and increasing the risk of limb loss, PAD may be associated with increased risk of acute coronary events, strokes and cardiovascular and all-cause mortality.1,2,3 Diabetes is a strong risk factor for PAD.1 In the Framingham Heart Study, about a fifth of patients with intermittent claudication had diabetes.4 About 30% of diabetic patients aged >50 years in primary care practice had PAD in a study reported from United States.5 PAD is often asymptomatic and its diagnosis may be more challenging in diabetic patients as peripheral neuropathy may interfere with pain perception. Presentation with ischaemic foot ulcers and gangrene is more common in diabetic PAD.6 Diagnosis of PAD in diabetes assumes importance, as it not only identifies a subset of patients who benefit most from aggressive cardiovascular risk reduction interventions but also facilitates optimal PAD management thereby reducing foot complications.
Ankle brachial index (ABI) is a frequently employed noninvasive test for PAD, with reasonable sensitivity and specificity.7 ABI can also assess PAD severity and independently predict cardiovascular morbidity and mortality. However, ABI has lower sensitivity in diabetic patients and can give rise to false negative results.6,8 When the resting (R) ABI is > 0.9 and there is still a clinical suspicion of PAD, postexercise (PE) ABI has been recommended to be useful for PAD diagnosis.8,9 Transcutaneous partial pressure of oxygen (TcPO2), which assesses tissue perfusion, has been used for predicting wound/ulcer healing and determining amputation level in diabetic foot and PAD. TcPO2 has also been suggested for PAD diagnosis in diabetes.10 TcPO2 has good reproducibility and can also be a potential predictor of future cardiovascular events in type 2 diabetes mellitus (T2DM).11 PE testing may increase the sensitivity of TcPO2 and may be particularly useful in diabetic patients in whom medial arterial calcification may give rise to falsely normal R-ABI.8,9,11
Though conventional angiography is the gold standard test for PAD diagnosis, it is not routinely employed as it is invasive and requires radiocontrast use. Colour duplex ultrasound (CDU) is a fairly reliable noninvasive test for diagnosing PAD, with sensitivity and specificity of >90%.12 However, CDU is highly operator-dependent, cumbersome to do (more time consuming) and needs expensive equipment and expertise. ABI and TcPO2 are more simple, easy to carry out noninvasive tests which can be reliably done by laboratory technicians with lesser training compared to CDU. There are no studies from India comparing the performance of R/PE ABI and TcPO2 for PAD diagnosis. There is also paucity of data on the application of TcPO2 for PAD diagnosis in T2DM. With this background, the aim of this study was to evaluate and compare the performance of (R + PE) ABI and TcPO2 for PAD diagnosis among T2DM patients at increased PAD risk, considering CDU as reference standard.
Materials and methods
This diagnostic accuracy study was carried out prospectively between January 2017 and July 2018 at a tertiary care teaching hospital located in southern India. The study was approved by the Institute Ethics Committee (Human Studies). Written informed consent was obtained from all patients before enrolment.
Adult (age ≥18 years) T2DM patients having risk factors for PAD9 and attending medicine, diabetes and cardiology outpatient clinics of our hospital were included in this study. Convenience sampling method was adopted. Those enrolled had one or more of the following: age ≥65 years, diabetes duration ≥10 years, smoking, hypertension, dyslipidemia (defined as fasting serum low density lipoprotein cholesterol ≥100 mg/dl with or without statins), coronary artery disease (CAD), cerebrovascular disease, nephropathy (defined by 24 h urine protein excretion >300 mg and/or serum creatinine ≥1.5 mg/dl), diabetic retinopathy and diabetic foot complications (current or past foot ulcers). CAD diagnosis was based on history of stable angina, previously documented acute coronary syndrome, coronary angiography showing >50% stenosis (in those who had undergone angiography earlier), electrocardiographic changes suggestive of ischaemia or echocardiography showing left ventricular regional wall motion abnormalities. Cerebrovascular disease was diagnosed based on prior episode of stroke (ischaemic/haemorrhagic) or transient ischaemic attack. The following were the exclusion criteria for the study: age >80 years, previously diagnosed PAD, patients who were nonambulatory or had acute limb threatening ischaemia, prior lower extremity deep vein thrombosis or amputation, gross lower limb oedema and serum creatinine >2 mg/dl.
