Abstract
Considerable evidence indicates the important role of gut microbiota in human health. Through the interaction with the host and diet, it secretes a myriad of metabolites to modulate biological processes essential for health. Cognitive impairment is a common feature of psychiatric and neurological disorders, which may seriously damage the quality of patients' life. Studies have found that cognitive impairment has a close relationship with gut microbiota, and plant polysaccharides intervention to maintain intestinal micro-ecological balance has a great impact on ameliorating cognitive impairment. This review introduced the interaction between gut microbiota and plant polysaccharides, and focused on signaling pathogenesis of gut microbiota in cognitive impairment. The effect of plant polysaccharides intervention on regulation of gut microbiota was also discussed, so as to provide a promising strategy for ameliorating cognitive impairment.
Keywords: Gut microbiota, Human health, Metabolites, Cognitive impairment, Plant polysaccharides
Graphical abstract
Highlights
-
•
Plant polysaccharides consumption can shape the diversity of the gut microbiota.
-
•
Plant polysaccharides intervention to maintain intestinal micro-ecological balance may ameliorate cognitive impairment.
-
•
The interaction between plant polysaccharides and gut microbiota may be beneficial to the cognition.
1. Introduction
Psychiatric and neurological disorders, such as Alzheimer's disease (AD), Parkinson's disease (PD), depression, and so forth, usually accompanied by a series of symptoms involving alterations in mood, behavior and, most pertinently, cognitive impairment.1 Cognitive impairment is sometimes neglected but common, which has a profound effect on daily life of patients. Persons with cognitive impairment have overt changes in thinking and memory, accompanied by aphasia or loss of recognition or misbehavior, but without significantly twisting the capacity of social relationships or the activities of daily life.2 Evidence shows that patients with mild cognitive impairment have a far higher rate of developing chronic neurodegenerative diseases than cognitively normal persons.1 Currently, the treatment of cognitive impairment mainly focuses on pharmacotherapeutic cure. While, medications that are available merely slow the process of decline but cannot reverse it. Thus, alternative treatment approaches for mitigating cognitive impairment are greatly needed.
Polysaccharides are natural active macromolecular substances widely exist in plants, animals, microorganisms and algae.3 Among them, plant polysaccharides are one of important ingredients with prominent physiological functions. Pharmacological and clinical studies have shown that plant polysaccharides perform a wide variety of biological functions, such as immune regulation, anti-inflammatory activity, antiviral and a hypoglycaemic effect.4 Recent studies have revealed their benefits to the brain. For example, Ganoderma lucidum could promote neural progenitor cell proliferation to enhance neurogenesis and alleviate cognitive deficits in transgenic AD mice.5 More intriguingly, dietary polysaccharides that reach the human intestine have a major impact on gut microbial ecology and brain function.6 The complexity of these interactions is enclosed in the denomination of “gut-brain axis”.
Gut microbiota is being called the “second brain” of the human, as it produces most neurotransmitters in the human brain, and is identified as a significant role in neural development, cognition and behavior.6 It is estimated that gut microbiota composes of approximately 1014 bacteria, and is 100–150 times that of the human genome.7 Previous studies suggested that healthy adults share certain gut microbiota species, which are characterized by a relatively high representation of Bacteroides, Prevotella and Ruminococcus.8 Gut microbiota is increasingly recognized as a critical organ within the human body, essential for extracting nutrients and energy from our diets, regulating immune system and cognitive function.9 In some instances, Astragalus membranaceus polysaccharides intervention significantly decreases the relative abundance of Firmicutes but increases the relative abundance of Bacteroidetes, as well as alleviates the hyperglycemia, tissue impairment, and inhibits cognitive impairment on diabetic mice.10
The pathogenesis of cognitive impairment is complex and multifactorial, but the role of the gut microbiota is not excluded. Studies attest a pivotal role of gut microbiota in orchestrating brain development. Modification of the gut microbial environment, including increase the diversity of gut microbiota, facilitate the growth of beneficial bacteria (Bifidobacterium, Lactobacillus, Allobaculum, etc), may prevent cognitive impairment. The intervention of alterations induced by cognitive impairment using dietary supplements, such as plant polysaccharides, is a possible therapeutic strategy for a healthy life. In this review, the changes of gut microbiota characteristics in cognitive impairment are discussed. We also described the current understanding of plant polysaccharides and cognitive impairment mechanisms, with a focus on the gut microbiome-derived metabolites, anti-inflammatory effects as well as immune regulation, and offered new insights for ameliorating cognitive impairment by plant polysaccharides intervention.
