Abstract
Triphala is a mixture of tree fruits obtained from Terminalia chebula, Terminalia bellerica, and Phyllanthus emblica. It is one of the Ayurveda medicinal recipes used to treat health diseases such as obesity. The chemical composition analysis of Triphala extracts obtained from an equal portion of three fruits was performed. The contents of total phenolic compounds (62.87 ± 0.21 mg gallic acid equivalent/mL), total flavonoids (0.24 ± 0.01 mg catechin equivalent/mL), hydrolyzable tannins (177.27 ± 10.09 mg gallotannin equivalent/mL), and condensed tannins (0.62 ± 0.11 mg catechin equivalent/mL) were observed in Triphala extracts. The 1 mg/mL of Triphala extracts was applied to batch culture fermentation which contained the feces from voluntarily obese female adults (body mass index of 35.0–40.0 kg/m2) for 24 h. The extraction of DNA and metabolites was each conducted on the samples obtained from batch culture fermentation within and without Triphala extracts treatment. The 16S rRNA gene sequencing and untargeted metabolomic analysis were carried out. There was no statistically significant difference between Triphala extracts and control treatments on the changes in microbial profiles (p-value <0.05). While the metabolomic analysis showed statistically significant differences of 305 up-regulated and 23 down-regulated metabolites in the treatment of Triphala extracts when compared with the control (p-value <0.05 and fold-change ≥2) belonging to 60 pathways. The pathway analysis revealed that Triphala extracts play an important role in the activation of phenylalanine, tyrosine and tryptophan biosynthesis. In this study, phenylalanine and tyrosine were identified metabolites which involve in the regulation of energy metabolism. The treatment of Triphala extracts possesses the induction of phenylalanine, tyrosine and tryptophan biosynthesis in fecal batch culture fermentation of obese adults and therefore it can be suggested as a probable herbal medicinal recipe for obesity treatment.
Keywords: Feces, Metabolome, Microbiome, Obesity, Triphala extracts
Graphical abstract
Highlights
-
•
Triphala extracts treatment showed statistically significant differences in metabolite changes when compared with the control.
-
•
Triphala extracts employed the biosynthesis of phenylalanine, tyrosine and tryptophan as a major pathway in human gut model.
-
•
Triphala extracts are involved in the regulation of energy metabolism and obesity management.
1. Introduction
Excess body weight has been found in every generation of ages. The excess body weight complied with the body mass index (BMI) levels could indicate the stages of obesity. One of the most important medical problems is obesity which associates with high economic costs in the healthcare system.1 Obesity is a leading risk factor for many health problems such as the risks of mortality in the medical wards during COVID-19,2 coronary heart disease,3 type-2 diabetes,4 including dysbiosis of gut microbiota.5
Gut microbiota plays an important role in human health and physiology. Microbial organisms in the colon can produce several beneficial metabolites such as short-chain fatty acids functioning in the maintenance of gut microbial homeostasis.6 The alterations of microbial profiles in the gut have been associated with various health problems.7 The pattern of gut microbiota depends on the environment, habitat, and food. The previous study presented the different patterns of gut microbiota between obese and healthy people.8,9 The higher abundance of Firmicutes and lower abundance of Bacteroidetes were observed in obese adults when compared with normal-weight adults.8 The variety and percentage of microorganisms existing in the gut affect the energy balance of the host10 which might relate to obesity. Maintaining the balance of the gut is one of the approaches to maintaining the host's normal health and preventing numerous health problems.
Plant extracts, one of the functional ingredients, were used as an alternative method for the manipulation of the gut microbiome. The previous study reported the efficacy of plant extracts on inhibition of the Escherichia/Shigella genus which relate to gastrointestinal diseases during fecal fermentation.11 Emblica officinalis extracts enhanced the proportion of the Eubacterium genus in the family of Eubacteriaceae belonging to the Firmicutes phylum.12 Tea extracts were reported to enrich the growth of Faecalibacterium, which is considered as a butyrate producer.11 Plant extracts that contain polyphenols as a major component, were reported to promote the growth of Lactiplantibacillus plantarum 299v considered as a beneficial bacterium for the gut.13 Moreover, plant extracts have been used to modulate the abundance and composition of gut microbiota for obesity treatment.14
Triphala is a traditional medicinal recipe consisting of equal quantities of Terminalia chebula, Terminalia bellerica, and E. officinalis. Triphala is traditionally used for the treatment of digestive disorders.15 The contents of phenolic, flavonoid, and tannin compounds were contained in Triphala extracts.16 Polyphenols in Triphala presented the regulation activity of gut microorganisms, increased the growth of Bifidobacteria and Lactobacillus, and suppressed the growth of unpleasant microbes in the human gut.15 Triphala extracts were not only reported several pharmacological and ethnopharmacological properties such as antioxidant, antimicrobial, and antidiabetic but also revealed the effect on the gastrointestinal tract system such as constipation including the properties against obesity.17 Triphala has been reported the potential ability on reduction of low-density lipoprotein-cholesterol, total cholesterol and triglyceride, body weight, body mass index, and waist circumference in humans.18 However, the efficacy of Triphala extracts on the changes of gut microbiota and metabolome against obesity at the metabolic level is still needed.
To determine the microbial profile and metabolic changes in the gut, modern powerful technologies such as metagenomics and metabolomics are required. Shotgun metagenomic analysis was used to differentiate the changes of bacterial composition in the gut of pigs.19 Moreover, 16S rRNA gene sequencing was used to remark the alteration of gut microbial profiles in mice treated with calebin A for the anti-obesity property.20 The results of 16S rRNA profiling revealed Triphala's ability on the changes of human gut microbial composition by decreasing of Firmicutes to Bacteroidetes ratio.21
At the same time, metabolomics has exhibited the potential to profile the up-regulated and down-regulated metabolites in metabolic pathways22 and indicate the BMI-associated metabolites by untargeted metabolomic analysis.23 Metabolomics has been used to investigate the changes in metabolic responses to bitter melon in a high-fat diet-induced obesity model. The results of liquid chromatography-mass spectrometry (LC-MS)-based metabolomics presented an increase in glucose homeostasis and vitamin D metabolism in diet-induced obese male mice by lyophilized bitter melon juice extract.24 However, the understanding of Triphala extracts’ action on the metabolome alteration in obesity remains to be explained.
In vitro human gut model that simulates the gut conditions, has been developed to use for studying microbial composition and community in fermentation patterns. A bioreactor (batch) has been designed to culture microorganisms obtained from the human gut microbiota in a dynamic environment.25 The human gut model facilitates primary screening of the effects of many agents of gut microbiome modulators such as prebiotics and food ingredients on the gut microbial composition and metabolic activities such as alterations of short-chain fatty acids.26 The human gut model has been used to examine the effect of dietary fibers such as oat on fecal microbial compositions and production of short-chain fatty acids on in vitro fermentation for 24 h.27 Moreover, in vitro human gut model has been used to study trimethylamine production which is converted to trimethylamine-N-oxide relating to increased risk of metabolic diseases from dietary precursors for 24 h.28 This study aimed to evaluate the effect of Triphala extracts on microbiota and metabolic changes in fecal batch culture fermentation as the human gut model of obese adults. The 16S rRNA gene sequencing and untargeted metabolomic analysis were performed to analyze the microbial composition and metabolite alteration of in vitro batch culture fermentation. This study gains insight into the action of Triphala extracts on in vitro metabolic changes in obesity to support Triphala extracts as an alternatively medicinal recipe for obesity treatment.
