Abstract
The cholesterol-lowering and immunomodulatory effects and probiotic properties of 25 lactic acid bacteria (LAB) isolated from fermented fish (pla-paeng-daeng) in Thailand were examined in the present study. Based on their phenotypic and genetic characteristics, LAB were identified as Lactiplantibacillus pentosus (Group I, 6 isolates), Lactiplantibacillus argentoratensis (Group II, 1 isolate), Limosilactobacillus fermentum (Group III, 2 isolates), Companilactobacillus pabuli (Group IV, 4 isolates), Companilactobacillus farciminis (Group V, 5 isolates), Companilactobacillus futsaii (Group VI, 6 isolates), and Enterococcus lactis (Group VII, 1 isolate). Lactiplantibacillus pentosus PD3-1 and PD9-2 and Enterococcus lactis PD3-2 exhibited bile salt hydrolase (BSH) activities. The percentage of cholesterol assimilated by all isolates ranged between 21.40 and 54.07%. Bile salt hydrolase-producing isolates tolerated acidic and bile conditions and possessed adhesion properties. They also exerted immunomodulatory effects that affected the production of interleukin-12 (IL-12), interferon-γ (IFN-γ), human β-defensin-2 (hBD-2), and nitric oxide (NO). These isolates meet standard probiotic requirements and exert beneficial effects.
Keywords: lactic acid bacteria (LAB), Thai fermented fish, cholesterol-lowering effects, immunomodulation, probiotics
Pla-paeng-daeng (fermented fish) is a typical fermented food of Southern Thailand. It is a reddish, semi-solid, whole fish or pieces of fish (Nemotolosa nasus, Ta-pian-nam-khem) with a sour (pH 3.5–4.0) and salty taste (2.3–4.0% NaCl) that is fermented for 4–5 days. Saccharomyces cerevisiae, Staphylococcus sp., Bacillus sp., Vibrio sp., and Tetragenococcus halophilus strains have been identified in fermented fish products (Phithakpol et al., 1995; Tanasupawat and Komagata, 1995). Lactic acid bacteria (LAB) contribute to the flavor, texture, and odor of food and also enhance food preservation. They are used as probiotics in several Asian fermented foods (Ngasotter et al., 2020). Some LAB are generally regarded as safe (GRAS) microorganisms (Nasrollahzadeh et al., 2022). Previous studies suggested the beneficial effects of probiotics as part of a healthy diet in addition to their health-promoting effects, such as reductions in cholesterol and immunomodulation (Ohashi and Ushida, 2009; Angmo et al., 2016).
The administration of LAB has been shown to reduce the risk of coronary heart disease (CAD) (De Vries et al., 2006). Furthermore, a slight (1%) reduction in serum cholesterol decreased the risk of CAD by 2 to 3% (Albano et al., 2018). The immunomodulatory effects of LAB have recently been attracting increasing attention. Probiotic LAB regulate immunity, exert immune-boosting effects, and are used to treat unique disorders, such as immunodeficiency and autoimmune diseases. Interferon-γ (IFN-γ) enhances a host’s defenses against intracellular infections. Interleukin-12 (IL-12) is a pro-inflammatory cytokine that plays a role in preventing infection and cancer and induces the production of IFN-γ (Thamacharoensuk et al., 2017). Previous studies demonstrated that Lactobacillus and other LAB modulated the synthesis of IL-12 and IFN-γ (Chen et al., 2013; Thamacharoensuk et al., 2017; Moon et al., 2019; Nakai et al., 2019). Defensins are human antimicrobial peptides, which play important roles in host defenses. Human β-defensin-2 (hBD-2) is activated by infection or inflammation. The expression of BD is up-regulated by several LAB strains, which may prevent infections (Kobatake and Kabuki, 2019). Nitric oxide (NO) is an endogenously synthesized molecule that plays a vital role in defenses against infection and immunomodulation (Wang et al., 2009). LAB-induced NO production has been extensively examined (Kmonickova et al., 2012; Surayot et al., 2014).
