Abstract
Autism is a complex neurodevelopmental disorder which disrupts communication, social and interactive skills followed by appearance of repetitive behavior. The underlying etiology remains incomprehensible but genetic and environmental factors play a key role. Accumulated evidence shows that alteration in level of gut microbes and their metabolites are not only linked to gastrointestinal problems but also to autism. So far the mix of microbes that is present in the gut affects human health in numerous ways through extensive bacterial-mammalian cometabolism and has a marked influence over health via gut-brain-microbial interactions. Healthy microbiota may even ease the symptoms of autism, as microbial balance influences brain development through the neuroendocrine, neuroimmune, and autonomic nervous systems. In this article, we focused on reviewing the correlation between gut microbiota and their metabolites on symptoms of autism by utilizing prebiotics, probiotics and herbal remedies to target gut microflora hence autism.
Keywords: Autism, Gut dysbiosis, Gut-brain-axis, Dietary intervention, Probiotics and prebiotics, Herbal remedies
Graphical abstract
Highlights
-
•
Autism is the fastest growing developmental disability in the world.
-
•
Gut microbiota is either directly or indirectly linked to autism.
-
•
Therapeutic approaches which target microbiota can help in management of autism.
Abbreviations
- ASD
Autism Spectrum Disorder
- CDC
Centers for Disease Control and Prevention
- FMT
Fecal Microbial Transportation
- US-
United States
- GI
Gastrointestinal
- ENS
Enteric nervous system
- CNS
Central nervous system
- SCFA
short chain fatty acids
- HPA
hypothalamic pituitary-adrenal
- GABA
gamma-Aminobutyric acid
- LPS
Lipopolysaccharide,
- BBB
blood brain barrier
- CLDN
claudin
- OCLN
Occludin
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- INF-γ-
Interferon-γ,
- TNF-α-
Tumor necrosis factor-α,
- LC-MS
Liquid chromatography–mass spectrometry
- GC-MS
Gas chromatography–mass spectrometry
- NMDA
N-Methyl-d-Aspartate
- IL-8
Interleukin-8
- CRH
Corticotropin releasing hormone
- NT
Neurotensin
- 5-HT
5-Hydroxytryptamine,
- IBD
Inflammatory bowel disease
- IBS
Inflammatory bowel syndrome
- MTT
Microbiota transfer therapy
- ADOS
Autism Diagnostic Observation Schedule
- B-GOS
®-Bimuno ®galactooligosaccharide,
- BCP
Bovine Colostrum Product
- GFCF
Gluten free Casein free diet,
- SAM
S-adenosylmethionine,
- CYS
Cysteine,
- MET
Methionine,
- NADH
Nicotinamide adenine dinucleotide,
- NAD
Nicotinamide dinucleotide,
- PUFA
Polyunsaturated fatty acids
- TCM
Traditional Chinese medicine,
- BHT
BuyangHuanwu Tang
- ROS
reactive oxygen species
- ARE
antioxidant response element
- ETC
Electron transport chain
- scGOS
Short chain glactooligosacharide,
- lcFOS
long chain fructo-oligosaccharide,
- ATEC
Autism Treatment Evaluation Checklist
- CFU
Colony forming unit
1. Introduction
Autism is a neurodevelopmental abnormality characterized by shortfalls in social communication, reiterative and obstinate behavior which leads to consequential loss in quality of life.1 According to statistics, autism ratio stands at 1:3 in male children to 1:4 in female children.2The Centers for Disease Control and Prevention (CDC) estimated that about 1 in 59 (1.68%) children of the United States (US) aged 8 years are diagnosed with Autism Spectrum Disorder (ASD). In the US, parent-reported autism investigations in 2016 equated slightly higher at 2.5% than previously reported data.3 The etiology of autism is heterogenous. Multiple causative factors including genetic, environmental and a complex interplay between these factors have been proposed. Although inherited (genetic) factors might largely contribute to the occurrence of autism, but these genetic aberrations cannot fully account for all the cases. Therefore, in addition to genetic factors (combination of autism related genes), specific environmental factors (maternal infections, dietary factors, gut dysbiosis, exposure to pesticides, stress, medications, antibiotic consumption during pregnancy) might act as risk factors that can trigger the development of autism.4 In recent times, the role of gut microbiota in autism has become a subject of interest. Literature clearly indicates that human gut microbiota plays a promising role in health and diseases, thus any alteration in composition can inturn perturb the coordination between gastrointestinal microflora and brain.5
Many studies have highlighted a two way connection between the gut and brain, popularly known as the “gut-brain-axis.” The nexus between gut microbiota and autism is arguably one of the most fascinating areas of microbiome research. As we know humans have long viewed the contents of the bowel as a waste product only, but with progressing technology, we realized that the gut has a complex nervous system, which interferes directly with the brain and allows bidirectional flow of information. This interaction gives us the idea that function, mood, and cognition may be influenced by our gastrointestinal contents. In lieu of this, the role of gut microbiota has been studied in relation to development of ASDs.5
Studies have unveiled that gut bacterial dysbiosis is directly correlated to changes in an organism's overall health. However, it is not clear if there is a specific group of dysbiotic bacteria in autism. A major hurdle comes in the way while selecting the typical gut bacteria which participates in autism. There is an “n” number of bacteria present in the gut, where some bacterial species are generally considered good and some bad, there is no one size fits all solution to microbiome therapy. This heterogeneity makes it troublesome for the researchers to know how the microbiota precisely influences the disease. The basic therapy can be stabilization of microbiome balance using various remedies such as probiotics, prebiotics, herbal treatment, and Fecal Microbial Transportation (FMT) process of a healthy subject.5, 6, 7 The aforementioned treatments give symptomatic relief as the popular dictum goes: "fix your gut, fix your brain”. Subsequently, the aim of this review is to utilize the present-day knowledge about gut-bacterial dysbiosis and ASD and evaluate the role of prebiotics, probiotics, diet and herbal medicines in the treatment of ASD.
Articles related to the subject were collected from the search engines i.e. Pub Med, Science Direct, Google scholar and Scopus. The searched terms were: autism, ASD, gut microbiota, gut-brain axis, dysbiosis, herbal medicine, probiotics, FMT, antibiotics, and other related terms. Initial assessment of the articles was assessed by researchers from the title, abstract and full text. Those articles were selected and included into the study which met the predefined inclusion and exclusion criteria. In this review, literature from 1985 to 2021 was included.
2. Gut microbiota and its role in autism
2.1. Gut microbiota
Microbiota in general refers to commensal, pathogenic and symbiotic microbes that are found in multicellular organisms which include not just bacteria but also other microbes such as protista, fungus and viruses.8 Gut acts as the major habitat of human microbiota and depicts microbial population inhabiting the length and width of mammalian gastrointestinal (GI) tract. The human gut consists of approximately 500–1000 bacterial species and healthy gut flora is largely responsible in maintaining individual's overall health status.9 There are manifold functions performed by the gut microbiota that includes; maintaining the integrity of intestinal barrier, stimulating regeneration of epithelium through the production of short chain fatty acids (SCFAs) and some other metabolites, leading to production of mucus, fermentation of non-digestible carbohydrates and a lot more.10,11The gut microbes also takes part in the maturation of immune system, in the synthesis and metabolism of certain essential nutrients, vitamins and hormones, and in excretion of some drugs from the body. Microbiota interactions with the brain are likely to begin early in the developing fetus. The present evidence strongly claims that there is transportation of bacteria and their metabolites between mother and fetus through the amniotic fluid. After delivery, the newborn gets exposed to environmental microbes and the gut begins to start true colonization.12The mode of delivery, vaginal or cesarean, mother's health status will influence the microbes that first colonized in the gut. During early childhood, the factors that determine the gut microbial composition include breastfeeding, diet and antibiotic treatment.13Two major phyla i.e. Bacteroidetes and Firmicutes make the major portion of gut microflora.14Many studies have shown that a maternal high fat diet during pregnancy decreases the levels of Bacteroides in neonates. Furthermore, maternal obesity during pregnancy and gestational diabetes alter the gut microbiota and might be associated with autism.15,16As shown by Buffington et al., dysbiosis and autism like phenotypes in mice can be induced by high fat maternal diet and Lactobacillus reuteri helps to restores these alterations.17
2.2. Microbiota Gut-brain axis
The gut-brain-axis is regarded as the biochemical bidirectional signaling that takes place between the gut and the brain acting through the neuroendocrine system, neuroimmune system, hypothalamic pituitary-adrenal (HPA) axis, sympathetic and parasympathetic nervous system, the enteric nervous system (ENS) and vagus nerve.18,19In recent studies, the gut-brain-axis has been related to the gut microbiota and its role has been studied in different diseases like obesity, Alzheimer's disease, type 2 Diabetes.20Microbiota has emerged as the key regulators of gut brain functioning thus leading to distinct “Microbiota-Gut-Brain Axis.”Multiple approaches have been used to investigate this axis including the use of Germ Free (GF) animals, probiotic agents, antibiotics induced depletion of gut microbes etc.19,21,22,23,24The study on GF mice (mice raised without any exposure to microbes) showed effects on brain like alterations in sociability, locomotor activity and repetitive stereotyped behavior depicting the vital role of gut microbiota in normal stress responsivity, sociability, cognition and maintenance of central nervous system homeostasis.21,22 Another study was done on healthy mice that were given B.longum 1714 strain (probiotic) that showed anti-stress effect and increase in neurocognitive function in healthy mice.23In another study on mice (two groups consisted of non-treated control males and antibiotic treated males), microbiota depletion from weaning onwards by means of chronic treatment with antibiotics [antibiotic cocktail consisted of Ampicillin (1 mg/ml), Vancomycin (5 mg/ml), Neomycin (10 mg/ml), Metronidazole (10 mg/ml) and supplemented with Amphotericin-B (0.1 mg/ml)] impacts on anxiety, cognitive behavior and key neuromodulators (tryptophan, monoamines and neuropeptides) of gut-brain-axis suggesting that dysregulation of this axis may contribute to the pathogenesis of disorders associated with altered anxiety and cognition.24Moreover, literature showed that gut microbiota exerts its action on the brain through its influence on production and expression of neurotransmitters like serotonin, gamma-Aminobutyric acid (GABA) and sensory afferents, production of various bacterial metabolites and mucosal immune regulation. On the other hand, the Central Nervous System (CNS) exerts its action on microbiota through metamorphosis in mucous and biofilm production, modulation in the motility of gastrointestinal tract (GIT), alteration in the equilibrium of intestinal permeability and reorganization in immune functions.25
2.3. Dysbiosis in gut leading to ASD
Imbalance in the composition of the microbiota can result in disturbed host-microbiota homeostasis which can lead to ASD as summarized in Table 1. Evidence showed that autistic subjects harbor an altered gut microbiota which can influence the immune system and cause secretion of its metabolites, thus establishing an association between dysbiotic gut microbiota and autism. Furthermore, there is an alteration of intestinal permeability (referred to as “leaky gut”) in autistic patients due to altered gut microbiota or dysbiosis that results in the production and spread of a potent proinflammatory endotoxin, namely Lipopolysaccharide (LPS). This molecule can modulate CNS by increasing the activity of areas like amygdala that control emotions and behavior.26 It also results in the inflammatory cytokines production from the immune cells that can alter the normal brain physiology and can modulates neuropeptide synthesis.27Fiorentino et al., demonstrated that both the gut barrier integrity and blood brain barrier (BBB) were impaired in individuals having autism. This is evidenced by the increased expression of claudin CLDN-5, CLDN-12 in the brain, decreased amount of barrier forming intestinal tight junction components like CLDN-1, Occludin (OCLN), TRIC gene protein and increased levels of pore forming CLDN (CLDN-2, CLDN-10, CLDN-15) in autistic patients as compared with control ones. Claudins (CLDN-1, CLDN-3, CLDN-5, CLDN-12) are tight junction components present abundantly in brain and their increased expression suggested a compensatory mechanism for disrupted blood brain barrier integrity.Occludin (OLCN), TRIC gene protein are the other components of tight junctions associated with increased epithelial tightness and decreased permeability to macromolecules. Pore forming CLDN are also there like CLDN-2, CLDN-10, CLDN-15. Decrease in the levels of barrier forming components and increase in pore forming components leads to impaired intestinal integrity).28The Microbiota-Gut-Brain-Axis plays a key role in the pathogenesis of autism by different ways; foremost and most prominently by contributing in maintaining the gut permeability and formation of leaky gut in autism due to microbiota alteration. Secondly, microbiota plays a role in immune system maturation and dysbiosis in autism leading to immune system dysregulation.29, 30, 31The activated immune system releases chemokines and cytokines such as interleukin-1β (IL-1β), interleukin-6(IL-6), interferon-γ (INF-γ), tumor necrosis factor-α (TNF-α) which crosses the BBB. These mediators bind to endothelial cells of brain and induce immune responses in the brain.32, 33, 34Ashwood et al., investigated cytokine levels in plasma samples obtained from 2 to 5 year old children with ASD in comparison to age matched typically developing (TD) children. They found significant increase in plasma level of cytokines including IL-1β, IL-6, IL-8 in ASD group compared with TD controls.33
Table 1.