2. Materials and methods
2.1. Preparation of Triphala extracts and composition of Triphala extracts' constituents
2.1.1. Preparation of Triphala extracts
Each equally dried component of T. bellirica, T. chebula, and P. emblica16 which were obtained from Songkla province, Thailand, was ground to the fine powder of Triphala. The 15 g of Triphala fine powder was boiled in 100 mL sterile distilled water for 30 min. The supernatant was filtered and then evaporated with the CentriVap Complete vacuum concentrator (Labconco, USA). The dried form was kept as the Triphala extracts and stored at room temperature (28 °C) before performing the next experiments.
2.1.2. Composition of Triphala extracts’ constituents
The total flavonoid content of Triphala extracts was determined following the previous study.29 Briefly, 90 μL of extract solution was mixed with 360 μL of distilled water and 30 μL of 5% sodium nitrite solution (NaNO2) in a microcentrifuge tube and incubated for 6 min. Then, 30 μL of 10% aluminium chloride solution (AlCl3) was added and incubated for 5 min. The 180 and 210 μL of 1 M sodium hydroxide solution (NaOH) and sterile distilled water were added in a microcentrifuge tube, respectively. The mixture was filled to a 96-well plate. The total flavonoid content was measured under the absorbance at 510 nm by Synergy HTX Multi-Mode Reader (BioTek, USA) using the standard curve of catechin (CAS: 225937-10-0, Sigma-Aldrinch, USA). The analysis of total phenolic content and total hydrolyzable tannin content using the Folin-ciocalteu method including total condensed tannin content using hydrochloric acid-vanillin assay was carried out by Center of Excellent in Natural Products Innovation, Mae Fah Luang University, Chiang Rai, Thailand. The total amount of phenolic, flavonoid, hydrolyzable tannin, and condensed tannin contents was calculated and expressed as mg gallic acid equivalent/mL, mg catechin equivalent/mL, mg gallotannin equivalent/mL, and mg catechin equivalent/mL, respectively.
2.2. Microbial and metabolite profiling
2.2.1. Study subjects and fecal sample collection
The study design of this study was collecting feces from three Thai female volunteers with obesity conditions to ferment in batch culture fermentation as the human gut model for 24 h within and without Triphala extracts co-fermentation. Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University, Bangkok, Thailand was the place for settings and location. Fresh fecal samples were obtained from 3 Thai female volunteers. The females with the inclusion criteria (23–33 years old with a BMI of 35.0–40.0 kg/m2) and the exclusion criteria (no history of antibiotic usage, no diarrhea or gastrointestinal tract diseases and do not use probiotics and prebiotics products in daily life for 3 months, no congenital diseases such as diabetes, heart disease, liver disease, kidney disease, thyroid disease, adrenal gland disease, cancer, high blood pressure under taking antihypertensive drugs as well as high blood fats or cholesterol under taking blood lipid/cholesterol-lowering drugs, no received medication, no drinking alcoholic or caffeinated beverages within 24 h, no taking medications that suppress the immune system such as steroids, no undergone major surgery on the gastrointestinal tract, no pregnant or lactating women, and no injury or severe illness during the study period of the research project) were required as the volunteers. The written informed consent was obtained from all volunteers before sample collection. From the recorded questionnaire, each volunteer had the same portions of carbohydrates, proteins, and fats including fruits and vegetables of eating history. Each fresh fecal sample was transported under anaerobic conditions and used within 1 h. Each fecal sample of 5 g was mixed with 4 vol of sterile phosphate-buffered saline (PBS). Then, the fecal solution was transferred into the batch fermentation which was used as the human gut model. This research was approved by Kasetsart University Ethics Committee on Human Research (KUREC-HS64/025).
2.2.2. Simulation of the human gut model
Batch fermentation under anaerobic conditions (37 °C and pH 6.8–6.9) and basal medium (pH 6.8–6.9) were prepared following the previous study30 to simulate the human gut model. The fecal slurry was incubated in the human gut model for 1% (v/v) of the total working volume. From the preliminary experiments, the concentration of Triphala extracts at 1 mg/mL was the minimum inhibitory concentration which did not inhibit the growth of 4 probiotic strains (Limosilactobacillus reuteri KUB-AC5, L. fermentum KUB-D18, Pediococcus acidilactici HM04-3, and P. acidilactici HM04-25) by minimum inhibitory concentration assay. Thus, Triphala extracts at 1 mg/mL were added to the human gut model. The model which contained only fecal solution with the basal medium was used as a control. All contents from the human gut model at 24 h of fermentation time were collected as samples. For DNA extraction, the samples were centrifuged at 15,000 g for 10 min to discard the supernatant and washed bacterial cells (pellet) with PBS 3 times before storing at −20 °C. For metabolite extraction, the samples were kept at −20 °C directly.
2.2.3. DNA extraction, 16s rRNA gene sequencing, data processing, and visualization of microbial compositions
DNA extraction was performed using the QIAamp PowerFecal Pro DNA Kit (QIAGEN, Germany) with a minor modification of the manufacturer's methods according to the previous work.31 Briefly, 250 μL of each fermented sample was vortexed and added into the PowerBead Pro tube. The lysis buffer (800 μL) was added to the tube. Physical cell lysis was conducted at 6.5 m/s for 1 min, taken rest for 5 min, and repeated 3 times using FastPrep-24 benchtop instrument (MP Biomedicals, USA). Cell debris and bead were centrifuged at 15,000 g, 25 °C for 1 min. The supernatant (500 μL) was transferred to a new clean microcentrifuge tube and then followed the protocol kit directly. The quality and quantity of DNA were measured using NanoDrop 2000/2000c spectrophotometers (ThermoFisher Scientific, USA). DNA samples at 15 ng/mL with A260/A280 absorbance ratios of 1.8–2.0 were submitted to Novogene (Beijing, China) for 16S rRNA gene sequencing using Illumina NovaSeq 6000 (Illumina, USA). Before providing the DNA samples for 16S rRNA gene sequencing, each DNA sample was amplified from 16s rRNA genes at the V3–V4 region using polymerase chain reaction (PCR) for quality measurement. The primers of 341F (forward: CCTAYGGGRBGCASCAG) and 806R (reverse: GGACTACNNGGGTATCTAAT) were used in this study. The PCR amplification was performed by ThermalCycler (Biometra, Germany). The integrity and size of DNA in each PCR product fragment were evaluated by 1% agarose gel electrophoresis. The raw data of 16S rRNA gene sequences obtained from all samples were trimmed the low-quality bases and adapters to create the clean sequences. Usearch version 11.0.667 (https://drive5.com/usearch/) was used to analyze clean sequences of all samples for operational taxonomic units (OTUs) and microbial compositions. The alpha diversity index, beta diversity index, and the relative abundance at the phylum level of each sample were analyzed and visualized using the MicrobiotaProcess package in R version 4.2.0.
2.2.4. Metabolite extraction
All contents from the human gut model were collected after 24 h of fermentation time. The metabolite extraction was modified from the previous study.32 Briefly, 1 mL of each sample was mixed with cold methanol (LC-MS grade) in a 1:1 v/v ratio and incubated at 4 °C for 24 h. The centrifugation was performed at 14,000 g, 4 °C for 10 min on all samples. The supernatant (1 mL) of each sample was transferred to a new tube, filtered with a 0.22 μm syringe filter, and kept at −20 °C until untargeted metabolomic analysis.