Thai traditional fermented food is an excellent source of potentially novel probiotic isolates. There is currently no information on the cholesterol-lowering and immunomodulatory effects of LAB in pla-paeng-daeng. Therefore, we herein attempted to characterize LAB from Thai fermented fish (pla-paeng-daeng) based on phenotypic and genotypic characteristics and examined their bile salt hydrolase (BSH) activities, cholesterol assimilation capacities, immunomodulatory effects, and probiotic properties.
Materials and Methods
Sources and isolation
Twelve samples of fermented fish (pla-paeng-daeng) were collected from various local markets in the southern part of Thailand (Table 1). Twenty-five grams or 25 mL of each sample was enriched in 225 mL of MRS broth (de Man, Rogosa and Sharpe; Difco) (De Man et al., 1960) and incubated at 30°C for 72 h. One loopful of the culture broth was then streaked over MRS agar supplemented with 0.3% (w/v) CaCO3 and incubated under the same conditions. Colonies surrounded by a clear zone were selected for purification. Pure cultures were stored at –20°C in 40% (v/v) glycerol and lyophilized with 10% (w/v) skim milk. The number of viable LAB in samples was assessed by counting with colony-forming units on MRS agar plates as described above.
Table 1.
Sample number, province, total count, and isolate number of LAB from fermented fish.
| Sample no. | Province | LAB count (CFU g–1) | Isolate no. | Number of isolates |
|---|---|---|---|---|
| PD1 | Nakhon Si Thammarat | 1.1×108 | PD1-1, PD1-2 | 2 |
| PD2 | Nakhon Si Thammarat | 5.7×108 | PD2-1, PD2-2 | 2 |
| PD3 | Nakhon Si Thammarat | 1.2×108 | PD3-1, PD3-2 | 2 |
| PD4 | Nakhon Si Thammarat | 2.2×107 | PD4-1, PD4-2 | 2 |
| PD5 | Nakhon Si Thammarat | 1.2×108 | PD5-1, PD5-2 | 2 |
| PD6 | Nakhon Si Thammarat | 2.3×107 | PD6-1, PD6-2, PD6-3 | 3 |
| PD7 | Nakhon Si Thammarat | 1.6×104 | PD7-1, PD7-2 | 2 |
| PD8 | Songkhla | 1.4×106 | PD8-1, PD8-2 | 2 |
| PD9 | Songkhla | 1.3×108 | PD9-1, PD9-2 | 2 |
| PD10 | Songkhla | 4.3×105 | PD10-1, PD10-2 | 2 |
| PD11 | Satul | 2.2×109 | PD11-1, PD11-2 | 2 |
| PD12 | Satul | 1.4×107 | PD12-1, PD12-2 | 2 |
| Total | 25 |
Identification methods
Phenotypic characterization
After incubation on MRS agar plates at 30°C for 48 h, colony appearance, cell shape, cell organization, catalase activity, and Gram staining were examined. The following physiological and biochemical characteristics were assessed according to the methods described by Tanasupawat et al. (1998): growth in 2, 4, 6, and 8% (w/v) NaCl, growth at temperatures of 15, 30, and 45°C, growth at pH 3.0, 6.0, and 9.0, nitrate reduction, gas production, aesculin hydrolysis, arginine hydrolysis, and acid production from carbohydrates. A hierarchical cluster analysis to group isolates using SPSS version 22.0 was performed based on phenotypic characteristics.
Genotypic characterization
The 16S rRNA gene sequences of isolates were amplified by PCR as previously described by Phuengjayaem et al. (2017). PCR products were sequenced using a DNA sequencer (Macrogen) with universal primers, as reported by Lane (1991). Sequence similarity values between the isolates and their related reference isolates were calculated using the EzBiocloud tool (Yoon et al., 2017). MEGA 7 constructed a phylogenetic tree using the neighbor-joining (NJ) approach (Saitou and Nei, 1987; Kumar et al., 2016). A bootstrap analysis with 1,000 replicates was used to assess the confidence values for each branch in the phylogenetic tree (Felsenstein, 1985). The sequences identified were deposited in the DNA Data Bank of Japan (DDBJ, Mishima, Japan) and accession numbers in the DDBJ database are shown in Table 2.
Table 2.