Effect of different microbes in autistic patients.
| Name of microbes | Microbial level in autistic patients | Effect in autistic patients | References |
|---|---|---|---|
| Proteobacteria | Increases | It caused host inflammation and reduction in levels of GSH. It also led to the production of LPS which is the major cause of immune dysregulation in autism. | 36,37 |
| Bacteroides | Increases | It produces short chain fatty acids and their metabolites especially propionic acid which may influence autism behavior by gut brain axis. | 51 |
| Clostridium | Increases | It produces endotoxins and propionate that may be associated with severity of ASD symptoms. | 40, 41, 42, 43 |
| Faecalibacterium prausnitzii | Increases | It produces anti-inflammatory butyrate which is regarded as commensal or even beneficial in children with autism. | 47 |
| Candida albicans | Increases | It results in absorption of carbohydrates and releases ammonia which leads to excess of GABA production that can lead to the appearance of autistic behavior. | 49 |
| Bifidobacterium | Decreases | Bifidobacterium synthesize GABA, as its level decreases in autism so children with autism have low levels of GABA. | 52 |
| Blautia | Decreases | This bacterium has role in synthesis of Tryptophan and bile acid that acts as a precursor of Serotonin. Hence, its lower levels leads to less serotonin in brain and can be correlated to autistic behavior. | 53 |
| Prevotella | Decreases | Involved in metabolism of saccharides due to which autistic patients are thought to have impaired Carbohydrate metabolism. | 54 |
GSH- Glutathione; LPS- Lipopolysaccharide; ASD- Autism Spectrum Disorder; GABA- Gamma aminobutyric acid.
Reports suggest that autism patients had elevated abundance of Proteobacteria, Lactobacillus, Bacteroides, Desulfovibrio, Clostridium while levels of Bifidobacterium, Blautia, Dialister, Prevotella, Veillonella and Turicibacter were consistently lower in them relative to healthy controls.35Proteobacteria, abundantly present in the gut of autistic patients is associated with host inflammation.36Animal studies suggested that Proteobacteria produces LPS which can reduce level of glutathione (GSH) in brain, which is an antioxidant.37,38Another important gut microbe is Bacteroides which is the main producer of propionate in the gut and abundance of propionate in feces correlates strongly with the abundance of Bacteroides in patients suffering from autism.39 The level of another microbe i.e. Clostridium is increased in autistic patients, that is the main producer of propionate which is further used for gluconeogenesis in liver.40Clostridium produces endotoxins and propionate that may be associated with severity of ASD symptoms.41 Some species of Clostridium produces p-cresol which can cause reduction of GSH and reported to be a possible urinary biomarker for autism0.42,43It has been suggested that, reduction of Clostridium yields improvement in the behavioral and GI symptoms like diarrhea as demonstrated by Sandler et al.; in which short term improvement in children with autism was observed after the oral vancomycin (a poorly absorbed antibiotic known to destroy Clostridia and other gram positive organism)treatment. Short term improvement can be explained by the fact that vancomycin treatment reduces the Clostridium population but due to the persistence of spores, Clostridium levels returns once treatment was ceased. Thus it can be concluded that the short term improvement from vancomycin treatment might be due to temporary elimination of neurotoxins producing microbes like Clostridium.44
There are some conflicting results about the alterations of Akkermansia and Sutterella in autistic patients. The effects of Akkermansia, a mucin-degrading bacterium residing in the gut were studied in children with ASD. Lower levels of abundance of Akkermansia was taken as an indication of thin GI mucus barrier; indirectly stating impairment in gut permeability in children suffering from ASD.45Sutterella can control the mucosal metabolism and integrity of intestinal epithelial cells.46 Changes of mucus degrading microbes may impact the mucosal barrier in the gut. Another bacterium, Faecali bacterium is regarded as beneficial in autism due to its function of producing butyrate having anti-inflammatory action.47 Yeast present in gut like Candida albicans results in absorption of carbohydrates and releases toxins. Candida can interact with the assembly of microbiota and can contribute to dysbiosis. Kantarcioglu et al., in a study has isolated 338 yeast strains from 415 stool samples of individuals having autism. 81.4% were Candida albicans among the yeast strains .48Strati et al., found that Candida was two times more abundant in individuals with ASD as compared to the normal ones. It has been hypothesized that Candida albicans when present in excess may be associated with autism as it produces ammonia that reacts with propionic acid in the gastrointestinal tract and gets converted to beta-alanine, somewhat structurally comparable to GABA. Beta alanine can cross BBB and acts as partial GABA antagonist which partially blocks GABA receptor sites, enough for brain to overproduce GABA in order to attain homeostatic equilibrium. Therefore an excess of GABA (inhibitory neurotransmitter) produced may partially help explain the appearance of autistic behaviors.49,50
3. Pathogenesis
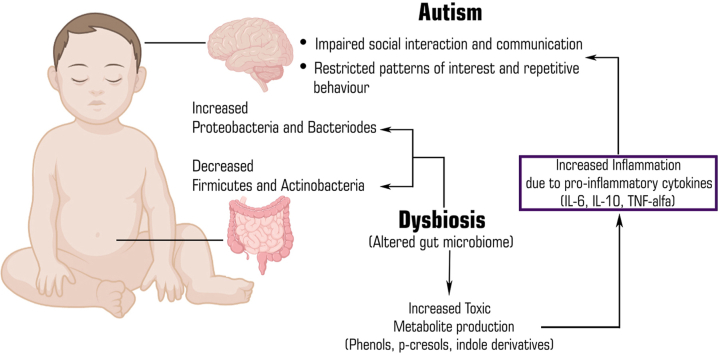
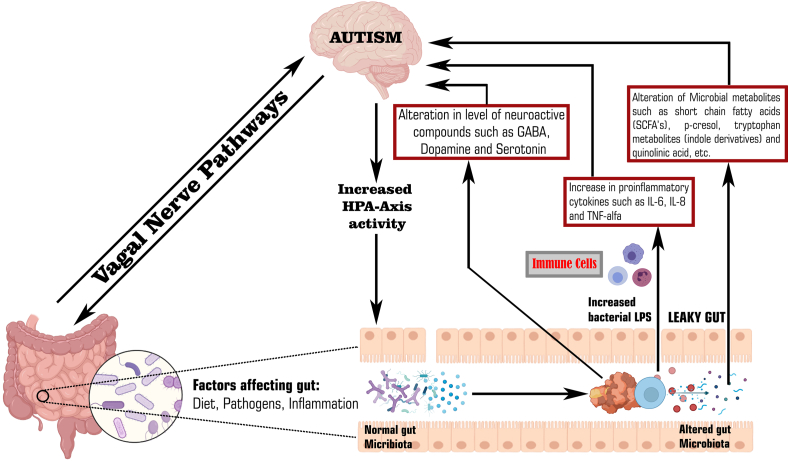
A large number of emerging evidences demonstrate that the gut-brain-axis plays a major role in the pathogenesis of autism as shown in Fig. 1. The gut microbiota can alter the brain physiology through neuroendocrine, neuroimmune pathway and via its metabolites.5,28The GI mucosa containing millions of neurons makes up the Enteric Nervous System (ENS) referred to as “Second brain” as it regulates all the gastrointestinal functions. The ENS can acts as a communication mediator between the gut microbiota and the CNS. Due to leaky gut and impaired BBB (as mentioned above), there is increased access of bacterial products into bloodstream that enters brain and alters its function.5,29The gut microbiota can alter the brain functions by three main pathways which are as followed:
Fig. 1.
Potential relationship between gut dysbiosis, its metabolites and autism.