2.2.5. Untargeted metabolomic analysis
Water (LC-MS grade) and formic acid (LC-MS grade) were purchased from Fisher Scientific, USA. To check the contamination of sample preparation, a blank sample of water was analyzed. The quality control (QC) samples were prepared by mixing an equal aliquot of extracted metabolite supernatant from all of the treatments for monitoring the stability of the acquisition system. The metabolomics profiling was performed using an ultra-high-performance liquid chromatography (UHPLC) system (Vanquish Flex UHPLC, Thermo Scientific, Germany) consisting of a binary pump with built-in degasser (Binary Pump F), a column compartment (Column Compartment H) and an autosampler (Split Sampler FT) coupled with an Orbitrap Exploris 120 system equipped with heated electrospray ionization (H-ESI) source and a hybrid quadrupole-orbitrap mass spectrometer (Thermo Scientific, Bremen, Germany). The UHPLC column was a Hypersil Gold C-18 column (2.1 × 150 mm, 1.9 μm) (Thermo Fisher Scientific, USA). Two modes of the analytical system (positive and negative ion modes of electrospray ionization) and mass calibration were executed using Thermo Scientific Pierce FlexMix Calibration Solution (Thermo Fisher Scientific, USA). The 5 μL of each metabolite supernatant was randomly injected in no particular order to the UHPLC system. Ten QC samples were run before the samples to equilibrate the detection system, and a QC sample was injected every ten samples during analysis.22 The setup for MS analysis followed the previous work.33 The mobile phase under positive ionization consisted of solvent A (0.1% (v/v) formic acid in water), and solvent B (methanol), and under negative ionization consisted of solvent A (water), and solvent B (methanol). The analysis was carried out with elution gradient as follows: t = 0 min, 2% B; t = 8 min, 40% B; t = 12 min, 98% B; t = 15.5 min, 2% B. The flow rate was 300 μL/min. The positive and negative spray voltages were 3.3 kV and 2.7 kV, respectively.34 Two acquisition ion modes of electrospray ionization were done with a range of mass-to-charge ratio (m/z) from 70 to 1200. The resolution was set at 60,000 and 15,000 for full MS and data-dependent MS,2 respectively. The collision energy of 20, 40, and 60 eV was set to acquire the MS2 spectrometry data. For positively identified metabolites, Compound Discover software version 3.3 (Thermo Fisher Scientific, USA) against mzCloud (https://www.mzcloud.org) and ChemSpider compound (http://www.chemspider.com) databases were used. An identification of putatively identified metabolites was performed via MyCompoundID MS software (https://mcid.chem.ualberta.ca) against the Human Metabolome Database (HMDB) library and the Evidence-based Metabolome Library (EML) database.35 R program with the packages of ggpubr and mixOmics were conducted to create principal component analysis (PCA). MetaboAnalyst 5.0 was used to perform differential analysis via a volcano plot and pathway analysis of the LC-MS data.36
3. Results
3.1. Constituents of Triphala extracts
Total phenolic content, total flavonoid content, and total hydrolyzable and condensed tannin content were performed on Triphala extracts for determination of constituents. It was found that the total hydrolyzable tannin content was 177.27 ± 10.09 mg gallotannin equivalent/mL which was the majority content of Triphala extracts. Total phenolic content was 62.87 ± 0.21 mg gallic acid equivalent/mL. The contents of flavonoids and total condensed tannins of Triphala extracts were 0.62 ± 0.11 mg catechin equivalent/mL and 0.24 ± 0.01 mg catechin equivalent/mL, respectively.
3.2. Microbial profile analysis of human gut model
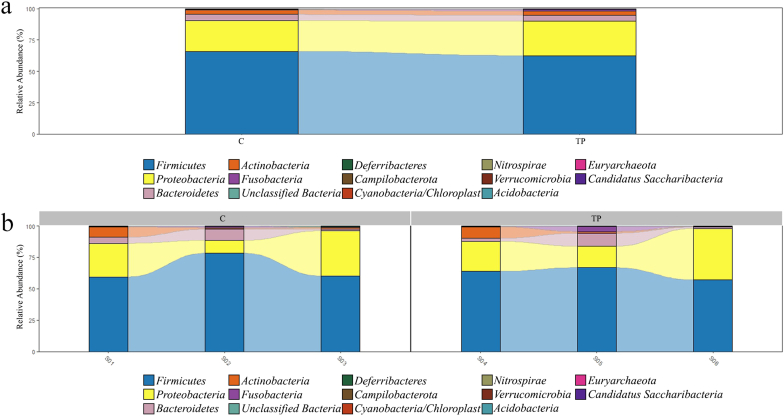
The 16S rRNA gene sequencing was performed to determine the microbial profiles of the human gut model in each sample. After data processing, alpha and beta diversity indexes including the relative abundance of microbial profiles in the Triphala extracts-treated group were analyzed and compared with the control group. It was found that there was no statistically significant difference between both groups. The results of the alpha diversity index and principal coordinate analysis (beta diversity index) were presented in Supplementary Figs. S1-S2. The relative abundance in both groups at the phylum level which presented Firmicutes, Proteobacteria, and Bacteroidetes as 3 main phyla were revealed in Fig. 1.
Fig. 1.
The relative abundance of microbial profiles at the phylum level in each group at 24 h of fermentation time in the human gut model. (a) The average of relative abundance. (b) The relative abundance of each replication in the experiments. TP, Triphala extracts group; C, control group.
3.3. Submetabolome profiling and metabolite identification
A total of 3205 metabolites were detected in the duplicate analysis of fecal samples in both treatments. For metabolite identification, 4 databases (mzCloud, Chemspider Compound, HMDB, and EML) were used. There were 37 metabolites which are matched by m/z and retention time to both mzCloud and ChemSpider compound databases. While 14 and 326 metabolites were each matched by m/z and retention time to mzCloud and ChemSpider compound databases, respectively (Supplemental Tables S1-S2). In the HMDB library and the EML library, the m/z of detected metabolites were mass-matched to 308,182 and 599,882 metabolites, respectively, which were considered as putatively identified metabolites (Supplemental Tables S3-S4). The submetabolome composition in Triphala extracts-treated group and the control group appeared to be complicated. The positively identified metabolites coupled with many more putatively identified metabolites covered a broad range of metabolic pathways, which will be discussed in the section of pathway analysis.