Isolate number, nearest relatives, 16S rRNA gene sequence similarity (%), cholesterol assimilation, and BSH activity of isolates.
| Isolate no. | Nearest relatives | Similarity (%) | Length (bp) | Accession no. | Cholesterol assimilation (%) | BSH activity |
|---|---|---|---|---|---|---|
| Group I | ||||||
| PD3-1 | Lactiplantibacillus pentosus DSM 20314T | 100 | 1,353 | LC706744 | 28.07±5.03 | + |
| PD6-2 | Lactiplantibacillus pentosus DSM 20314T | 100 | 1,410 | LC706746 | 43.40±10.39 | – |
| PD11-1 | Lactiplantibacillus pentosus DSM 20314T | 99.71 | 1,376 | LC706745 | 29.40±4.00 | – |
| PD8-1 | ND | ND | ND | 26.07±8.08 | – | |
| PD9-2 | Lactiplantibacillus pentosus DSM 20314T | 100 | 1,354 | LC706743 | 27.40±2.00 | + |
| PD6-1 | Lactiplantibacillus pentosus DSM 20314T | 100 | 1,368 | LC706742 | 45.40±5.29 | – |
| Group II | ||||||
| PD9-1 | Lactiplantibacillus argentoratensis DSM 16365T | 99.85 | 1,356 | LC706741 | 47.40±8.72 | – |
| Group III | ||||||
| PD10-1 | Limosilactobacillus fermentum CECT 562T | 99.49 | 1,369 | LC706738 | 48.07±4.16 | – |
| PD8-2 | ND | ND | ND | 46.73±4.16 | – | |
| Group IV | ||||||
| PD12-2 | Companilactobacillus pabuli NFFJ11T | 99.71 | 1,356 | LC706740 | 46.07±5.03 | – |
| PD7-1 | Companilactobacillus pabuli NFFJ11T | 99.71 | 1,370 | LC706739 | 46.73±6.11 | – |
| PD6-3 | Companilactobacillus pabuli NFFJ11T | 99.63 | 1,343 | LC706622 | 54.07±13.32 | – |
| PD4-2 | ND | ND | ND | 45.40±6.00 | – | |
| Group V | ||||||
| PD11-2 | Companilactobacillus farciminis KCTC 3681T | 99.85 | 1,354 | LC706629 | 21.40±4.00 | – |
| PD12-1 | Companilactobacillus farciminis KCTC 3681T | 99.85 | 1,368 | LC706628 | 27.40±3.46 | – |
| PD5-2 | Companilactobacillus farciminis KCTC 3681T | 99.85 | 1,368 | LC706627 | 46.07±4.16 | – |
| PD7-2 | Companilactobacillus farciminis KCTC 3681T | 99.77 | 1,323 | LC706626 | 32.07±3.06 | – |
| PD10-2 | ND | ND | ND | 40.07±9.87 | – | |
| Group VI | ||||||
| PD5-1 | Companilactobacillus futsaii JCM 17355T | 100 | 1,369 | LC706624 | 32.73+4.62 | – |
| PD4-1 | ND | ND | ND | 25.40±8.00 | – | |
| PD1-1 | Companilactobacillus futsaii JCM 17355T | 100 | 1,353 | LC706625 | 45.40±2.00 | – |
| PD1-2 | ND | ND | ND | 40.07±9.02 | – | |
| PD2-2 | Companilactobacillus futsaii JCM 17355T | 100 | 1,356 | LC706623 | 34.07+5.03 | – |
| PD2-1 | ND | ND | ND | 26.73±5.03 | – | |
| Group VII | ||||||
| PD3-2 | Enterococcus lactis BT159T | 99.54 | 1,313 | LC706621 | 32.40±9.17 | + |
Data on cholesterol assimilation ability are represented as the mean±SD. ND, not determined for the 16S RNA gene sequence. Bile salt hydrolase activity: +, positive reaction; –, negative reaction.