2. Structures and host benefits of polysaccharides
The range of polysaccharides that reach the gut from the diet is massive, and one of the significant components is generally insoluble plant fiber, such as dietary fiber, including inulin, fructooligosaccharides, pectin and so forth.11 According to World Health Organization, the recommended intake of total dietary fiber ranges from 25 to 35 g/2000 kcal/day.12 Specific polysaccharides have been widely proven as prebiotic dietary additives that are designed to selectively stimulate the growth of a subset of beneficial gut bacteria, and consequently to sustain the homeostasis of gut microbial community as well as the host health.13
The physicochemical property analysis identified that different polysaccharides have a discordant structure, with difference in molecular weight monosaccharide compositions linkage type, configuration and branch structure.14 Here, we summarize polysaccharides from different plants and their effects on host health.
2.1. Inulin
Inulin, a natural fructose polymer linked by β-(2, 1) bonds, exists in many plants, including wheat, onion, banana, garlic and chicory. Inulin is extensively used as industrial food ingredients owing to its unique functional characteristics along with health benefits. An expanding array of trials shows that inulin could benefit individuals with type 2 diabetes via promoting the growth of gut microflora and enhancing the release of SCFAs.15
2.2. Pectin
Pectin is an intricate polysaccharide linked galacturonic acid-rich, which mainly appears in plant cell walls. Pectins are resistant to digestive enzymes, however, can be easily degraded by the gut microbiota. One mechanism by which pectin is considered to provide beneficial effects is in terms of stimulating the growth and diversity of microbiota communities, improving intestinal integrity and mucosal proliferation, as well as favoring adhesion of probiotic Lactobacillus strains to the epithelial cells.16 Furthermore, a research indicated that pectin could strengthen the mucus layer, which would limit the passage of harmful substances into the underlying tissues and thereby prevent activation of inflammatory responses.
2.3. Purple sweet potato polysaccharide
Purple sweet potato polysaccharide (PSPP) composes of 1,4-α-d-glucopyranosyl residues backbone with side chains substituted at O-6 position consisting of terminal α-d-mannopyranosyl, α-d-rhamnopyranosyl, α-l-arabinofuranosyl and β-d-glucopyranosyl residues.17 The biological activities of PSPP have received an increasing amount of attention, including of anti-tumor, hepato-protective and immune-enhancing activities.18, 19, 20 A recent study demonstrated that PSPP could alleviate colonic inflammation via blocking pro-inflammatory cytokines and modulate the structure of gut microbiota in dextran sulfate sodium-induced colitis mice.17
2.4. Lycium barbarum polysaccharide
Lycium barbarum polysaccharide (LBP) is the main active component of Lycium barbarum. The backbone of LBP composes of (1 → 4)-α-GalA, and branches contained α-1,2-Rha, α-1,4-Rha and α-1,5-Ara. A large number of researches have been reported that LBP exhibited neuroprotective activity in various central nervous system (CNS) diseases via its anti-inflammatory and antioxidant properties.21,22 A recent study revealed that LBP could ameliorate memory and neurogenesis impairments via preventing aberrant neuronal activity and microglial activation in the lateral habenula. Meanwhile, this study also demonstrated LBP administration could prevent neuroblast differentiation from scopolamine toxicity in the dentate gyrus as well as ameliorate the cognitive and memory function.22
3. Potential signaling pathogenesis of gut microbiota in cognitive impairment
