Abstract
Gut microbiota contributes to diverse mammalian processes including the metabolic functions of drugs. It is a potential new territory for drug targeting, especially for dietary natural compounds such as tannins, flavonoids, steroidal glycosides, anthocyanins, lignans, alkaloids, and others. Because most herbal medicines are orally administered, the chemical profile and corresponding bioactivities of herbal medicines may be altered and implication to ailments by specific microbiota through gut microbiota metabolisms (GMMs) and gut microbiota biotransformations (GMBTs). In this review, briefly introducing the interactions between different categories of natural compounds and gut microbiota produced countless microbial degraded or fragmented metabolites with their biological significance in rodent-based models. From natural product chemistry division, thousands of molecules are produced, degraded, synthesized, and isolated from natural sources but exploited due to lack of biological significance. In this direction, we add a Bio-Chemoinformatics approach to get clues of biology through a specific microbial assault to (Natural products) NPs.
Keywords: Gut microbiota, Dietary natural compounds, Gut-microbial diversity, Mutualism strategies, GMM-GMBTs reactions, Bio-chemoinformatics, Gut microbiota-interplay disorders
Graphical abstract
1. Introduction
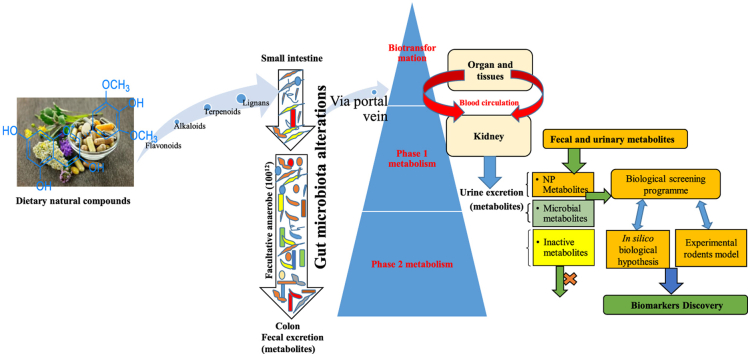
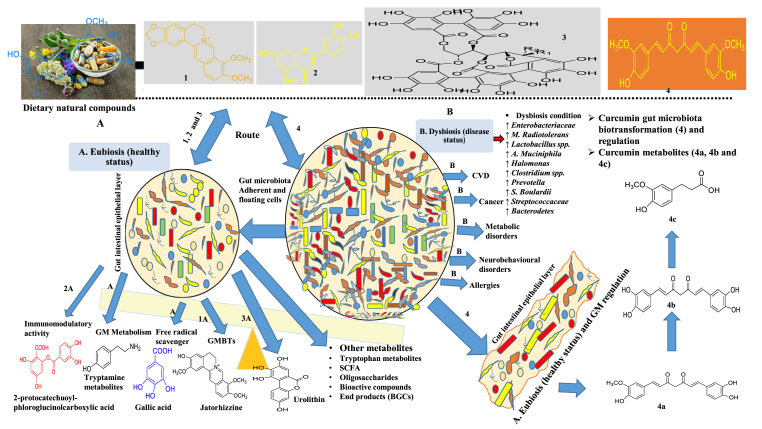
The human gut is colonized by an extremely dense population of bacteria, collectively termed as microbiota or gut “flora” and is a site where they exert strong influences on human biology as well as drug fate.1,2 The entire system of human gut microbiota can be pictured as a microbial organ, which is closely associated with diverse processes including the metabolic function of drugs.3 Gut microbiota might be a potential new territory for drug targeting, especially for dietary (Natural products) NPs.4 A better understanding of the mechanisms of these interactions has led to greater interest in the corresponding secondary metabolites.5 Gut microbiota produces NPs in two distinct ways. Firstly, the metabolites are produced by gut microbiota from dietary components, such as tryptophan metabolites,6 short-chain fatty acids, oligosaccharides and, others. Secondly, the production of uncharacterized end products from the unique biosynthetic gene clusters (BGCs) of the gut microbiota. The past three decades have witnessed of great progress in BGC-derived natural product discovery from the mammalian gut microbiota. These NPs have potential for novel bioactive functions,7 such as the antibiotic microcin M/H47,8 the genotoxin colibactin,9 the cytotoxic compound tilivalline,10 and the protease inhibitors dipeptide aldehydes.11 These distinct secondary metabolites are played vital roles in understanding gut microbiota and in developing the pharmaceutical, agricultural, and food industries. As the concern of NPs, it shows a promising interplay host-microbiota functional interaction to the regulation of gut microbiota associated with several disorders like CVD, cancer, allergies, asthma, Crohn's disorder, and metabolic disorders mainly influenced under dysbiosis status. Pure compound curcumin gives eubiosis status and regulates gut microbiota-associated dysbiosis status from biotransformation with demethylation, hydrogenation, hydroxylation reactions as shown in (Fig. 1A and B).
Fig. 1.
(A) Roles and modulation of gut microbiota. In addition transforming natural product metabolites i.e. biologically active regulates dysbiosis-eubiosis status. (B) Bidirectional interaction between curcumin and gut microbiota. Curcumin biotransformed by gut microbiota to more bioactive metabolites by several reactions than parent curcumin. After administration of curcumin, it regulate the gut microbiota by transformation of metabolites that gives alteration of microbial abundance, diversity and composition, which may exert health benefits via indirect mechanism.
Trillions of microorganisms colonize the human body, maintaining homeostasis and significantly influence human health and disease. These organisms are collectively known as the gut microbiota. It comprises many different bacterial species that are in dynamic, localized, close association with each other and with the host and play a vital role in the progression of several diseases. These microbiota are responsible for the extensive breakdown of the original dietary natural compounds into a low molecular weight metabolites, being absorbable, may actually be responsible for the health effects, rather than the original compounds. In total, the intestinal microbiota consists of approximately 500–1,000 species that, interestingly, belong to only a few of the known bacterial phyla with relative (%) abundance as shown in (Supplementary materials, Table 1). Gut bacteria can have beneficial effects such as the digestion of complex carbohydrates, colonization resistance against invading pathogens, maturation of the adaptive mucosal immune system, and the production of secondary metabolites, including vitamins. The evidence for host benefits comes both from our understanding of the metabolic services that the gut microbiota provides and studies of germ-free animal models.12 The ecosystem of the gastrointestinal tract is characterized by a great number of microbial species living in balance (eubiosis/dysbiosis) by adopting mutualistic strategies. Dysbiosis and chronic, subclinical, low-grade inflammation in the bowel wall contribute to and may even initiate the development of CRC, IBD to other extra-intestinal disorders13 as shown in (Supplementary materials, Table 2).