All patients underwent detailed clinical evaluation for diabetic complications and PAD. Edinburgh Claudication Questionnaire13 was used to assess intermittent claudication. ABI and TcPO2 measurements were done on different days by a person well trained in these procedures. CDU was performed after measurement of ABI and TcPO2. Smokers were strictly instructed not to smoke for at least 24 h before scheduled appointments for ABI, TcPO2 and CDU.
Resting and postexercise ABI measurement
Standard procedure8 was followed and room temperature was maintained between 26°C and 28°C while determining ABI. After 5 min rest in supine position, ABI was measured with arms and legs at the same level as the heart. A handheld portable Doppler probe (Doppler Hadeco Smartdop® 30EX by Koven Technology Inc., United States) was used to locate the brachial, posterior tibial and dorsalis pedis pulses in upper and lower limbs. Brachial systolic pressures of both arms were obtained and the higher of the two values was chosen as the denominator. Similarly, dorsalis pedis and posterior tibial systolic pressures in both legs were obtained at ankle and the higher of the two was selected as the numerator. ABI was calculated separately for each leg14 and the lower of the two was considered as the final R-ABI. R-ABI ≤0.9 diagnosed PAD.8,9 Patients who had R-ABI between 0.91 and 1.40 in both legs underwent PE-ABI measurement (after brisk walk to the extent possible on level ground for 5 min14 or as limited by symptoms) and those who had >20% reduction from R-ABI value in either of the two legs were also diagnosed to have PAD.8
Resting and postexercise TcPO2 measurement
TcPO2 was measured using a multichannel TcPO2 monitor TCM400 (by Radiometer™, Denmark). R-TcPO2 was measured with patients in supine position, at a controlled room temperature of 26°C–28°C. During this procedure, the TcPO2 electrode was calibrated for 15 min to a barometric pressure of 760 mmHg (standard calibration at the geographic location). TcPO2 was measured on the dorsum of each foot. The measuring site was cleaned with saline and transducers were fixed to the skin with double-sided adhesive rings and contact liquid was applied. The electrode was heated to 45°C and TcPO2 measurement was taken at 15 min. The lower of the two R-TcPO2 values recorded in a given patient was considered for PAD diagnosis. Unlike ABI, there is no consensus regarding TcPO2 cutoff for PAD diagnosis in diabetes.11 R-TcPO2 <30 mm Hg14,15 in either of the feet was considered diagnostic of PAD. In those with R-TcPO2 ≥30 mm Hg in both feet, PE-TcPO2 measurements were done after exercise (as described under ABI). PE-TcPO2 <30 mm Hg in either of the feet was also considered diagnostic of PAD.
Colour duplex ultrasound assessment
CDU, which combines two-dimensional grey scale ultrasound and colour flow imaging to study arteries and pulse-wave doppler to assess the velocity of arterial blood flow, was used to evaluate the common iliac, external iliac and other lower extremity arteries. CDU was performed by well-trained radiologists on Acuson Antares PE ultrasound system (Siemens Medical Solutions, CA, USA). High frequency (5–10 MHz) transducers were used to obtain better image resolution of the vessels. The degree of arterial stenosis was based on the peak systolic velocity and velocity ratio, as determined by pulse-wave Doppler. PAD was diagnosed when the stenosis was >50%16 or in presence of monophasic flow distal to arterial calcification or total arterial occlusion in either of lower limbs. Following features were used for diagnosing arterial occlusion: segmental signal loss in the vessel insonated, dampened signal distally compared with the proximal signal and collaterals exiting proximally and re-entering distally. CDU was considered as the reference standard for final diagnosis.
Statistical analysis
Assuming a PAD prevalence of 14%17 in T2DM, ABI sensitivity of 71%16 for PAD diagnosis in T2DM and a maximal margin of error of difference between ABI and CDU of 20%, the sample size calculated for ABI was 141, with 95% confidence level. Similarly, assuming the sensitivity of TcPO2 for PAD diagnosis in T2DM to be ≈ 48%,15 the sample size calculated for TcPO2 was 171. The higher of the two samples calculated (i.e., 171) was taken as the final required sample size. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of ABI and TcPO2 for PAD diagnosis were calculated against CDU. The agreements between ABI and CDU and TcPO2 and CDU were assessed using Cohen's kappa. Statistical analysis was performed using IBM SPSS software (version-19) and p < 0.05 was considered significant.