BSH activity
BSH activity was assessed with slight modifications to the method described by Shehata et al. (2016). Twenty microliters of the overnight culture broth was spotted on MRS agar supplemented with 0.037% (w/v) calcium chloride (CaCl2) and 0.5% (w/v) taurodeoxycholic acid (TDCA) (sodium salt hydrate). Plates were incubated anaerobically at 37°C for 72 h. BSH activity was indicated by the formation of halos around colonies or white opaque colonies. Non-modified MRS served as the control. BSH-producing LAB were selected to assess probiotic characteristics.
Cholesterol assimilation
The assimilation of cholesterol by LAB was examined in MRS broth supplemented with cholesterol-polyethylene glycol (PEG) 600 (Sigma) at a final concentration of 100 μg mL–1. Each inoculum (1% [v/v]) was inoculated into MRS-cholesterol-PEG 600 and incubated anaerobically at 37°C for 24 h. Cholesterol in MRS broth was extracted using the technique described by Tomaro-Duchesneau et al. (2014). The residual cholesterol content was measured using the modified method of Rudel and Morris (1973). The following cholesterol concentrations were used to construct a standard absorbance curve: 0.000, 3.1250, 6.250, 12.50, 25.00, 50.00, 75.00, 100.0, and 125.0 μg mL–1 in MRS. Cholesterol concentrations were compared to the standard curve constructed using the cholesterol stock solution. All experiments were performed in triplicate. The abilities of all LAB to assimilate cholesterol in MRS were shown as the percentage of cholesterol assimilated in each incubation as follows:
Evaluation of probiotic properties
Preparation of LAB cell suspensions
LAB cell suspensions were prepared as described by Pithva et al. (2014) to examine probiotic characteristics. The selected isolates were propagated twice in MRS broth at 30°C for 24 h. LAB cells were then collected by centrifugation at 9,000×g at 4°C for 10 min, washed twice with phosphate buffer (0.1 M, pH 7.2, containing 0.85% [w/v] NaCl), and resuspended in phosphate buffer (0.1 M, pH 7) to obtain a cell suspension with OD600=1 and 109 CFU mL–1.
Acid and bile tolerance
The selected LAB isolates were subjected to the acid tolerance test using a modified method of Thamacharoensuk et al. (2017). Briefly, a LAB cell suspension was inoculated into MRS broth pH 2 and 3 or supplemented with 0.3 and 0.8% (w/v) bile salt and then incubated anaerobically at 37°C for 3 h. The number of viable LAB was then quantified using a serial 10-fold dilution and the spot plate technique, as described by Whitmire and Merrell (2012). Viable bacteria were expressed as logarithms of colony-forming units per milliliter (logCFU mL–1).
Adhesion assay
The human intestinal epithelial cell line Caco-2 was used to investigate the adhesion properties of selected LAB isolates, with slight modifications to the procedure described by Han et al. (2017). Caco-2 cells were routinely grown at 37°C in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin (PS) under humidified conditions of 95% air and 5% CO2. Caco-2 cells were seeded on 24-well tissue culture plates at a concentration of 5×105 cells mL–1 for the adhesion test. Tissue plates were incubated at 37°C in a 5% CO2 incubator until Caco-2 cells developed a confluent monolayer of differentiated cells. Caco-2 cells were then washed twice in PBS. The LAB cell suspension was obtained by centrifugation at 12,100×g at 4°C for 10 min and re-suspended in DMEM without antibiotics. Each LAB cell suspension was seeded on wells and incubated at 37°C for 90 min in a 5% CO2 incubator. Caco-2 cells were washed three times with PBS after the incubation to remove unbound LAB cells. Cells were then lysed with 0.05% Triton X-100 solution. The number of adhering bacteria was counted using the spot plate method on MRS agar and then incubated at 37°C for 48 h. Lacticaseibacillus rhamnosus GG was used as the control. The adherent abilities of the selected LAB were calculated according to the following equation;
where Nt=the number of LAB cells adhering to Caco-2 cells
N0=the total number of inoculated LAB cells
Immunomodulatory effects
The immunomodulatory effects of the selected LAB were examined according to the method of Hosaka et al. (2021).