Some orally administered NPs by their alteration of chemical structures by GMMs and GMBTs to get into >1 fragmented metabolites, which exert better bioavailability and therapeutic effects than their parent compounds against ailments. Despite these NPs, others remain without biological significance. The components of these NPs regarding special computational biological screening through Bio-Cheminformatic tools are fewer reported. Three bioflavonoids such as indigocarpan, mucronulatol, indigocarpan diacetate, and two diterpenes namely erythroxydiol X and Y were derived from I. aspalathoides as PDGFRβ and VEGFR2 inhibitors nicely reported.14 Similarly, natural flavonoids and synthetic indole-chalcones were tested for in silico pharmacokinetic properties for validated as drug-like nature.15 Moreover, the analysis of therapeutic target for SARS-CoV-2 and discovery of potential drugs reported by computational methods.16 In the presented report, efforts have been made to discover and highlight strategies for further exploring the biosynthetic capacity of the human microbiome with distinct NPs. Besides, also briefly discusses the potential biological roles of these metabolites. From, Bio-Chemoinformatics approach that relies on in silico biological hypothesis with well-defined ADME protocol, which recommends for all probable degraded NP metabolites biopredictory role by the action of gut microbiota. At last, we reported some biology of NPs with gut microbiota modulation with the special context of biological disorders like cancer, NAFLD, GIP, metabolic, and neurobehavioral disorders. So, this report harnessing of NP metabolites predicted with in silico biological hypothesis. This tool protects the exploitation and makes use of degraded metabolites for the Natural Product Chemistry division for a further drug-discovery innovation program.
2. Gut microbiota functional transformation of NPs from GMM and GMBTS
Gut bacteria can hydrolyze glycosides, glucuronides, amides, esters, sulfates, and lactones. They undergo reduction, de-carboxylation, de-methylation, ring cleavage, and dihydroxylation reactions.17 The report aimed to summarize the current information about the microbial degradation metabolites and their formation pathways obtained from the different groups of dietary NPs, identifying their differences and similarities. Recent progress in the identification of colonic microbial species responsible for NPs and novel tools used to identify them and the impact of NPs microbial metabolism and biotransformation on their bioavailability and bioactivity were also reviewed.
2.1. Gut microbiota metabolism
The metabolic crosstalk between the host and gut microbiota modulates the pharmacokinetic and pharmacodynamic properties of NPs.18 Direct and indirect mechanism of gut microbiota is very much renowned for the promising effect of pharmacokinetic by participating in the direct metabolism of NPs or through its indirect interaction with the host enzymatic system mechanistically. The indirect interaction is facilitated through the production of microbial or mammalian microbial co-metabolites that compete for the metabolism of xenobiotics compounds found in our diet including NPs or act as signaling molecules that influence the host gene expression.19 All types of probable metabolic conversions of NPs with specific microbiota are shown in (Fig. 2).
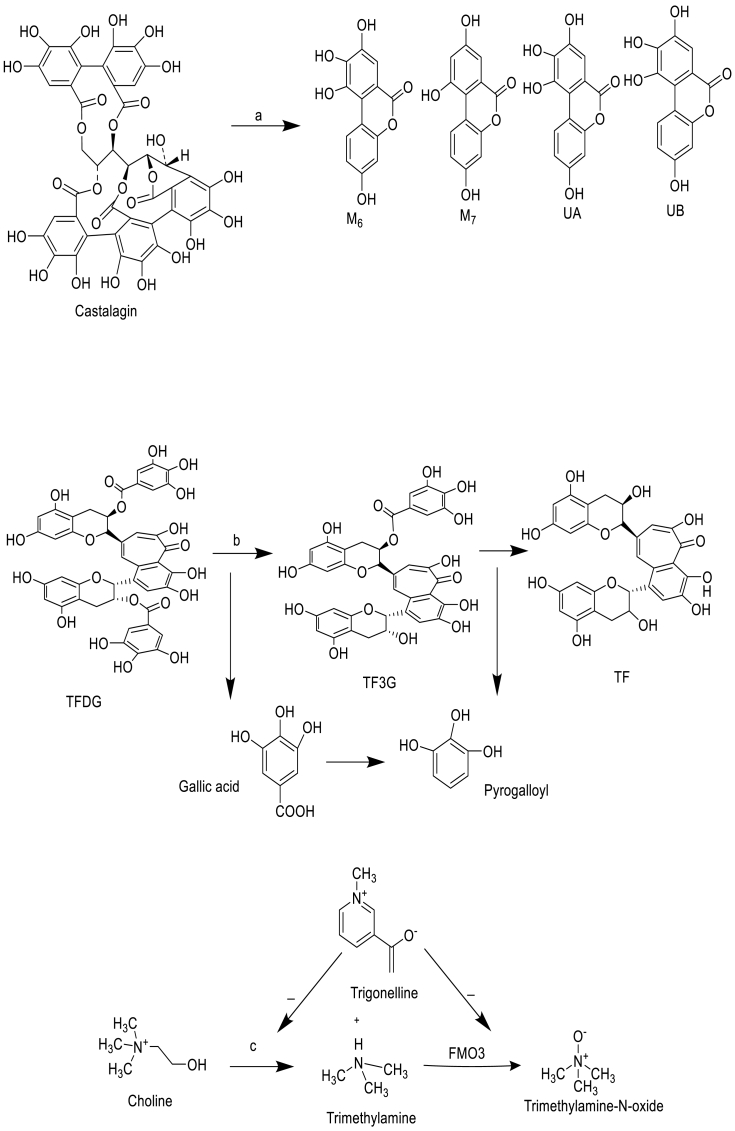
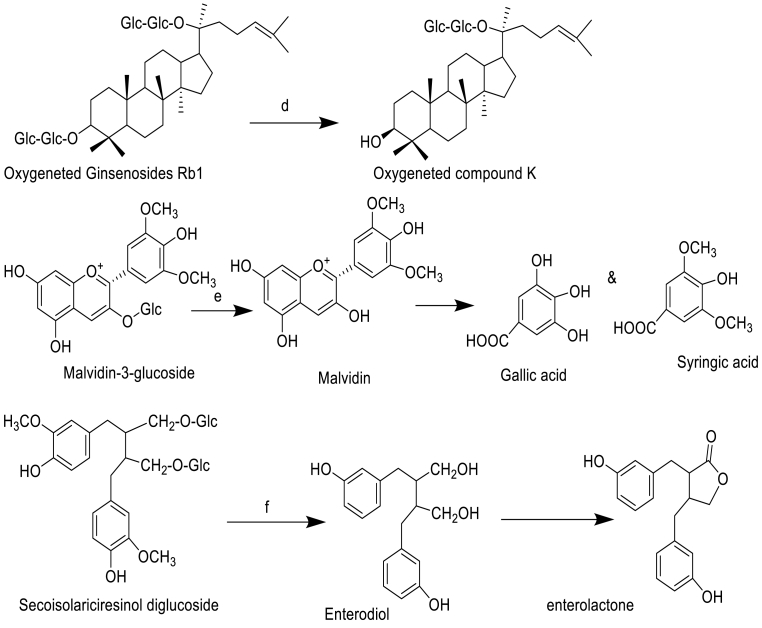
Fig. 2.
