Abstract
Influenza, also known as “flu”, is an infectious disease caused by influenza viruses. Three types of influenza virus, A, B, and C, are able to infect humans. In most people, influenza causes mild symptoms, but it can also induce severe complications and death. Annual influenza vaccines are currently the main intervention used to minimize mortality and morbidity. However, vaccination frequently fails to provide adequate protection, especially in the elderly. Traditional flu vaccine targets hemagglutinin to prevent virus infection, but the constant mutation of hemagglutinin means that it is a challenge to develop vaccines quickly enough to keep up with mutations. Thus, other methods of curbing influenza incidence would be welcomed, especially for vulnerable populations. Although influenza viruses primarily infect the respiratory tract, influenza virus infection also induces intestinal dysbiosis. Through gut microbiota-derived secreted products and the circulating immune cells, gut microbiota can affect pulmonary immunity. The crosstalk between the respiratory tract and gut microbiota, termed the “gut-lung axis”, is observed in the regulation of immune responses against influenza virus infection or inflammation-induced lung damage, indicating the possibility of using probiotics to prevent influenza virus infection or alleviate respiratory symptoms. In this review, we summarize the current findings on the antiviral functions of particular probiotics and/or combinations and discuss the antiviral mechanisms and immunomodulatory activities of probiotics in vitro, in mice, and in humans. Clinical studies show probiotic supplements can provide health benefits, not only to the elderly or children with compromised immune systems, but also to young- and middle-aged adults.
Keywords: Influenza, Microbiota, Gut-lung axis, Probiotics, Immunomodulatory
Graphical abstract
Highlights
-
•
The “gut-lung axis” means the crosstalk between the respiratory and intestinal tract.
-
•
Probiotics can confer protection against influenza or respiratory virus infections.
-
•
Potential probiotics inhibit influenza virus infection through different mechanisms.
1. Introduction
Human influenza viruses A, B, and C are RNA viruses. Influenza A and B viruses can cause seasonal epidemics, but only influenza A viruses are known to cause flu pandemics, i.e., global epidemics of influenza. Influenza C viruses, by contrast, usually cause mild respiratory symptoms and minor localized outbreaks.1 For most people, influenza usually causes mild symptoms, such as cough, sore throat, and rhinitis. However, influenza infection can also cause serious diseases such as bronchitis, pneumonia, myocarditis, and encephalitis, and result in high morbidity and mortality in children, pregnant women, and the elderly. Recent estimates find that 291,243–645,832 global seasonal influenza-associated respiratory deaths (4·0–8·8 per 100,000 individuals) occur annually,2 suggesting an urgent need to prevent influenza infection, especially in the aforementioned vulnerable populations.
Influenza A viruses can be found in human and animal populations. The classification of influenza A viruses is based on two glycoproteins on the surface of the virus: hemagglutinin (HA) and neuraminidase (NA). To date, 18 HA subtypes (H1 to H18) and 11 NA subtypes (N1 to N11) have been identified as antigenically distinct. HA attaches to cell surface sialyloligosaccharide receptors, initiating virus entry into host cells. NA enables the virus to be released from the host cell by cleaving sialyloligosaccharide residues from the host cell surface.3 Thus, both HA and NA play an important role in the virulence and pathogenesis of the influenza virus.
Annual influenza vaccines are the main intervention used to minimize the mortality and morbidity of influenza. Traditional flu vaccines target the surface HA to prevent the virus from binding to human cells. However, vaccination frequently fails to provide adequate protection from infection, especially in people older than 65 years. Its protective effects only range from 30% to 40% in elderly adults.4 In addition to immunosenescence that accounts in part for the reduced vaccine efficacy observed in the elderly,4 the constant mutation of HA through antigenic drift and reassortment also means that it is challenging to keep vaccine development up to date with the latest mutations. A vaccine formulated for any given year may be ineffective in the following year because the rapid mutation and evolution of influenza viruses mean that different strains may become dominant every year. The antibodies induced by a specific influenza virus strain or subtype cannot usually effectively neutralize other strains or subtypes. Moreover, the traditional method of influenza vaccine production is through culturing the virus using embryonated hens’ eggs. Some vaccine virus strains grow poorly in eggs, and can cause safety concerns in people with allergy to chicken eggs.5 Under these circumstances, probiotic-based supplements may provide an alternative choice to protect against influenza virus infection, especially in the elderly or those who are allergic to chicken eggs.
Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts. They have long been known for their microbiota-modulatory properties and immunomodulatory activities.6 Probiotics can stimulate antigen-presenting cells (APCs), modulate cytokines or immunoglobulin release, build a barrier lining on the gut epithelial cells, alter mucus secretion and defend against pathogenic bacteria and virus infection.7 This review highlights and provides updated information about current probiotic-based supplements with respect to their biological function in the prevention or alleviation of influenza virus infection and infection-induced tissue damage. Advances made over the past 11 years regarding how probiotics inhibit influenza virus infection, and the underlying modulatory mechanisms in vitro, in vivo, and in clinical studies are summarized. The interplay and communication between the gastrointestinal (GI) tract and respiratory system is also discussed.
2. The gut-lung axis in the pathogenesis or regulation of influenza virus infection
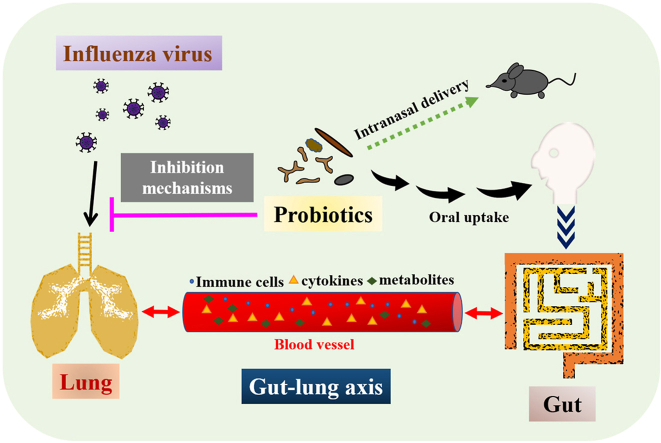
“Microbiota” means the community of microorganisms, including bacteria, viruses, fungi, and protozoans, which live in a host. The gut microbiota constitutes a complex ecosystem inhabited and regulated by microorganisms that have co-evolved in mutualistic relationships in the gut. Commensal microbiota produce a variety of metabolites, hormones, essential vitamins, and bioactive products in the host gut system,8 and the GI tract is constantly communicating with the immune system. Through the secreted products derived from gut microbiota and the circulating immune cells, gut microbiota can influence the local pulmonary immunity. The “gut-lung axis” refers to the crosstalk between the respiratory tract and GI tract.9
For example, gut microbiota-derived endotoxins, microbiota metabolites, and cytokines were able to reach the lung niche through circulation in the blood stream, forming a bidirectional gut-lung axis crosstalk. Short-chain fatty acids (SCFAs) released from the gut were able to reach the bone marrow through circulation to affect hematopoiesis, resulting in an increase in Ly6c− monocytes in the bone marrow. After moving to the lung, these patrolling Ly6c− monocytes differentiated into alternatively activated macrophages expressing lower amounts of C-X-C motif chemokine ligand 1 (CXCL1), a neutrophil chemoattractant.10 Therefore, neutrophil influx was reduced, limiting influenza-triggered lung immunopathology. Moreover, SCFAs directly promoted the antiviral activity of influenza-specific CD8+ T cells by enhancing their metabolism. Thus, SCFAs released from the gut balanced innate and adaptive immunity, leading to the resolution of influenza infection and prevention of immune-associated pathology.10 Pretreatment with bacterial lipopolysaccharide (LPS), which constitute the exterior surface and are shed by all Gram-negative bacteria, triggered an antiviral response through the Toll-like receptor 4 (TLR4) pathway to protect mice from lethal infection with influenza A virus.11 Antibiotic treatment induced gut dysbiosis and impaired the generation of influenza virus-specific CD4+ T and CD8+ T cells and antibody responses following respiratory influenza virus infection. Both antibody and T-cell responses were completely restored by a single inoculation of LPS either intranasally or intrarectally.12 Rectal inoculation was used to mimic the effect of the high levels of commensal bacteria present in the colon. Furthermore, intrarectal injection of other TLR ligands, such as CpG (TLR9 agonist) or Poly I:C (TLR3 agonist), were also able to restore immunity in response to influenza virus infection. Intact commensal microbiota were required to provide signals that induce inflammasome activation in the lung, leading to migration of dendritic cells (DCs) from the lung to the mesenteric lymph node for T-cell priming.12 It has also been reported that antibiotic treatment suppressed lung stromal cells from expressing anti-viral type I interferon (IFN) signature, resulting in the acceleration of influenza virus replication in the lung epithelia. Fecal transplantation from control mice to antibiotic-treated mice reversed the antibiotic-induced reduction of IFN signature in lung stromal cells, suggesting that commensal microbiota could drive the IFN signature expression specifically in lung stroma cells.13 These findings together indicated that commensal microbiota were able to regulate adaptive immunity in the respiratory mucosa against influenza virus infection (Fig. 1a).