Results
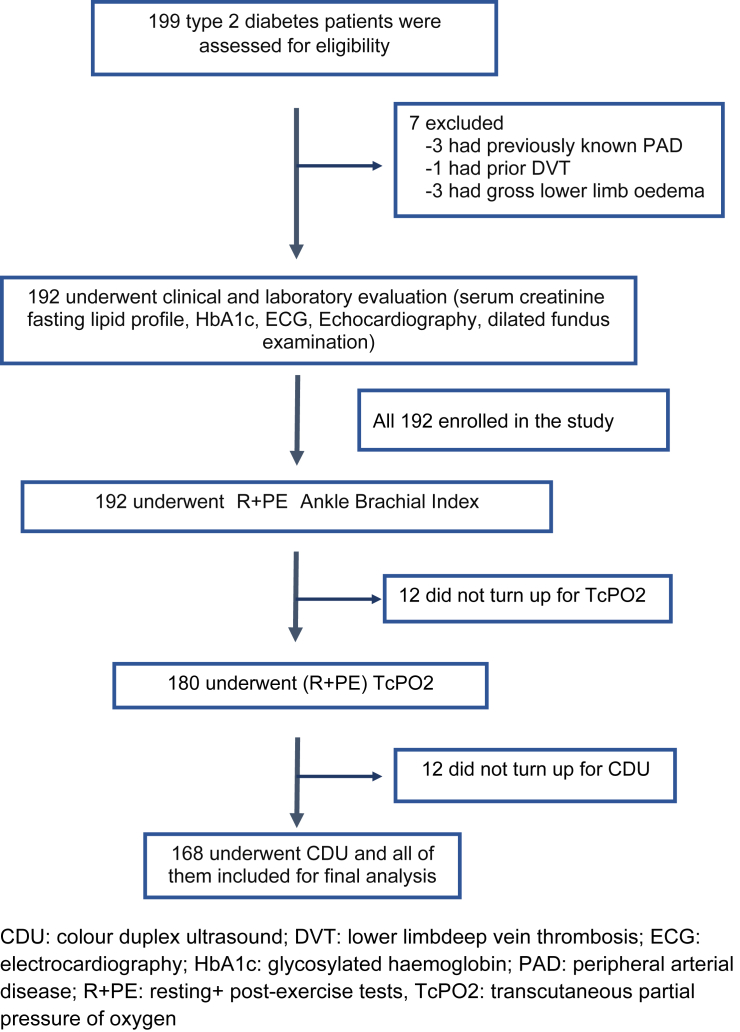
Fig. 1 shows enrolment of patients for the study. Finally, 168 patients (age range:35–78 years) completed ABI, TcPO2 and CDU (done in 336 lower limbs among 168 patients) and were included for analysis. Characteristics and risk factors for PAD among these patients are shown in Table 1. Majority (78%) of patients were aged >50 years.
Fig. 1.
Showing enrolment of study patients.
Table 1.
Characteristics and risk factors for PAD among patients.
| Parameter | Value |
|---|---|
| Age (years) mean ± SD | 56.9 ± 8.7 |
| Males No. (%) | 82 (49) |
| Diabetes duration (years) mean ± SD | 11.1 ± 4.2 |
| ≤10 No. (%) | 60 (35.7) |
| 10.1–20 No. (%) | 98 (58.3) |
| ≥20 No. (%) | 10 (6) |
| Lower limb intermittent claudicationa No. (%) | 8 (4.8) |
| Smokingb No. (%) | 75 (44.6) |
| Hypertension No. (%) | 96 (57) |
| Coronary artery disease No. (%) | 52 (31) |
| Cerebrovascular disease No. (%) | 4 (2.4) |
| BMI (kg/m2) mean ± SD | 24.1 ± 2.5 |
| <23 No. (%) | 46 (27.4) |
| 23.0–24.9 No. (%) | 68 (40.5) |
| ≥25 No. (%) | 54 (32) |
| Weak lower limb arterial pulses/trophic foot changes/foot ulcers No. (%) | 23 (13.7)c |
| Peripheral neuropathyd No. (%) | 62 (37) |
| Diabetic retinopathy No. (%) | 57 (34) |
| Dyslipidemiae No. (%) | 54 (32) |
| HbA1cf mean ± SD | 9.07 ± 1.5 |
| ≤7% No. (%) | 10 (6.7) |
| 7.1–8.0% No. (%) | 26 (17.4) |
| 8.1–10.0% No. (%) | 77 (51.6) |
| ≥10.0% No. (%) | 36 (24.2) |
| Nephropathyg No. (%) | 13 (7.7) |
| Treatment for type 2 diabetes No. (%) | |
| Oral drugs alone | 72 (42.6) |
| Oral drugs + insulin | 84 (49.7) |
| Insulin alone | 9 (5.3) |
| Non-pharmacological therapy | 3 (2) |
| Concomitant drug therapies No. (%) | |
| Statins | 102 (60.7) |
| Antiplatelet drugs | 81 (48.2) |
BMI: body mass index; HbA1c: glycosylated haemoglobin; SD: standard deviation.