Gut microbiota metabolism of selected NPs with respect to name of microbiota: a, Gordonibacter urolithinfaciens and G. pamelaeae DSM 19378; b, Lactobacillus plantarum 299, Bacillus subtilis; c, Citrobecter freundii and flavin monooxygenase inhibition; d, Eubacterium, Clostridiales; e, Lactobacillus plantarum, Lactobacillus casei, and Bifdobacterium lactis; f, Peptostreptococcus and Eubacterium species.
The metabolic fate of ETs molecules with gut microbiota using in vivo cultures was nicely reported.20 This depends upon a rate-limiting manner with promising yield of microbial metabolites and other derivatives of ellagic acid. Two species of bacteria, Gordonibacter urolithinfaciens and G. pamelaeae DSM 19378, being able to sequentially metabolize 1 to urolithins M5, M6, and UC (Fig. 2). The species of microbiota metabolizing UA and UB and those responsible for alternative modes of deoxygenation remain unidentified.20
Theaflavins (TF), theaflavin-3-gallate (TF3G), theaflavin-3′-gallate (TF3′G), and theaflavin-3, 3′-digallate (TFDG) are the major bioactive polyphenols present in black tea. From fecal microbiota from healthy human volunteers involved in the metabolism of TFDG, and found that TFDG is metabolized to TF, TF3G, TF3′G, gallic acid, and pyrogallol. Moreover, both TF3G and TF3′G are metabolized to TF, gallic acid, and pyrogallol by human microbiota. Lactobacillus plantarum 299v and Bacillus subtilis (Fig. 2) can metabolize to TFDG.21
Trigonelline significantly reduced levels of TMAO and was estimated due to flavin mono-oxygenase (FMO3) enzyme inhibition under ex vivo conditions. So, the gut microbiota in choline metabolism and consequently the production of metabolites was causing CVD. The growth of isolated bacteria C. freundii strain was checked by Kirby-Bauer disk diffusion susceptibility test in the presence and absence of trigonelline. Further, in vitro conversion of trimethylamine (TMA) to trimethylamine oxide (TMAO) through isolated liver FMO3 enzyme was studied and in absence of trigonelline, TMAO (Fig. 2) was formed.22
The conversion of ginsenosides (Rb1) in the gastrointestinal tract has been largely studied using in vitro and in vivo studies. The de-glycosylation reactions occurred by intestinal bacteria Eubacterium, Clostridiales via stepwise cleavage of the sugar moieties. The metabolic product of Rb1 i.e. compound K has been reported to have potential antitumor effects and stronger than its parent compound ginsenosides Rb1 and Rd (Fig. 2).
Anthocyanins as anthocyanidin aglycons (cyanidin, pelargonidin, and malvidin) have very low bioavailability so do not appear to undergo extensive metabolism. Large amounts of these compounds enter the colon, where undergo de-glycosylated by gut microbiota. Malvidin-3-glucoside (from grape extracts) with L. plantarum, L. casei (Fig. 2) under incubation to the formation of syringic, gallic, and p-coumaric acids.
Lignans (1,4 diarylbutane structure such as secoisolariciresinol, syringaresinol, and others) metabolism includes glucuronidation and to a lesser degree of sulfation. The biological activity of lignans is related to the activation of these compounds by Bacteroides and Clostridium species (in the gut microbiota) to enterolactone and enterodiol (Fig. 2). This transformation of lignans was carried out after de-methylation and de-hydroxylation reactions (carried out by Peptostreptococcus and Eubacterium species) and probable derivatives reached circulation.23
2.2. Gut microbiota biotransformation
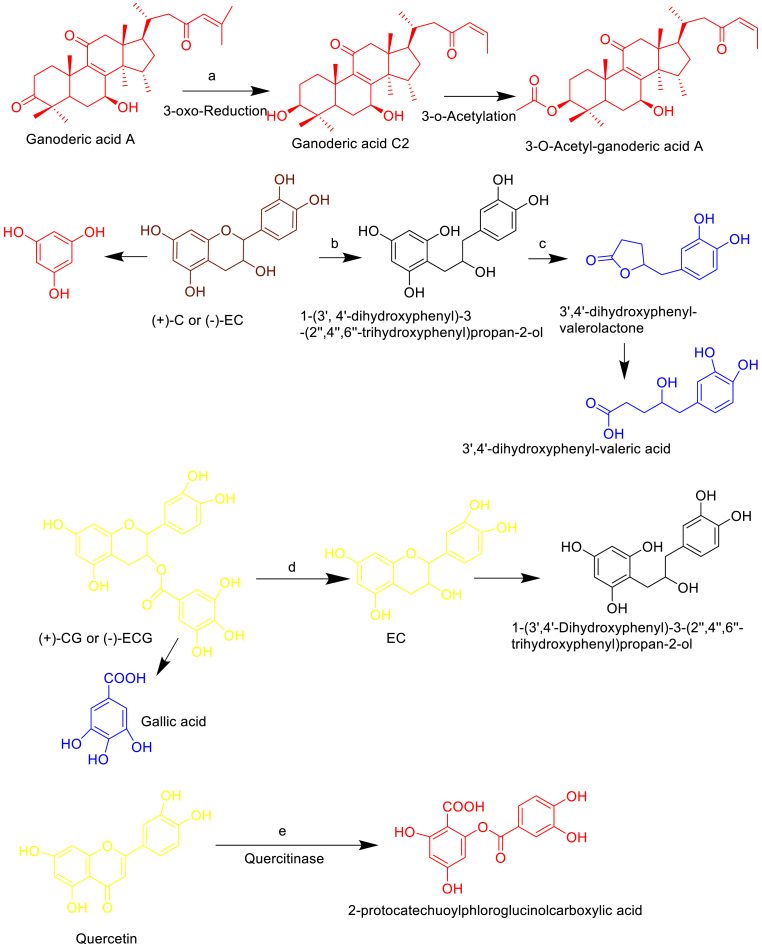
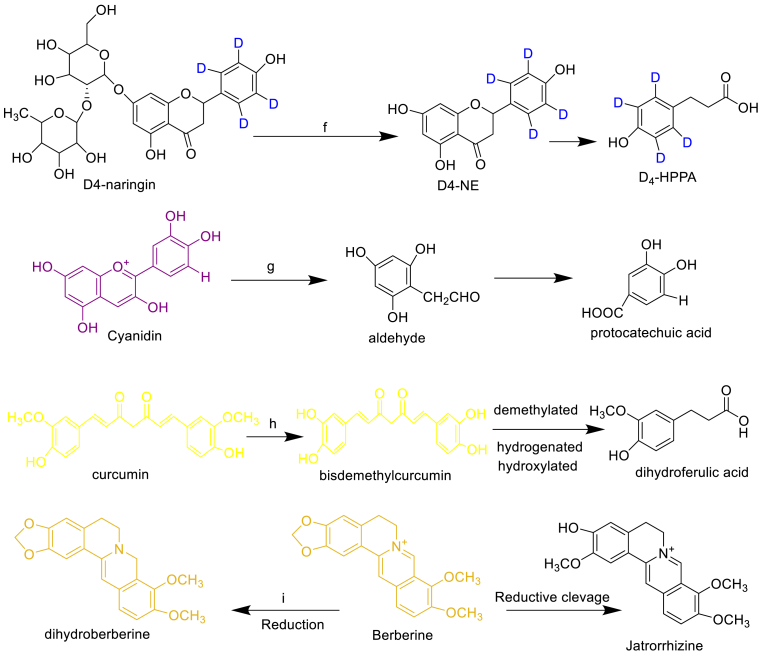
Some well-known investigations might be substantially inspired by the gut microbiota biotransformation of selected NPs as shown in (Fig. 3).