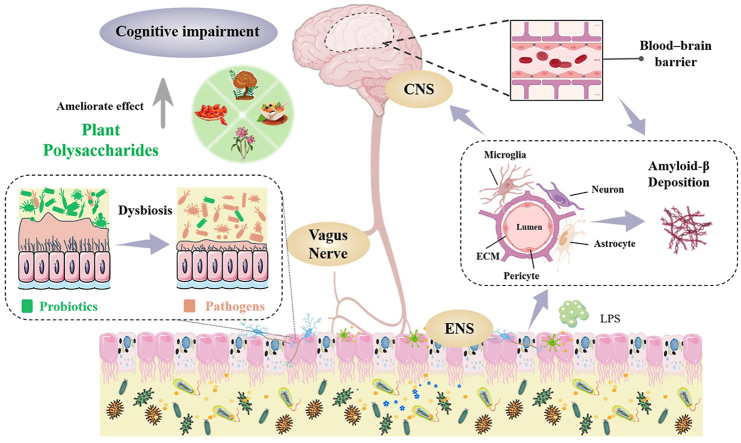
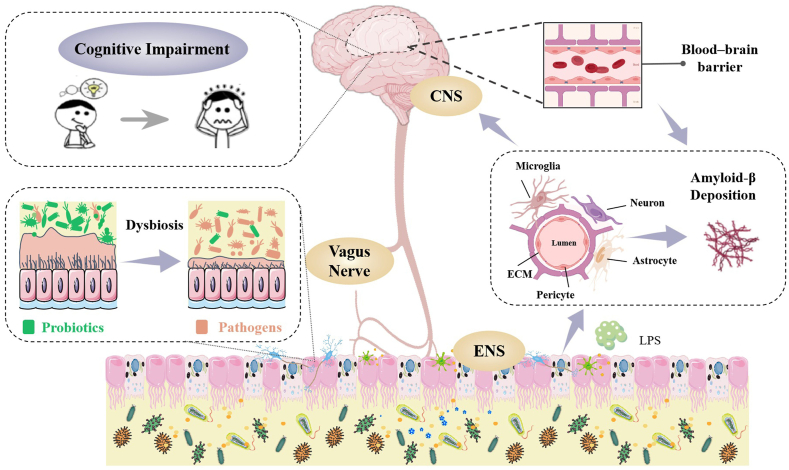
Physiological studies of cognitive impairment have shown that cognitive functions are subject to neuronal loss, decrease in neurogenesis and synaptic plasticity, and neuroinflammation, while these conditions may result from an alteration in the microbiota.2 In addition, evidence also indicates that cognitive impairment is critically driven by specific personality traits, cardiac dysfunction, malnutrition, chronic inflammation and hormonal dysregulation.23 In particular, most of these factors have related to the dysbiosis of gut microbiota, involving in receding gut microbial diversity, increasing gut permeability and depleting of bacteria-derived metabolites (Fig. 1).2 The results of high-throughput sequencing showed that gut microbiota of patients with cognitive impairment related diseases were significantly different from that of healthy people (Table 1).
Fig. 1.
The relationship between gut microbiota disturbance and cognitive impairment.
Table 1.
Microbes changed in patients with cognitive impairment.
| Study design | Microbes changed in the gut | Results | References |
|---|---|---|---|
| Patients with the PD |
Escherichia/Shigella ↑ Eubacterium rectale ↓ |
Gut microbiota may drive peripheral inflammation, contributing to brain amyloidosis cognitive symptoms in AD. | 11 |
| Patients with the PD |
Faecalibacterium ↑ Proteobacteria ↓ |
The dysbiosis of gut microbiota may play a role in neuronal loss by perpetuating inflammatory cascades and oxidative injury in the brain through an LPS involvement. | 12 |
| Patients with the AD |
Firmicutes ↓ Actinobacteria ↓ Bacteroidetes ↑ |
There is a correlation between the gut microbial alterations and ccerebrospinal fluid biomarkers of AD. | 13 |
| Patients with dementia | Clostridium difficile ↑ | Patients with dementia have substantial dysbiosis of the gut microbiota. | 14 |
| Patients with mild cognitive impairment |
Lachnospiraceae ↓ Clostridiaceae ↓ Ruminococcus ↓ |
Cognitive function is related to specific bacterial taxal populations comprising the gut microbiota | 15 |
| Patients with post-stroke comorbid cognitive impairment and depression |
Gammaproteobacteri ↑ Enterobacteriales ↑ Enterobacteriaceae ↑ Fusicatenibacter ↓ |
The number of pathogenic bacteria increased, while several SCFAs-producing bacteria decreased. | 16 |
The dysbiosis of gut microbiota may also affect the blood-brain barrier (BBB) formation, which controls over the vital nutrients that leave or enter the brain precisely and ensures an optimal microenvironment for neuronal growth and specification as well as prevents the entry of pathological agents.24 It is composed of microglia, astrocytes, pericytes, neurons, brain microvascular endothelial cells, and extracellular matrix, which together innervate the pathogenesis of cognitive impairment.25 On the one hand, diseases associated with cognitive impairment are always involving the accumulation of amyloidogenic proteins, whereas pericyte damage and loss will propel amyloid-β (Aβ) deposition.26 Infiltrating immune cells, microglia, and astrocytes may reciprocally activate each other, facilitating chronic inflammation and preventing BBB repair, with pericyte damage and neuronal loss further promoting cognitive decline. On the other hand, increasing of the BBB permeability, resulting from the dysbiosis of gut microbiota allows ingress of bacteria and other components such as toxins and lipopolysaccharides (LPS), triggering these substances to be translocated to the central nervous system and propelling cognitive impairment.27 Thus, the permeability of the BBB is vital for the support of gut health and the modulation of cognitive functions through the regulation of gut microbiota imbalance.