Preparation of sterilized LAB powder
Selected isolates were inoculated into MRS broth and incubated at 30°C with shaking at 120 rpm for 24 h. After sterilizing the culture medium at 100°C for 20 min, the selected isolates were collected by centrifugation at 1,000 rpm for 10 min. The selected LAB powders were washed with sterile distilled water and then lyophilized to obtain sterilized LAB powders. LAB powders were suspended in PBS at a concentration of 200 μg mL–1 to prepare test samples.
Cell culture and cell differentiation
RAW264.7 cells were cultured in DMEM supplemented with 5% FBS and 0.25% PS at 37°C in a 5% CO2 incubator. Professor Shinichi Yokota of Sapporo Medical University School of Medicine donated Caco-2 cells. Caco-2 cells were grown in DMEM supplemented with 5% FBS and 0.25% PS at 37°C in a 5% CO2 incubator. THP-1 cells were grown in RPMI 1640 media supplemented with 10% FBS and 0.20% PS at 37°C in a 5% CO2 incubator.
Caco-2 cells (1.5×105 cells) were cultivated for 72 h in cell culture inserts (24-well hanging inserts 0.4 m; Falcon). After 72 h, the solution containing 5 mM sodium butyrate was replaced, and cells were cultured for 96 h to promote differentiation. Differentiated cells were evaluated by transepithelial electrical resistance (TEER) using Millicell-ERS (Merk), and differentiated cells (TEER values >400 Ω cm2) were used. THP-1 cells were seeded on a multi-well plate (24-well; Falcon) and incubated for 72 h in media supplemented with 100 ng mL–1 cholecalciferol (Vitamin D3) and 10 nM phorbol 12-myristate13-acetate (PMA) to differentiate into macrophage-like cells. Caco-2 and THP-1 cells were then co-cultured in transwells.
Measurement of NO production
The production of NO was measured as described by Yang et al. (2018). RAW264.7 cells were suspended in DMEM medium (5% FBS+0.2% PS) at a concentration of 3×105 cells mL–1, seeded on a 24-well plate, and incubated at 37°C for 24 h in a 5% CO2 incubator. Samples were examined at a final concentration of 20 μg mL–1 to stimulate cells. PBS was used as a negative control and LPS (10 μg mL–1) (Fujifilm Wako) as a positive control. After a 24-h stimulation, the supernatant was collected, centrifuged at 12,100×g for 20 min, and subjected to the Griess reaction as reported by Baek et al. (2015). One hundred microliters each of Griess reagent, medium supernatant sample, and 1.56 to 100 μM of sodium nitrite standard solution were added to 96-well microplates and incubated at room temperature for 20 min. Absorbance at 550 nm was measured by a microplate reader. The quantity of nitrite in the medium supernatant was enumerated using a calibration curve generated from sodium nitrite standard solution.
Intestinal immunity model
An in vitro intestinal immune model was generated by a co-culture of cell culture inserts (apical side) and multi-well plates (basal side). Samples were suspended in RPMI 1640 medium on the apical side (final concentration 20 μg mL–1), and cells were stimulated at 37°C for 2 days in a 5% CO2 incubator. The basal side of the medium was then collected, and after centrifugation at 12,100×g for 20 min, the supernatant was harvested to eliminate foreign compounds. Regarding IL-12 and IFN-γ, proteins were precipitated by adding a 25% volume of 100% trichloroacetic acid (TCA) to the culture medium supernatant. Precipitates were cleaned with acetone to eliminate TCA and then dissolved in 1× sample buffer for protein enrichment after a heat treatment at 100°C for 2 min. SDS-PAGE were used to separate proteins and was performed according to Laemmli (1970). Target proteins were detected by Western blotting as previously described by Towbin et al. (1979). Calibration curves were prepared with known concentrations of the IL-12 standard (Gibco) and IFN-γ standard (Gibco) to calculate the production of IL-12 and IFN-γ. Production levels were corrected by measuring β-actin as an endogenous control. Regarding hBD-2, an unenriched medium supernatant was analyzed by a dot blot, and the amount of hBD-2 produced was corrected from the amount of total protein by CBB staining. Values were evaluated relative to the non-stimulated test section with PBS.