Fig. 3.
Gut microbiota biotransformation of selected NPs with respect to name of microbiota: a, Streptomyces sp. AI 045; b, Eggerthella lenta rK3; c, Eggerthella lenta rK3; d, Bifidobacterium and Clostridium; e, Streptomyces urythermus, Aspergillus niger, A. flavus, Penicillium olsonii, P. decumbens; f, Clostridum spp., E. ramulus; g, L. plantaurum and L. casei; h, Blautia sp. Escherichia fergusonii, Escherichia coli; i, Escherichia coli.
Although numerous triterpenoids have been identified from the medicinal fungus G. lucidum,24 few studies have been published on the microbial biotransformation of ganoderma triterpenoids. Gut microbiota biotransformation of ganoderma triterpenoid GA-A and with soil-isolated Streptomyces sp. AI 045, making it the first case in which a microbe was found to biotransform triterpenoid to its 3-O-acetyl derivative. This is the first time that the acetylation metabolite 3-O-acetyl GA-A has been identified. Although numerous triterpenoids of G. lucidum were reported for several biological disorders.25
Catechins, revealed that both (+)-C and (–)-EC biotransformed to 1-(3′, 4′-dihydroxyphenyl)-3-(2'',4'',6''-trihydroxyphenyl)propan-2-ol by Eggerthella lenta rK3. However, the conversion of (+)- proceeded five times faster than that of (–)-EC. Flavonifractorplautii aK2 further converted 1-(3′,4′-dihydroxyphenyl)-3-(2'',4'',6''- trihydroxyphenyl) propan-2-ol to δ-(3′,4′-dihydroxyphenyl)-γ-valerolactone and δ-(3′,4′-dihydroxyphenyl)-γ-valeric acid and other small metabolites. (Fig. 3).26
The (+)-catechin gallate (CG) and (–)-epicatechin gallate (ECG), (+)-CG and (–) ECG are the 3-O-gallate products of (+)-C and (–)-EC, respectively. Since (–)-ECG is the major constituent of tea, its biotransformation has extensively been investigated, while the biotransformation of (+)-CG has not been reported. The biotransformation of (–)-ECG is discussed. The first step of microbial metabolism of (–)-ECG is hydrolysis, giving gallic acid and (–)-EC. Gallic acid is metabolized to pyrogallol, and the metabolism of (–)-EC follows that discussed and reported that 13 metabolites were found after incubation of (–)-ECG with human intestinal microbiota (Fig. 3) (Bifidobacterium strain). Epicatechin (+)-C, (–)-EC and epigallocathechin gallate (+)-CG and (–) ECG biotransformed to 3-(3-hydroxypjenyl) propionic acid and 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone by Bifidobacterium and Clostridium.
Flavanones and flavonols in hops are mainly represented by derivatives of naringenin (flavanone) and quercetin (flavonol), and are subjected to microbial transformations in the heterocyclic C ring. Quercetin is converted via oxygenolytic ring-cleavage, catalyzed by flavonol 2, 3- di-oxygenase (quercetinase), leading to the formation of 2-protocatechuoylphloroglucinol carboxylic acid (Fig. 3). Quercetinase activity was found in several fungal strains such as Streptomyces urythermus, Aspergillus niger, A. flavus, Penicillium olsonii and P. decumbents.27
In plants, most flavanones are found as glycosides (e.g. D4-naringin) and they are initially de-glycosylated followed by C-ring cleavage with β-glucosidase enzyme responsible microbiota to give D4-NE ([2′, 3′, 5′, 6′-D4] naringenin) and D4-HPPA (3-(4′-hydroxyphenyl)-[2′, 3′, 5′, 6′-D4] propanoic acid). Clostridum strains and E. ramulus (Fig. 3) are responsible for this conversion. In the colon, flavone C-multiglucosides undergointestinal absorption without any degradation, translocate to the liver through portal circulation, and then re-circulates intact to the intestine through enterohepatic circulation.
Anthocyanins undergo de-glycosylation by bacterial enzymes (e.g. α, l-rhamnosidase and β, d-glucosidase), and then cleavage of C-ring at various sites results in formation of aldehydes (Fig. 3) derived from A-ring and small phenolic acids from B-ring. This report concluded that the first step of the bacterial biotransformation (L. plantaurum and L. casei) to yield tentative small metabolites. De-carboxylation and de-hydroxylation products of protocatechuic acid, catechol, and phenol, were identified as metabolites.