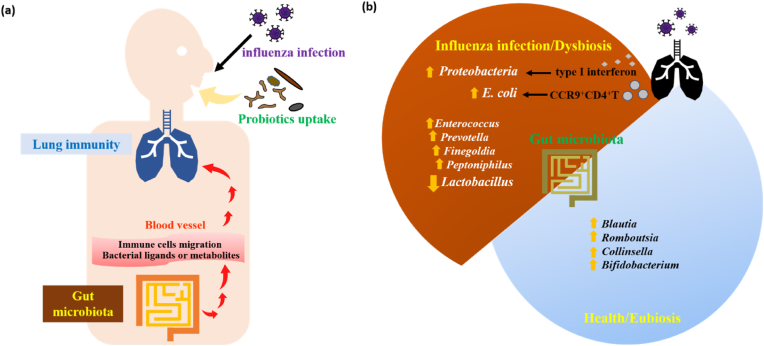
Fig. 1.
The gut-lung axis in the pathogenesis or regulation of influenza virus infection.
(a) Explanations about how gut microbiota might affect the lung immunity against influenza infection. The probiotics are colonized in the gut after one orally uptakes. Intestinal microbiota can release secreted products, such as bacterial ligands and metabolites, which enter the blood stream to affect the immune cells in the circulation. Immune cells can migrate to the lung through circulation in the blood vessels, further regulating the antiviral response and/or lung inflammation.
(b) Explanations about how influenza virus infection might affect the composition of gut microbiota. After influenza virus infection, lung-derived CCR9+CD4+ T cells were recruited by intestinal epithelial cells and induced the outgrowth of E. coli. Type I IFN produced in the lung after influenza infection also influenced the intestinal microbiota, resulting in the enrichment of Proteobacteria. In mice infected with influenza virus, the richness of Lactobacillus were decreased in the gut microbiota. In H1N1 patients, fecal microbiome was enriched by Enterococcus, Prevotella, Finegoldia, and Peptoniphilus, while the microbiome of healthy controls was dominated by Blautia, Romboutsia, Collinsella, Bifidobacterium, and other beneficial bacteria.
Although influenza viruses primarily adhere to and replicate within epithelial cells spanning from the upper (nasal cavity, oral cavity, pharynx, larynx) to the lower respiratory tract (trachea, bronchi, bronchioles and alveoli),1 influenza virus infection also affects the gut microbiota. Influenza A virus infection was found to have minor effects on the global lower respiratory tract microbiome in mice. In comparison, influenza A viruses induced robust depletion of bacterial content and disruption of mucus layer integrity in the intestine.14 Another study also reported that influenza virus infection resulted in significantly altered gut microbiota diversity in mice.15 Both studies reported decreases in the richness of Firmicutes (as shown by Lactobacillus) after infection with influenza virus in mice.14,15 It was found that the fecal microbiome of the H1N1 patients was enriched by Enterococcus, Prevotella, Finegoldia, and Peptoniphilus, while the microbiome of healthy controls was dominated by Blautia, Romboutsia, Collinsella, Bifidobacterium, and other beneficial bacteria. Six biomarkers (Fusicatenibacter, Romboutsia, Anaerostipes, E. hallii group, Ruminococcus torques group, and Blautia) could be used to differentiate the H1N1 patients and healthy people.16 Indeed, influenza infection in humans is often accompanied by GI symptoms, such as nausea, vomiting, and diarrhea, although no evidence has shown that the influenza virus replicates in the intestine in humans. Intranasal administration of influenza viruses did not infect the small intestine directly but caused lung and intestinal immune injury in mice. Lung-derived CC-chemokine receptor 9 positive (CCR9+) CD4+ T cells were recruited by C–C motif ligand 25 (CCL25) expressed on intestinal epithelial cells. After migrating into the GI tract, lung-derived CD4+ T cells induced the outgrowth of E. coli, aberrant T-helper 17 (Th17)-dependent inflammation, and intestinal damage by secreting IFN-γ.17 In addition to IFN-γ, type I IFN produced in the lung after respiratory infection of influenza virus also influenced the intestinal microbiota, resulting in the enrichment of Proteobacteria and the depletion of obligate anaerobic bacteria in the gut. Moreover, type I IFN-mediated dysbiosis inhibited the antimicrobial inflammatory responses in the gut against secondary Salmonella infection, further enhancing Salmonella intestinal colonization and systemic dissemination.18 These findings together suggest that respiratory infection by influenza viruses may not only affect the local pulmonary immunity but also induce the distal intestinal dysbiosis (Fig. 1b).