3.1. Gut microbial metabolites pathway
The metabolites produced by gut microbiota affect the neural processes based on their levels. Metabolomic studies using Liquid chromatography–mass spectrometry (LC-MS), Gas chromatography–mass spectrometry (GC-MS) in the serum, urine and fecal samples of patients with autism showed an increased level of metabolites such as SCFA, Free amino acids (FAA), p-cresol and ammonia which can lead to behavioral symptoms in autism and these metabolites exert autism like symptoms through the vagal pathways0. 55, 56, 57Among these, the SCFA are the key metabolites that includes acetic acid, propionic acid, butyrate, isobutyric acid, valeric acid and isovaleric acid, that are produced via the gut bacterial fermentation of non-digestible carbohydrates.58 Although short chain fatty acids do not belong to the class of neuroactive substances, but these metabolic products play a significant role in maintaining the neurotransmitter phenotype after birth and in modulating biosynthesis of catecholamines throughout the lifespan. Several studies demonstrated that these SCFA played a crucial role in autism patients. Wang et al., detected high amounts of these SCFA (acetic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid) in fecal matter of children with ASD as compared to fecal matter of controls.59On contrary some studies proved that the children with autism have lower levels of these short chain fatty acids except propionic and acetic acid. Propionic acid is a SCFA produced by Clostridia and Bacteroidetes. It can cross BBB thus induce autism like behaviors by modulating serotonin and dopamine in brain.60Propionic acid is responsible for the generation of pro-inflammatory cytokines and results in the reduction of intracellular antioxidants like GSH and superoxide dismutase.61
A study in which the role of propionic acid was studied in a rodent model of ASD concluded that although propionic acid and related SCFA are largely derived in the gut but they can still gain an easy access within the brain. Moreover, on entering the brain, propionic acid or other SCFA can induce modulation in neurophysiological processes, thus changing the functioning of brain and cause alteration in behavior.62 Butyrate can modulate catecholaminergic synthesis (dopamine, epinephrine, norepinephrine) by the alteration of gene expression of tyrosine hydroxylase gene.63 A study by Xiong et al., analyzed urinary samples from sixty two autistic and sixty two non-autistic controls and found higher levels of 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, 3-hydroxyphenylacetic acid, 3-hydroxyhippuric acid in samples of autistic children. 3-(3-hydroxyphenyl)-3-hydroxypropionic acid induced autistic symptoms by decreasing brain catecholamine levels.64,65Free fatty acids derived from the hydrolysis of dietary proteins and peptides were also found to be associated with autism. It was found from some studies that indole and 3-methylindole levels are increased in children with autism.64Several commensal or beneficial bacteria like Alistipes that colonize the human gastrointestinal tract synthesize indole, a metabolite of tryptophan that acts as a precursor of important molecules like serotonin and dopamine.66 The gut microbiota is responsible for regulating tryptophan metabolism directly through the degradation of tryptophan to indole containing derivatives and indirectly through the serotonin and kynurenine pathways. The primary pathway for tryptophan metabolism is kynurenine pathway, resulting in the kynurenic acid synthesis.67 But in children with autism, due to dysbiosis, there is decrease in kynurenine pathway and alternative pathway increases which makes quinolinic acid, a compound that shows excitotoxic N-Methyl-d-Aspartate (NMDA) receptor agonistic activity in children.68,69
3.2. Neuroimmune pathway
The gut microbiota plays a significant role in the maturation of immune system of host by modulating the innate and adaptive immune system, more specifically T-cells, which helps in preventing inflammation, but dysbiosis in autistic children leads to an over activation of the immune system, release of inflammatory cytokines and chemokines that regulate the CNS through the vagal system. Among these markers TNF-α, Interleukin-8 (IL-8) and IL-6 are linked to autism.33 Studies indicate that brain inflammation or neuroinflammation has a significant role in the pathophysiology of autism. Alzghoul et al., conducted a study in Jordanian children to measure and compare levels of TNF- α, IL -8, IL-6 in autistic children, their unaffected siblings and unrelated healthy controls. After investigations, they reported an elevated plasma level of IL-8 and TNF-in plasma of autistic children in contrast to their siblings and unaffected healthy control group.70An endothelial cell of BBB makes a tight junction between blood and brain and regulates the infiltration of immune factors. Cytokines stimulates the endothelial cells and in turn these cells produce more cytokines themselves and worsen the immune response. This could be the reason for the rise in the above mentioned factors (TNF-α, IL-8, IL-6 etc.). Theoharides et al., reported that mast cells are involved in the development of autism, which worsens with stress. Stimulation of mast cells by neuropeptides like the corticotropin releasing hormone (CRH) and neurotensin (NT) can lead to the release of neurotoxic and inflammatory mediators. These mediators will lead to the disruption of blood brain barrier, microglia stimulation and focal inflammation.71 Immune response to toxins produced by pathogenic microbiota and this focal inflammation increases gut permeability, which allows the gut bacteria to translocate across intestinal wall and into the mesenteric lymphoid tissue, where they can activate the immune system through mucosal immune cells.72 The activated immune cells releases inflammatory cytokines which have been found responsible for development of autism.
3.3. Neuroactive compound pathway
This pathway involves the role of neurotransmitters in the microbiota-gut-brain axis. The gut microbes synthesize neuroactive molecules such as 5-Hydroxytryptamine (5-HT), dopamine, GABA and histamine. These chemicals can activate or inhibit central neurons through the vagus nerve.73Researchers reported that various microbes are involved in production of neurotransmitters like GABA and serotonin.74The level of these neurotransmitters e.g. serotonin, GABA and dopamine are altered in autism patients.75Blood serotonin was the first biomarker identified in children with ASD. An interesting recent study demonstrated the role of neurotransmitter serotonin as an important link between gut-brain-axis in autism. In addition to this, serotonin is also involved in the development of both CNS and ENS.76,77Hyperserotonemia in children with autism has been identified since 1970's and its relationship with gastrointestinal symptoms has been found by Marler and colleagues. As most of the serotonin (about 90%) of body is synthesized in gastrointestinal tract by enterochromaffin cells so it has been postulated that high serotonin levels in autism patients is may be due to the hypersecretion of serotonin from gastrointestinal enterochromaffin cells.77 Recent animal studies have found that dysbiosis in ASD mouse models were associated with gastrointestinal problems and increased level of serotonin, thus supporting a link between enteric serotonin production and dysbiosis. However, in the brain, hyposerotonemia i.e., lower levels of serotonin in the brain has been reported in autistic children.78GABA is an inhibitory neurotransmitter in the CNS and its activity gets altered in autistic patients. One hypothesis could be, gut microbes like Lactobacillus brevis and Bifidobacterium dentium produces GABA, and therefore dysbiosis can lead to alteration in its synthesis and activity.74
Dysbiosis in children leads to an over activation of the immune system, release of inflammatory cytokines and chemokines such as IL-6, IL-8, TNF-α that can regulate the CNS.33,70 Level of neuroactive compounds like 5-HT, GABA and dopamine produced by bacteria gets altered due to gut dysbiosis.74, 75, 76 Moreover, gut microbial metabolites such as SCFAs, and other metabolites can cross leaky gut to affect the brain function and thus cause autism.55, 56, 57 On the other hand, gram negative bacteria like proteobacteria produces LPS that can cross leaky gut, increases permeability of BBB and lead to neuroinflammation which is another reason to potentiate autism in children.26 Furthermore, the neuroactive compounds stimulates HPA axis and can lead to increased circulating cortisol levels which can have harmful effects on brain development.71,72
4. Therapeutic approaches to target gut microbes in ASD
4.1. Antibiotics
Treatment of gastrointestinal infections can be managed by the usage of antibiotics as they have a tendency to alter the composition of gut microbiota. Research suggests that early exposure to antibiotics may trigger autism but there are certain results where antibiotics served as a treatment for autism, such as aminoglycosides which help in relieving symptoms of autism to certain extent. The hypothesis that is put forth by some researchers is that aminoglycoside antibiotics could improve autism symptoms by correcting the premature stop codon mutation in a hypothetical polymorphic gene linked to autism.79 In addition to aminoglycosides, we have a few other FDA-approved medications for treating autism such as glutamate antagonists. Ceftriaxone, a beta-lactam antibiotic, enhances the expression of the glutamate transporter 1 which limits extracellular glutamate levels. Another hypothesis is that modulating astrocyte glutamate transporter expression by ceftriaxone or cefixime which in turn might improve some symptoms of autism.80Antibiotics in some studies showed benefits in alleviating ASD symptoms via selectively eliminating residual microbiota. Vancomycin and Metronidazole have been used in the treatment of ASD symptoms. However, Metronidazole is not preferred due to causation of systemic side effects. A study was conducted on 11 ASD affected children who were treated with Vancomycin (an antibiotic mainly acts against gram positive bacteria like Clostridia), communication and behavioral improvements were observed after the planned 8 week treatment. According to one more study on the administration of Amoxicillin over a 10 day course deemed to improve autism symptoms in a child as reported by his parents. There are some studies suggesting that early exposure to antibiotics can trigger Autism. A study in rodent models suggested that the maternal use of oral antibiotics (non-absorbable Sulfonamide, Neomycin, Bacitracin) yielded offspring with impaired social interactions. Faecal samples of these offspring exposed to antibiotics showed 50% reduction in Lactobacillus abundance and increased Clostridium. This implies that there is negative impact on the behavioral outcome in offspring after early exposure to antibiotics. The association between gut microbiota and antibiotic treatment has yet to be fully confirmed.81,82
4.2. FMT and MTT
Another treatment methodology for the treatment of ASD is FMT technique, in which the fecal microbiota from a healthy human being is transferred to a patient suffering from gut dysbiosis. FMT technique acts as a rich source of thousands of bacterial species which are congenital to the gut in comparison to probiotic treatment which contains only a few bacterial species found in milk culture. FMT treatment helps in eradicating perennial Clostridium difficile infection and is an encouraging therapeutic approach for tackling chronic inflammatory diseases like insulin sensitivity and inflammatory bowel disease (IBD). Moreover, research indicates that FMT can improve signs and symptoms of constipation and normalize the gut micro flora in patients suffering from IBD and inflammatory bowel syndrome (IBS).83Therefore, researchers are taking keen interest in using FMT to treat children suffering from ASD. Recently, an improved modification of FMT protocol called Microbiota transfer therapy (MTT) has gained attention of many scientists. In this technique, a 2-week antibiotic treatment followed by bowel cleansing, and then an FMT using a high initial dose of standardized human gut microbiota for 7–8 weeks is adapted.84Additionally to support this, a clinical trial was conducted which showed that MTT shows promising results in improving GI indications such as constipation, indigestion, abdominal pains and diarrhea. Furthermore it was also concluded that MTT ameliorated ASD coupled symptoms and accustomed the gut microbiota in ASD patients. Another report showed that MTT is approximately 92% effective in curing recurrent Clostridium difficile infections, but recently new-onset obesity was observed after giving FMT to recipient for recurrent C. difficle infection after transplantation from obese donor.85Kang et al., performed an open model clinical trial on 18 autism diagnosed children to study the impact of microbiota transplant therapy on composition of gut microbes and alteration of gastrointestinal and autism symptoms and it was found that there was an 80% reduction of gastrointestinal symptoms and significant improvement in the behavior. The researchers observed improvement in behavioral symptoms of children having autism that continued for 8 weeks after treatment.84
4.3. Probiotic and prebiotic therapy
Probiotic therapy has been proposed as a non-pharmacological treatment which can diminish the degree of ASD and GI tract related symptoms. It is recommended as an adjuvant therapy in children with ASD as it possesses no potential side effects. Literature suggests that patients suffering from ASD suffer from GI tract complaints frequently and it is postulated that probiotics can be used to treat gut inflammation, reduce gastrointestinal symptoms in children with IBD and improve behavioral symptoms in children with autism.39,86Several studies narrated that the consumption of probiotics best owes innumerable health benefits by supporting favorable modifications of gut microflora. The exact mechanism by which probiotics function is unknown but a few mechanisms have been proposed. Firstly, probiotics alter the functioning and composition of microbes residing in the gut and have a direct interaction with the host via which it can contact the immune system.39And secondly, probiotics can sustain mucosal barrier by elevating levels of mucin expression, abating overgrowth of bacteria, promoting synthesis of antioxidants, and modulating secretion of Immunoglobulin A to stimulate immunity of mucosa.87
Studies have suggested that severe cases of ASD are characterized by bowel dysfunction and inflammation of GI tract due to gut microbial dysbiosis. Thus, in order to restore the balanced levels of gut microbiota and improve ASD symptoms, usage of probiotics is recommended.36 In lieu of this, influence of probiotics as a therapeutic approach was studied on health status of autistic patients. A study showed that probiotic supplementation could prevent Candida colonization in the gut by decreasing levels of D-arabinitol (a metabolite of Candida species) in the urine of autistic patients. Another study concerning D-arabinitol suggested that the ratio of d-arabinitol/l-arabinitol can act as a biomarker in detection of candidiasis.88 Furthermore, a probiotic supplement comprising of Lactobacillus acidophilus, not only decreased d-arabinitol/l-arabinitol ratio but also boosted the concentration and aptitude to acknowledge on order in ASD children with major behavioral problems.89Another case study employing a mixture of live cells (VSL#3) which showed massive improvement in autistic fundamental symptoms and GIT symptoms in a severe case of ASD. Moreover, during the study it was also reported that VSL#3 supplementation minimized the social affect domain of “Autism Diagnostic Observation Schedule” (ADOS) score and no changes were observed in ADOS score up to a follow up period of 10 months.90 ADOS score is an element for diagnosis for autistic disorder by means of direct monitoring that evaluates social interaction, communication, stereotypical behavior and imaginative utility of objects.91 Under observation, a score is given to the examinee in order to classify the conditions as autism spectrum, autism, or non-autism. ADOS consist of four modules which are directed to the examinee on the basis of age and level of language.92 Furthermore, it was reported that on administration of a probiotic strain (L. plantarum WCSF1) to autistic patients, remarkable changes were observed such as increased levels of enterococci and lactobacilli group and lowering in the levels of Clostridium cluster XIVa. Moreover, bowel functioning also improved in the placebo group but other GIT symptoms showed no marked changes. On conducting an examination based on “The Development Behavior Checklist questionnaire” it was found that behavior profile improved immensely on giving probiotic therapy in comparison to placebo group. The study showed that the usage of WCSF1 altered gut microbiota and behavior in autistic children.90There are evidences from some studies that probiotic supplementation can also results in the behavioral improvements in children with ASD. The probiotics via the gut-brain axis influence several neuroactive compounds such as Serotonin and GABA.76,93,94Due to hyperactivation of gene that codes for serotonin reuptake transporters, gut bacteria influences brain serotonin levels .76Probiotics improves serotonin levels and thus exerts behavioral improvements in ASD. It has been observed that some probiotics improves oxytocin levels and thus improves social behavior. Due to gut dysbiosis, there is also dysregulation of immune system that leads to autoantibodies formation in ASD patients.