3.4. Fecal metabolome profiles and comparison
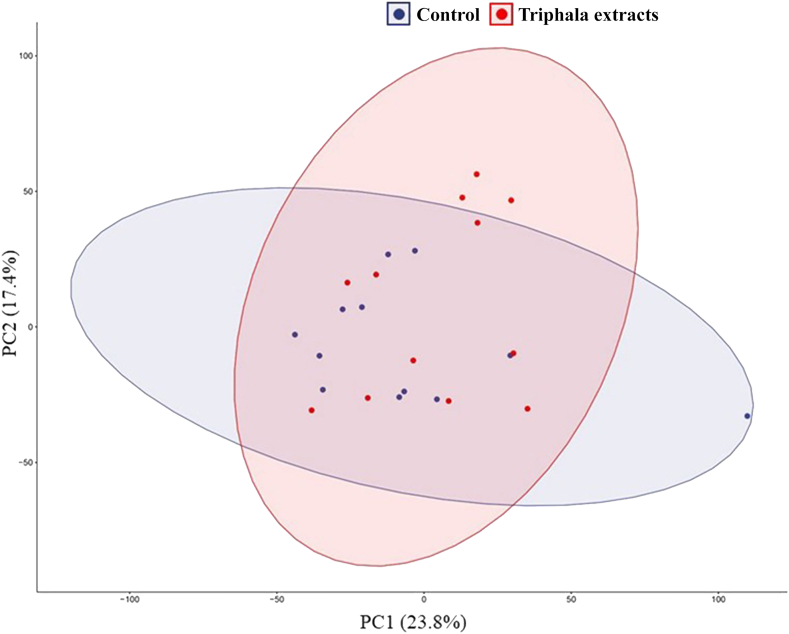
To indicate the metabolome differences in the samples of the Triphala extracts-treated group compared with the control group, statistical tools were used to analyze the data set of LC-MS/MS runs in both positive and negative ion modes of electrospray ionization. The distinct separations of the Triphala extracts-treated group and the control group were presented by PCA plot. In Fig. 2, the metabolome data could be divided into 2 groups plus the QC group (Supplementary Fig. S3) shown in different colors. The clustering of QC runs determined the reproducibility of the analysis. The treatment of Triphala extracts and the control group were labeled with orange and blue colors, respectively. The metabolome data of Triphala extracts treatment was closely clustered together with the metabolome data of the control via PCA plot. However, some distinct patterns were observed to separate the metabolome data between Triphala extracts-treated samples and the control group. There were some data from the Triphala extracts-treated group that stepped away from the control group in the oval shape of the PCA plot.
Fig. 2.
The principal component analysis (PCA) of the metabolomic data obtained from Triphala extracts and the control groups.
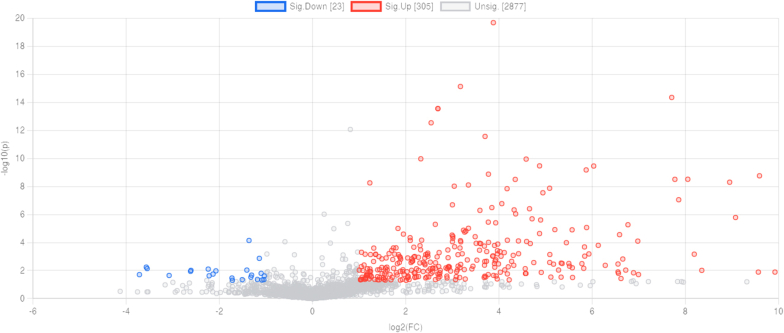
To designate the fecal metabolome changes in the human gut model, a binary comparison via volcano plot was conducted to analyze the differential analysis. The volcano plot showed the metabolic changes of fecal samples after being treated with Triphala extracts, as compared to the control group (Fig. 3). The effect of the control group was deducted by binary comparison of the submetabolome in the Triphala extracts-treated group versus the control group in each ion mode of electrospray ionization. Any change of more than 2-fold and a p-value of below 0.05 in metabolite concentration was contemplated to be significant. The volcano plot analysis of the Triphala extracts group versus the control group showed that out of 2305 metabolites, 305 metabolites were up-regulated (i.e., higher metabolite concentration in the treated samples than the control group) shown in red dots and 23 metabolites were down-regulated (i.e., lower metabolite concentration in the treated samples) shown in blue dots (Fig. 3). The grey dots represent no statistically significant difference.
Fig. 3.
The volcano plot of binary comparison of metabolites in Triphala extracts vs. control. The significant metabolites are shown in red dots (up-regulated metabolites) or blue (down-regulated metabolites) with fold change ≥2 and p-value <0.05. The grey dots represent no statistically significant difference of metabolites.
3.5. Fecal metabolite regulation in the human gut model of obese adults
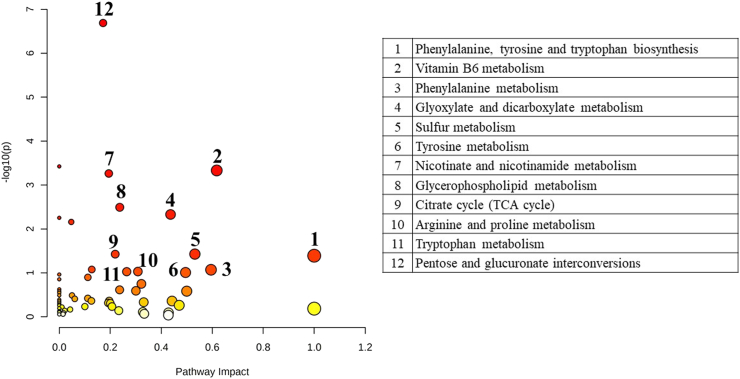
Triphala extracts revealed the action to trigger the changes of fecal metabolites better than the control group in the human gut model (Fig. 3). To indicate the related pathways which might be the action of Triphala extracts on alterations of fecal metabolites, the positively identified metabolites were submitted to MetaboAnalyst 5.0 software based on the database of Homo sapiens. The positively identified metabolites were matched to 60 pathways as shown in Fig. 4. Individual pathways contained an impact and a p-value according to the numbers of ‘hits’ recorded and the significant factors of the detected metabolites. The results showed that the metabolism of phenylalanine, tyrosine and tryptophan biosynthesis was found to be the greatest pathway impact and statistical significance. The induction of some other metabolites was also detected in pathways that play a role in associated mechanisms such as vitamin B6 metabolism, phenylalanine metabolism, glyoxylate and dicarboxylate metabolism, sulfur metabolism, tyrosine metabolism, nicotinate and nicotinamide metabolism, glycerophospholipid metabolism, citrate cycle (tricarboxylic acid cycle), arginine and proline metabolism, tryptophan metabolism, and pentose and glucuronate interconversions.
Fig. 4.
Overview of metabolic pathway analysis relating to stress responses.