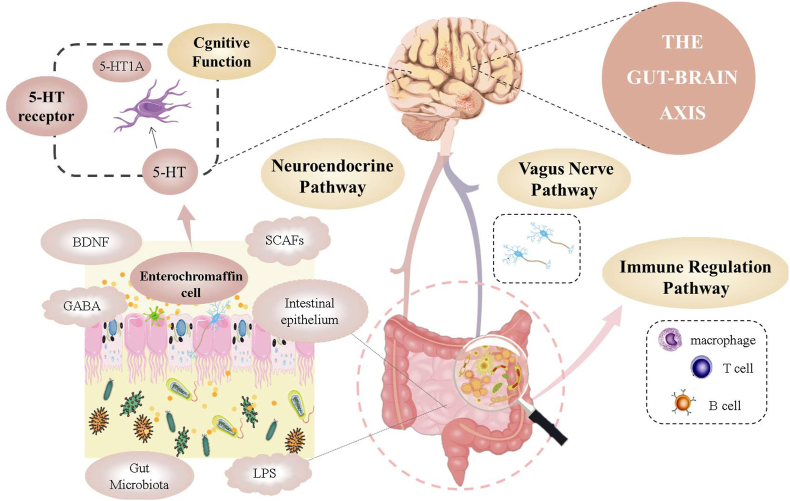
In addition, gut microbiota (e.g., Bifidobacterium adolescentis, Lactobacillus rhamnosus, Citrobacter rodentium and Campylobacter jejuni) also affects cognitive functions through three pathways mainly involved in gut-brain axis.28 The first is the neuroendocrine pathway, in which gut microbiota may influence the CNS via regulation of the secretion of neurotransmitters, for instance, cortisol, serotonin (5-hydroxytryptamine, 5-HT), and γ-aminobutyric acid (GABA). The second is the immune regulation pathway. Recent evidence shows that gut microbiota has the capacity to modulate the function of tissue-resident immune cells in the CNS, and then regulate responses to brain cognition.29 And the third is the vagus nerve pathway. We reasoned that this main neural pathway may not interact with the gut microbiota directly, but indirectly sense microbial signals through various bacterial metabolites, thereby affect brain function.30 Thus, targeting of gut microbiota could hold the key to uncouple pathological interactions between the gut and brain (Fig. 2).
Fig. 2.
Three pathways of gut microbiota affecting cognitive function.
4. Interactions between polysaccharides and intestinal micro-ecology: A new cue to ameliorate cognitive impairment
Cognitive impairment is a chronic disease, which is the major cause of progressive functionality loss and disability. It is noteworthy that several strategies to improve gut microbiota imbalance have been raised, including fecal microbiota transplants, the utilization of probiotics, and dietary intervention.13 The gut microbiota depends largely on dietary polysaccharides as energy sources. Moreover, dietary polysaccharides that reach the human large intestine present a significant effect in gut microbial ecology and health.11 Thus, it is an effective diet intervention strategy to improve intestinal micro-ecology and then ameliorate cognitive impairment by polysaccharides.
4.1. Polysaccharides shape the diversity of gut microbiota
Take the difference in diet between the East and the West as an example, Oriental populations who consume far less refined diets and significantly higher proportions of plant polysaccharides exhibited higher richness in microbiota. However, western populations have lower gut microbiota abundance.31 Reducing polysaccharides intake periodically does not seem to have long-lasting effects on the microbial richness, however, long-term reduction in polysaccharides intake may lead to permanent disappearance of some important microbial.32 Filippo compared the fecal microbiota from European children (EU, the diet is characterized by high fat, high protein and low fiber) with that of the children from Burkina Faso (BF, the diet is characterized by low fat, low protein and high fiber). BF children showed a significant enrichment in Bacteroidetes and depletion in Firmicutes, with a unique abundance of microbiota from the genus Prevotella and Xylanibacter, which were completely lack in the EU children.33 Further, a study about the health effects of compound polysaccharides supplementation on the intestinal microecosystem and metabolism of mice reported that the relative abundances of four bacterial genera significantly increased, including Bifidobacterium, Lactobacillus, Allobaculum and Oligella, whereas the relative abundance of Enterococcus declined in the compound polysaccharide-treated groups compared with the control group. Meanwhile, dietary compound polysaccharide treatment benefited the functional maturation of intestinal bacterial community, mainly manifested in the increase of amino acid metabolism, energy metabolism, SCFAs-related metabolism and nucleotide metabolism.34
Notably, these intestinal microbes include Lactobacillus fermentum, Lactobacillus acidophilus, Bifidobacterium lactis and so forth are of significance to improve cognitive impairment. It has recently been proposed that Lactobacillus fermentum could provide beneficial effects on cognitive function, physiological behavior and immune responses in aged mice.35 Lactobacillus plantarum MTCC 1325 might ameliorate memory impairment and cognition deficits in AD-model mice.36 Bifidobacterium breve (B. breve) could reverse impairment of working memory in mice in a Y maze test, based on evidence that suggests B. breve was a possible therapeutic strategy for improving cognitive impairment.37 Whilst, these data are mainly concentrated on animal experiments, and more convincing clinical data involved remain to be studied.