Statistical analysis
All experiments were performed in triplicate, and the results obtained are shown as the mean±standard deviation (SD). Results on acid and bile tolerance as well as adhesion ability were examined by ANOVA using SPSS 22.0 software. Duncan’s Multiple Range Test (DMRT) was used for comparisons of mean values at a significance level of P<0.05. Immunomodulatory effects were assessed by Welch’s t-test at a significance level of P<0.05.
Results and Discussion
Isolation and identification of isolates
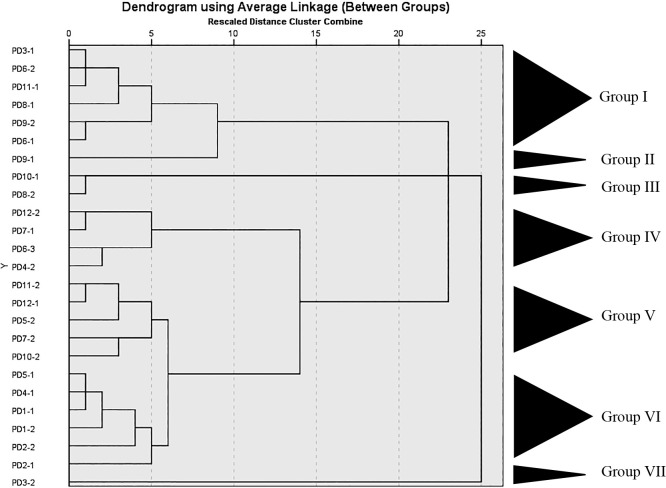
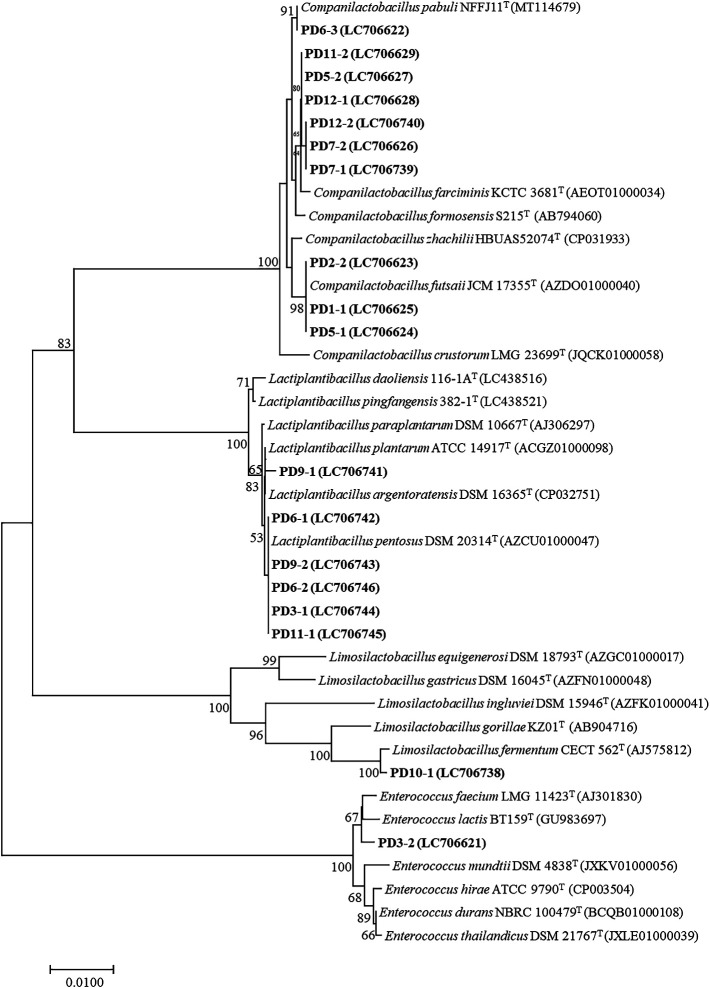
The total number of LAB detected in 6 samples collected from Nakhon Si Thammarat, 3 from Songkhla, and 2 from Satul Province ranged between 1.6×104 and 5.7×108, 4.3×105 and 1.3×108, and 1.4×107 and 2.2×109 CFU g–1, respectively. Twenty-five LAB isolates were isolated from Thai fermented fish (pla-paeng-daeng) samples based on differences in colony appearance and cell form (Table 1). All isolates were Gram-positive, catalase-negative, and facultatively anaerobic bacteria. They were members of the genera Lactiplantibacillus, Limosilactobacillus, Companilactobacillus, and Enterococcus, were divided into 7 groups according to the results of a hierarchical cluster analysis of their phenotypic characteristics, and 16S rRNA gene sequence similarities among the representative isolates were examined (Fig. 2, 3, and Table 2).