Some of the microbes such as Lactobacillus species have been found to down regulate hypersensitive responses and improves behavioral symptoms.94L. reuteri was found to influence the pituitary gland to produce oxytocin (secreted by hypothalamus) that positively influences social behavior.95In another study after the Bacteroides fragilis treatment, the mice appeared more socialized and appeared less anxious and obsessive.96
Apart from probiotics, the role of prebiotics on gut microbiota cannot be neglected. Prebiotics such as galactooligosaccharides, resistant starch, xylooligosaccharides, oligosaccharides and non-starch polysaccharides are indigestible food materials which potentiate the growth of host bacterial strains, altering their functioning and composition, thus benefiting health and well-being of host.97Although the effects of prebiotics on ASD patients is still under investigation but recently a few studies have been conducted to understand the impact of prebiotics in ASD patients. In a prebiotic intervention study, the consequences of exclusion diet and a 6-week Bimuno ®galactooligosaccharide (B-GOS ®) was studied in 30 autistic children. It was inferred that the combination therapy showed synergistic effects and significantly impacted gut microbiota composition and psychological traits in autistic patients. The results showed that prebiotic supplementation reduced GI tract discomfort by lowering the scores with respect to abdominal pain. Moreover, on analyzing the anxiety and ASD-related behavior questionnaires, social behavioral scores were improved on B-GOS ® intervention. Another study tested the effects of two prebiotics namely fructo-oligosaccharides and B-GOS ®in healthy volunteers with respect to their hormonal and behavioral profiles. The results revealed that B-GOS ®containing prebiotic formulation minimized salivary-cortisol secretions indicating arresting of neuroendocrine stress response, followed by improvement in patient's attention duration. Since the above mentioned behavioral domains are highly impaired in ASD patients, it was concluded that the prebiotic supplementation may be useful in treatment of ASD clinical signs and symptoms.98
Another novel study demonstrated that GI tract abnormalities prevail in a considerable number of autistic children. Thus, a pilot study using probiotic B.infantis and prebiotic oligosaccharide (Bovine Colostrum Product, BCP) supplementation on the functioning of the gut was performed in ASD children having GIT symptoms. In this study, a controlled trial using a combination treatment (BCP + B infantis) vs BCP only group was evaluated in children (2–11 years) with ASD and GI tract problems. This 12 week study based on usage of both probiotic and prebiotic supplementation was done in order to assess changes in GI co-morbidities and atypical behaviors. The findings revealed that the group to which only BCP was administered showed significant decrease in irritability, hyperactivity, lethargy and stereotypical behavior.99
Although, the findings from studies suggest an evident link that alteration in the composition of gut microbiota in children suffering from autism can affect the co-ordination of microbiota-gut-brain axis. However, due to the extraordinary microbiome profile and heterogeneity in patients, a lot more research needs to be performed in this area. Concrete evidence to prove a solid potential effect of probiotics and prebiotics on the symptoms of ASD needs more supporting research, but a few studies using prebiotics and prebiotics with their respective dose, type of study, duration of intervention and outcomes have been enlisted in Table 2.
Table 2.
Various probiotics and prebiotics used in the management of autism.
| Category | Name of strain and marketed formulations | Study type | Dose | Duration of intervention | Dosage form | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Probiotic | 1) Children Dophilus: blend of 3 strains of lactobacillus (60%), 2 strains of Bifidobacterium (25%), 1 strain of Streptococcus (15%) | A non- controlled trial conducted in Slovakia on 29 Children: 10 ASD, 9 non-ASD siblings, 10 ASD controls | 3 times/day | 4 months | – | Probiotic supplementation elicited: | 39,100 |
| |||||||
| |||||||
| |||||||
| The following diagnostic scales were used for assessment of these symptoms: Autism Diagnostic Interview (ADI): Childhood Autism Rating Scale (CARS); Total Behavior Problem Score (TBPS) | |||||||
| 2) Delpro capsule: Lactobacillus delbrueckii, L. acidophilus, Lactobacillus casei, B. longum, Bifidobacteriumbifidum (along with an additional 8 mg Del-immune V powder) | A noncontrolled trial conducted in USA on 33 Children with ASD in age group of 3–16 years. | 10 billion Colony forming units (CFUs) total (1 capsule, 3 times/day) | 21 days | Capsule | A significant decrease across all ATEC (Autism Treatment Evaluation Checklist) domain scores including speech, communication language, sociability, sensory/cognitive awareness, health/physical/behavior. Childhood Autism Rating Scale was used for the evaluation of symptoms. | 100 | |
|
3) Vivomix probiotic: S. thermophilus, B. breve, B. longum |
Randomized placebo controlled trial of a group of 100 children | – | 5 months | Water soluble formula for oral consumption |
|
39,101 | |
| |||||||
| 4) Protein R: mixture of Bifidobacteria and Lactobacilli strain. | 50 young male hamsters weighing between 60 and 70 g in which autistic like behaviors were induced by clindamycin and propionic acid administration. | 2 × 108 CFU/day | 27 days | – |
|
13,102 | |
| |||||||
| 5) 3 strain: L. acidophilus, Lactobacillus rhamnosus, B. Longum | Trial in Egypt of 60 children, aged between 5 and 9 years. | 5 g of powder/day (each gram contains 100 × 106 CFUs of each strain. | 1 time/day for 3 months. | – |
|
18,25,90,103 | |
| |||||||
| |||||||
| |||||||
| 6) VISBIOME probiotic: S. thermophillus, B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, Lactobacillus paracasei, Lactobacillus delbruecki subsp. Bulgaricus. |
Randomized crossover trial with 13 children. | – | 19 weeks | Available in powder packets (each containing 900 billion bacteria). |
|
13,60,87,103, 104, 105 | |
| |||||||
| |||||||
| 7) L. acidophilus | A non- controlled trial in Poland of 22 children with ASD aged between 4 and 10 years. | 5 × 1010 CFUs 2 times/day |
2 months | – |
|
88,100 | |
| |||||||
| 8) Lactobacillus rhamnosus | Randomized trial, placebo control in Finland on 15 infants for 6 months of life and followed for 13 years starting at the age of 6 month. | – | First 6 months of life. | Capsule |
|
13,106 | |
| |||||||
| Prebiotics | 1(B-GOS) Bimuno-galactooligosaccharide | Double blind placebo controlled, crossover study, 83 subjects from general population with GI symptoms. | 2.75 g/day | 2 weeks | Supplied in sachets containing powder form. | Resulted in lower scores of abdominal pain and bowel movement as well as lower abundance of Bifidobacterium, higher presence of Faecali bacterium prausnitzii and Bacteroides spp. | 107 |
| 2) Short chain glactooligosacharide (scGOS), long chain fructooligosaccharide (lcFOS). | Trial on healthy BALB/c mice from day of birth | 9 parts of scGOS:1 part of lcFOS (w/w) administered from the day of birth | – | – | Reduce anxiety, stereotypic behavior and improve social behavior in adulthood. | 108 |
4.4. Dietary intervention
Many environmental factors could affect the microbiota-gut brain axis, one of which is daily food intake. Food can alter the composition of gut microbiota and could influence the serum metabolites thus modulating the brain activity in the host. Therefore many food supplements can restore the microbial balance in the gut and have therapeutic effects on ASD related deficits. Dietary interventions in children with autism are very popular but these can impart many adverse effects because restrictive diets can lead to other nutritional deficiencies. Children with autism tolerate only a narrow range of foods and exhibit more feeding issues than normal children. Many parents of children with autism complain of their selective eating habits. At present a variety of dietary interventions are in use, including Gluten and Casein free diet, ketogenic diet, yeast free diet, food allergens restrictions, supplementation with vitamins A, C, B6, folic acid B12, minerals like magnesium and omega-3-fatty acids.89,90
4.4.1. Gluten free casein free diet (GFCF)
The most frequently used intervention for ASD is the GFCF diet. This includes elimination of diet/all foods containing gluten (present in wheat, barley) and casein (found in milk and dairy products) from a child's daily food intake. Current theories suggests that gluten and casein peptides trigger an immune responses resulting in inflammation of GI.109 Investigators conducted some studies to test the hypothesis that autism is linked with the absorption of “exorphins” contained in gluten and casein.110It is hypothesized that some symptoms of autism like stereotypical behaviors, excessive activity, speech delays may result from opioid peptides. On the basis of opioid excess theory, digestion of gluten and casein results in the production of peptides with an opioid activity. Due to leaky gut in autism (due to gut dysbiosis), these opioid peptides enters blood stream and shows systemic actions by binding to opioid receptors and speculated to affect processes in the CNS and have negative effects on attention, brain maturation, social communication and learning.111,112These opioid peptides includes exorphins such as gluteomorphin and caseomorphin.