4.2. Repair of intestinal barrier
Intestinal barrier depends on interactions among several barrier components including the adhesive mucous layer, immunoglobulin A, antibacterial peptides, and tight intrinsic junctions, which acts as a multitasking mediator between bodily function and a vast diversity of microbial.38 Impaired integrity and structure of the intestinal barrier will result in the invasion of harmful substances, causing a series of adverse reactions and even diseases. In some instances, research indicated that LPS could stimulate systemic inflammation and neuroinflammation, and then cause an elevation in Aβ levels and neuronal cell death, finally resulting in cognitive impairment.39 Remarkably, polysaccharides can protect and repair the intestinal barrier via maintaining the permeability and integrity of intestinal mucous and up-regulating the expression of tight junction proteins.40 For instance, Dendrobium huoshanense polysaccharide supplementation could improve the intestinal barrier function by modulating mucous structures and up-regulating the expression of tight junction proteins.41 And a recent study demonstrated that the loss of polysaccharides could lead to mucous layer degradation and reduce mucus-producing goblet cells in the epithelial tissue.42
4.3. Beneficial effects of interaction between plant polysaccharides and gut microbiota on cognitive impairment
Convincing evidence exists that different polysaccharides have a regulatory effect on cognitive impairment (Table 2). A study performed by Liu, assessed the effect of astragalus membranaceus polysaccharides on cognitive dysfunction in diabetic mice. And results showed that Astragalus membranaceus polysaccharide could alter the gut microbiota and modulate the composition of metabolites like SCFAs, which was the potential mechanism of inhibiting cognitive impairment.10 Another study showed that Dendrobium polysaccharides had the potential to provide neuroprotection against AD-related cognitive impairment via modulation of microglial activation.43 In addition, polysaccharides from ganoderma lucidum could promote cognitive function in mouse model of AD by reducing Aβ deposits.5 To guide future targeted dietary therapies, we provide an overview of what is currently known regarding underlying mechanisms of action by which plant polysaccharides may ameliorate cognitive impairment from the perspective of gut microbiota. Of course, the mechanisms of action associating polysaccharides, gut microbiota with cognitive impairment are complex, multifaceted, interacting, and not restricted to any one pathway.44
Table 2.
The regulation of polysaccharides on cognitive impairment.
| Polysaccharide | Duration of intervention | Results | References |
|---|---|---|---|
| Schisandra chinensis fructus | Seven weeks AD mice |
Reduce the deposition of Aβ; Downregulate the expression of pro-inflammatory cytokines and the activation of glial cells in the hippocampus. |
52 |
| Astragalus membranaceus | Ten weeks Diabetic mice |
Lactobacillus ↑ Sutterella ↑ Oscillospira ↓ Mucispirillum↓ Butyric acid ↑ Alleviate the hyperglycemia; Inhibit cognitive impairment. |
10 |
| Dendrobium polysaccharides | Three months AD mice |
Modulate microglial activation; Reduce the deposition of Aβ; Attenuate cognitive impairment. |
43 |
| Ganoderma lucidum | Three months AD mice |
Reduce the deposition of Aβ; Enhance neurogenesis along with reducing cognitive deficits in AD mice. |
5 |
| Inulin | Four months Mice with cognitive impairment |
Prevotella ↑ Lactobacillus ↑ Escherichia ↓ Turicibacter ↓ Akkermansia ↓ Acetate ↑ Butyrate ↑ propionate ↑ Improve immune function in the periphery and CNS; Reduce neuroinflammation. |
44 |
4.3.1. Gut microbiota-derived metabolites pathway
Gut microbiota possesses a series of polysaccharides degrading enzymes, which largely complement the few expressed by the human host.45 Therefore, microbiota can be considered as a “digestive partner” which has co-evolved with the human host and allows the host to benefit from food components that would otherwise be lost as waste.46 In the cecum and colon, polysaccharides can produce a series of metabolites after being hydrolyzed and utilized by gut microbiota, and the most important fermentation products are SCFAs, which are speculated to have a key role in microbiota-gut-brain crosstalk.47