Diagnosed based on Edinburgh Claudication Questionnaire.
Both current and former smokers included.
Five among these had intermittent claudication.
Diagnosed based on symptoms (numbness, paraesthesias, tingling, weakness, etc.) and/or objective signs of sensory loss, trophic changes in feet, loss of ankle jerk and wasting of small muscles of feet.
Defined by fasting low density lipoprotein-cholesterol ≥100 mg/dl, with or without statins.
HbA1c was available for 149 patients only.
Diagnosed based on 24-h urine protein excretion >300 mg and/or serum creatinine ≥1.5 mg/dl.
Final diagnosis of PAD based on CDU
Based on CDU, 17 (10%) were finally confirmed to have PAD [8 had proximal (common femoral/superficial femoral/profunda femoris artery) and 9 distal (popliteal/anterior tibial/posterior tibial/dorsalis pedis artery) stenoses]. Five (29.4%) patients with PAD reported intermittent claudication, 2 (11.8%) had poorly palpable lower extremity pulses and one (5.9%) had foot ulcer. There was significant association between PAD and age, male sex, smoking and CAD (p < 0.05 for all). However, the observed associations between PAD and age, sex, smoking and CAD might have been confounded by selection bias. Despite selection bias, there was no association between PAD and diabetes duration, hypertension, dyslipidemia, nephropathy, cerebrovascular disease, body mass index and HbA1c. Among 52 CAD patients, 11 (21.2%) had co-existing PAD.
Diagnosis of PAD based on ABI
Mean R-ABI among patients was 1.1 ± 0.2. Based on R-ABI ≤0.9, 16 (10 had ABI between 0.81 and 0.9 and 6 between 0.41 and 0.8) were initially diagnosed to have PAD (Table 2 and Fig. 2). Of these 16, 11 were finally confirmed to have PAD based on CDU and the remaining 5 who did not have PAD as per CDU had ABI between 0.8 and 0.9. On subjecting 145 with R-ABI between 0.91 and 1.40 to PE-ABI testing, 3 had postexercise decline of ABI by >20% (these 3 had R-ABI between 0.91 and 1.0) and all of them had PAD finally confirmed by CDU as well. Of the 7 patients with ABI >1.4 (suggests medial arterial calcification with noncompressible arteries), 3 (43%) had PAD diagnosed with CDU. R-ABI had sensitivity, specificity, PPV and NPV of 64.7% (11/17), 96.7% (146/151), 68.8% (11/16) and 96% (146/152), respectively, for PAD diagnosis (Table 2). PE-ABI increased the sensitivity of ABI by ≈ 18% and had PPV of 100% for PAD diagnosis. The sensitivity, specificity, PPV and NPV of R + PE ABI for PAD diagnosis were 82.3% (14/17), 96.7% (146/151), 73.7% (14/19) and 98% (146/149), respectively (Table 2). There was substantial agreement between R + PE ABI and CDU for PAD diagnosis (Cohen's kappa = 0.75, percentage of agreement = 95.3%, Table 2 and Fig. 2).
Table 2.