3. Possible mechanisms through which probiotics may modulate influenza virus infection
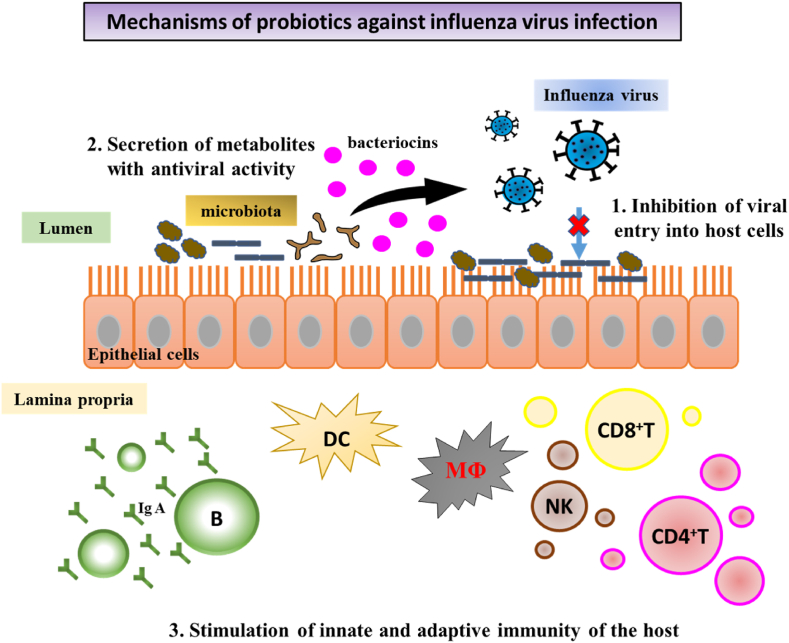
The microbiota-modulatory effect of probiotics is achieved mainly through the inhibitory activity of probiotics against pathogens, which increases the populations of beneficial microorganisms and decreases the levels of pathogens in the GI tract.19 The immunomodulatory effect of probiotics on the innate and adaptive immune systems may provide host protection against a wide variety of pathogens.20 Several probiotic strains have shown anti-influenza virus activities.20,21 Possible mechanisms for the antiviral activity of probiotics have been proposed (Fig. 2), including (1) inhibition of viral entry into host cells; (2) secretion of metabolites with antiviral activity; (3) stimulation of innate and adaptive immunity of the host.22
-
(1)
Inhibition of influenza virus entry into host cells
Fig. 2.
Mechanisms of probiotics against influenza virus infection.
1. Inhibition of viral entry into host cells: some probiotics directly bind to influenza virus, blocking virus adsorption on host epithelial cells. 2. Secretion of metabolites with antiviral activity: antiviral metabolites such as organic acids, bacteriocins, and hydrogen peroxide produced by some probiotics can disrupt virial protein structure or inhibit virus replication.3. Stimulation of innate and adaptive immunity of the host: administration of some probiotics induced dendritic cells (DCs) or lung stromal cells to produce type I and/or type II IFNs, which further activate NK cells. In addition, microbe-associated molecular patterns such as TLR ligands stimulate activation of antigen-presenting cells, including DCs, macrophages (MΦ), and B cells, further priming CD4+ T, and CD8+ T cells. Cytokines release from T cells can induce B cells production of secretory IgA to neutralize viral infectivity. Moreover, probiotics may alleviate viral infection induced lung inflammation.
Some probiotics have been found to bind to influenza virus, blocking virus adsorption on host cells.23 Enterococcus faecium NCIMB 10415 inhibited influenza virus A H1N1 and H3N2 via direct physical interaction between bacterial cells and viral particles.24 Besides the direct interaction between probiotics and viruses, some probiotics were able to adhere to cells and compete with viruses for attachment to cell adhesion receptors, thereby preventing viral invasion into host cells.23 Pretreatment of DCs with the S-layer protein of Lactobacillus acidophilus ATCC 4356 was able to prevent the invasion of influenza A H9N2 into DCs.25
-
(2)
Secretion of metabolites with anti-influenza virus activity
Some probiotics can synthesize antiviral metabolites, such as organic acids, bacteriocins, and hydrogen peroxide.22 The bacteriocin from Lactobacillus delbrueckii subsp. bulgaricus 1043 showed inhibitory effects against influenza virus A H7N1 and H7N7.26 The enterocin B originating from Enterococcus faecium L3 reduced the reproduction of influenza virus A H3N2 in cells and had a protective effect against influenza viral infection in mice.27
-
(3)
Stimulation of innate and adaptive immunity of the host
-
(a)
Effects of probiotics on innate and adaptive immune response against influenza virus infection
Innate immunity functions as the first line of host defense against viral infection. The magnitude of innate response activated by viral infection may influence the adaptive immunity induction. It is now widely accepted that some highly pathogenic influenza viral strains hyperstimulate the immune system, leading to an event called “cytokine storm”, which induces tissue damage and causes systemic disease.28 Therefore, the risk of viral infection can be significantly reduced if innate immunity is enhanced but the excessive production of inflammatory cytokines is prevented. Numerous studies show that some probiotics, most of them belonging to the Lactobacillus and Bifidobacterium genera, provide host protection against viral infection through their modulatory effects on innate and adaptive immunity.22
Upon viral infection, innate immunity functions as the first line of host defense. Type I IFNs are induced and secreted upon host cell recognition of viral nucleic acids, and are important mediators that exert their antiviral activities though the induction of hundreds of IFN-stimulated genes (ISGs). The products of ISGs exert numerous antiviral effector functions, including blocking viral transcription, degrading viral nucleic acids, inhibiting translation, and modifying protein function to control all steps of viral replication.29 The classical ISGs include myxovirus resistance (Mx), 2ʹ,5ʹ-oligoadenylate synthetases (OAS), IFN-stimulated protein of 15 kDa (ISG15), and so on.30 Mx proteins are dynamin-like GTPases that appear to target viral nucleocapsids, resulting in the inhibition of viral RNA polymerase activity, effectively blocking both transcription and replication of the virus.30 OAS are activated by double-stranded RNA binding and can catalyze the formation of 2ʹ–5ʹ oligoadenylates to activate cellular ribonuclease L (RNase L), which in turn, degrade cellular and viral RNA.31 ISG15 is a small, ubiquitin-like molecule that has numerous antiviral functions including inhibition of virus release, ISGylation of both viral and host proteins, and immunomodulatory cytokine-like properties in its unconjugated form.30 Previous studies reported that administration of probiotics induced the expression of IFNs and ISGs. Prophylactical administration of Lactobacillus brevis KB290 significantly alleviated the clinical symptoms induced by influenza virus infection, which could be attributed to the enhancement of IFN-α production induced by L. brevis KB290 consumption.32 Takeda et al. found that oral administration of Lactobacillus plantarum 06CC2 alleviated influenza symptoms in mice, which was associated with the enhancement of IFN-α production through intestinal immunity, as revealed by the increased expressions of IL-12 receptor and IFN-γ in Peyer's patches.33 Nakayama et al. reported that oral administration of Lactobacillus gasseri SBT2055 prevented body weight loss and decreased viral load in the lung of influenza virus infected mice by the downregulation of viral replication through the induction of antiviral genes Mx1 and OAS1a expression.34 Similarly, pretreatment of DCs with the S-layer protein of Lactobacillus acidophilus ATCC 4356 increased Mx1 and ISG15 mRNA expression to inhibit the influenza virus infection.25 Lu et al. suggested that oral administration of Lactobacillus mucosae 1025 and Bifidobacterium breve CCFM1026 reduced the viral load in the lungs of influenza virus infected mice, which could be associated with an increase in MxA expression in the lungs resulting from the alteration of gut microbiota.35 As the ISGs are crucial components of the IFN responses and play a key role in establishing an antiviral state for virus clearance and restriction of spread, the anti-influenza virus effects of probiotics could be attributed to their up-regulatory effect on the expression of ISG genes of host cells.