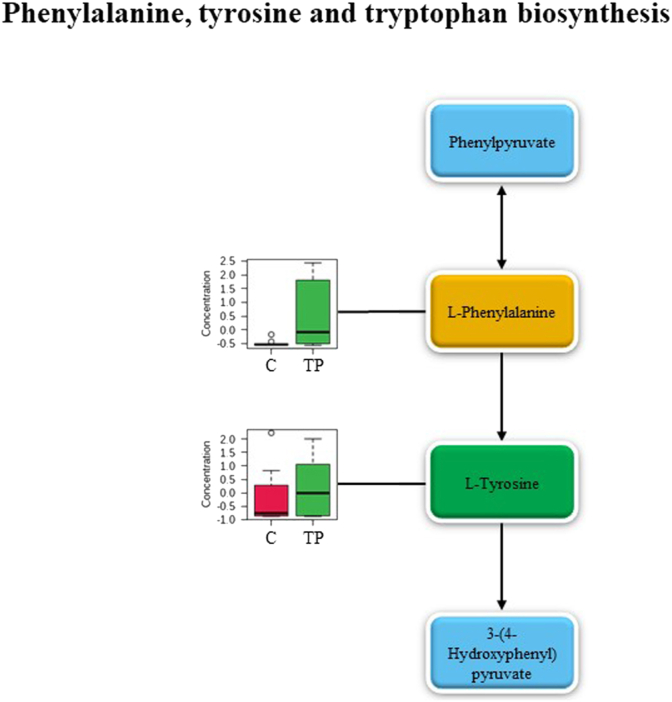
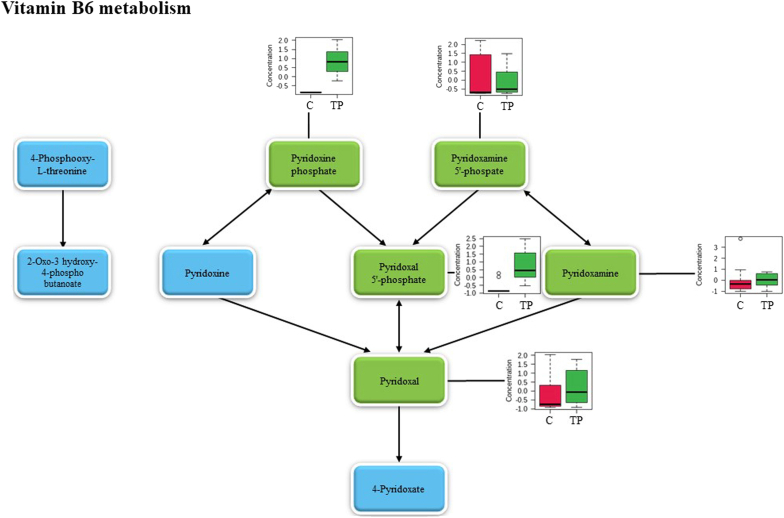
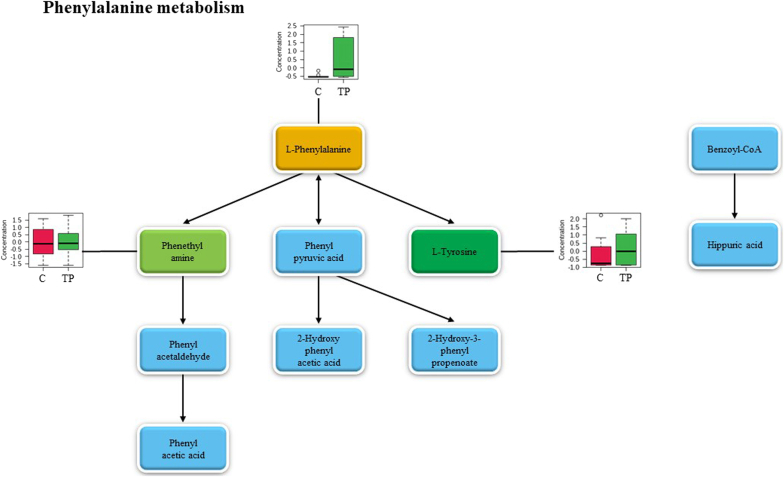
The top three pathways which presented the highest pathway impact and statistical significance were phenylalanine, tyrosine and tryptophan biosynthesis, vitamin B6 metabolism, and phenylalanine metabolism. Fig. 5, Fig. 6, Fig. 7 reveal the metabolites in phenylalanine, tyrosine and tryptophan biosynthesis, vitamin B6 metabolism and phenylalanine metabolism, respectively. The light blue boxes represented the metabolites yet to be identified. The dark green boxes acted for the positively identified metabolites which matched both mzCloud and ChemSpider compound databases. The light green boxes were positively identified metabolites that matched to mzCloud or ChemSpider compound databases. While the orange boxes displayed the putatively identified metabolites. To present the significant level changes compared to the control group, the positively and putatively identified metabolites in each pathway were exhibited via box plots.
Fig. 5.
Metabolic pathway of phenylalanine, tyrosine and tryptophan biosynthesis. The metabolite in the dark green box represents the positively identified metabolite that matched to both mzCloud and ChemSpider compound databases, the orange box represents the putatively identified metabolite, the blue box represents the metabolite that relates to this pathway (not found in this study), and the box plots of identified metabolites are displayed beside the corresponding metabolites. TP, Triphala extracts group; C, control group.
Fig. 6.
Metabolic pathway of vitamin B6 metabolism. The metabolite in the light green box represents the positively identified metabolite that matched to mzCloud or ChemSpider compound databases, the orange box represents the putatively identified metabolite, the blue box represents the metabolite that relates to this pathway (not found in this study), and the box plots of identified metabolites are displayed beside the corresponding metabolites. TP, Triphala extracts group; C, control group.
Fig. 7.
Metabolic pathway of phenylalanine metabolism. The metabolite in the dark green box represents the positively identified metabolite that matched to both mzCloud and ChemSpider compound databases, the metabolite in the light green box represents the positively identified metabolite that matched to mzCloud or ChemSpider compound databases, the orange box represents the putatively identified metabolite, the blue box represents the metabolite which relates to this pathway (not found in this study) and the box plots of identified metabolites are displayed beside the corresponding metabolites. TP, Triphala extracts group; C, control group.
l-tyrosine was labeled with green color while l-phenylalanine was highlighted in orange color in phenylalanine, tyrosine and tryptophan biosynthesis (Fig. 5). Pyridoxine phosphate, pyridoxamine 5′-phosphate, pyridoxal 5′-phosphate, pyridoxamine, and pyridoxal were labeled with green color in vitamin B6 metabolism (Fig. 6). l-tyrosine and phenethyl amine were highlighted in green color while l-phenylalanine was labeled in orange color in phenylalanine metabolism (Fig. 7). Markedly, l-tyrosine which was a positively identified metabolite, played an important role in both phenylalanine, tyrosine and tryptophan biosynthesis and phenylalanine metabolism pondered as a key metabolite to link both pathways together. Regarding the box plots, most of the metabolites are the precursor or substrate to generate the following metabolites in the pathway, therefore, metabolite concentration in the Triphala extracts-treated group disclosed a higher level than those found in the control group (Fig. 5, Fig. 6, Fig. 7) indicating the higher activity of metabolism activation in the human gut model of obese adults.
4. Discussion
Obesity is one of the main health problems affecting life quality and the health care system. Not only modern medicine but alternative medicine is also required to prevent the factors which generate obesity. Medicinal plant extracts have been introduced for obesity treatment.14 The present study provided the action of Triphala extracts on the changes of microbial profiles and metabolites in feces obtained from obese volunteers during batch fermentation as the human gut model compared with the control. The contents of Triphala extracts were characterized to determine total phenolic content, total flavonoid content, and total hydrolyzable and condensed tannin content. The gut microbial profiles were analyzed by 16S rRNA gene sequencing. Untargeted metabolomic analysis coupled with pathway analysis was carried out to profile the metabolite alterations linked to metabolic pathways.
To determine the main compounds in Triphala extracts that might play an important role in obesity treatment, the constituent analysis was performed. The main component of Triphala extracts in this study was total hydrolyzable tannin content which is the same manner as the previous study37 that reported a great number of hydrolyzable tannins in Triphala when extracted using hot water. Hydrolyzable tannins have reported the effect on reduction of body weight, weight gain, cholesterol, low-density lipoprotein, and triglycerides in rats.38 Ellagitannins which is a member of hydrolyzable tannins, revealed the inhibitory properties on the activity of carbohydrate digestive enzymes39 relating to obesity treatment. Moreover, hydrolyzable tannins could decrease the relative abundance of Vibrio and Carboxylicivirga in the gut microbiota of Pacific white shrimp.40 The minor content of Triphala extracts was phenolic compounds that appeared as the effective agents on decreasing of lipogenesis, increasing of lipolysis, stimulating fatty acids beta-oxidation, and inhibiting adipocyte differentiation.41 During digestion, phenolic compounds was reported to involve in inhibition of lipase activity, resulting in aggregation of the fat globules and long-chain fatty acids generation.42 In this study, the contents of flavonoids and total condensed tannins were little amount in Triphala extracts. Flavonoids have been reported the ability in stimulation of the 5′adenosine monophosphate-activated protein kinase, which is an important enzyme to monitor lipid metabolism and adipogenesis. Furthermore, 5′adenosine monophosphate-activated protein kinase phosphorylation and activation advocate catabolic processes such as glycolysis including suppress anabolic processes such as fatty acid synthesis,43 resulting in obesity management. In a previous work, dietary condensed tannins showed the potential on reducing of lipid accumulation, blocking of adipocyte differentiation, and increasing of short-chain fatty acid accumulation,44 affecting lipid metabolism. To determine the effect of polyphenols such as phenolic compounds, flavonoids, hydrolyzable tannins, and condensed tannins in Triphala extracts on metabolic regulation, the concentration and dose of each component should be focused on further experiments.