Shows diagnostic performance of ABI and TcPO2 against CDU.
| Final diagnosis based on CDU |
Total | |||
|---|---|---|---|---|
| No PAD | PAD | |||
| Diagnosis based on resting ABI alone | No PADa | 146 | 6 | 152 |
| PADb | 5 | 11 | 16 | |
| Total | 151 | 17 | 168 | |
| Diagnosis based on resting + post-exercise ABIc | No PAD | 146 | 3 | 149 |
| PAD | 5 | 14 | 19 | |
| Total | 151 | 17 | 168 | |
| Diagnosis based on resting TcPO2 alone | No PADd | 111 | 5 | 116 |
| PADe | 40 | 12 | 52 | |
| Total | 151 | 17 | 168 | |
| Diagnosis based on resting + post-exercise TcPO2f | No PAD | 103 | 4 | 107 |
| PAD | 48 | 13 | 61 | |
| Total | 151 | 17 | 168 | |
ABI: ankle brachial index; CDU: colour duplex ultrasound; PAD: peripheral arterial disease; TcPO2: transcutaneous partial pressure of oxygen.
Resting ABI >0.90 in both lower limbs.
Resting ABI ≤0.90 in either of the lower limbs.
Postexercise ABI decline of >20% from resting value in either of the limbs diagnosed PAD in those with resting ABI between 0.91 and 1.40 in both lower limbs.
Resting TcPO2 ≥30 mm Hg in both feet.
Resting TcPO2 <30 mm Hg in either of the feet.
Postexercise fall of TcPO2 to <30 mm Hg in either of the feet was diagnostic of PAD in those with resting TcPO2 ≥30 mm Hg in both feet.
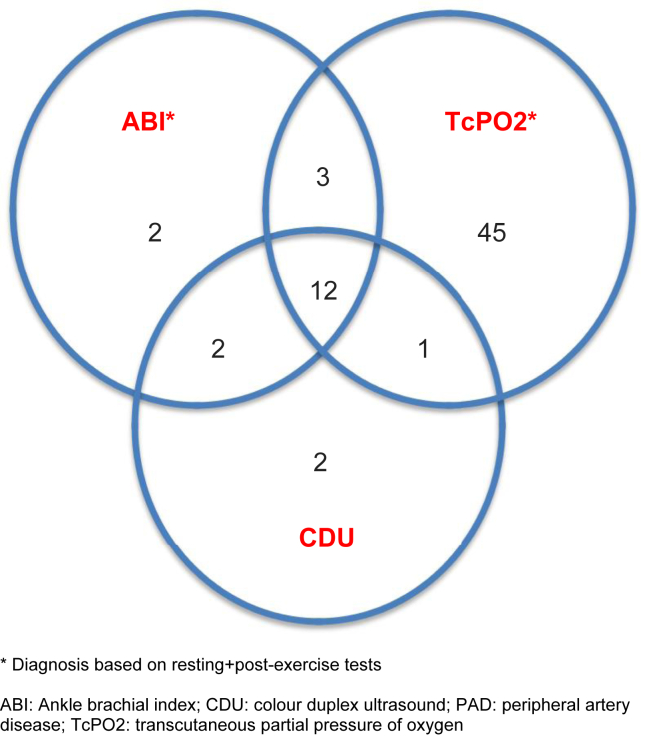
Fig. 2.
Venn diagram showing PAD diagnosis made by ABI, TcPO2, and CDU.
Diagnosis of PAD based on TcPO2
Mean R-TcPO2 among patients was 32.5 ± 12.8 mm Hg. Of the 52 patients who were diagnosed to have PAD based on R-TcPO2 <30 mm Hg, 12 were confirmed to have PAD with CDU. R-TcPO2 had sensitivity, specificity, PPV and NPV of 70.6% (12/17), 73.5% (111/151), 23% (12/52) and 95.7% (111/116), respectively (Table 2). Out of 116 patients with R-TcPO2 ≥30 mm Hg (13 had RTcPO2 of >50 mm Hg and 103 between 30 and 50 mm Hg), 9 were additionally diagnosed to have PAD based on fall of PE-TcPO2 to <30 mm Hg. However, only one among these nine was confirmed to have PAD based on CDU. Though postexercise TcPO2 increased the sensitivity of TcPO2 marginally by ˜ 6%, it had poor specificity. The sensitivity, specificity, PPV and NPV of R + PE TcPO2 for PAD diagnosis were 76.5% (13/17), 68.2% (103/151), 21.3% (13/61) and 96.2% (103/107), respectively (Table 2). There was poor agreement between R + PE TcPO2 and CDU for PAD diagnosis (Cohen's kappa = 0.20, percentage of agreement = 69%, Table 2 and Fig. 2). Of the 17 patients with PAD confirmed by CDU, only 2 (≈12%) had both ABI and TcPO2 (R + PE) within normal range (Fig. 2).