In addition to the upregulation of type 1 IFN and ISG expression, probiotics may alleviate viral infection through the modulation of the production of certain cytokines by immune cells. Oral administration of heat-killed Lactobacillus plantarum 06CC2 alleviated influenza virus infection in mice in correlation with the enhancement of IL-12 and IFN-γ production and the decrease in TNF-α levels in the lung.33 Goto et al. demonstrated that oral administration of Lactobacillus acidophilus L-92 showed protective effects against influenza virus infection in mice, which was associated with an increase in expression of various cytokines and chemokines, including IFN-α, IL-1β, macrophage colony-stimulating factor (M-CSF), eotaxin, and regulated on activation, normal T cell expressed and secreted (RANTES).36 Park et al. indicated that intranasal or oral administration of Lactobacillus plantarum DK119 conferred protection against influenza virus infection in mice, probably by enhancing the innate immunity of DCs and macrophages and in turn increasing the production of IL-12 and IFN-γ.37
Moreover, probiotics are also able to improve the immune system against viral infection by increasing the production of viral-specific antibodies. Nasal administration of Lactobacillus fermentum CJL-112 upregulated the expression of T-helper 1 (Th1) cytokines such as IL-2 and IFN-γ and reduced or left unchanged the levels of Th2 cytokines like IL-4, IL-5, and IL-10 in the lung of influenza virus infected mice. Moreover, nasal administration of L. fermentum CJL-112 also significantly increased the specific anti-influenza IgA levels in the lungs of influenza virus infected mice.38 In comparison, nasal administration of Lactobacillus rhamnosus CRL1505 significantly increased the levels of IFN-γ and specific anti-influenza IgA and IgG in the serum and/or respiratory tract of influenza virus infected mice.39
-
(b)
Putative mechanisms for the immunomodulatory activities of probiotics
Numerous putative mechanisms for the immunomodulatory activities of probiotics have been proposed. Some extracellular polysaccharides produced by probiotics possess immunomodulatory activities. For instance, the extracellular polysaccharides produced by Lactobacillus delbrueckii OLL1073R-1 increased the expression levels of IFN-α, IFN-β, MxA, and RNase L in porcine intestinal epithelial cells,40 and significantly protected against influenza virus infection.41 Besides extracellular polysaccharides, some cellular components, such as DNA, RNA, and bacterial cell-wall components, including peptidoglycan, S-layer proteins, teichoic acids, capsule, and pellicle can modulate the innate antiviral immune response. The anti-influenza virus effects of Enterococcus faecalis KH2 and Lactobacillus plantarum SNK12 were mediated by the bacterial RNAs.42 Gao et al. found that the S-layer protein of L. acidophilus ATCC 4356 helped control the exacerbated inflammation caused by influenza virus infection. Secretion of the anti-inflammatory cytokine IL-10 by DCs treated with the S-layer protein was significantly higher than secretion from DCs infected with only the H9N2 virus, whereas the pro-inflammatory cytokine TNF-α secretion exhibited an opposing trend.25 Inflammation can promote the recruitment of immune cells, but uncontrolled and exacerbated inflammation will induce “cytokine storm”, leading to systemic edema and extensive tissue damage.28
4. Clinical findings regarding preventive or relief effects of probiotics against influenza or upper respiratory tract infections
Probiotics provided in capsules, milk or yogurt are often used for application in humans. The clinical effects of probiotics in the prevention of influenza or upper respiratory tract infections (URTIs) and/or on the alleviation of respiratory symptoms have been examined in humans of different ages. Nursing home residents aged 65 and older received Lactobacillus rhamnosus GG capsules daily for six months. Respiratory viral infections as examined by real-time polymerase chain reaction (RT-PCR) were observed in 15.0% of residents with probiotic treatment and in 22.9% of residents with placebo treatment, suggesting that probiotic uptake might reduce the risk for influenza and other respiratory virus infections in residents of long-term and chronic care facilities.43 Test yogurt containing L. bulgaricus OLL1073R-1 and S. thermophilus OLS3059 may help prevent infection with influenza A virus subtype H3N2 in the elderly with weakened immunity, probably by increasing the production of H3N2-specific IgA in saliva.44 Milk with or without L. casei strain Shirota was given to the elderly aged 74–92 years, showing that the total number of acute URTI events and the severity of symptoms were not significantly different between the two test groups. Nevertheless, probiotic intervention significantly reduced the mean duration of infection per infection event as compared to the placebo treatment.45 Yogurt supplemented with a selected strain of Lactobacillus paracasei N1115 could protect middle-aged and elderly people (aged ≥ 45 years) from acute URTIs, probably due to the increased percentage of total CD3+ T cells induced by probiotic intervention.46
For healthy male office workers aged 30–49 years, daily intake of L. casei strain Shirota-fermented milk significantly reduced the incidence and duration of URTIs. The protective effects of probiotic-fermented milk might be through modulation of the immune system, as revealed by the significantly enhanced nature killer (NK) cell activity and decreased salivary levels of stress marker cortisol at 6 weeks after probiotic intervention versus control milk supplementation. Both control and probiotic milk was able to increase the salivary IgA secretion at 6 and 12 weeks after intervention, but the secreted levels of IgA did not vary significantly between different treatments.47 For adults aged 25–45 years, test yogurt drink with probiotics, including L. paracasei, L. casei 431, and L. fermentium PCC, significantly reduced the incidence of URTIs and flu-like symptoms, and increased levels of IFN-γ in the serum and secretory IgA in the gut as compared to placebo yogurt drink. There were no significant differences in the productions of IL-4, IL-10, IgA, IgG and IgM in the serum between the probiotic and placebo treatments.48 In comparison, L. salivarius administrated in powder form to athletes aged 18–35 years could not provide health benefit in reducing the frequency of URTIs and did not affect blood leukocyte counts or levels of salivary IgA and lysozyme during a spring period of training and competition.49 College students aged 18–25 years received a probiotic stick containing L. rhamnosus GG and Bifidobacterium lactis BB-12 for 12 weeks, showing that the duration and severity of URTIs were significantly decreased by the combined probiotic intervention.50 As compared to placebo treatment, L. plantarum DR7 administrated to adults aged 30–60 years could significantly reduce the duration of nasal symptoms of URTIs, while it marginally reduced the duration of general flu symptoms (P = 0.062). L. plantarum DR7 administrated to young adults aged < 30 years marginally reduced the duration of general flu symptoms (P = 0.066). L. plantarum DR7 supplement significantly decreased plasma proinflammatory cytokines [tumor necrosis factor (TNF)-α, IFN-γ, IL-1β] in middle-aged adults, and significantly increased anti-inflammatory cytokines (IL-10, IL-4) in young adults. For both populations, L. plantarum DR7 intervention significantly reduced plasma peroxidation and oxidative stress levels. Therefore, the protective effects of L. plantarum DR7 might be related to its immunomodulatory activities, exerting differential effects on different aged populations.51
Combined treatment of vitamin C and probiotics, including Lactobacillus acidophilus CUL21 (NCIMB 30156), Lactobacillus acidophilus CUL60 (NCIMB 30157), Bifidobacterium bifidum CUL20 (NCIMB 30153) and Bifidobacterium animalis subsp. lactis CUL34 (NCIMB 30172), significantly reduced the incidence rate of URTIs and the number of days with URTI symptoms in children attending preschool facilities, even though the children did not receive a flu vaccine during the study period.52 In comparison, children receiving L. rhamnosus GG probiotic supplement in milk had fewer days with respiratory symptoms than those receiving control milk without probiotics. However, probiotic intervention was not effective in reducing the occurrence of respiratory viruses and the number of respiratory symptoms observed during viral episodes.53 Another study reported that long-term daily intake of milk containing L. rhamnosus GG could significantly decrease the occurrence of respiratory illness in children with recovery of L. rhamnosus GG in their fecal samples. Strain-specific real-time quantitative PCR assay of fecal samples helped confirm the successful colonization of the uptake probiotic in the intestine, providing useful information through which to analyze the association of respiratory illness and probiotic intake in this particular group of children (n = 128).54 Tablets with or without B. animalis subsp. lactis BB-12 were administrated to 1 or 2-month-old infants, showing that probiotic intervention in early childhood significantly reduced the number of respiratory infections.55
Based on the clinical findings from populations of different ages (Table 1), we know that specific probiotics or their combinations can reduce the duration or risk of influenza and/or other respiratory virus infections and/or relieve the respiratory symptoms after infection. The protective and relief effects of specific probiotics or their combinations can be observed in the elderly with weakened immunity due to immunosenescence,4 in infants and children with incomplete development of the immune system,56 and in office workers and college students. These findings suggest that probiotic supplements can provide health benefits, not only to the elderly or children with compromised immune systems, but also to young- and middle-aged adults.