SCFAs mainly act on the colon, but they are also absorbed into the blood and act on other organs, including the brain.33 SCFAs produced by the bacterial fermentation of dietary polysaccharide can improve the growth and development of brain microglia, which can enhance brain immune defense function and act as the most important immune barrier of CNS, but their insufficient may induce CNS diseases.48 Maintenance of epithelial integrity has been associated with butyrate, which is the main energy source for colonocytes and is able to improve intestinal tight junction integrity by regulating the expression of tight junction proteins.27 Moreover, SCFAs can cross the BBB, modulate brain development and behavior and have been implicated in the development of cognitive function.47
4.3.2. Immune regulation pathway
The immune system, by protecting the host against invading pathogens and internal damage, is fundamental for the survival of all species.48 It can also be considered as the gatekeeper and master regulator of gut-brain communication. Probiotics are co-evolved with the immune system to maintain a symbiotic relationship, provide colonization resistance to pathogens, and educate the immune system.49 The immune system consists of a series of immune cells, such as macrophages, monocytes, neutrophils and microglia. Among them, microglia are the innate immune cells of the CNS, which are crucial source of cytokines during neuroinflammation and has essential effects on CNS tissue maintenance, injury response, and pathogen defense.50 Interestingly, gut microbiota plays a pivotal role in the healthy development, maturation and activation of microglia. Preclinical evidence suggested that GF mice usually displayed defects in microglia, including the alteration of cell proportions and immature phenotype, resulting to impaired innate immune responses. In contrast, microglia features could be partially restored by recolonization with a complex microbiota.51 Additionally, immune response mediated chronic neuroinflammation may damage brain tissue and brain volume through the proliferation of inflammatory cells, leading to neurodegeneration and cognitive impairment.52
Intriguingly, it is becoming apparent that plant polysaccharides have a profound effect on anti-inflammatory functions and improving cognitive impairment. In some instances, Inulin had the capacity to propel systemic metabolism and reduce neuroinflammation through modulating gut microbiota even before the development of Aβ in the cognitive impairment mice.44 Polysaccharides from Schisandra chinensis fructus also have a good therapeutic effect on anti-inflammation, significantly improving the cognition and histopathological changes of AD mice by reducing the deposition of Aβ and the release of pro-inflammatory cytokines.52
For the mechanism of anti-inflammatory effects of polysaccharides, studies have manifested that polysaccharides intervention can significantly regulate the expression and release of pro-inflammatory cytokines. Gut microbiota can regulate the permeability of the intestines. With multiple detrimental components from pathogenic bacteria, including LPS, cell capsule carbohydrates and other endotoxins, may promote a “leaky gut".27 This leakiness phenomenon may produce and activate inflammatory cytokines to a certain extent, leading to local inflammation. LPS also binds to toll-like receptor 4 find on immune cells, and in doing so can propel inflammation on both a local and systemic scale.26 Moreover, Astrocytes and microglia are activated excessively under inflammatory stress, can produce a wide range of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6.53 Meanwhile, plant polysaccharides could attenuate LPS-mediated inflammatory responses via nuclear factor-κB or mitogen activated protein kinases signaling pathway (the main signaling pathways to control immune responses in macrophages), decrease Aβ deposition, reduce the release of these pro-inflammatory cytokines, improve astrocyte and microglia activation, as well as have a resistance to the cognitive impairment related diseases.52
5. Conclusion
With the development of intestinal genomics and metabolomics, the establishment of intestinal flora genomic database has improved gut microbiota information system, which makes gut microbiota no longer mysterious, and its effect on human health is more prominent. There is a certain relationship between gut microbiota and cognitive function. Improving the dysbiosis of gut microbiota by dietary polysaccharide intervention has been believed to be a promising research area for developing novel preventive or curative strategies against cognitive impairment, but the potential mechanism is still in its infancy, the intervention time is short, and the sample size is small, so no recommendations for clinical practice have been made. Therefore, further elucidation of the mechanism of the interaction between intestinal flora and nervous system will help us understand cognitive impairment and find the best treatment.