Discussion
The burgeoning epidemic of T2DM and the resultant increase in atherosclerotic cardiovascular disease burden pose significant challenges to the healthcare delivery systems worldwide. Although identification of asymptomatic lower extremity atherosclerotic PAD by ABI is important for identifying patients at high risk of cardiovascular events, ABI has limitations in diabetic patients.18 False negative results have been reported more often among diabetic patients, especially when there is significant ankle oedema, peripheral neuropathy, nephropathy/chronic kidney disease and in advanced age. This is probably the first Indian study to evaluate PE-ABI and R + PE TcPO2 for PAD diagnosis.
About 15% of patients in this study had clinical features (symptoms suggestive of vascular claudication, poorly palpable arterial pulses, trophic foot changes/ulcers, Table 1) to suggest possibility of PAD in them. However, despite presence of these features and risk factors for PAD9 (Table 1), the prevalence of PAD in the present study was only 10%. Clinical features have been reported to be neither sensitive nor specific for PAD in diabetes.6,15 Moreover, studies have reported lower prevalence of PAD among Indian diabetic patients (3.9–18%)17,19,20,21 compared to Westerners (16–29%).5,22 A study from northern India,17 involving T2DM patients with risk profile similar to present study, has reported prevalence of Intermittent claudication and PAD (4% and 14%, respectively) similar to present study (4.8% and 10%). Although selection bias might have confounded the observed associations between PAD and age, male sex, smoking and CAD in the present study, previous studies have documented higher risk of PAD associated with advanced age,1,2,17,20 male sex,21 smoking2,17 and CAD.1,2,6,17 About a fifth of patients with CAD had concomitant PAD in this study. A previous community-based study20 from India has reported 7% prevalence of PAD among CAD patients. The prevalence of CAD among PAD patients (≈65%) in the present study was higher compared to previous Indian studies (25–52%)17,21 most probably because of selection bias.
A study from southern India16 which excluded ABI >1.4 as uninterpretable ABI for final analysis has reported sensitivity and specificity of R-ABI of ≈71% and 89%, respectively, similar to present study (≈65% and 97%, respectively). Another study from northern India has reported high R-ABI sensitivity, specificity, PPV and NPV of 92%, 88%, 84% and 95%, respectively.23 The employment of PE-ABI, in addition to R-ABI, increased the sensitivity by ˜ 18%. The sensitivity of R + PE ABI in this study (82%) was higher compared to previous studies employing R-ABI alone.16,18,24 Moreover, PE-ABI had much higher PPV for PAD diagnosis compared to R-ABI (100% vs. 69%) in this study. Previous studies have also reported the utility of abnormal PE-ABI in predicting lower extremity revascularizations25,26 and major adverse cardiovascular events26 in diabetic patients. However, PE-ABI did not add to the accuracy of R-ABI among diabetic patients with suspected claudication in another study.27 The surprisingly high NPV of R-ABI (96%) in this study, contrary to several previous reports,6,8,9,18 can be explained by the confounding effect of much lower PAD prevalence (≈10%) in this study compared to previous studies. There was substantial agreement between ABI and CDU for PAD diagnosis in the present study, similar to findings in a previous study.28 Another Indian study found poor agreement between ABI and CDU for PAD diagnosis.16 Nearly 43% of patients with ABI >1.4 had PAD diagnosed by CDU in this study. In a previous study involving diabetic patients at high risk for atherosclerotic arterial disease, 58% with ABI >1.3 were diagnosed to have PAD by CDU.24 Peripheral neuropathy leads to false overestimation of ABI among diabetic patients, thereby reducing its sensitivity for PAD diagnosis.18 Despite presence of peripheral neuropathy in about a third of patients in this study (Table 1), the ABI sensitivity was still >80% most probably because of employment of PE-ABI.