Table 1.
Probiotics contribute to prevention of influenza virus infection or alleviation of respiratory symptoms observed in cells, mice, and humans.
| Disease | Probiotics | Assay model | Treatment | Biological functions | Ref. |
|---|---|---|---|---|---|
| Influenza A/chicken/Germany/Weybridge (H7N7) Influenza A/chicken/Germany/Rostock (H7N1) |
Lactobacillus delbrueckii | Cell | The bacteriocins from L. delbrueckii were incubated with virus and then the virus-induced cytopathic effect was determined. | The bacteriocin from L. delbrueckii showed a pronounced influenza virus-inhibitory effect. | 26 |
| Influenza A/Puerto Rico/8/34 (H1N1) | L. plantarum 06CC2 | Mouse | Oral administration of killed probiotics at a concentration of 20 mg/mouse twice daily, starting 2 days before infection and lasting for 7 days after infection. | After 2 days of viral infection, the NK cell activity and levels of IFN-α, IL-12, and IFN-γ in the BALF were increased; meanwhile the levels of TNF-α in the BALF were decreased in the mice administrated with probiotics. After 6 days of viral infection, the viral yields in lungs were decreased. | 33 |
| Influenza A/Swine/Greven/IDT2889/2004 (H1N1) Influenza A/Swine/Bondelum/IDT5959/2007 (H3N2) | Enterococcus faecium NCIMB 10415 | Cell | Live probiotics (106 CFU/ml) were mixed with virus (0.01 MOI) in a total of 1 ml medium for 90 min before viral infection assay. | Direct adsorptive trapping of swine influenza viruses by E. faecium. | 24 |
| Influenza A/Puerto Rico/8/34 (H1N1) | L. plantarum DK119 | Mouse | Intranasal co-administration of live probiotics at a concentration of 108 CFU/mouse and influenza virus. | After 4 days of viral infection, the titer of virus in the lung was decreased, the levels of IL-12 and IFN-γ in BALF were increased, and the levels of IL-6 and TNF-α in the BALF were decreased in the mice administrated with probiotics. | 37 |
| Influenza A/Puerto Rico/8/34 (H1N1) | L. acidophilus L-92 | Mouse | Oral administration of live probiotics at a concentration of 10 mg/mouse/day for 15 days before viral infection and for 6 days after viral infection. | After 6 days of viral infection, the titer of virus in the lung was decreased, the NK cell activity and the levels of eotaxin, M-CSF, IL-1β, RANTES, and IFN-α in lung were increased in the mice administrated probiotics. | 36 |
| Influenza A/NWS/33 (H1N1) | L. fermentum CJL-112 | Mouse | Intranasal administration of live probiotics at a concentration of 108 CFU/mouse 6 times 21 days before viral infection. | After 48 h of viral infection, the expression levels of IL-1β, IL-2, IFN-α, and IFN-γ in lung were increased, the expression levels of IL-10 and TNF-α in the lung were decreased, and the specific anti-influenza IgA levels in the lung were increased in the mice administrated with probiotics. | 38 |
| Influenza A/Puerto Rico/8/34 (H1N1) | L. brevis KB290 | Mouse | Oral administration of live probiotics at a concentration of 109 CFU/mouse/day for 14 days before viral infection. | After 7 days of viral infection, the clinical symptoms were alleviated, the levels of IFN-α in sera were increased, and the specific anti-influenza IgA levels in the BALF were increased in the mice administrated with probiotics. | 32 |
| Influenza A/Puerto Rico/8/34 (H1N1) | L. gasseri SBT2055 | Mouse | Oral administration of live probiotics at a concentration of 1.0 × 108, 1.0 or 1.6 × 109 CFU/mouse/day for 7 or 21 days before the virus infection. | After 5 days of viral infection, the viral load in the lung was reduced, and the expression levels of Mx1 and Oas1a in the lung were increased in the mice treated with probiotics. | 34 |
| Influenza A/Duck/Nan-Jing/01/1000 (H9N2) | L. acidophilus ATCC 4356 | Cell | DCs (106) were pretreated with S-layer proteins (400 μg/ml) from L. acidophilus for 1 h before viral infection. | Pretreatment of DCs with the S-layer protein increased the Mx1 and ISG15 mRNA expressions in DCs and inhibited the influenza virus infection. | 25 |
| Influenza A/South Africa/3626/2013 (H1N1) | E. faecium L3 | Mouse | Oral administration of live probiotics at a concentration of 107 CFU/mouse/day, starting the day before viral infection and 10 days after infection. | Probiotic treatment significantly prolonged mouse survival. | 27 |
| Influenza A/Puerto Rico/8/34 (H1N1) | L. rhamnosus CRL1505 | Mouse | Intranasal administration of live or killed probiotics at a concentration of 108 CFU/mouse/day for 2 days before viral infection. | After 5 days of viral infection, the levels of IL-4, IFN-γ, and specific anti-influenza IgA and IgG in the BALF and/or serum were increased; meanwhile the levels of IL-17 in the BALF and sera were decreased in the mice treated with probiotics. | 39 |
| Influenza A/Puerto Rico/8/34 (H1N1) | L. delbrueckii ssp.bulgaricus OLL1073R-1 | Cell | Exopolysaccharide from L. delbrueckii was incubated with virus for 1 h. | After 6 h of viral infection, the virus titer was decreased in the mice treated with probiotics. | 41 |
| Influenza A/NWS/33 (H1N1) | E. faecalis KH2 and L. plantarum SNK12 | Mouse | Oral administration of killed probiotics at a concentration of 5 mg/mouse/day for 7 days before viral infection until 14 days after viral infection. | After 3 days of viral infection, the viral load in BALF was reduced; 14 days after viral infection, the levels of virus-neutralizing antibody and specific anti-virus IgA in BALF were increased in the mice treated with probiotics. | 42 |
| Influenza A/Fort Monmouth/1/47 (H1N1) | L. mucosae 1025 and Bifidobacterium breve CCFM1026 | Mouse | Oral administration of live probiotics at a concentration of 109 CFU/mouse/day for 15 days before viral infection and for 4 days after viral infection. | After 5 days of viral infection, the viral load in the lung was reduced, and the expression levels of MxA in the lung were increased in the mice administrated with probiotics. | 35 |
| Clinical findings | |||||
| Influenza A and B as well as other respiratory viruses (2017) (NCT01720329) |
Lactobacillus rhamnosus GG | Adults aged 65 years and older (n = 209) | Participants received 2 capsules (1010 CFU of L. rhamnosus GG per capsule) or a placebo daily for 6 months. | Probiotic uptake might reduce the risk for influenza and other respiratory virus infections in residents of long-term and chronic care facilities. | 43 |
| Influenza A virus (H3N2) (2019) |
Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 | Adults of mean age 86 years (n = 96) | Participants consumed 100 g of test (1.8–3.5 × 108 CFU/g of L. bulgaricus OLL1073R-1 and 8.1–10.8 × 108 CFU/g of S. thermophilus OLS3059) or control (1.7 × 108 CFU/g of L. bulgaricus OLL1256 and 2.6 × 108 CFU/g of S. thermophilus OLS3295) yogurt daily for 12 weeks. | Test yogurt supplement may help prevent from infection with influenza A virus subtype H3N2 in the elderly with weakened immunity, probably by increasing the production of H3N2-bound salivary IgA. | 44 |
| Upper respiratory tract infection (URTI) (2013) |