The levels of GSH, an intracellular antioxidant and detoxifying agent and SAM (S-adenosylmethionine), a methyl donor for many metabolic reactions are significantly lower in ASD. Because metabolic pathways that provides GSH and SAM are supported by nutritional factors (eg sulfur amino acid, Cysteine (CYS)and Methionine (MET), folate and vitamin B12), they are critical for ASD diets.113The rationale for the use of GFCF diets in ASD patients stems from the effects of opioid peptides that are synthesized from incomplete breakdown of Casein and Gluten. Opioid peptides derived from gluten and casein decreases the uptake of cysteine by the cells, which is required in intracellular GSH synthesis. Therefore, decreased CYS uptake leads to decreased GSH levels. Furthermore, opioid peptides also decreases the methylation index (i.e. the ratio of methyl donor SAM to its inhibitory product S-adenosylhomocysteine), leading to altered patterns of DNA methylation and gene expression.113 Lower GSH (antioxidant) levels leads to increase GI inflammation and contributes to GI discomfort. Therefore, GFCF diet may provide clinical benefit by improving CYS absorption and GSH levels. Knivensberg et al., conducted a single blind study which evaluated the effect of GFCF diet on a group of 20 children with a diagnosis of autism and urinary peptide abnormalities. Evaluation test was conducted before and after a treatment of one year, and treatment shows significant improvement in the areas of communication, language and motor skills.114
4.4.2. Ketogenic diet
Ketogenic diet, also known as long chain triglyceride diet is a low-carbohydrate, moderate protein, high-fat diet that has emerged as a potential dietary intervention for ASD. Ketogenic diet mimics the fasting state and causes shift in host metabolism by promoting ketone body production and utilization. There is paucity of data in the evaluation of ketogenic diet but a few studies are available. Newell et al., conducted a study to examine the impact of ketogenic diet on the gut microbiota of a mouse model of ASD.115,116The key findings of that study were: (1) As compared to control mice, gut microbiota composition of cecal and fecal samples were altered in BTBR mice, (2) Ketogenic diet causes anti-microbial like effect, hence decreases host bacterial abundance in cecal and fecal matter, and (3) Ketogenic diet reversed the elevated Akkermansia muciniphilia content in fecal matter of BTBR animals.115,116Experimental studies demonstrated that ketogenic diet increases ketone bodies which maintains high GABA levels.117 There is one more hypothesis that autistic individuals may have deficient oxidation of glucose, therefore ketone bodies acts as an alternative energy fuel.117A study was conducted by Ruskin et al., on BTBR mice model. Ketogenic diet improves various autistic behaviors in mice and ketogenic diet fed mice have shown increased sociability.89Still a lot more clinical research is required to test the efficacy of ketogenic diet in autism treatment.
4.4.3. Polyunsaturated fatty acids(PUFA)
PUFA plays a key role in the normal brain growth and development, memory formation and cognitive function. Several studies identified Eicosapentaenoic acid (EPA; omega-3) and Docosahexaenoic acid (DHA; omega-3) as essential fatty acids, hence required in diet. Omega-3 PUFA is important constituent of all cell membranes so gets incorporated into it and this incorporation takes place in high levels in brain cells especially in the gray matter. These PUFA play important role in neurogenesis, neurotransmission and protection against oxidative stress. These starts accumulating in the brain during pregnancy and level continues to increase up to two years after the birth. Once high levels are achieved in brain, they are then maintained during later life depending on the optimal dietary supply. They also exhibit anti-inflammatory and immunomodulatory activity as there supplementation was associated with decrease in IL-12, IL-13, IL-6, IL-17A and tumor necrosis factor-α(TNF-α)gene expressions.118PUFA deficiency may serve as a mechanistic pathway for ASD development. Deficiency of DHA has been associated with instability of neuronal membrane and dysfunction in the transmission of serotonin, norepinephrine and dopamine. Deficiency of Omega-3 PUFAs(especially in early stages of life) may causes changes of myelination, neurotransmitter turnover, neurogenesis, inflammatory reactions and cognitive functions.118Recently researchers have suggested that effect of omega-3 PUFAs on the CNS can also takes place via gut-brain axis.Their deficiency may leads to the alteration of gut microbiota and SCFA produced by microbiota and transit signals to affect functioning of brain through the vagus nerve from gut to brain.118 Investigators found that children assigned to treatment with omega-3-supplementation exhibited a significant reduction in ASD symptoms.112Cheng et al., conducted a preliminary meta-analysis that suggested that Omega-3 fatty acids may improve hyperactivity, lethargy and stereotypy in ASD patients.119Pusceddu and colleagues demonstrated that long term EPA/DHA supplementation can lead to anti-inflammatory effect associated with restoration of altered gut microbiota on maternal separated rats.120Maternal-separated rats showed an increase in Bacteroidetes, and non-separated rats showed decreased Firmicutes. Anti-inflammatory effect is due to increase in butyrate producing bacteria and decreased levels of pro-inflammatory bacterial genera, such as Akkermansia and Flexibacter120
4.4.4. Vitamins and minerals
Due to gut dysbiosis there are decreased levels of vitamin-producing microbiota in the intestines that could lead to poor nutritional status.Vitamins and mineral supplements supports the body's basic physiological processes and impact various metabolic processes i.e. sulfation, methylation, oxidative stress, mitochondrial dysfunction.112A recent study examined the effect of vitamin A deficiency on ASD children and concluded that vitamin A deficiency can exacerbate the core ASD symptoms in children with ASD.121Furthermore, it was noted that vitamin-C plays a vital role in brain development, functional maturation and has antioxidant action. Hence, its deficiency can lead to the aggravation of ASD symptoms.122Hatice et al., examined the relationship between vitamin-D, homocysteine, vitamins B6, B12, and folate in 60 children with ASD and 45 children as control group. The study concluded that the total score of the Childhood Autism Rating Scale was positively linked to low serum levels of vitamins-D, B6, B12, folate and high homocysteine levels which implies that these nutrients may be involved in pathogenesis of autism.123
4.4.5. Specific carbohydrate diet
Coleman and Blass in 1985 reported first evidence that autism might be linked to carbohydrate digestion.124Researchers reported that D-lactic acidosis was seen in autistic children. d-lactic acid is a by-product of bacterial fermentation (process by which microbes get their energy from carbohydrates). They reported that carbohydrates were not being digested or absorbed due to the mucosal damage (bearing disaccharides enzymes) in autism patients that prevent the digestion of disaccharides therefore intestinal microbiota got flooded with surplus of carbohydrates. As a result of bacterial fermentation, d-lactic acid along with other toxic metabolites is produced in large amounts. High amounts of d- Lactic acid has been found to cause bizarre behavioral symptoms that includes sudden disorientation, blurred vision, blunted judgment, rolling of the eye ball, confusion etc.124,125Due to this reason the Specific Carbohydrate diet comes into picture. The diet includes administration of monosaccharide's whose sources are fruits, vegetables, honey etc. On the other hand the consumption of complex carbohydrates is restricted because they take longer time to digest than monosaccharides. Therefore, undigested or partially digested carbohydrates stays for longer period in GIT and can acts as a breeding source (acts as energy source) for pathogenic bacteria.126Therefore the main goal of specific carbohydrate diet is to heal the intestinal tract and to rid it of bacterial and fungal overgrowth. Not many studies have been conducted on this protocol but one such study examined the implementation of specific carbohydrate diet in a child with ASD. Results showed that the specific carbohydrate diet was well tolerated and led to improvement in GI and behavioral symptoms.126Further research is required to evaluate implementation of specific carbohydrate diet in young children with ASD.
5. Herbal medicine: A ray of hope for autism therapy
Over the ages, it has been recognized that herbal medications help in ameliorating the signs and symptoms associated with many psychiatric disorders. It is a renowned fact that the administration of herbs can benefit the immune system, assist in detoxifying the body, or modulate brain activity with fewer side effects.127 Due to these aforementioned advantages, the usage of herbal medicine concerning autism is gaining momentum and can be studied under two categories:
5.1. Herbal medicines acting via gut microbiota
5.1.1. Traditional Chinese medicine (TCM)
As stated earlier, the root cause of autism might be oxidative stress, inflammation, neuronal degeneration and dysbiosis of gut microbiota and to curb these, a few herbal therapies have shown promising results. TCM is one such herbal medicine that can help in the treatment of autism by targeting gut microbiota.128 A study reported that the use of TCM, such as a diet constituted of Chinese medicinal foods and targeted therapies of Chinese herbal medicine may propose new hopes for ASD management by accentuating the gut microbiome.129 In a novel investigation, a dietary scheme based on whole grains and traditional Chinese medicinal foods led to notable improvement of gut microbiome profile.129 During the study it was also observed that pyrosequencing of fecal specimens explicated that phylotypes associated with endotoxin-producing opportunistic pathogens were reduced significantly, while those having gut barrier-protection action like Bifidobacteria were increased.129 Other maladies such as gut permeability and inflammation also showed significant positive effect when treated with TCM. Although this study was conducted to examine the use of medicinal Chinese foods for the treatment of obesity, it was concluded that the underlying mechanism may apply to ASD.
In another investigation, comparative bioinformatics analysis was done to identify the effect of TCM on overall changes in gut microbiota in relation to ASD by comparing the intestinal microbiome level before and after treatment with TCM and comparing the level of intestinal flora with healthy controls. Buyang Huanwu Tang (BHT) was the traditional Chinese medicine employed during the course of bioinformatics analysis. This study showed that level of proteobacteria notably increased whereas Blautia and Coprococcus significantly decreased in children with autism, leading to an imbalance of healthy gut microbiome level.130After treating with BHT it was seen that the imbalance of gut microbiome level in autistic children was normalized. The activity of BHT decoction was due to Astragalus membranaceus which consists of saponins, flavonoids and polysaccharides. After oral administration of Astragalus membranaceus, its active ingredient, calycosin-7-o-b-d-glucoside (C7G), interacted with gut microbiome and acted as a regulator of intestinal microflora hence ameliorating ASD.130
Another study revealed that the ‘Decoction of Four Nobles’, a clinically utilized antiquated formula for the treatment of both constipation and diarrhea can also be used for curbing ASD.129This formula comprises of a blend of Radix Ginseng, Rhizoma Atractylodis Macrocephalae, Poria, and Radix glycyrrhizae praeparatae. The chemical constituents of the formula were found to be capable of interacting with gut microflora, while vitamins and lactones present in it acted as nutrients for the gut bacteria. In another study, the extract from Ginkgo biloba (EGB) was manifested to normalize gut flora henceforth combating the ASD symptoms through the gut-brain axis.129The above cited findings render us with novel insights of using TCM into the diagnosis and therapy of ASD.130
5.1.2. Phytochemicals
Abnormal gut microbiome balance may lead to increased production of SCFA such as propionic acid by Clostridia, Desulfovibrio, and Sutterella species. This propionic acid can easily cross blood brain barrier and further leads to mitochondrial dysfunction by destroying Electron transport chain (ETC) leading to generation of reactive oxygen species (ROS) as an outcome of oxidative stress.131As we are already aware of the fact that oxidative stress might work as one of the reasons for autism, hence curbing the oxidative stress by scavenging ROS can work as a therapy. We also know that body's combat mechanism for fighting against mild-moderate oxidative stress is the activation of the Nrf2/ARE pathway which will lead to dissociation of Nrf2 from its inhibitor Keap1 and translocating Nrf2 in the nucleus which will further bind to the antioxidant response element (ARE). The binding of Nrf2 with ARE will lead to transcription of antioxidant genes and phase II enzymes which will eventually inhibit ROS hence curbing autistic symptoms. Now as we are clear of the fact that Nrf2/ARE pathway is basically responsible for scavenging from ROS, here comes the role of dietary phytochemicals such as curcumin, resveratrol, naringenin, and sulforaphane which basically enhance the activation of Nrf2/ARE pathway by interacting with Keap1 which eventually leads to the release of various pro-inflammatory cytokines and other stress mediators which decrease the ROS generation hence restraining autism.131
5.2. Herbal medicines having direct impact over autism
Few herbs belonging to TCM also have direct effect over autism without interfering with gut microbiome.Ukgansan, a herbal remedy composed of Atractylodis Rhizoma White, Poria, Angelicae, Gigantis Radix, Cnidii Rhizoma, Uncariae Ramulus et Uncus, Bupleuri Radix, and Glycyrrhizae Radix was invented by XueJi in 1556 for handling children's liver dysfunction and epilepsy.132 It repressed hypersensitivity and hyperactivity of ASD through anti-inflammatory effects and also helped in control of serotonin and glutamate upregulation.133 Ukgansan prevented behavioral anomalies and enhanced neuroplasticity signaling in a social isolation model in mice.134 One more study of ukgansan was done on a 12-year-old boy with ASD and the results were marvelous. It improved irritability, which had not been successfully treated even with antipsychotic drug.134
After TCM, Ayurvedic and Unani system also came into frame for treating autistic symptoms as mentioned in Table 3. Talking about ayurvedic system first, the most imminent association of autism with Ayurvedic diagnosis is Unmada (Insanity).135The Lakshanas (features) specified in Unmada are a mixture of features of Vata, Pitta and Kapha singularly or collectively.135 The most widely used ayurvedic therapy is composed of CNS rejuvenators (Medhya Rasayana) which help to maintain and restores the body's harmony which further leads to improvement of balance between brain and nervous system hence curbing the autistic symptoms.135 Coming down to Unani system as we know in this system several plants, medications have been employed widely in numerous neurological ailments because of their neuroprotective properties.136The neuroprotective effect is a broad picture of several effects like prevention against oxidative stress, mitochondrial dysfunction, inflammation, and immune dysregulation. These drugs with the attributes of curing neurological disorders can be utilized in the management of autistic symptoms.