Although the microbial profiles of Triphala extracts-treated treatment in the human gut model did not show a statistically significant difference when compared with the control group. But it was not surprising because the previous study21 also exhibited the same trend of results which presented no difference in gut microbial taxa between the Triphala-treated and placebo-treated participants. In addition, this study measured the efficacy of Triphala extracts on gut microbial profile changes at 24 h after fermentation time which might be too early for the evaluation of Triphala extracts’ action. In this study, the same microbial and metabolite profiles between both groups were observed because the short duration of the interference (24 h) and dosing of Triphala extracts might have contributed to the results acquired. However, Triphala has been reported the ability to increase the growth of Bifidobacteria and Lactobacillus which are beneficial microbes in the human gut.15 The previous work exhibited that the average relative abundance of Firmicutes and Bacteroidetes was changed to decrease the average ratio of Firmicutes:Bacteroidetes in Triphala treatment compared to control group although no taxa were uniformly changed; however, this was highly personalized.21
From the results of this study, the three main phyla in Triphala extracts and the control groups were Firmicutes, Proteobacteria, and Bacteroidetes, respectively. A previous study reported that Firmicutes, Bacteroidetes, and Proteobacteria were significant phyla alterations in obese. Firmicutes and Proteobacteria were obesity-associated, while Bacteroidetes was lean-associated.45 A high ratio of Bacteroidetes to Firmicutes was found in healthy human gut microbiota, while a high ratio of Firmicutes to Bacteroidetes was shown in obese.46 Although the microbial profiles on the Triphala extracts and the control treatment were not significantly different, there were some phyla that relate to anti-obesity activity. Bacteria in the phylum of Bacteroidetes can contribute acetate and propionate. Bacteria in the phylum of Firmicutes can produce butyrate. Acetate, propionate, and butyrate, which are short-chain fatty acids, have been reported to modulate the host metabolism by regulating energy harvest, fat accumulation, and appetite,46 affecting anti-obesity activity.
Even Triphala extracts had no statistically significant difference in microbial profile changes, but they presented a statistically significant difference in fecal metabolic alteration when compared with the control group. To explain the understanding of Triphala extracts’ action on the metabolic alteration and mechanism of metabolism regulation in fecal obesity during batch fermentation, metabolomic analysis has been performed. According to untargeted metabolomic analysis, Triphala extracts revealed the effect on the fecal metabolite changes better than the control group in the human gut model via volcano plot. This work found that Triphala extracts induced the accumulations of metabolites that were linked to 12 major pathways including biosynthesis of phenylalanine, tyrosine, and tryptophan, metabolism of vitamin B6, and metabolism of phenylalanine.
Pathways analysis exhibited the property of Triphala extracts on metabolite induction related to phenylalanine, tyrosine, and tryptophan biosynthesis as a major pathway with the highest impact score. l-tyrosine and l-phenylalanine were 2 identified metabolites observed in phenylalanine, tyrosine, and tryptophan biosynthesis. While phenylalanine metabolism contained 3 identified metabolites (phenethylamine, l-tyrosine, and l-phenylalanine). Tyrosine plays an important role in the regulation of energy metabolism. The previous work47 reported that tyrosine supplementation elicited the normalization of triglycerides and low-density lipoprotein in rats with the obese model. Furthermore, phenylalanine supplementation was reported to reduce energy intake in overweight and obese women.48 Phenethylamine has been documented the ability to reduce body weight in the patients who took phenethylamine compared with the control patients introduced for the treatment of obesity.49 From the box plots which displayed the concentrations of l-tyrosine and l-phenylalanine in the Triphala extract-treated group higher than the control group, it could be suggested that Triphala extracts triggered the fecal metabolite accumulation of obese adults during batch fermentation resulting in health benefits.
However, the produced concentration of l-tyrosine and l-phenylalanine which reaches the effective dose for health benefits by Triphala extracts should be considered. Previous works47,50 demonstrated the effective dose of tyrosine on modulation of energy and lipid metabolism in rats with high-fat-carbohydrate diet condition at 1250 mg/kg of body weight. In human, the bioactive supplements which contain 1200 mg per day of tyrosine as an ingredient showed the body fat loss property after eight weeks.51 l-phenylalanine with 10 g before an ad libitum lunch and dinner meal in obese women reduced energy intake but increased ratings of nausea.48 In this study, Triphala extracts increased the relative accumulation of l-tyrosine and l-phenylalanine in obese fecal during fermentation period when compared with the control group. The quantitative concentration of l-tyrosine and l-phenylalanine produced by Triphala extracts reaching the effective dose should be focused on the future work.
Not only phenylalanine, tyrosine, and tryptophan biosynthesis and phenylalanine metabolism but vitamin B6 metabolism is also activated by Triphala extracts treatment which consisted of 5 identified metabolites (pyridoxal, pyridoxal 5′-phosphate, pyridoxamine, pyridoxamine 5′-phosphate, and pyridoxine phosphate). For several cellular metabolisms, vitamin B6 is very crucial. A deficiency of vitamin B6 has been linked to several adverse health effects.52 Vitamin B6 covers 6 general forms called pyridoxal, pyridoxamine, pyridoxine, and their related 5′-phosphate derivatives.53 The greater level of vitamin B6 has publicized the association with a higher fat-free mass percentage in overweight/obese women during periods of weight loss.54 In mice with a high-fat diet, vitamin B6 supplementation noticeably reduced random blood glucose, and improved the tolerance of glucose and insulin including decreased hepatic lipid abundance pondered as an alternative prevention of metabolic syndrome.52 Most identified metabolites of vitamin B6 metabolism in the Triphala extracts-treated group revealed a greater accumulation than the control group via the box plots indicating the higher effect of Triphala extracts on the induction of fecal metabolite changes in the human gut model.
During batch fermentation of obese adults’ feces, Triphala extracts influenced several metabolites, especially, amino acids and vitamin B6 compounds which relate to various metabolic pathways involved in obesity management. The induction of metabolites affecting phenylalanine, tyrosine and tryptophan biosynthesis, vitamin B6 metabolism and phenylalanine metabolism by Triphala extracts was explained in this study for obesity manipulation. Thus, it could be suggested that Triphala extracts had effects on the changes of the fecal metabolome in obesity during the human gut model period considered as an alternative medicinal recipe for obesity treatment.