Tan et al., investigated the biotransformation of three curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) by human fecal microbiota (Fig. 3) using a model in vitro. Three main metabolites, including tetrahydrocurcumin, dihydroferulic acid and 1-(4-hydroxy- 3-methoxyphenyl)-2-propanol, were detected through the use of ultrahigh-pressure liquid chromatography (UHPLC), coupled with a linear ion trap mass spectrometer after 24 h of fermentation. It was employed ultra-performance liquid chromatography coupled with quadrupole time of flight mass spectrometry (UPLC-Q-TOF-MS) with automated data analysis software to identify curcumin metabolites produced by human intestinal flora. A total of 23 metabolites were registered and several novel human gut microbiota curcumin metabolic pathways, via demethylation, reduction, hydroxylation, and acetylation, or the combination of these, were revealed. Burapan et al., reported their findings of demethylation as an important metabolism pathway of curcuminoids (a mixture of curcumin (1), demethoxycurcumin (2), and bisdemethoxycurcumin (3), by the human intestinal bacterium Blautia sp. MRG-PMF1. It was found that curcumin was converted to two new metabolites, demethylcurcumin and bisdemethylcurcumin through the methyl aryl ether cleavage reaction. Furthermore, Shen et al. studied, the biotransformation of curcuminoids by Escherichia fergusonii (ATCC 35469) and two Escherichia coli strains (ATCC 8739 and DH10B).28
The major problem of berberine with poor oral bioavailability (<5%) and a quiet low concentration in plasma while its metabolites were usually maintained at high plasma concentrations.29 Nitroreductases (≥90%, recombinant, expressed in Escherichia coli) produced by gut microbiota (Fig. 3) could convert BBR into dihydroberberine (dhBBR), which had an intestinal absorption rate that was five-fold higher than that of BBR. However, an unstable form of dhBBR and would be oxidized to BBR in intestines before the final absorption. This summarizes the role of the gut microbiota biotransformation in regulating the conversion-absorption-reversion process of BBR in the intestine system. In another study, anaerobic cultured gut microbiota of rats could also transform BBR into other metabolites, including berberrubine (BRB), demethyleneberberine (DMB) and jatrorrhizine (JAT). BRB, DMB and JAT possessed higher lipophilicity than BBR, and were of great significance for many therapeutic activities of BBR.30,31
3. The experimental perspective of NP metabolites via computational hypothesis approach
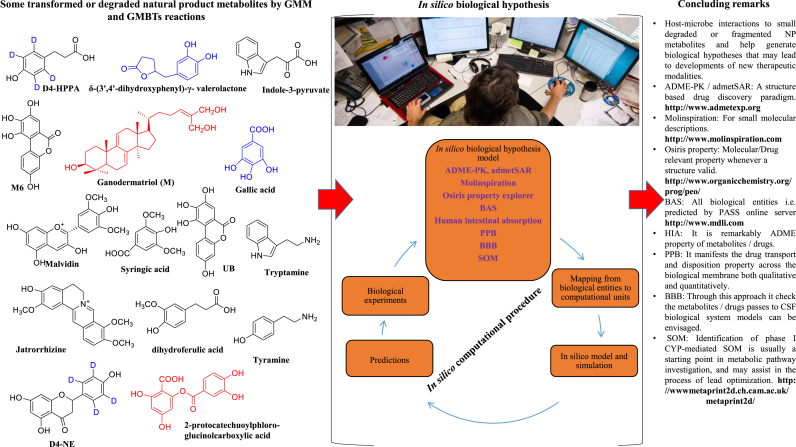
The discovery of new pharmaceutical drugs is one of the preeminent tasks—scientifically, economically, and socially in biomedical research. Advances in bioinformatics and computational biology have increased productivity at many stages of the drug discovery pipeline. So, several procedures of biochemoinformatics via computational tool or free web server to identify and predicts the significant NP metabolites in terms of the score of specific pharmacological activity by well-known models and databases (Fig. 4). Nowadays these in silico computational approaches play a vital role in drug discovery development of natural products via routine experimental analysis in the laboratory and in the future to prevent the exploitation of valuable metabolites and molecules.32
Fig. 4.
Schematic illustrations of the GMM and GMBTs metabolite undrgoes in silico biological hypothesis through computational chemistry to biology models of valid natural product metabolites gives full remarks via biochemoinformatics tools-drug discovery, chemical structure, biological targets, biological activity fingerprint, toxicity profiling, site of metabolism and data visualization to predicts possible chemistry and biology before wet lab experimental procedure.
3.1. In silico biological hypothesis
Such methods have been integrated approach to frequent use in the discovery and optimization of plant, microbe derived molecules and metabolites with affinity to a target, the clarification of absorption, distribution, metabolism, excretion, toxicity properties (ADME-PK models), as well as physicochemical and biological characterization like drug likeliness, biological activity spectrum, ligand-receptors based and metabolic predictions (SOM).32 So that utility of drug discovery will become functionalized.
3.1.1. In silico pharmacokinetics
These tools to investigate absorption, distribution, metabolism, excretion, and pharmacokinetics (ADME-PK) properties of new chemical entities (NCEs) i.e. new metabolites are an integral part of the current industrial drug discovery paradigm.32
3.1.2. Molinspiration
Molecular descriptors and drug likeliness properties of metabolites and molecules were analyzed by the Molinspiration server (http://www.molinspiration.com), based on Lipinski Rules of five. It states that most “drug like” molecules must have log P ≤ 5, molecular weight ≤500, number of hydrogen bond acceptors ≤10, and number of hydrogen bond donors ≤5. It calculates the molecular properties such as (LogP, number of hydrogen bond donors, acceptors, and polar surface area) with a prediction of bioactivity score with respective drug targets (GPCR ligands), kinase inhibitors, ion channel modulators, enzymes, and nuclear receptors.
3.1.3. Toxicity risk assessment
This assesses the undesirable toxic properties of our metabolites and compounds from Osiris Property Explorer (http://www.organicchemistry.org/prog/peo) was used. It shows in terms of mutagenic, tumorogenic, irritant, reproductive effects, drug-relevant properties [c Log P, Log S (Solubility)], molecular weight, and overall drug-score were calculated with different color codes.
3.1.4. Biological activity spectrum
It describes the intrinsic properties of the compound depends on its structures with high particularities. The set of pharmacological effects, mechanisms of action, and specific toxicities that might be exhibited by a particular compound in its interaction with biological entities are predicted by PASS (http://www.mdli.com). PASS uses Sdffile (.sdf) or MOL file (.mol) formats as an external source of structure and activity data. Their values vary from 0.000 to 1.000. Only those activity types for which Pa > Pi was considered possible.
3.1.5. Human intestinal absorption (HIA)
A large amount of data regarding HIA has been produced rapidly by in vivo and in vitro experimental assays. Many computational classification and correlation models have been developed to predict the HIA-based data. The most influential substructure patterns and metabolites are recognized by an information gain analysis.
3.1.6. Plasma Protein Binding (PPB)
These models can also be classified as ligand and receptor-based models, as shown in (Fig. 4). The characteristics of small molecules can be directly used for binding sites and affinity prediction, forming some ligand-based HSA binding models.