Table 3.
Active constituent and mechanism of action of different herbal drugs pertaining to Ayurvedic and Unani system.
| S.no. | Biological name | Common name | Active constituent | Mechanism of action | Chemical structure | Reference |
|---|---|---|---|---|---|---|
| 1. | Camellia Sinensis | Green tea | l-Theanine | l-Theanine the active constituent of Camellia sinensis has structural similarity with l-Glutamic Acid. Being structurally similar to l-Glutamate, l-Theanine binds to ionotropic Glutamate receptor protein, kainate1, by binding to this receptor and mimicking the same neuroprotective effect as l-Glutamic Acid. l-Theanine or Camellia Sinensis subdues the symptoms of autism. | 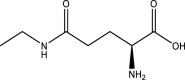 |
137, 138, 139 |
| 2. | Piper nigrum L | Black pepper | Piperine | It has been found that piperine demonstrated a positive effect on the cure of behavioral alterations and oxidative stress in autism. It showed neuroprotective effect on glutamate at a concentration of 20 mg/kg, which led to the refurbishment of viable cells. Due to this antioxidant and neuroprotectant property piperine is augmented in autism therapy. | 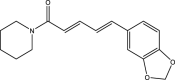 |
140,141 |
| 3. | Curcuma longa | Turmeric | Curcumin | Curcumin was found to target several cell signaling pathways which led to an increase in intracellular levels of GSH; reduce inflammatory components, mitochondrial dysfunction, oxidative/nitrosative stress, and protein aggregation. It also helped in fighting against the damage caused by heavy metals. Above-stated mechanisms suggested that curcumin has a direct or indirect positive effect over the course of autism. | 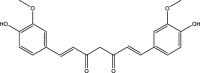 |
140,142 |
| 4. | Zinigiber officinale | Ginger | 6-gingerol | Antioxidant effects of 6-gingerol rich fraction of Z. officinale and its protective action against the cerebral cortical damage have been reported. It counteracted the reduced levels of GSH in brain and tend to reduce levels of pro-inflammatory cytokines (which were increased in autism) and thus impart beneficial effects in ASD. | 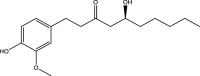 |
143,144 |
| 5. | Emblica officinalis | Amla | Vitamin-C | Neuroprotective and antioxidant effect by improving the levels of GSH and TNF-α in the brain. | 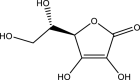 |
145, 146, 147 |
| 6. | Prunus amygdalus | Badam | Vitamin-E | Vitamin E possesses neuroprotective and antioxidant properties. Pre-treatment with Prunus amygdalus extract prevented neurochemical, histopathological, and behavioral changes to a significant extent. | 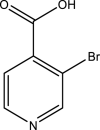 |
148 |
6. Conclusion
In the foregoing review, we compile the information from numerous articles and various studies confirming that gut microbiota is either directly or indirectly linked to autism. As the awareness and recognition of autism is on the rise, the fraction of people diagnosed with the ailment has progressed a lot in the recent years. This has made research and treatments for autism more relevant and important. GI issues are common in people with autism, Understanding the link between gut microbiota and the brain through the gut-brain axis will help the researchers and clinicians to improve the neurological symptoms related to autism. In the present review, we talked about the pathogenesis of ASD and various therapies to address autism concerning the gut microbiome which can help in addressing the problems and symptoms associated with autism. Research has shown prosperous results in a variety of treatments including probiotics, prebiotics, FMT, MTT, dietary changes. We also reviewed new hopes in herbal remedies which can mitigate autism either via gut brain axis or directly having neuroprotective or antioxidant action but still randomized controlled trial should be carried out more explicitly for significant outcomes in autism research.
Declaration of competing interest
The authors declare that they have no conflicts of interest to disclose.
Acknowledgements
The authors are thankful to the Department of Pharmaceutical Sciences, Guru Nanak Dev University, Amritsar, Punjab, India, for providing all the necessary facilities to carry out this study.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
LIST OF ABBREVIATIONS
- ASD
Autistic Spectrum Disorder
- CDC
Centers for Disease Control and Prevention
- FMT
Fecal Microbial Transportation
- US-
United States
- GI
Gastrointestinal
- ENS
Enteric nervous system
- CNS
Central nervous system
- SCFA
Short chain fatty acids
- HPA
Hypothalamic pituitary-adrenal
- GF
Germ free
- GABA
gamma-Aminobutyric acid
- LPS
Lipopolysaccharide
- BBB
Blood brain barrier
- CLDN
Claudin
- OCLN
Occludin
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- INF-γ-
Interferon-γ
- TNF-α-
Tumor necrosis factor-α
- LC-MS
Liquid chromatography–mass spectrometry
- GC-MS
Gas chromatography–mass spectrometry
- NMDA
N-Methyl-d-Aspartate
- IL-8
Interleukin-8
- CRH
Corticotropin releasing hormone
- NT
Neurotensin
- 5-HT
5-Hydroxytryptamine
- IBD
Inflammatory bowel disease
- IBS
Inflammatory bowel syndrome
- MTT
Microbiota transfer therapy
- ADOS
Autism Diagnostic Observation Schedule
- B-GOS ®
Bimuno ®galactooligosaccharide
- BCP
Bovine Colostrum Product
- GFCF
Gluten free Casein free diet
- SAM
S-adenosylmethionine
- CYS
Cysteine
- MET
Methionine
- NADH
Nicotinamide adenine dinucleotide
- NAD
Nicotinamide dinucleotide
- PUFA
Polyunsaturated fatty acids
- TCM
Traditional Chinese medicine
- BHT
Buyang Huanwu Tang
- ROS
Reactive oxygen species
- ARE
Antioxidant response element
- ETC
Electron transport chain
- scGOS
Short chain glactooligosacharide
- lcFOS
Long chain fructo-oligosaccharide
- ATEC
Autism Treatment Evaluation Checklist
- CFU
Colony forming unit
- PPA
Propionic Acid
References
- 1.Bölte S., Girdler S., Marschik P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76(7):1275–1297. doi: 10.1007/s00018-018-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rezapour S., Bahmani M., Afsordeh O., Rafieian R., Sheikhian A. Herbal medicines: a new hope for autism therapy. J HerbmedPharmacol. 2016;5(3):89–91. [Google Scholar]
- 3.Hodges H., Fealko C., Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9(Suppl 1):S55–S65. doi: 10.21037/tp.2019.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grabrucker A.M. Environmental factors in autism. Front Psychiatr. 2013;3:118. doi: 10.3389/fpsyt.2012.00118. Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q., Han Y., Dy A.B.C., Hagerman R.J. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. 2017;11:120. doi: 10.3389/fncel.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang M., Lee S.H., Cho S.H., et al. Herbal medicine treatment for children with autism spectrum disorder: a systematic review. Evid Based Complement Alternat Med. 2017;2017:8614680. doi: 10.1155/2017/8614680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao L., Yan J., Yang T., et al. Fecal microbiome transplantation from children with autism spectrum disorder modulates tryptophan and serotonergic synapse metabolism and induces altered behaviors in germ-free mice. mSystems. 2021;6(2) doi: 10.1128/mSystems.01343-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group N.H.W., Peterson J., Garges S., et al. The NIH human microbiome project. Genome Res. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 10.Burger-van Paassen N., Vincent A., Puiman P.J., et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420(2):211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 11.Mangiola F., Ianiro G., Franceschi F., Fagiuoli S., Gasbarrini G., Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22(1):361–368. doi: 10.3748/wjg.v22.i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toh M.C., Allen-Vercoe E. The human gut microbiota with reference to autism spectrum disorder: considering the whole as more than a sum of its parts. Microb Ecol Health Dis. 2015;26:26309. doi: 10.3402/mehd.v26.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdellatif B., McVeigh C., Bendriss G., Chaari A. The promising role of probiotics in managing the altered gut in autism spectrum disorders. Int J Mol Sci. 2020;21(11):4159. doi: 10.3390/ijms21114159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinninella E., Raoul P., Cintoni M., et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J., Prince A.L., Bader D., et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5(1):3889. doi: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu D.M., Antony K.M., Ma J., et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. doi: 10.1186/s13073-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buffington S.A., Di Prisco G.V., Auchtung T.A., Ajami N.J., Petrosino J.F., Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Kasper L.H. The role of microbiome in central nervous system disorders. Brain. Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinan T.G., Cryan J.F. The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr Opin Clin Nutr Metab Care. 2015;18(6):552–558. doi: 10.1097/MCO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 20.Naseer M.I., Bibi F., Alqahtani M.H., et al. Role of gut microbiota in obesity, type 2 diabetes and Alzheimer's disease. CNS Neurol Disord - Drug Targets. 2014;13(2):305–311. doi: 10.2174/18715273113126660147. [DOI] [PubMed] [Google Scholar]
- 21.Luczynski P., McVey Neufeld K.A., Oriach C.S., Clarke G., Dinan T.G., Cryan J.F. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. 2016;19(8):1–17. doi: 10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cryan J.F., O'Riordan K.J., Cowan C.S., et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 23.Allen A.P., Hutch W., Borre Y.E., et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6(11):1–7. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desbonnet L., Clarke G., Traplin A., et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;1:165–173. doi: 10.1016/j.bbi.2015.04.004. 48. [DOI] [PubMed] [Google Scholar]
- 25.Tengeler A.C., Kozicz T., Kiliaan A.J. Relationship between diet, the gut microbiota, and brain function. Nutr Rev. 2018;76(8):603–617. doi: 10.1093/nutrit/nuy016. [DOI] [PubMed] [Google Scholar]