5. Conclusion
Obesity is closely associated with metabolic syndrome caused health problems. To provide a preventive option for obesity, Triphala extracts have been introduced. Determination of Triphala extracts' actions on gut microbial profiles and metabolic pathways could provide a better understanding of Triphala extracts’ properties for obesity treatment. The 16S rRNA gene sequencing and untargeted metabolomic analysis were carried out to indicate the effects of Triphala extracts on the alterations of gut microbial profiles and metabolites in batch fermentation used as the human gut model of fecal obesity adults. Although no statistically significant difference was observed in gut microbial profile changes in Triphala extracts treatment, but statistically significant difference in metabolite changes was detected when compared with the control group. Triphala extracts employed the biosynthesis of phenylalanine, tyrosine and tryptophan as a major pathway for their actions resulting in the regulation of energy metabolism and obesity management. Further study should be focused on the long periods of Triphala extracts supplementation in the human gut model to detect the gut microbial profile changes. Therefore, this study provided an alternative approach for obesity management using the Triphala extracts.
Human ethical approval
We are confirming that the study was approved by Kasetsart University Ethics Committee on Human Research (KUREC-HS64/025).
Funding
This research is funded by Kasetsart University through the Graduate School Fellowship Program and the Kasetsart University Research and Development Institute (KURDI) (FF (KU)13.65).
Data availability
16S rRNA gene sequences of all samples were privately deposited at Sequence Read Archive (BioProject ID: PRJNA901375) The supplementary data including LC-MS data presented in this study are privately deposited in FigShare at https://figshare.com/s/fb48fa6dc51b7fedb0e9.
Declaration of competing interest
The authors declared no conflict of interest.
Acknowledgements
We would like to thank the Department of Biotechnology, Faculty of Agro-Industry, and Specialized Research Unit of Prebiotics and Probiotics for Health, Kasetsart University for all support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2023.02.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Ward Z.J., Bleich S.N., Long M.W., Gortmaker S.L. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0247307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soffer S., Zimlichman E., Glicksberg B.S., et al. Obesity as a mortality risk factor in the medical ward: a case control study. BMC Endocr Disord. 2022;22(1):13. doi: 10.1186/s12902-021-00912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckel R.H., Krauss R.M. American heart association call to action: obesity as a major risk factor for coronary heart disease. Circulation. 1998;97(21):2099–2100. doi: 10.1161/01.cir.97.21.2099. [DOI] [PubMed] [Google Scholar]
- 4.Barnes A.S. The epidemic of obesity and diabetes: trends and treatments. Tex Heart Inst J. 2011;38(2):142–144. [PMC free article] [PubMed] [Google Scholar]
- 5.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26 doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin R., Kuo H.C., Hudlikar R., et al. Gut microbiota, dietary phytochemicals and benefits to human health. Curr Pharmacol Rep. 2019;5:332–344. doi: 10.1007/s40495-019-00196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Argenio V., Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451(Pt A):97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Koliada A., Syzenko G., Moseiko V., et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan M., Wang Y., Zhang Q., Zou R., Guo M., Zheng H. Characteristics of gut microbiota in people with obesity. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowiak-Kopec P., Slizewska K. The Effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12(4):1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Burillo S., Hinojosa-Nogueira D., Pastoriza S., Rufian-Henares J.A. Plant extracts as natural modulators of gut microbiota community structure and functionality. Heliyon. 2020;6(11) doi: 10.1016/j.heliyon.2020.e05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brochot A., Azalbert V., Landrier J.F., Tourniaire F., Serino M. A two-week treatment with plant extracts changes gut microbiota, caecum metabolome, and markers of lipid metabolism in ob/ob mice. Mol Nutr Food Res. 2019;63(17) doi: 10.1002/mnfr.201900403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milutinovic M., Dimitrijevic-Brankovic S., Rajilic-Stojanovic M. Plant extracts rich in polyphenols as potent modulators in the growth of probiotic and pathogenic intestinal microorganisms. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.688843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng G., Duan Y., Zhong Y., et al. Plant extracts in obesity: a role of gut microbiota. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.727951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson C.T., Denniston K., Chopra D. Therapeutic uses of triphala in ayurvedic medicine. J Alternative Compl Med. 2017;23(8):607–614. doi: 10.1089/acm.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westfall S., Lomis N., Prakash S. A novel polyphenolic prebiotic and probiotic formulation have synergistic effects on the gut microbiota influencing Drosophila melanogaster physiology. Artif Cell Nanomed Biotechnol. 2018;46(sup2):441–455. doi: 10.1080/21691401.2018.1458731. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S., Ding X., Sharma A. Exploring scientific validation of Triphala Rasayana in ayurveda as a source of rejuvenation for contemporary healthcare: an update. J Ethnopharmacol. 2021;273 doi: 10.1016/j.jep.2021.113829. [DOI] [PubMed] [Google Scholar]
- 18.Phimarn W., Sungthong B., Itabe H. Effects of Triphala on lipid and glucose profiles and anthropometric parameters: a systematic review. J Evid Based Integr Med. 2021;26 doi: 10.1177/2515690X211011038. 2515690X211011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunsagool P., Mhuantong W., Tangphatsornruang S., et al. Metagenomics of antimicrobial and heavy metal resistance in the cecal microbiome of fattening pigs raised without antibiotics. Appl Environ Microbiol. 2021;87(8) doi: 10.1128/AEM.02684-20. e02684-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee P.S., Lu Y.Y., Nagabhushanam K., Ho C.T., Mei H.C., Pan M.H. Calebin-A prevents HFD-induced obesity in mice by promoting thermogenesis and modulating gut microbiota. J Tradit Complement Med. 2022 doi: 10.1016/j.jtcme.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson C.T., Pourang A., Dhaliwal S., et al. Modulatory effects of Triphala and Manjistha dietary supplementation on human gut microbiota: a double-blind, randomized, placebo-controlled pilot study. J Alternative Compl Med. 2020;26(11):1015–1024. doi: 10.1089/acm.2020.0148. [DOI] [PubMed] [Google Scholar]
- 22.Tunsagool P., Wang X., Leelasuphakul W., et al. Metabolomic study of stress responses leading to plant resistance in Mandarin fruit mediated by preventive applications of Bacillus subtilis cyclic lipopeptides. Postharvest Biol Technol. 2019;156 [Google Scholar]
- 23.Hsu Y.H., Astley C.M., Cole J.B., et al. Integrating untargeted metabolomics, genetically informed causal inference, and pathway enrichment to define the obesity metabolome. Int J Obes. 2020;44(7):1596–1606. doi: 10.1038/s41366-020-0603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed D., Kumar D., Kumar S., et al. Transcriptome and metabolome changes induced by bitter melon (Momordica charantia)- intake in a high-fat diet induced obesity model. J Tradit Complement Med. 2022;12(3):287–301. doi: 10.1016/j.jtcme.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biagini F., Daddi C., Calvigioni M., et al. Designs and methodologies to recreate in vitro human gut microbiota models. Bio-des Manuf. 2022 doi: 10.1007/s42242-022-00210-6. [DOI] [Google Scholar]