3.1.7. Blood-brain Barrier (BBB)
Most in silico models that are devoted to this aim are based on the assumption that compounds are transported across the BBB by passive diffusion. Lanevskij et al.,33 developed a simple QSAR model based on log P, pKa, and fraction unbound on the plasma for log BB prediction, also considering the influence of brain tissue binding by estimating the negative logarithm of the fraction that is unbound in the brain (−log fu, br) with a non-linear ionization-specific model that is based on log P and pKa. As a result, the model demonstrated good predictive power for both internal and external validations.
3.1.8. Metabolism
Currently, metabolism-related prediction models have mainly focused on the following studies: (1) the interaction models of enzymes with xenobiotics/molecules, which were often used to distinguish whether a xenobiotic is a substrate or inhibitor of Cytochrome P450 monooxygenase system, and then to evaluate DDIs; (2) the clearance models of the liver that could quantitatively predict the metabolic stability of xenobiotics.
The interaction of dietary natural compounds (like polyphenols, triterpenoids, polysaccharides and others) and gut microbiota plays a substantial role in human health considerations. To solve this complex category of natural compounds, the genome scale metabolic networks and bioinformatic tool predicts the novel bioactive metabolites through gut microbiota transformations. Recently, AGORA-based REconstruction for Diet Analysis (AGREDA), is thus more agreeable to analyze the role of the human gut microbiota in diet metabolism. This revealed degradation pathways of 209 compounds present in the human diet, mainly phenolic compounds, a family of metabolites highly relevant for human health and nutrition.34 At present, very few scientific measures are reported for the qualitative and quantitate analysis of biomarkers in the context of category of natural compounds vs gut microbiota is a key question for balancing in host health.
3.1.9. Metabolite prediction
The purpose of metabolite prediction is to identify the primary metabolites for xenobiotics. This type of study can also provide insight into the mechanisms that are involved in metabolite related toxicity or other pharmacological research. The methods that are available for metabolite prediction can be divided into expert systems and statistical-based methods. Ahlstrom et al.,35 was used MetaSite, a metabolism site prediction program, to optimize the metabolic stability of celecoxib. Most analogs may retain their inhibitory activities, and their metabolic stabilities toward CYP2C9 were also improved. The consensus score was developed based on five metabolism prediction methods, including MetaPrint2D, SmartCYP, MetaDrug, MetaSite, and SOM.
4. Role of gut microbiota vs natural products associated with several diseases
The experimental perspective via wet lab of dietary NPs by gut microbiota modulation nowadays is the novel potential therapeutic target in drug discovery and development approach. These experimental procedures are increasingly applied as a powerful method to identify bioactive compounds from gut microbiota and to identify the microbiota-driven biological mechanism with the rodents-based model.
4.1. Metabolic disorders
The gut microbiome actively mediates the pathogenesis in a myriad of metabolic disorders28 viz., obesity, CVD, T2DM, and inflammatory bowel diseases. The synergistic effect of isoquercetin and inulin was completed from two metabolically related ways (i) direct prebiotic effect of inulin to improve gut microbiota profiles, and (ii) this improved microbiome with isoquercetin facilitating the rate of absorption and metabolism, thereby mutually enhancing its direct beneficial effects.36 The combination diet with mice model also exhibited much abundance of Faecalibaculum rodentium, mainly known to produce high quantities of lactic acid as a source of lactate. F. rodentium dependent biotransformation reaction to produce small metabolites propionate and butyrate and gut-derived propionate is used in hepatic synthesis of odd-chain fatty acids, which are associated with a reduced risk of T2DM. Supplementation of G. lucidum polysaccharide strain S3 (GLPS3) increased the relative abundance of the beneficial bacteria such as lactobacillus, roseburia, and lachnospiraceae. GLPS3 inhibits pancreatitis through microbiota regulation.37 Curcumin with antimicrobial activities should be further investigated as novel adjunctive therapies for NAFLD. In addition, NAFLD patients were found with a significant over-representation of Lactobacillus species and some phylum Firmicutes (Lachnospiraceae; genera Dorea, Robinsoniella, and Roseburia), as well as a significant under-representation in phylum Firmicutes (Ruminococcaceae; genus Oscillibacter). Also, a significant elevation of volatile organic compounds (VOC) was observed in their feces.38
4.2. Gastrointestinal physiology (GIP)
Dietary polyphenols affect the microbiome, resulting in health promotion by the activation of short-chain fatty acids (SCFA) excretion and intestinal immune function.39 Curcumin and resveratrol also decrease the Firmicutes:Bacteroidetes40 ratio and have anti-inflammation and anti-carcinogenesis effects by modifying colonic microbial ecology in animal experiments. Procyanidins (flavan-3-ol moieties) and are subject to metabolization by gut microbiota in experimental animals disease models like cancer, heart disease, and diabetes. The metabolic products of procyanidins are majorly absorbed in the system and have shown a variety of pharmacological activities in vitro and experimental animals.41 In addition to these prebiotic-like effects, mainly preclinical studies indicate that these polyphenols can shape gut microbiota by increasing the Firmicutes:Bacterioides ratio and by positively influencing the abundance of certain microbial species that may confer health beneficial effects to the human host, such as Akkermansia muciniphila, Faecalibacterium prausnitzii, and Roseburia sp.42
4.3. Cancer
Modulation of gut microbiota by glycyrrhizinic acid (GA) suppresses HFD-enhanced pre-metastatic niche formation, recolonizes the Desulfovibrio vulgaris and Clostridium sordellii, and prevents the metastasis of 4T1 breast cancer and B16F10 melanoma.43 Colonization with lignin-metabolizing microbial community protected germ-free rats from 7, 12-dimethylbenz(a)anthracene-induced cancer and tumor. Both urolithins A and B, the most representative microbial metabolites of dietary ETs by Gordonibacter urolithinfaciens and G. pamelaeae DSM 19378, have shown oestrogenic activity in a dose-dependent manner, without antiproliferative or toxic effects towards MCF-7 breast cancer cells.