- 26.Haba R., Shintani N., Onaka Y., et al. Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: possible role of activation of the central amygdala. Behav Brain Res. 2012;228(2):423–431. doi: 10.1016/j.bbr.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Kastin A.J., Pan W. Concepts for biologically active peptides. Curr Pharmaceut Des. 2010;16(30):3390–3400. doi: 10.2174/138161210793563491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiorentino M., Sapone A., Senger S., et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7:49. doi: 10.1186/s13229-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doenyas C. Gut microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience. 2018;374:271–286. doi: 10.1016/j.neuroscience.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao E.Y., McBride S.W., Hsien S., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onore C., Careaga M., Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W., Dowd S.E., Scurlock B., Acosta-Martinez V., Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav. 2009;96(4-5):557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Theije C.G., Wu J., da Silva S.L., et al. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur J Pharmacol. 2011;668(Suppl 1):S70–S80. doi: 10.1016/j.ejphar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Liu F., Li J., Wu F., Zheng H., Peng Q., Zhou H. Altered composition and function of intestinal microbiota in autism spectrum disorders: a systematic review. Transl Psychiatry. 2019;9(1):43. doi: 10.1038/s41398-019-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin N.R., Whon T.W., Bae J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y., Carvey P.M., Ling Z. Altered glutathione homeostasis in animals prenatally exposed to lipopolysaccharide. Neurochem Int. 2007;50(4):671–680. doi: 10.1016/j.neuint.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chauhan A., Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13(3):171–181. doi: 10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Navarro F., Liu Y., Rhoads J.M. Can probiotics benefit children with autism spectrum disorders? World J Gastroenterol. 2016;22(46):10093–10102. doi: 10.3748/wjg.v22.i46.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finegold S.M., Molitoris D., Song Y., et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35(Suppl 1):S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 41.Frye R.E., Rose S., Slattery J., MacFabe D.F. Gastrointestinal dysfunction in autism spectrum disorder: the role of the mitochondria and the enteric microbiome. Microb Ecol Health Dis. 2015;26:27458. doi: 10.3402/mehd.v26.27458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altieri L., Neri C., Sacco R., et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers. 2011;16(3):252–260. doi: 10.3109/1354750X.2010.548010. [DOI] [PubMed] [Google Scholar]
- 43.Persico A.M., Napolioni V. Urinary p-cresol in autism spectrum disorder. Neurotoxicol Teratol. 2013;36:82–90. doi: 10.1016/j.ntt.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Sandler R.H., Finegold S.M., Bolte E.R., et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15(7):429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 45.Xu M., Xu X., Li J., Li F. Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front Psychiatr. 2019;10:473. doi: 10.3389/fpsyt.2019.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4(1):42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding H.T., Taur Y., Walkup J.T. Gut microbiota and autism: key concepts and findings. J Autism Dev Disord. 2017;47(2):480–489. doi: 10.1007/s10803-016-2960-9. [DOI] [PubMed] [Google Scholar]
- 48.Kantarcioglu A.S., Kiraz N., Aydin A. Microbiota-gut-brain Axis: yeast species isolated from stool samples of children with suspected or diagnosed autism spectrum disorders and in vitro susceptibility against nystatin and fluconazole. Mycopathologia. 2016;181(1-2):1–7. doi: 10.1007/s11046-015-9949-3. [DOI] [PubMed] [Google Scholar]
- 49.Strati F., Cavalieri D., Albanese D., et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5(1):24. doi: 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burrus C.J. A biochemical rationale for the interaction between gastrointestinal yeast and autism. Med Hypotheses. 2012;79(6):784–785. doi: 10.1016/j.mehy.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 51.Finegold S.M., Dowd S.E., Gontcharova V., et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16(4):444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Iglesias-Vázquez L., Van Ginkel Riba G., Arija V., Canals J. Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients. 2020;12(3):792. doi: 10.3390/nu12030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golubeva A.V., Joyce S.A., Moloney G., et al. Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine. 2017;24:166–178. doi: 10.1016/j.ebiom.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang D.W., Park J.G., Ilhan Z.E., et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimmura C., Suda S., Tsuchiya K.J., et al. Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0025340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macfabe D.F. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012;23:19260. doi: 10.3402/mehd.v23i0.19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forsythe P., Bienenstock J., Kunze W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 58.Van de Wouw M., Boehme M., Lyte J.M., et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596(20):4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57(8):2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 60.Thomas R.H., Meeking M.M., Mepham J.R., et al. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation. 2012;9:153. doi: 10.1186/1742-2094-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wajner M., Latini A., Wyse A.T., Dutra-Filho C.S. The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis. 2004;27(4):427–448. doi: 10.1023/B:BOLI.0000037353.13085.e2. [DOI] [PubMed] [Google Scholar]
- 62.Shultz S.R., MacFabe D.F. In: Comprehensive Guide to Autism. Patel V.B., Preedy V.R., Martin C.R., editors. Springer New York; New York, NY: 2014. Propionic acid animal model of autism; pp. 1755–1778. [Google Scholar]
- 63.Patel P., Nankova B.B., LaGamma E.F. Butyrate, a gut-derived environmental signal, regulates tyrosine hydroxylase gene expression via a novel promoter element. Brain Res Dev Brain Res. 2005;160(1):53–62. doi: 10.1016/j.devbrainres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Xiong X., Liu D., Wang Y., Zeng T., Peng Y. Urinary 3-(3-Hydroxyphenyl)-3-hydroxypropionic acid, 3-hydroxyphenylacetic acid, and 3-hydroxyhippuric acid are elevated in children with autism spectrum disorders. BioMed Res Int. 2016;2016:9485412. doi: 10.1155/2016/9485412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabit H., Tombuloglu H., Rehman S., et al. Gut microbiota metabolites in autistic children: an epigenetic perspective. Heliyon. 2021;7(1) doi: 10.1016/j.heliyon.2021.e06105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Angelis M., Piccolo M., Vannini L., et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boccuto L., Chen C.F., Pittman A.R., et al. Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol Autism. 2013;4(1):16. doi: 10.1186/2040-2392-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Gevi F., Zolla L., Gabriele S., Persico A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol Autism. 2016;7:47. doi: 10.1186/s13229-016-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alzghoul L., Abdelhamid S.S., Yanis A.H., Qwaider Y.Z., Aldahabi M., Albdour S.A. The association between levels of inflammatory markers in autistic children compared to their unaffected siblings and unrelated healthy controls. Turk J Med Sci. 2019;49(4):1047–1053. doi: 10.3906/sag-1812-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lungba R.M., Khan S.Z.A., Ajibawo-Aganbi U., et al. The role of the gut microbiota and the immune system in the development of autism. Cureus. 2020;12(10):e11226. doi: 10.7759/cureus.11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yarandi S.S., Peterson D.A., Treisman G.J., Moran T.H., Pasricha P.J. Modulatory effects of gut microbiota on the central nervous system: how gut could play a role in neuropsychiatric health and diseases. J Neurogastroenterol Motil. 2016;22(2):201–212. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eisenstein M. Microbiome: bacterial broadband. Nature. 2016;533(7603):S104–S106. doi: 10.1038/533S104a. [DOI] [PubMed] [Google Scholar]
- 74.Cenit M.C., Sanz Y., Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. 2017;23(30):5486–5498. doi: 10.3748/wjg.v23.i30.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoban A.E., Moloney R.D., Golubeva A.V., et al. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience. 2016;339:463–477. doi: 10.1016/j.neuroscience.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Israelyan N., Margolis K.G. Reprint of: serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol Res. 2019;140:115–120. doi: 10.1016/j.phrs.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaspar P., Cases O., Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 78.Meyza K.Z., Defensor E.B., Jensen A.L., et al. The BTBR T+ tf/J mouse model for autism spectrum disorders-in search of biomarkers. Behav Brain Res. 2013;251:25–34. doi: 10.1016/j.bbr.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manev R., Manev H. Aminoglycoside antibiotics and autism: a speculative hypothesis. BMC Psychiatr. 2001;1:5. doi: 10.1186/1471-244X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghanizadeh A., Berk M. Beta-lactam antibiotics as a possible novel therapy for managing epilepsy and autism, a case report and review of literature. Iran J Child Neurol. 2015;9(1):99–102. [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson D., Letchumanan V., Thurairajasingam S., Lee L.H. A revolutionizing approach to autism spectrum disorder using the microbiome. Nutrients. 2020;12(7):1983. doi: 10.3390/nu12071983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tochitani S., Ikeno T., Ito T., Sakurai A., Yamauchi T., Matsuzaki H. Administration of non-absorbable antibiotics to pregnant mice to perturb the maternal gut microbiota is associated with alterations in offspring behavior. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0138293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang D.W., Adams J.B., Gregory A.C., et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang D.W., Adams J.B., Coleman D.M., et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9(1):5821. doi: 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frye R.E., Slattery J., MacFabe D.F., et al. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis. 2015;26:26878. doi: 10.3402/mehd.v26.26878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jonkers D., Penders J., Masclee A., Pierik M. Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs. 2012;72(6):803–823. doi: 10.2165/11632710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Critchfield J.W., van Hemert S., Ash M., Mulder L., Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract. 2011;2011:161358. doi: 10.1155/2011/161358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kałużna-Czaplińska J., Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition. 2012;28(2):124–126. doi: 10.1016/j.nut.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Cekici H., Sanlier N. Current nutritional approaches in managing autism spectrum disorder: a review. Nutr Neurosci. 2019;22(3):145–155. doi: 10.1080/1028415X.2017.1358481. [DOI] [PubMed] [Google Scholar]