Taxonomy (classification by EVISE)
Chinese Herbal Medicine, Diet In Clinical Trials, Food Technology, Functional Disorder, Traditional Medicine.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
This work was sponsored by Zhejiang Provincial Key Research and Development Program (2020C02037) and People-benefit Project of Ningbo (202002N3078).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Chi-Tang Ho, Email: ctho@sebs.rutgers.edu.
Xin Zhang, Email: zhangxin@nbu.edu.cn.
References
- 1.Goldman J.G., Holden S.K., Litvan I., McKeith I., Stebbins G.T., Taylor J.P. Evolution of diagnostic criteria and assessments for Parkinson's disease mild cognitive impairment. Mov Disord. 2018;33:503–510. doi: 10.1002/mds.27323. [DOI] [PubMed] [Google Scholar]
- 2.Romo-Araiza A., Ibarra A. Prebiotics and probiotics as potential therapy for cognitive impairment. Med Hypotheses. 2020;134:109410. doi: 10.1016/j.mehy.2019.109410. [DOI] [PubMed] [Google Scholar]
- 3.Sun Q., Cheng L., Zeng X., Zhang X., Wu Z., Weng P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int J Biol Macromol. 2020;164:1484–1492. doi: 10.1016/j.ijbiomac.2020.07.208. [DOI] [PubMed] [Google Scholar]
- 4.Yuan L., Zhong Z.C., Liu Y. Structural characterisation and immunomodulatory activity of a neutral polysaccharide from Sambucus adnata Wall. Int J Biol Macromol. 2020;154:1400–1407. doi: 10.1016/j.ijbiomac.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Huang S., Mao J., Ding K., et al. Polysaccharides from Ganoderma lucidum promote cognitive function and neural progenitor proliferation in mouse model of Alzheimer's disease. Stem Cell Reports. 2017;8:84–94. doi: 10.1016/j.stemcr.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinan T.G., Cryan J.F. Microbes, immunity, and behavior: psychoneuroimmunology meets the microbiome. Neuropsychopharmacology. 2017;42:178–192. doi: 10.1038/npp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. 2015;73:32–40. doi: 10.1093/nutrit/nuv039. [DOI] [PubMed] [Google Scholar]
- 8.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song D., Yang C.S., Zhang X., Wang Y. The relationship between host circadian rhythms and intestinal microbiota: a new cue to improve health by tea polyphenols. Crit Rev Food Sci Nutr. 2021;61:139–148. doi: 10.1080/10408398.2020.1719473. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Liu W., Li J., et al. A polysaccharide extracted from Astragalus membranaceus residue improves cognitive dysfunction by altering gut microbiota in diabetic mice. Carbohydr Polym. 2019;205:500–512. doi: 10.1016/j.carbpol.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y., Cheng L., Zeng X., et al. The intervention of unique plant polysaccharides - dietary fiber on depression from the gut-brain axis. Int J Biol Macromol. 2021;170:336–342. doi: 10.1016/j.ijbiomac.2020.12.164. [DOI] [PubMed] [Google Scholar]
- 12.Adamberg K., Jaagura M., Aaspollu A., Nurk E., Adamberg S. The composition of faecal microbiota is related to the amount and variety of dietary fibres. Int J Food Sci Nutr. 2020;71:845–855. doi: 10.1080/09637486.2020.1727864. [DOI] [PubMed] [Google Scholar]
- 13.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 14.Nie S.P., Xie M.Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocolloids. 2011;25:144–149. [Google Scholar]
- 15.Birkeland E., Gharagozlian S., Birkeland K.I., et al. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: a randomised controlled trial. Eur J Nutr. 2020;59:3325–3338. doi: 10.1007/s00394-020-02282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkar S.G., Redgate E.L., Wibisono R., Luo X.X., Koh E.T.H., Schroder R. Gut health benefits of kiwifruit pectins: comparison with commercial functional polysaccharides. J Funct Foods. 2010;2:210–218. [Google Scholar]
- 17.Sun J., Chen H., Kan J., et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int J Biol Macromol. 2020;153:708–722. doi: 10.1016/j.ijbiomac.2020.03.053. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q., Qu H., Jia J., et al. Characterization, antioxidant and antitumor activities of polysaccharides from purple sweet potato. Carbohydr Polym. 2015;132:31–40. doi: 10.1016/j.carbpol.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 19.Sun J., Zhou B., Tang C., et al. Characterization, antioxidant activity and hepatoprotective effect of purple sweetpotato polysaccharides. Int J Biol Macromol. 2018;115:69–76. doi: 10.1016/j.ijbiomac.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Tang C., Sun J., Liu J., et al. Immune-enhancing effects of polysaccharides from purple sweet potato. Int J Biol Macromol. 2019;123:923–930. doi: 10.1016/j.ijbiomac.2018.11.187. [DOI] [PubMed] [Google Scholar]
- 21.Bie M., Lv Y., Ren C., et al. Lycium barbarum polysaccharide improves bipolar pulse current-induced microglia cell injury through modulating autophagy. Cell Transplant. 2015;24:419–428. doi: 10.3727/096368915X687453. [DOI] [PubMed] [Google Scholar]