4.4. Gut-brain disorders
Curcumin, a nontoxic, naturally occurring polyphenol, has been recently proposed for the management of neurological and neurodegenerative diseases. The experimental study indicated that curcumin favors brain health by modulating specific pathways such as the PI3K/Akt (Phosphatidylinositol 3-kinase/Protein Kinase B) pathway, MAPK (Mitogen-Activated Protein Kinase)/Akt pathways, Akt/Nrf2 (Nuclear factor-E2-related factor 2), and AMP (AMP-activated protein) kinase pathway.44 The blackberry anthocyanins can prevent some of the features of HFD-induced dysbiosis. In addition, anthocyanin-induced changes in the gut microbiota composition are related to their anti-neuroinflammatory properties. LW-AFC—Liuwei Dihuang a saccharide-enriched fraction, some small molecules such as rhein (the main rhubarb ingredient) was also demonstrated to improve the recognition memory capability (Alzheimer's disease) in HFD-fed mice through increasing the abundance of Lactobacillus and Bifidobacterium.45
5. Conclusion and future directions
Ethnopharmacological relevance's point of view, personalized dietary NPs have been fewer documented and used for several medical disorders like asthma, immunomodulatory, arthritis, cough, malaria, COPD, CVD, diabetes, and cancer. Regulation and restoring the aesthetic value of NPs concerns nowadays look interesting with special remarks on the diversity of human gut microbiota populations. Because a specific population of gut microbiota and associated reactions have the caliber to transform ingested NPs to most bioactive metabolites i.e. prone to biology for specific ailments, which regulates the dysbiosis to eubiosis status of gut microbiota niche. These metabolites are nowadays the most promising for phytopharmacology considerations with specified animal models through gut microbiota metabolism i.e. phase I and II reactions. Health benefiting bacteria F/B, Akkermansia, Bifidobacterium, Lactobacillus, and Faecalibacterium could be the most important targets for the treatment in case of illness associated with dysbiosis. This approach channelizes host physiology with ingested bioactive and efficient NPs via regulating gut microbiota composition and plays a vital role in therapeutic significance in the upcoming era.
So, over here one of the most promising tools i.e. Bio-Chemoinformatics applied for degraded molecules that procured from gut microbiota modulation with several biochemical reactions. This tool gives the prediction of the biological activity of degraded molecules and plays a vital role in the preliminary biological hypothesis before the rodents-based experimental model. This gut microbiota produced NP metabolites whose biological significances predicts by in silico Bio-Chemoinformatics based models. This report gives keen information to harnessing the dietary natural product-metabolites versus host-microbiota interplay disorders with in silico bioactivity consideration. Which leads to a better understanding the experimental and computational pharmacology. Hopefully, this combined approach surely gives positive sound for the improvement of host health and prevention or mitigation of several kinds of ailments.
Novelty
-
-
Gut microbiota might be a potential new territory for drug targeting, especially for dietary natural products
-
-
Gut microbiota vs dietary natural product metabolism and biotransformation to potential metabolite implications to several type of ailments
-
-
The experimental perspective of NP metabolites via computational hypothesis approach
-
-
Role of gut microbiota vs dietary natural products associated in several diseases
Declaration of competing interest
The authors have no conflict of interest.
Acknowledgements
This publication was made possible by CSIR-SRF fellowship grant with file number 09/591(0153)/2018-EMR-I of the council of scientific and industrial research (CSIR), ministry of human resources and development (MHRD) for financial support to Mohd Hafizur Rehman Ansari to carry out of his partial research work. The authors are especially thankful to Bioactive Natural Product Laboratory, Department of Pharmacognosy and Phytochemistry, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi-110062, India, for providing necessary facilities to support and guidance to complete the whole manuscript and its contents are the sole responsibility of authors.
List of abbreviations
- ADME-PK
Absorption Distribution Metabolism Excretion and Pharmacokinetic
- Akt/Nrf2
Protein kinase B/Nuclear factor-E2-related factor 2
- AMP
AMP-activated protein pathway
- BBB
Blood Brain Barrier
- BBR
Berberine
- BRB
Berberubine
- dhBBR
Dihydroberberine
- CVD
Cardiovascular disorders
- CYP450s
Cytochrome P450 monooxygenase system
- DMB
Demethyleneberberine
- ETs
Ellagitannins
- GA
Glycyrrhizinic acid
- GIP
Gastrointestinal physiology
- GLPS3
G. lucidum polysaccharide strain S3
- GMBTs
Gut microbiota biotransformation
- GMM
Gut microbiota metabolism
- GPCR
G-protein coupled receptor
- HIA
Human Intestinal absorption
- HPLC
High Performance Liquid Chromatography
- HAS
Human serum albumin
- JAT
Jatrorrhizine
- log BB
log blood brain
- MAPK
Mitogen-Activated Protein Kinase)
- NAFLD
Nonalcoholic fatty liver disease
- NCEs
New chemical entities
- NPs
Natural Products
- PDGFRβ
Platelet-derived growth factor receptor-beta
- PI3K/Akt
Phosphatidylinositol 3-kinase/Protein Kinase B
- PPB
Plasma Protein Binding
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SOM
Site of metabolism
- T2DM
Type-2 diabetes mellitus
- VEGFR2
Vascular endothelial growth factor receptor-2
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2022.03.005.
Contributor Information
Imran Ahmad Khan, Email: imranakhan@jamiahamdard.ac.in.
Sayeed Ahmad, Email: sahmad_jh@yahoo.co.in.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Glenwright A.J., Pothula K.R., Bhamidimarri S.P., et al. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature. 2017;541(7637):407–411. doi: 10.1038/nature20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H.Y., Qi L.W., Wang C.Z., Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am J Chin Med. 2011;39:1103–1115. doi: 10.1142/S0192415X11009433. 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., Brown P.O. In: Ruan Y., editor. vol. 5. 2007. Development of the human infant intestinal microbiota. (PLoS Biol). 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia W., Li H., Zhao L., Nicholson J.K. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7(2):123–129. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- 5.Donia M.S., Fischbach M.A. Small molecules from the human microbiota. Science. 2015;349(80, 6246) doi: 10.1126/science.1254766. 1254766-1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan S., Ding Y., Saedi N., et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23(4):1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milshteyn A., Colosimo D.A., Brady S.F. Accessing bioactive natural products from the human microbiome. Cell Host Microbe. 2018;23(6):725–736. doi: 10.1016/j.chom.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sassone-Corsi M., Nuccio S.P., Liu H., et al. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016;540(7632):280–283. doi: 10.1038/nature20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balskus E.P. Colibactin: understanding an elusive gut bacterial genotoxin. Nat Prod Rep. 2015;32(11):1534–1540. doi: 10.1039/C5NP00091B. [DOI] [PubMed] [Google Scholar]