L. casei strain Shirota | Adults aged 74–92 years (n = 154) | Milk with or without 4 × 1010 CFU of the probiotic was given to participants daily for 5 months. | Fermented milk containing the probiotic significantly reduced the duration of acute URTIs. | 45 |
| Acute URTI (2017) (ChiCTR-IOR-16010164) |
Lactobacillus paracasei N1115 | Adults aged 45 years and older (n = 205) | Participants received 3.6 × 107 CFU/mL in yogurt for 12 weeks. The control group did not receive this yogurt. | Yogurt with the selected probiotic reduced the risk of acute URTIs and enhanced the T-cell-mediated immune defense in the elderly. | 46 |
| URTI (2017) | L. casei strain Shirota | Male office workers aged 30–49 years (n = 96) | 1011 CFU of L. casei Shirota probiotic or control milk was given to the participants once daily for 12 weeks during the winter season. | Daily intake of L. casei strain Shirota-fermented milk significantly reduced the risk of URTIs in healthy middle-aged male workers, probably through modulation of the innate and adaptive immunity. | 47 |
| Influenza and URTI (2018) | L. paracasei, L. casei 431, and L. fermentium PCC | Adults aged 25–45 years (n = 134) | Subjects received once-daily doses of yogurt drink (150 mL) with or without probiotics (3 × 107 CFU/ml of L. paracasei, 3 × 107 CFU/ml of L. casei 431, and 3 × 106 CFU/ml of L. fermentium PCC) for 12 weeks. | As compared to placebo yogurt drink, test yogurt drink with probiotics significantly reduced the incidence of URTIs and flu-like symptoms, but increased higher levels of IFN-γ in the serum and secretory IgA in the gut. | 48 |
| URTI (2012) |
Lactobacillus salivarius | Endurance athletes aged 18–35 years (n = 54) | 2 × 1010 CFU of L. salivarius or placebo in powder form was given to participants daily for 16 weeks. | Probiotic intervention could not provide health benefit in reducing the frequency of URTIs in the athletes and did not affect blood leukocyte counts or levels of salivary IgA and lysozyme. | 49 |
| URTI (NCT01657643) (2013) |
L. rhamnosus GG and Bifidobacterium lactis BB-12 | College students aged 18–25 years (n = 198) | Participants consumed the placebo or probiotic (109 CFU of each probiotic strain in powder form) stick daily for 12 weeks. | Probiotic combination could decrease the duration and severity of infection, improving the health-related quality of life during upper respiratory infections. | 50 |
| URTI (2019) |
L. plantarum DR7 | Adults aged < 30 or 30–60 years (n = 109) | 109 CFU of L. plantarum DR7 was given to participants in powder form daily for 12 weeks. | For adults aged 30–60 years, probiotic supplements significantly reduced the duration of nasal symptoms, while marginally reducing the duration of general flu symptoms. For young adults aged <30 years, probiotic intervention marginally reduced the duration of general flu symptoms. | 51 |
| Respiratory tract infections (RTI) (2014) |
L. acidophilus CUL21 (NCIMB 30156) and CUL60 (NCIMB30157), plus B. bifidum CUL20 (NCIMB 30153) and B. animalis subsp. lactis CUL34 (NCIMB 30172) | Children aged 3–6 years (n = 57) | One chewable tablet containing 1.25 × 1010 CFU (Lactobacillus sp. 1 × 1010 and Bifidobacterium sp. 0.25 × 1010) and 50 mg vitamin C or a placebo was given to participants daily for 6 months. | Combined treatment reduced the incidence of respiratory tract infection symptoms, even though the children did not receive the flu vaccine during the study period. | 52 |
| Influenza A (H1N1, H3N2) and other respiratory viruses (2013) |
L. rhamnosus GG (ATCC 53103) | Children aged 2–6 years (n = 194) | Milk with or without 108 CFU of the probiotic supplement was given to children daily for 28 weeks. | Probiotic supplement reduced the number of days with respiratory symptoms during the intervention, but did not have a significant effect on the occurrence of viruses in the nasopharynx nor the symptoms during viral episodes. | 53 |
| Respiratory symptoms (2012) | L. rhamnosus GG (ATCC 53103) | Children aged 2–6 years (n = 501) | Children received milk with or without the probiotics (2.0 × 105 to 1.9 × 106 CFU/ml) at three daily meals (total 400 ml of study milk daily) for 28 weeks. | Probiotic intake significantly reduced the occurrence of respiratory illness in the children with recovery of L. rhamnosus GG in their fecal samples. | 54 |
| RTI (2011) | B. animalis subsp. lactis BB-12 | 1 or 2-month-old infants (n = 69) | Tablets with or without the probiotic (109 CFU) were given to infants twice daily for 6–7 months. | Probiotic administration in early childhood significantly reduced the number of respiratory infections. | 55 |
Abbreviation: dendritic cells (DCs), bronchoalveolar lavage fluid (BALF), interferon (IFN), interleukin (IL), myxovirus resistance (Mx), nature killer (NK), 2′,5′-oligoadenylate synthetase (OAS), regulated on activation, normal T cell expressed and secreted (RANTES), tumor necrosis factor-α (TNF-α), colony-forming unit (CFU), multiplicity of infection (MOI), upper respiratory tract infection (URTI), respiratory tract infections (RTI).
5. Conclusions and future perspectives
The translocation of bacteria from the GI tract to the respiratory system can be observed in sepsis and acute respiratory distress syndrome when barrier integrity is compromised.57 Bacteria from the gut can also travel to the lung through aspiration of vomit or esophageal reflux.58 There is less evidence to show the direct transfer of microorganisms between the gut and lung under normal conditions. However, there is evidence to demonstrate the crosstalk between the respiratory tract and the GI tract, a phenomenon termed the “gut-lung axis”. The mucosal surfaces in the lung and gut regulate the immune responses by combating pathogens and preventing excessive inflammation-induced tissue damage. Both the gut and lung are able to influence each other's immune responses through the secreted products released in the blood and the immune cells circulating between sites, which might explain why we can observe the protective effects of probiotics administrated through the oral route against influenza infection in mice and humans. The in vitro cell assay of influenza virus infection reveals the direct inhibition of viral entry or replication by probiotics, which might explain why the intranasal administration of probiotics, but not oral uptake of probiotics, can reduce viral titers and relieve lung inflammation in mice. These studies illustrate the direct use of the nasal spray technique for possible application of probiotics in humans to prevent influenza virus infection or to alleviate respiratory symptoms after infection. The antiviral effects of probiotics through nasal delivery might be faster than through the oral intake. It is possible that nasal administration of probiotics may affect the nasal microbiota. Actually, influenza infection in humans can affect the nasal microbiota,59 and the nasal commensal Staphylococcus epidermidis is reported to serve as a defense mechanism against influenza virus infection.60 The relevant studies about the regulation of nasal microbiota are not mentioned in the current review due to space and word limitations.