The sensitivity, specificity, PPV and NPV of R-TcPO2 were 70.6%, 73.5%, 23% and 95.7%, respectively, which were inferior to that of R + PE ABI. A previous study15 has evaluated R-TcPO2 vis-à-vis CDU for PAD diagnosis among diabetic patients with foot ulcers and has reported much lower R-TcPO2 sensitivity, specificity, PPV and NPV of 28%, 66%, 28% and 66%, respectively. PE-TcPO2, unlike PE-ABI, did not improve the performance of R-TcPO2 in this study. Lower accuracy of TcPO2 for PAD, compared to ABI, is understandable given the fact that the former assesses tissue microcirculation (which may be affected by PAD or diabetic microvasculopathy) and the latter macrovascular disease. Moreover, TcPO2 values can be affected by diabetic microvasculopathy or peripheral neuropathy,29 even when there is no PAD. When both ABI and TcPO2 (R + PE) were normal, PAD could be safely excluded in nearly 90% of diabetic patients in the present study (Fig. 2).
Strengths and limitations
Employment of both R + PE ABI as well as TcPO2 in a relatively large number of T2DM patients helped us to ascertain their utility for PAD diagnosis. However, the present study has some limitations. The conduct of this study in hospital setting and enrolment of patients at moderate to high PAD risk may limit the generalisability of its results. A uniform, standardised exercise protocol such as treadmill test8 (TMT) could not be used in this study because of logistic constraints. However, simpler exercise protocols similar to the one used in this study have also been recommended to be useful.14 As PE-TcPO2 has been studied poorly so far, the use of PE-TcPO2 <30 mm Hg for PAD diagnosis in this study is not backed by evidence and might have been arbitrary.
Conclusion
PE-ABI increased the sensitivity of ABI from 64% to 82% and should be routinely considered for PAD diagnosis in T2DM patients at increased risk for developing PAD. TcPO2 (R/PE) was unreliable for PAD diagnosis as a stand-alone test because of its poor specificity. However, when both ABI and TcPO2 (R + PE tests) were normal, PAD could be safely excluded in nearly 90%.
Patient consent and confidentiality
Informed written consent has been obtained from all participating patients, and we declare that anonymity of data has been maintained, ensuring that patient confidentiality has been protected.
Ethics approval
This study was approved by the Institutional Ethics Committee (Human Research) of JIPMER, Pondicherry [Ethics Committee Approval Ref. No. JIP/IEC/SC/2016/33/982, approved on 15th December 2016]. This study involving humans has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Disclosure of competing interest
The authors have none to declare.
Acknowledgments
We gratefully acknowledge the help and guidance of Dr. R Amala, Assistant Professor, Dept. of Biostatistics, JIPMER, Puducherry while carrying out statistical analysis for this study.
This study was funded by Intramural Research Grant from Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India. Grant reference number - JIP/Res/Intra-MD-MS/phs1/01/grant 10/2017-18 dated 13th September 2017.
References
- 1.Criqui M.H. Peripheral arterial disease--epidemiological aspects. Vasc Med. 2001;6:3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- 2.Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 3.Resnick H.E., Lindsay R.S., McDermott M.M., et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 4.Murabito J.M., D'Agostino R.B., Silbershatz H., Wilson W.F. Intermittent claudication. A risk profile from the Framingham heart study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch A.T., Criqui M.H., Treat-Jacobson D., et al. Peripheral arterial disease detection, awareness, and treatment in primary care. J Am Med Assoc. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 7.Xu Dachun, Li Jue, Zou Liling, et al. Sensitivity and specificity of the ankle--brachial index to diagnose peripheral artery disease: a structured review. Vasc Med. 2010;15:361–369. doi: 10.1177/1358863X10378376. [DOI] [PubMed] [Google Scholar]
- 8.Aboyans V., Criqui M.H., Abraham P., et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Meijer V.E., Van't Sant H.P., Spronk S., Kusters F.J., den Hoed P.T. Reference value of transcutaneous oxygen measurement in diabetic patients compared with nondiabetic patients. Vasc Surg. 2008;48:382–388. doi: 10.1016/j.jvs.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Gazzaruso C., Coppola A., Falcone C., et al. Transcutaneous oxygen tension as a potential predictor of cardiovascular events in type 2 diabetes: comparison with ankle-brachial index. Diabetes Care. 2013;36:1720–1725. doi: 10.2337/dc12-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelan J.F., Barry M.H., Moir J.D. Color flow Doppler ultrasonography: comparison with peripheral arteriography for the investigation of peripheral vascular disease. J Clin Ultrasound. 1992;20:369–374. doi: 10.1002/jcu.1870200602. [DOI] [PubMed] [Google Scholar]