- 10.Dornisch E., Pletz J., Glabonjat R.A., et al. Biosynthesis of the enterotoxic pyrrolobenzodiazepine natural product tilivalline. Angew Chem Int Ed. 2017;56(46):14753–14757. doi: 10.1002/anie.201707737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo C.J., Chang F.Y., Wyche T.P., et al. Discovery of reactive microbiota-derived metabolites that inhibit host proteases. Cell. 2017;168(3):517–526. doi: 10.1016/j.cell.2016.12.021. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schluter J., Foster K.R. The evolution of mutualism in gut microbiota via host epithelial selection. Ellner S.P., editor. PLoS Biol. 2012;10(11) doi: 10.1371/journal.pbio.1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iebba V., Totino V., Gagliardi A., et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39(1):1–12. [PubMed] [Google Scholar]
- 14.Paramashivam S.K., Elayaperumal K., Natarajan B., Ramamoorthy M., Balasubramanian S., Dhiraviam K. In silico pharmacokinetic and molecular docking studies of small molecules derived from Indigofera aspalathoides Vahl targeting receptor tyrosine kinases. Bioinformation. 2015;11(2):73–84. doi: 10.6026/97320630011073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijayakumar B.G., Ramesh D., Joji A., Jayachandra prakasan J., Kannan T. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur J Pharmacol. 2020;886:173448. doi: 10.1016/j.ejphar.2020.173448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Liu Y., Yang Y., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lampe J., Chang J. Interindividual differences in phytochemical metabolism and disposition. Semin Cancer Biol. 2007;17(5):347–353. doi: 10.1016/j.semcancer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haiser H.J., Turnbaugh P.J. Developing a metagenomic view of xenobiotic metabolism. Pharmacol Res. 2013;69(1):21–31. doi: 10.1016/j.phrs.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spanogiannopoulos P., Bess E.N., Carmody R.N., Turnbaugh P.J. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol. 2016;14(5):273–287. doi: 10.1038/nrmicro.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selma M.V., Beltrán D., García-Villalba R., Espín J.C., Tomás-Barberán F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014;5(8):1779–1784. doi: 10.1039/C4FO00092G. [DOI] [PubMed] [Google Scholar]
- 21.Chen H., Hayek S., Rivera Guzman J., et al. In: Smidt H., editor. vol. 7. 2012. The microbiota is essential for the generation of black tea theaflavins-derived metabolites. (PLoS One). 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anwar S., Bhandari U., Panda B.P., Dubey K., Khan W., Ahmad S. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. J Pharm Biomed Anal. 2018;159:100–112. doi: 10.1016/j.jpba.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 23.Wang L.Q. Mammalian phytoestrogens: enterodiol and enterolactone. J Chromatogr B. 2002;777(1-2):289–309. doi: 10.1016/S1570-0232(02)00281-7. [DOI] [PubMed] [Google Scholar]
- 24.Ansari M.H.R., Khan W., Parveen R., Saher S., Ahmad S. Pharmacokinetic, metabolomic, and stability assessment of ganoderic acid h based triterpenoid enriched fraction of Ganoderma lucidum P. Karst. Metabolites. 2022;12(2):97. doi: 10.3390/metabo12020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang T.S., Ko H.H., Wang T.Y., Lee C.-H., Wu J.-Y. Biotransformation of ganoderic acid a to 3-o-acetyl ganoderic acid a by soil-isolated streptomyces sp. Fermentation. 2018;4(4):101. doi: 10.3390/fermentation4040101. [DOI] [Google Scholar]
- 26.Kutschera M., Engst W., Blaut M., Braune A. Isolation of catechin-converting human intestinal bacteria. J Appl Microbiol. 2011;111(1):165–175. doi: 10.1111/j.1365-2672.2011.05025.x. [DOI] [PubMed] [Google Scholar]
- 27.Karabin M., Hudcova T., Jelinek L., Dostalek P. Biotransformations and biological activities of hop flavonoids. Biotechnol Adv. 2015;33(6):1063–1090. doi: 10.1016/j.biotechadv.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Shen L., Ji H.F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit Rev Food Sci Nutr. 2019;59(18):2896–2902. doi: 10.1080/10408398.2018.1478388. [DOI] [PubMed] [Google Scholar]
- 29.Pirillo A., Catapano A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: from in vitro evidence to clinical studies. Atherosclerosis. 2015;243(2):449–461. doi: 10.1016/j.atherosclerosis.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Wang K., Feng X., Chai L., Cao S., Qiu F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab Rev. 2017;49(2):139–157. doi: 10.1080/03602532.2017.1306544. [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Feng W., Tang F., Ao H., Peng C. Gut microbial transformation, a potential improving factor in the therapeutic activities of four groups of natural compounds isolated from herbal medicines. Fitoterapia. 2019;138 doi: 10.1016/j.fitote.2019.104293. [DOI] [PubMed] [Google Scholar]
- 32.Ekins S., Mestres J., Testa B. In silico pharmacology for drug discovery: applications to targets and beyond. Br J Pharmacol. 2007;152(1):21–37. doi: 10.1038/sj.bjp.0707306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanevskij K., Dapkunas J., Juska L., Japertas P., Didziapetris R. QSAR analysis of blood–brain distribution: the influence of plasma and brain tissue binding. J Pharmacol Sci. 2011;100(6):2147–2160. doi: 10.1002/jps.22442. [DOI] [PubMed] [Google Scholar]
- 34.Blasco T., Pérez-Burillo S., Balzerani F., et al. An extended reconstruction of human gut microbiota metabolism of dietary compounds. Nat Commun. 2021;12(1):4728. doi: 10.1038/s41467-021-25056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahlström M.M., Ridderström M., Zamora I., Luthman K. CYP2C9 Structure−metabolism relationships: optimizing the metabolic stability of COX-2 inhibitors. J Med Chem. 2007;50(18):4444–4452. doi: 10.1021/jm0705096. [DOI] [PubMed] [Google Scholar]
- 36.Tan S., Caparros-Martin J.A., Matthews V.B., et al. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-28521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li K., Zhuo C., Teng C., et al. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int J Biol Macromol. 2016;93:904–912. doi: 10.1016/j.ijbiomac.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 38.Raman M., Ahmed I., Gillevet P.M., et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11(7):868–875. doi: 10.1016/j.cgh.2013.02.015. e3. [DOI] [PubMed] [Google Scholar]
- 39.Kawabata K., Yoshioka Y., Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24(2):370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McFadden R.-M.T., Larmonier C.B., Shehab K.W., et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm Bowel Dis. 2015;21(11):2483–2494. doi: 10.1097/MIB.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monagas M., Urpi-Sarda M., Sánchez-Patán F., et al. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1(3):233. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 42.Espín J.C., González-Sarrías A., Tomás-Barberán F.A. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017;139:82–93. doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 43.Qiu M., Huang K., Liu Y., et al. Modulation of intestinal microbiota by glycyrrhizic acid prevents high-fat diet-enhanced pre-metastatic niche formation and metastasis. Mucosal Immunol. 2019;12(4):945–957. doi: 10.1038/s41385-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 44.Wu J., Li Q., Wang X., et al. In: Arai K., editor. vol. 8. 2013. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. (PLoS One). 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S., Huang X.-F., Zhang P., et al. Chronic rhein treatment improves recognition memory in high-fat diet-induced obese male mice. J Nutr Biochem. 2016;36:42–50. doi: 10.1016/j.jnutbio.2016.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.