- 22.Chen W., Cheng X., Chen J., et al. Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ticinesi A., Tana C., Nouvenne A., Prati B., Lauretani F., Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497–1511. doi: 10.2147/CIA.S139163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene C., Hanley N., Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10:373. doi: 10.1038/s41398-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 26.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 27.Parker A., Fonseca S., Carding S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microb. 2020;11:135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 29.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Hao Y., Fan F., Zhang B. The role of microbiome in insomnia, circadian disturbance and depression. Front Psychiatr. 2018;9:669. doi: 10.3389/fpsyt.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makki K., Deehan E.C., Walter J., Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Sonnenburg E.D., Smits S.A., Tikhonov M., Higginbottom S.K., Wingreen N.S., Sonnenburg J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Filippo C., Cavalieri D., Di Paola M., et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M., Xie Z., Li L., et al. Supplementation with compound polysaccharides contributes to the development and metabolic activity of young rat intestinal microbiota. Food Funct. 2019;10:2658–2675. doi: 10.1039/c8fo02565g. [DOI] [PubMed] [Google Scholar]
- 35.Park M.R., Shin M., Mun D., et al. Probiotic Lactobacillus fermentum strain JDFM216 improves cognitive behavior and modulates immune response with gut microbiota. Sci Rep. 2020;10:21701. doi: 10.1038/s41598-020-77587-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nimgampalle M., Kuna Y. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer's disease induced albino rats. J Clin Diagn Res. 2017;11:KC01–KC05. doi: 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi Y., Sugahara H., Shimada K., et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease. Sci Rep. 2017;7:13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki T. Regulation of the intestinal barrier by nutrients: the role of tight junctions. Anim Sci J. 2020;91:e13357. doi: 10.1111/asj.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao J., Bi W., Xiao S., et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9:5790. doi: 10.1038/s41598-019-42286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Q., Wang Y., Huang L., et al. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res Int. 2021;140:109858. doi: 10.1016/j.foodres.2020.109858. [DOI] [PubMed] [Google Scholar]
- 41.Xie S.Z., Liu B., Ye H.Y., et al. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr Polym. 2019;206:149–162. doi: 10.1016/j.carbpol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Riva A., Kuzyk O., Forsberg E., et al. A fiber-deprived diet disturbs the fine-scale spatial architecture of the murine colon microbiome. Nat Commun. 2019;10:4366. doi: 10.1038/s41467-019-12413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng C.Z., Cao L., Luo D., et al. Dendrobium polysaccharides attenuate cognitive impairment in senescence-accelerated mouse prone 8 mice via modulation of microglial activation. Brain Res. 2019;1704:1–10. doi: 10.1016/j.brainres.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman J.D., Yanckello L.M., Chlipala G., et al. Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurokawa K., Itoh T., Kuwahara T., et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit Rev Food Sci Nutr. 2017;57:3543–3564. doi: 10.1080/10408398.2016.1180501. [DOI] [PubMed] [Google Scholar]
- 47.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 48.Sherwin E., Bordenstein S.R., Quinn J.L., Dinan T.G., Cryan J.F. Microbiota and the social brain. Science. 2019;366 doi: 10.1126/science.aar2016. [DOI] [PubMed] [Google Scholar]
- 49.Belkaid Y., Harrison O.J. Homeostatic immunity and the microbiota. Immunity. 2017;46:562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer's disease. J Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erny D., Hrabe de Angelis A.L., Jaitin D., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu M., Yan T., Fan K., et al. Polysaccharide of Schisandra Chinensis Fructus ameliorates cognitive decline in a mouse model of Alzheimer's disease. J Ethnopharmacol. 2019;237:354–365. doi: 10.1016/j.jep.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 53.Zhao H., Wang Q., Cheng X., et al. Inhibitive effect of resveratrol on the inflammation in cultured astrocytes and microglia induced by Abeta1-42. Neuroscience. 2018;379:390–404. doi: 10.1016/j.neuroscience.2018.03.047. [DOI] [PubMed] [Google Scholar]