- 26.Ahmadi S., Wang S., Nagpal R., et al. An in vitro batch-culture model to estimate the effects of interventional regimens on human fecal microbiota. JoVE. 2019;149 doi: 10.3791/59524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirkola L., Dicksved J., Loponen J., Marklinder I., Andersson R. Fecal microbiota composition affects in vitro fermentation of rye, oat, and wheat bread. Sci Rep. 2023;13(1):99. doi: 10.1038/s41598-022-26847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day-Walsh P., Shehata E., Saha S., et al. The use of an in-vitro batch fermentation (human colon) model for investigating mechanisms of TMA production from choline, l-carnitine and related precursors by the human gut microbiota. Eur J Nutr. 2021;60(7):3987–3999. doi: 10.1007/s00394-021-02572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewanto V., Wu X., Adom K.K., Liu R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50(10):3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 30.Ayimbila F., Siriwong S., Nakphaichit M., Keawsompong S. In vitro gastrointestinal digestion of Lentinus squarrosulus powder and impact on human fecal microbiota. Sci Rep. 2022;12(1):2655. doi: 10.1038/s41598-022-06648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plupjeen S.N., Chawjiraphan W., Charoensiddhi S., Nitisinprasert S., Nakphaichit M. Lactococcus lactis KA-FF 1-4 reduces vancomycin-resistant enterococci and impacts the human gut microbiome. 3 Biotech. 2020;10(7):295. doi: 10.1007/s13205-020-02282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song R., Wang J., Sun L., et al. The study of metabolites from fermentation culture of Alternaria oxytropis. BMC Microbiol. 2019;19(1):35. doi: 10.1186/s12866-019-1408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L., Han Y., Zheng Z., et al. Altered gut microbial metabolites in amnestic mild cognitive impairment and Alzheimer's disease: signals in host-microbe interplay. Nutrients. 2021;13(1):228. doi: 10.3390/nu13010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonato M., Fochi I., Vedovelli L., et al. Urinary metabolomics reveals kynurenine pathway perturbation in newborns with transposition of great arteries after surgical repair. Metabolomics. 2019;15(11):145. doi: 10.1007/s11306-019-1605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L., Li R., Zhou J., et al. MyCompoundID: using an evidence-based metabolome library for metabolite identification. Anal Chem. 2013;85(6):3401–3408. doi: 10.1021/ac400099b. [DOI] [PubMed] [Google Scholar]
- 36.Pang Z., Zhou G., Ewald J., et al. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat Protoc. 2022;17(8):1735–1761. doi: 10.1038/s41596-022-00710-w. [DOI] [PubMed] [Google Scholar]
- 37.Pakaweerachat P., Chysirichote T. Valorization of tannin rich triphala waste for simultaneous tannase and gallic acid production under solid state fermentation by Aspergillus niger. Chem Eng Commun. 2022:1–14. [Google Scholar]
- 38.Manzoor F., Nisa M.U., Hussain H.A., et al. Effect of hydrolysable tannin on nutrient intake obesity and other associated metabolic risk factors in polycystic rats. Transl Med Commun. 2021;6(1):10. [Google Scholar]
- 39.Prpa E.J., Bajka B.H., Ellis P.R., Butterworth P.J., Corpe C.P., Hall W.L. A systematic review of in vitro studies evaluating the inhibitory effects of polyphenol-rich fruit extracts on carbohydrate digestive enzymes activity: a focus on culinary fruits consumed in Europe. Crit Rev Food Sci Nutr. 2021;61(22):3783–3803. doi: 10.1080/10408398.2020.1808585. [DOI] [PubMed] [Google Scholar]
- 40.Zhu X.F., Guo H., Li G.L., Zhu C.H. Effects of dietary hydrolyzable tannins on growth performance, antioxidant capacity, intestinal microflora and resistance against Vibrio parahaemolyticus of juvenile Pacific white shrimp, Litopenaeus vannamei (Boone, 1931) Aquac Rep. 2021;19 [Google Scholar]
- 41.Rodríguez-Pérez C., Segura-Carretero A., del Mar Contreras M. Phenolic compounds as natural and multifunctional anti-obesity agents: a review. Crit Rev Food Sci Nutr. 2017;59(8):1212–1229. doi: 10.1080/10408398.2017.1399859. [DOI] [PubMed] [Google Scholar]
- 42.Kasprzak-Drozd K., Oniszczuk T., Stasiak M., Oniszczuk A. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int J Mol Sci. 2021;22(7):3175. doi: 10.3390/ijms22073715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandoval V., Sanz-Lamora H., Arias G., Marrero P.F., Haro D., Relat J. Metabolic impact of flavonoids consumption in obesity: from central to peripheral. Nutrients. 2020;12(8):2393. doi: 10.3390/nu12082393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y., Zhu L., Guo C., et al. Dietary intake of hydrolyzable tannins and condensed tannins to regulate lipid metabolism. Mini Rev Med Chem. 2022;22(13):1789–1802. doi: 10.2174/1389557522666211229112223. [DOI] [PubMed] [Google Scholar]
- 45.Xu Z., Jiang W., Huang W., Lin Y., Chan F.K.L., Ng S.C. Gut microbiota in patients with obesity and metabolic disorders — a systematic review. Genes Nutr. 2022;17(1):2. doi: 10.1186/s12263-021-00703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivamaruthi B.S., Kesika P., Suganthy N., Chaiyasut C. A review on role of microbiome in obesity and antiobesity properties of probiotic supplements. BioMed Res Int. 2019;2019 doi: 10.1155/2019/3291367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shipelin V.A., Trusov N.V., Apryatin S.A., et al. Effects of tyrosine and tryptophan in rats with diet-induced obesity. Int J Mol Sci. 2021;22(5):2429. doi: 10.3390/ijms22052429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pohle-Krauza R.J., Navia J.L., Madore E.Y.M., Nyrop J.E., Pelkman C.L. Effects of L-phenylalanine on energy intake in overweight and obese women: interactions with dietary restraint status. Appetite. 2008;51(1):111–119. doi: 10.1016/j.appet.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Bray G.A., Ryan D.H., Gordon D., Heidingsfelder S., Cerise F., Wilson K. A double-blind randomized placebo-controlled trial of sibutramine. Obes Res. 1996;4(3):263–270. doi: 10.1002/j.1550-8528.1996.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 50.Gmoshinski I.V., Shipelin V.A., Trusov N.V., et al. Effects of tyrosine and tryptophan supplements on the vital indicators in mice differently prone to diet-induced obesity. Int J Mol Sci. 2021;22(11):5956. doi: 10.3390/ijms22115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belza A., Frandsen E., Kondrup J. Body fat loss achieved by stimulation of thermogenesis by a combination of bioactive food ingredients: a placebo-controlled, double-blind 8-week intervention in obese subjects. Int J Obes. 2007;31(1):121–130. doi: 10.1038/sj.ijo.0803351. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z., Li P., Zhao Z.H., Zhang Y., Ma Z.M., Wang S.X. Vitamin B6 prevents endothelial dysfunction, insulin resistance, and hepatic lipid accumulation in apoe (-/-) mice fed with high-fat diet. J Diabetes Res. 2016;2016 doi: 10.1155/2016/1748065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mascolo E., Verni F. Vitamin B6 and diabetes: relationship and molecular mechanisms. Int J Mol Sci. 2020;21(10):3669. doi: 10.3390/ijms21103669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez-Rodríguez E., López-Sobaler A.M., Navarro A.R., Bermejo L.M., Ortega R.M., Andrés P. Vitamin B6 status improves in overweight/obese women following a hypocaloric diet rich in breakfast cereals, and may help in maintaining fat-free mass. Int J Obes. 2008;32(10):1552–1558. doi: 10.1038/ijo.2008.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
16S rRNA gene sequences of all samples were privately deposited at Sequence Read Archive (BioProject ID: PRJNA901375) The supplementary data including LC-MS data presented in this study are privately deposited in FigShare at https://figshare.com/s/fb48fa6dc51b7fedb0e9.