- 90.Sivamaruthi B.S., Suganthy N., Kesika P., Chaiyasut C. The role of microbiome, dietary supplements, and probiotics in autism spectrum disorder. Int J Environ Res Publ Health. 2020;17(8):2647. doi: 10.3390/ijerph17082647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lord C., Risi S., Lambrecht L., et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 92.Park H.S., Yi S.Y., Yoon S.A., Hong S.B. Comparison of the autism diagnostic observation schedule and childhood autism rating Scale in the diagnosis of autism spectrum disorder: a preliminary study. Soa Chongsonyon Chongsin Uihak. 2018;29(4):172–177. doi: 10.5765/jkacap.180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ng Q.X., Peters C., Ho C.Y.X., Lim D.Y., Yeo W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord. 2018;228:13–19. doi: 10.1016/j.jad.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 94.Ng Q.X., Loke W., Venkatanarayanan N., Lim D.Y., Soh A.Y.S., Yeo W.S. A systematic review of the role of prebiotics and probiotics in autism spectrum disorders. Medicina (Kaunas). 2019;55(5):129. doi: 10.3390/medicina55050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dinan T.G., Cryan J.F. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Srikantha P., Mohajeri M.H. The possible role of the microbiota-gut-brain-Axis in autism spectrum disorder. Int J Mol Sci. 2019;20(9) doi: 10.3390/ijms20092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davani-Davari D., Negahdaripour M., Karimzadeh I., et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3) doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grimaldi R., Gibson G.R., Vulevic J., et al. A prebiotic intervention study in children with autism spectrum disorders (ASDs) Microbiome. 2018;6(1):133. doi: 10.1186/s40168-018-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanctuary M.R., Kain J.N., Chen S.Y., et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patusco R., Ziegler J. Role of probiotics in managing gastrointestinal dysfunction in children with autism spectrum disorder: an update for practitioners. Adv Nutr. 2018;9(5):637–650. doi: 10.1093/advances/nmy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Santocchi E., Guiducci L., Fulceri F., et al. Gut to brain interaction in Autism Spectrum Disorders: a randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatr. 2016;16:183. doi: 10.1186/s12888-016-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.El-Ansary A., Bacha A.B., Bjørklund G., et al. Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab Brain Dis. 2018;33(4):1155–1164. doi: 10.1007/s11011-018-0212-8. [DOI] [PubMed] [Google Scholar]
- 103.Shaaban S.Y., El Gendy Y.G., Mehanna N.S., et al. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci. 2018;21(9):676–681. doi: 10.1080/1028415X.2017.1347746. [DOI] [PubMed] [Google Scholar]
- 104.Arnold L.E., Luna R.A., Williams K., et al. Probiotics for gastrointestinal symptoms and quality of life in autism: a placebo-controlled pilot trial. J Child Adolesc Psychopharmacol. 2019;29(9):659–669. doi: 10.1089/cap.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parracho H., Gibson G., Knott F., Bosscher D., Kleerebezem M., McCartney A. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int J Probiotics. 2010;5:69–74. [Google Scholar]
- 106.Pärtty A., Kalliomäki M., Wacklin P., Salminen S., Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res. 2015;77(6):823–828. doi: 10.1038/pr.2015.51. [DOI] [PubMed] [Google Scholar]
- 107.Vulevic J., Tzortzis G., Juric A., Gibson G.R. Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neuro Gastroenterol Motil. 2018;30(11) doi: 10.1111/nmo.13440. [DOI] [PubMed] [Google Scholar]
- 108.Szklany K., Wopereis H., de Waard C., et al. Supplementation of dietary non-digestible oligosaccharides from birth onwards improve social and reduce anxiety-like behaviour in male BALB/c mice. Nutr Neurosci. 2020;23(11):896–910. doi: 10.1080/1028415X.2019.1576362. [DOI] [PubMed] [Google Scholar]
- 109.Pennesi C.M., Klein L.C. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: based on parental report. Nutr Neurosci. 2012;15(2):85–91. doi: 10.1179/1476830512Y.0000000003. [DOI] [PubMed] [Google Scholar]
- 110.Elder J.H., Shankar M., Shuster J., Theriaque D., Burns S., Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord. 2006;36(3):413–420. doi: 10.1007/s10803-006-0079-0. [DOI] [PubMed] [Google Scholar]
- 111.Millward C., Ferriter M., Calver S., Connell-Jones G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD003498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taha Z., Abdalhai K.A. A review of the efficacy of the dietary intervention in autism spectrum disorder. Open Access Maced J Med Sci. 2021;9(F):88–94. [Google Scholar]
- 113.Karhu E., Zukerman R., Eshraghi R.S., et al. Nutritional interventions for autism spectrum disorder. Nutr Rev. 2020;78(7):515–531. doi: 10.1093/nutrit/nuz092. [DOI] [PubMed] [Google Scholar]
- 114.Knivsberg A.M., Reichelt K.L., Høien T., Nødland M. A randomised, controlled study of dietary intervention in autistic syndromes. Nutr Neurosci. 2002;5(4):251–261. doi: 10.1080/10284150290028945. [DOI] [PubMed] [Google Scholar]
- 115.Ruskin D.N., Svedova J., Cote J.L., et al. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Newell C., Bomhof M.R., Reimer R.A., Hittel D.S., Rho J.M., Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. 2016;7(1):37. doi: 10.1186/s13229-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Evangeliou A., Vlachonikolis I., Mihailidou H., et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol. 2003;18(2):113–118. doi: 10.1177/08830738030180020501. [DOI] [PubMed] [Google Scholar]
- 118.Chang J.P., Su K.P. Nutritional neuroscience as mainstream of psychiatry: the evidence-based treatment guidelines for using omega-3 fatty acids as a new treatment for psychiatric disorders in children and adolescents. Clin Psychopharmacol Neurosci. 2020;30(4):469. doi: 10.9758/cpn.2020.18.4.469. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng Y.S., Tseng P.T., Chen Y.W., et al. Supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in children with autism spectrum disorders: a meta-analysis of randomized controlled trials. Neuropsychiatric Dis Treat. 2017;13:2531. doi: 10.2147/NDT.S147305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Costantini L., Molinari R., Farinon B., Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18(12) doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng B., Zhu J., Yang T., et al. Vitamin A deficiency increases the risk of gastrointestinal comorbidity and exacerbates core symptoms in children with autism spectrum disorder. Pediatr Res. 2021;89(1):211–216. doi: 10.1038/s41390-020-0865-y. [DOI] [PubMed] [Google Scholar]
- 122.Qiu S., Li L., Weeber E.J., May J.M. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res. 2007;85(5):1046–1056. doi: 10.1002/jnr.21204. [DOI] [PubMed] [Google Scholar]
- 123.Altun H., Kurutaş E.B., Şahin N., Güngör O., Fındıklı E. The levels of vitamin D, vitamin D receptor, homocysteine and complex B vitamin in children with autism spectrum disorders. Clin Psychopharmacol Neurosci. 2018;16(4):383–390. doi: 10.9758/cpn.2018.16.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coleman M., Blass J.P. Autism and lactic acidosis. J Autism Dev Disord. 1985;15(1):1–8. doi: 10.1007/BF01837894. [DOI] [PubMed] [Google Scholar]
- 125.Gottschall E. Digestion-gut-autism connection: the specific carbohydrate diet. Med. Veritas J. Med. Truth. 2004;1:261–271. [Google Scholar]
- 126.Ristori M.V., Quagliariello A., Reddel S., et al. Autism, gastrointestinal symptoms and modulation of gut microbiota by nutritional interventions. Nutrients. 2019;11(11) doi: 10.3390/nu11112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sultan M.T., Butt M.S., Qayyum M.M., Suleria H.A. Immunity: plants as effective mediators. Crit Rev Food Sci Nutr. 2014;54(10):1298–1308. doi: 10.1080/10408398.2011.633249. [DOI] [PubMed] [Google Scholar]
- 128.Qu W., Liu S., Zhang W., et al. Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress-induced depression-like behaviors: intestinal microbiota and gut microbiome function. Food Funct. 2019;10(9):5886–5897. doi: 10.1039/c9fo00399a. [DOI] [PubMed] [Google Scholar]
- 129.Liu J., Zhang M., Kong X.J.N.A. Gut microbiome and autism: recent advances and future perspectives. N A J Med Sci. 2016;9(3):104–115. jom,science. [Google Scholar]
- 130.Zhang Y., Hu N., Cai Q., et al. Treatment with the traditional Chinese medicine BuYang HuanWu Tang induces alterations that normalize the microbiome in ASD patients. Biosci Microbiota Food Health. 2020;39(3):109–116. doi: 10.12938/bmfh.2019-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bhandari R., Paliwal J.K., Kuhad A. Dietary phytochemicals as neurotherapeutics for autism spectrum disorder: plausible mechanism and evidence. Adv Neurobiol. 2020;24:615–646. doi: 10.1007/978-3-030-30402-7_23. [DOI] [PubMed] [Google Scholar]
- 132.Cho K-h, Kim T.-H., Jin C., Lee J.E., Kwon S. The literary trends of herbal prescription ukgan-san and its application in modern traditional Korean medicine. J Korean Med. 2018;39(3):17–27. [Google Scholar]
- 133.Wake R., Miyaoka T., Furuya M., Hashioka S., Horiguchi J. Effects of yokukansan, a Japanese kampo medicine for symptoms associated autism spectrum disorder. CNS Neurol Disord - Drug Targets. 2016;15(5):551–563. doi: 10.2174/1871527315666160413120541. [DOI] [PubMed] [Google Scholar]
- 134.Wake R., Miyaoka T., Inagaki T., et al. Yokukansan (TJ-54) for irritability associated with pervasive developmental disorder in children and adolescents: a 12-week prospective, open-label study. J Child Adolesc Psychopharmacol. 2013;23(5):329–336. doi: 10.1089/cap.2012.0108. [DOI] [PubMed] [Google Scholar]
- 135.Sharma B., Chouhan K. Prevention & management of autism-an ayurvedic perspective. Journal of research in traditional medicine. J Res Tradit Med. 2016;2:117–121. [Google Scholar]
- 136.Shamsi Y., Nikhat S., Mukhgerjee A., Gombar V., Sinha S. Role of Unani neuroprotective herbal drugs in the management of autism. Int J Res & Rev. 2019;6:12–20. [Google Scholar]
- 137.Amin M., Khikmawati N.H., Suryadi, et al. Chemical interaction analysis of L-Theanine compounds from Camellia sinensis L. with kainate glutamate receptors and their toxicity effect as anti autism candidates based on in silico. AIP Conf Proc. 2020;2237(1) [Google Scholar]
- 138.Mondal T.K. In: Wild Crop Relatives: Genomic and Breeding Resources: Plantation and Ornamental Crops. Kole C., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2011. Camellia; pp. 15–39. [Google Scholar]
- 139.Namita P., Mukesh R., Vijay K. Camellia sinensis (green tea): a review. Global J Pharmacol. 2012;6:52–59. [Google Scholar]
- 140.Urdaneta K.E., Castillo M.A., Montiel N., Semprún-Hernández N., Antonucci N., Siniscalco D. Autism spectrum disorders: potential neuro-psychopharmacotherapeutic plant-based drugs. Assay Drug Dev Technol. 2018;16(8):433–444. doi: 10.1089/adt.2018.848. [DOI] [PubMed] [Google Scholar]
- 141.Nair H.H., Alex V.V., Anto R.J. In: Evolutionary Diversity as a Source for Anticancer Molecules. Srivastava A.K., Kannaujiya V.K., Singh R.K., Singh D., editors. Academic Press; 2021. 14 - significance of nutraceuticals in cancer therapy; pp. 309–321. [Google Scholar]
- 142.Sharma R.A., Gescher A.J., Steward W.P. Curcumin: the story so far. Eur J Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 143.Mao Q.Q., Xu X.Y., Cao S.Y., et al. Bioactive compounds and bioactivities of ginger (zingiber officinale roscoe) Foods. 2019;8(6) doi: 10.3390/foods8060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Farombi E.O., Abolaji A.O., Adetuyi B.O., Awosanya O., Fabusoro M. Neuroprotective role of 6-Gingerol-rich fraction of Zingiber officinale (Ginger) against acrylonitrile-induced neurotoxicity in male Wistar rats. J Basic Clin Physiol Pharmacol. 2018;30(3) doi: 10.1515/jbcpp-2018-0114. [DOI] [PubMed] [Google Scholar]
- 145.Golechha M., Bhatia J., Arya D.S. Hydroalcoholic extract of Emblica officinalis Gaertn. affords protection against PTZ-induced seizures, oxidative stress and cognitive impairment in rats. Indian J Exp Biol. 2010;48(5):474–478. [PubMed] [Google Scholar]
- 146.Bhandari P., Kamdod M. Emblica officinalis (Amla): a review of potential therapeutic applications. Int J Green Pharm. 2012;6:257. [Google Scholar]
- 147.Singh I., Sharma A., Jindal A., Soyal D., Goyal P. Fruit extract of emblica officinalis (amla) protects radiation induced biochemical lesions in the brain of Swiss albino mice. Ann Neurosci. 2006;13:65–71. [Google Scholar]
- 148.Arena A., Bisignano C., Stassi G., Mandalari G., Wickham M.S., Bisignano G. Immunomodulatory and antiviral activity of almond skins. Immunol Lett. 2010;132(1-2):18–23. doi: 10.1016/j.imlet.2010.04.010. [DOI] [PubMed] [Google Scholar]