Clinical trials in humans show promising data demonstrating that specific probiotics or their combinations can shorten the duration or reduce the risk of influenza and/or other respiratory virus infections, especially in the elderly and children. The clinical findings indicate that specific probiotics or related combinations can provide health benefits for the elderly and children, probably by strengthening their innate and/or adaptive immunity. This is particularly important for aged adults as immunosenescence can reduce the protection of the influenza vaccine.5 There are many clinical studies investigating the effects of probiotics as adjuvants to potentiate influenza vaccine efficacy in the elderly.61 These studies are not mentioned in the current review as, in this article, we aimed to search for probiotic studies pertinent to those who are not suitable to receive vaccination or have a poor response to vaccination. Based on this review, specific probiotics or combinations for which clinical findings are available, can also offer the general public, not only the elderly and children, alternative choices when the seasonal flu vaccine does not prevent against the currently circulating strain of epidemic influenza or cannot meet everyone's need due to vaccine production limit. In addition, the antiviral mechanisms of specific probiotics or combinations and possible explanations for the immunomodulatory activities of probiotics observed in cells, mice and humans have also been addressed, providing valuable information for understanding the mechanistic action of probiotics for further application of probiotics in the future.
Funding
This work was supported by grants from the Ministry of Science and Technology (MOST 111-2320-B-001 -012 -MY3 for T.-N. Wu; MOST 111-2811-B-001-018 for Y.-H. Wang) and institutional grants from Academia Sinica, Taiwan.
Declaration of competing interest
The authors declare no conflict of interest to report this work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Je-Ruei Liu, Email: jrliu@ntu.edu.tw.
Tai-Na Wu, Email: taina@gate.sinica.edu.tw.
References
- 1.Kalil A.C., Thomas P.G. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care. 2019;23(1):258. doi: 10.1186/s13054-019-2539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iuliano A.D., Roguski K.M., Chang H.H., et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paules C., Subbarao K. Influenza. Lancet. 2017;390(10095):697–708. doi: 10.1016/S0140-6736(17)30129-0. [DOI] [PubMed] [Google Scholar]
- 4.Reber A.J., Chirkova T., Kim J.H., et al. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging and disease. 2012;3(1):68–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Wong S.S., Webby R.J. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26(3):476–492. doi: 10.1128/CMR.00097-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso V.R., Guarner F. Linking the gut microbiota to human health. Br J Nutr. 2013;109(S2):S21–S26. doi: 10.1017/S0007114512005235. [DOI] [PubMed] [Google Scholar]
- 7.La Fata G., Weber P., Mohajeri M.H. Probiotics and the gut immune system: indirect regulation. Probiotics Antimicro. 2018;10(1):11–21. doi: 10.1007/s12602-017-9322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepage P., Leclerc M.C., Joossens M., et al. A metagenomic insight into our gut's microbiome. Gut. 2013;62(1):146–158. doi: 10.1136/gutjnl-2011-301805. [DOI] [PubMed] [Google Scholar]
- 9.Budden K.F., Gellatly S.L., Wood D.L., et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 10.Trompette A., Gollwitzer E.S., Pattaroni C., et al. Dietary fiber confers protection against flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism. Immunity. 2018;48(5):992–1005 e1008. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Shinya K., Ito M., Makino A., et al. The TLR4-TRIF pathway protects against H5N1 influenza virus infection. J Virol. 2012;86(1):19–24. doi: 10.1128/JVI.06168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichinohe T., Pang I.K., Kumamoto Y., et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U.S.A. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley K.C., Finsterbusch K., Schnepf D., et al. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28(1):245–256. doi: 10.1016/j.celrep.2019.05.105. e244. [DOI] [PubMed] [Google Scholar]
- 14.Yildiz S., Mazel-Sanchez B., Kandasamy M., Manicassamy B., Schmolke M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome. 2018;6(1):9. doi: 10.1186/s40168-017-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groves H.T., Cuthbertson L., James P., Moffatt M.F., Cox M.J., Tregoning J.S. Respiratory disease following viral lung infection alters the murine gut microbiota. Front Immunol. 2018;9:182. doi: 10.3389/fimmu.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu S., Chen Y., Wu Z., et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis : an official publication of the Infectious Diseases Society of America. 2020;71(10):2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Li F., Wei H., Lian Z.X., Sun R., Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211(12):2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deriu E., Boxx G.M., He X., et al. Influenza virus affects intestinal microbiota and secondary Salmonella infection in the gut through type I interferons. PLoS Pathog. 2016;12(5) doi: 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Cao S., Zhang X. Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J Agric Food Chem. 2015;63(36):7885–7895. doi: 10.1021/acs.jafc.5b02404. [DOI] [PubMed] [Google Scholar]
- 20.Villena J., Vizoso-Pinto M.G., Kitazawa H. Intestinal innate antiviral immunity and immunobiotics: beneficial effects against rotavirus infection. Front Immunol. 2016;7(DEC):563. doi: 10.3389/fimmu.2016.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arena M.P., Capozzi V., Russo P., Drider D., Spano G., Fiocco D. Immunobiosis and probiosis: antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl Microbiol Biotechnol. 2018;102(23):9949–9958. doi: 10.1007/s00253-018-9403-9. [DOI] [PubMed] [Google Scholar]
- 22.Kassaa I.A., Hober D., Hamze M., Chihib N.E., Drider D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicro. 2014;6(3-4):177–185. doi: 10.1007/s12602-014-9162-6. [DOI] [PubMed] [Google Scholar]
- 23.Lee N.-K., Paik H.-D. Prophylactic effects of probiotics on respiratory viruses including COVID-19: a review. Food Sci Biotechnol. 2021;30(6):773–781. doi: 10.1007/s10068-021-00913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z., Chai W., Burwinkel M., et al. Inhibitory influence of Enterococcus faecium on the propagation of swine influenza A virus in vitro. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0053043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao X., Huang L., Zhu L., Mou C., Hou Q., Yu Q. Inhibition of H9N2 virus invasion into dendritic cells by the S-layer protein from L. acidophilus ATCC 4356. Front Cell Infect Microbiol. 2016;6:137. doi: 10.3389/fcimb.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serkedjieva J., Danova S., Ivanova I. Antiinfluenza virus activity of a bacteriocin produced by Lactobacillus delbrueckii. Appl Biochem Biotechnol. 2000;88(1-3):285–298. [Google Scholar]
- 27.Ermolenko E.I., Desheva Y.A., Kolobov A.A., Kotyleva M.P., Sychev I.A., Suvorov A.N. Anti–influenza activity of enterocin B in vitro and protective effect of bacteriocinogenic enterococcal probiotic strain on influenza infection in mouse model. Probiotics Antimicro. 2019;11(2):705–712. doi: 10.1007/s12602-018-9457-0. [DOI] [PubMed] [Google Scholar]