- 13.Leng G.C., Fowkes F.G. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101–1109. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 14.Norgren L., Hiatt W.R., Dormandy J.A., et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33:S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Vriens B., D'Abate F., Ozdemir B.A., et al. Clinical examination and non-invasive screening tests in the diagnosis of peripheral artery disease in people with diabetes-related foot ulceration. Diabet Med. 2018;35:895–902. doi: 10.1111/dme.13634. [DOI] [PubMed] [Google Scholar]
- 16.Premalatha G., Ravikumar R., Sanjay R., Deepa R., Mohan V. Comparison of colour duplex ultrasound and ankle-brachial pressure index measurements in peripheral vascular disease in type 2 diabetic patients with foot infections. J Assoc Phys India. 2002;50:1240–1244. [PubMed] [Google Scholar]
- 17.Agarwal A.K., Singh M., Arya V., Garg U., Singh V.P., Jain V. Prevalence of peripheral arterial disease in type 2 diabetes mellitus and its correlation with coronary artery disease and its risk factors. J Assoc Phys India. 2012;60:28–32. [PubMed] [Google Scholar]
- 18.Potier L., Abi Khalil C., Mohammedi K., Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg. 2011;41:110–116. doi: 10.1016/j.ejvs.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Eshcol J., Jebarani S., Anjana R.M., Mohan V., Pradeepa R. Prevalence, incidence and progression of peripheral arterial disease in Asian Indian type 2 diabetic patients. J Diabet Complicat. 2014;28:627-31. doi: 10.1016/j.jdiacomp.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Premalatha G., Shanthirani S., Deepa R., Markovitz J., Mohan V. Prevalence and risk factors of peripheral vascular disease in a selected South Indian population: the Chennai Urban Population Study. Diabetes Care. 2000;23:1295–1300. doi: 10.2337/diacare.23.9.1295. [DOI] [PubMed] [Google Scholar]
- 21.Sosale B., Reddy Y., Nagbhushana M.V., Sosale A., Jude E.B. Peripheral arterial disease in patients with type 2 diabetes mellitus in South India: the urban vs rural divide. J Acad Med Sci. 2012;2:105–109. [Google Scholar]
- 22.Janka H.U., Standl E., Mehnert H. Peripheral vascular disease in diabetes mellitus and its relation to cardiovascular risk factors: screening with the Doppler ultrasonic technique. Diabetes Care. 1980;3:207–213. doi: 10.2337/diacare.3.2.207. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A., Tyagi V.K., Bansal N., Virmani S.K., Sirohi T.R. Comparison of ankle brachial pressure index to arterial Doppler USG in the diagnosis of peripheral vascular disease in diabetes mellitus. Int J Adv Med. 2017;4:1562–1565. [Google Scholar]
- 24.Potier L., Halbron M., Bouilloud F., et al. Ankle-to-brachial ratio index underestimates the prevalence of peripheral occlusive disease in diabetic patients at high risk for arterial disease. Diabetes Care. 2009;32(4):e44. doi: 10.2337/dc08-2015. [DOI] [PubMed] [Google Scholar]
- 25.Hammad T.A., Strefling J.A., Zellers P.R., et al. The effect of post-exercise ankle-brachial index on lower extremity revascularization. JACC Cardiovasc Interv. 2015;8:1238–1244. doi: 10.1016/j.jcin.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Diehm C., Darius H., Pittrow D., et al. Prognostic value of a low post-exercise ankle brachial index as assessed by primary care physicians. Atherosclerosis. 2011;214:364–372. doi: 10.1016/j.atherosclerosis.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Tehan P.E., Barwick A.L., Sebastian M., Chuter V.H. Diagnostic accuracy of the postexercise ankle-brachial index for detecting peripheral artery disease in suspected claudicants with and without diabetes. Vasc Med. 2018;23:116–125. doi: 10.1177/1358863X17751259. [DOI] [PubMed] [Google Scholar]
- 28.Alnaeb M.E., Crabtree V.P., Boutin A., Mikhailidis D.P., Seifalian A.M., Hamilton G. Prospective assessment of lower-extremity peripheral arterial disease in diabetic patients using a novel automated optical device. Angiology. 2007;58:579–585. doi: 10.1177/0003319707305685. [DOI] [PubMed] [Google Scholar]
- 29.Chao C.Y., Cheing G.L. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009;25:604–614. doi: 10.1002/dmrr.1004. [DOI] [PubMed] [Google Scholar]