- 28.Oslund K.L., Baumgarth N. Influenza-induced innate immunity: regulators of viral replication, respiratory tract pathology & adaptive immunity. Future Virol. 2011;6(8):951–962. doi: 10.2217/fvl.11.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoggins J.W., Rice C.M. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1(6):519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoggins J.W. Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol. 2014;6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi U.Y., Kang J.-S., Hwang Y.S., Kim Y.-J. Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases. Exp Mol Medicine. 2015;47(3) doi: 10.1038/emm.2014.110. e144-e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waki N., Yajima N., Suganuma H., et al. Oral administration of Lactobacillus brevis KB290 to mice alleviates clinical symptoms following influenza virus infection. Lett Appl Microbiol. 2014;58(1):87–93. doi: 10.1111/lam.12160. [DOI] [PubMed] [Google Scholar]
- 33.Takeda S., Takeshita M., Kikuchi Y., et al. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int Immunopharm. 2011;11(12):1976–1983. doi: 10.1016/j.intimp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama Y., Moriya T., Sakai F., et al. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci Rep-uk. 2014;4(1):4638. doi: 10.1038/srep04638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu W., Fang Z., Liu X., et al. The potential role of probiotics in protection against influenza A virus infection in mice. Foods. 2021;10(4):902. doi: 10.3390/foods10040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto H., Sagitani A., Ashida N., et al. Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity. Br J Nutr. 2013;110(10):1810–1818. doi: 10.1017/S0007114513001104. [DOI] [PubMed] [Google Scholar]
- 37.Park M.-K., Ngo V., Kwon Y.-M., et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo J.-M., Lee H.-J., Kim J.-W., et al. Lactobacillus fermentum CJL-112 protects mice against influenza virus infection by activating T-helper 1 and eliciting a protective immune response. Int Immunopharm. 2014;18(1):50–54. doi: 10.1016/j.intimp.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Tonetti F.R., Islam A., Vizoso-Pinto M.G., Takahashi H., Kitazawa H., Villena J. Nasal priming with immunobiotic lactobacilli improves the adaptive immune response against influenza virus. Int Immunopharm. 2020;78 doi: 10.1016/j.intimp.2019.106115. [DOI] [PubMed] [Google Scholar]
- 40.Kanmani P., Albarracin L., Kobayashi H., et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol Immunol. 2018;93:253–265. doi: 10.1016/j.molimm.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa H., Kuno Y., Kohda C., Sasaki H., Nagashima R., Iyoda M. Exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 prevent influenza virus infection and attenuate secondary bacterial infection risk. Lett Appl Microbiol. 2022;74:632–639. doi: 10.1111/lam.13649. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T., Hayashi K., Kan T., Ohwaki M., Kawahara T. Anti-Influenza virus effects of Enterococcus faecalis KH2 and Lactobacillus plantarum SNK12 RNA. Biosci Microbiota Food Heal. 2021;40(1):43–49. doi: 10.12938/bmfh.2020-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B., Hylwka T., Smieja M., Surrette M., Bowdish D.M.E., Loeb M. Probiotics to prevent respiratory infections in nursing homes: a pilot randomized controlled trial. J Am Geriatr Soc. 2018;66(7):1346–1352. doi: 10.1111/jgs.15396. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto Y., Saruta J., Takahashi T., et al. Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: a randomized controlled trial. Acta Odontol Scand. 2019;77(7):517–524. doi: 10.1080/00016357.2019.1609697. [DOI] [PubMed] [Google Scholar]
- 45.Fujita R., Iimuro S., Shinozaki T., et al. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: a multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am J Infect Control. 2013;41(12):1231–1235. doi: 10.1016/j.ajic.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Pu F.F., Guo Y., Li M., et al. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: a randomized controlled open-label trial. Clin Interv Aging. 2017;12:1223–1231. doi: 10.2147/CIA.S141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shida K., Sato T., Iizuka R., et al. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur J Nutr. 2017;56(1):45–53. doi: 10.1007/s00394-015-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H., Yeh C., Jin Z., et al. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol. 2018;3(2):113–120. doi: 10.1016/j.synbio.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gleeson M., Bishop N.C., Oliveira M., McCauley T., Tauler P., Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int J Sport Nutr Exerc Metabol. 2012;22(4):235–242. doi: 10.1123/ijsnem.22.4.235. [DOI] [PubMed] [Google Scholar]
- 50.Smith T.J., Rigassio-Radler D., Denmark R., Haley T., Touger-Decker R. Effect of Lactobacillus rhamnosus LGG(R) and Bifidobacterium animalis ssp. lactis BB-12(R) on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013;109(11):1999–2007. doi: 10.1017/S0007114512004138. [DOI] [PubMed] [Google Scholar]
- 51.Chong H.X., Yusoff N.A.A., Hor Y.Y., et al. Lactobacillus plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: a randomized, double-blind, placebo-controlled study. J Dairy Sci. 2019;102(6):4783–4797. doi: 10.3168/jds.2018-16103. [DOI] [PubMed] [Google Scholar]
- 52.Garaiova I., Muchova J., Nagyova Z., et al. Probiotics and vitamin C for the prevention of respiratory tract infections in children attending preschool: a randomised controlled pilot study. Eur J Clin Nutr. 2015;69(3):373–379. doi: 10.1038/ejcn.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumpu M., Lehtoranta L., Roivainen M., et al. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. J Med Virol. 2013;85(9):1632–1638. doi: 10.1002/jmv.23623. [DOI] [PubMed] [Google Scholar]
- 54.Kumpu M., Kekkonen R.A., Kautiainen H., et al. Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2012;66(9):1020–1023. doi: 10.1038/ejcn.2012.62. [DOI] [PubMed] [Google Scholar]
- 55.Taipale T., Pienihakkinen K., Isolauri E., et al. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr. 2011;105(3):409–416. doi: 10.1017/S0007114510003685. [DOI] [PubMed] [Google Scholar]
- 56.Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proceedings. Biological sciences. 2015;282(1821) doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dickson R.P., Singer B.H., Newstead M.W., et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nature microbiology. 2016;1(10) doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houghton L.A., Lee A.S., Badri H., DeVault K.R., Smith J.A. Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat Rev Gastroenterol Hepatol. 2016;13(8):445–460. doi: 10.1038/nrgastro.2016.91. [DOI] [PubMed] [Google Scholar]
- 59.Edouard S., Million M., Bachar D., et al. The nasopharyngeal microbiota in patients with viral respiratory tract infections is enriched in bacterial pathogens. Eur J Clin Microbiol Infect Dis : official publication of the European Society of Clinical Microbiology. 2018;37(9):1725–1733. doi: 10.1007/s10096-018-3305-8. [DOI] [PubMed] [Google Scholar]
- 60.Chen H.W., Liu P.F., Liu Y.T., et al. Nasal commensal Staphylococcus epidermidis counteracts influenza virus. Sci Rep. 2016;6 doi: 10.1038/srep27870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lei W.T., Shih P.C., Liu S.J., Lin C.Y., Yeh T.L. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9(11) doi: 10.3390/nu9111175. [DOI] [PMC free article] [PubMed] [Google Scholar]