Fig. 2.
Dendrogram of hierarchical cluster-based phenotypic characteristics.
Fig. 3.
Neighbor-joining tree based on the 16S rRNA gene of representative isolates from each group.
Group I included six rod-shaped isolates (PD3-1, PD6-2, PD11-1, PD8-1, PD9-2, and PD6-1). They did not produce gas from glucose. They grew at pH 3 and 9, in 6 and 8% NaCl, and at 15°C, but not at 45°C. They reduced nitrate, but did not hydrolyze arginine. The isolates contained meso-DAP in their cell walls. They produced DL-lactic acid. Acid production from L-arabinose, D-galactose, D-melibiose, D-raffinose, and D-ribose varied. The representative isolates in this group showed 99.71 to 100% 16S rRNA gene sequence similarity (Table 2) to Lactiplantibacillus pentosus DSM 20314T (Fig. 2). Therefore, they were closely related to Lb. pentosus and their differential phenotypic characteristics are shown in Table 3. Lb. pentosus strains have mostly been found in fermented fish products (Rodpai et al., 2021; Syafitri et al., 2022).
Table 3.
Phenotypic characteristics of isolates.
| Characteristics | I | II | III | IV | V | VI | VII |
|---|---|---|---|---|---|---|---|
| No. of isolates | 6 | 1 | 2 | 4 | 5 | 6 | 1 |
| Cell shape | Rods | Rods | Rods | Rods | Rods | Rods | Cocci in chains |
| Gas from glucose | – | – | + | – | – | – | – |
| Growth in 6% NaCl | + | + | + | + | + | + | + |
| Growth in 8% NaCl | + | + | + | + | + | + | + |
| Growth at pH 3 | + | + | + | + | + | + | + |
| pH 9 | + | + | – | + | + | + | + |
| Growth at 15°C | + | + | + | + | + | + | + |
| 45°C | – | + | + | + | + | + | + |
| Arginine hydrolysis | – | – | + | + | + | +(–1) | – |
| Nitrate reduction | + | – | – | – | – | – | – |
| Acid from: | |||||||
| Aesculin | + | – | – | +(–2) | –(+1) | –(+1) | + |
| L-Arabinose | +(–2) | + | + | + | – | –(+2) | + |
| D-Cellobiose | + | + | – | – | – | – | + |
| Fructose | + | + | + | + | + | + | + |
| D-Galactose | +(–2) | + | – | + | + | –(+2) | + |
| D-Glucose | + | + | + | + | + | + | + |
| Lactose | + | + | – | – | – | –(+1) | + |
| D-Mannose | + | + | – | + | + | + | + |
| D-Maltose | + | + | + | – | –(+2) | – | + |
| D-Mannitol | + | + | – | – | – | – | + |
| D-Melibiose | +(–2) | + | + | – | – | – | + |
| D-Raffinose | +(–3) | + | – | + | – | – | – |
| L-Rhamnose | + | + | – | – | – | – | – |
| D-Ribose | +(–1) | + | + | – | – | – | + |
| Salicin | + | + | – | + | +(–2) | + | + |
| D-Sorbitol | + | + | – | + | – | – | + |
| Sucrose | + | + | + | + | –(+1) | + | – |
| D-Trehalose | + | + | + | +(–2) | - | – | – |
| D-Xylose | + | + | + | +(–2) | –(+1) | –(+1) | + |
| meso-DAP | + | + | – | – | – | – | – |
| Isomer of lactic acid | DL | DL | DL | DL | L | L | L |
+, positive reaction; –, negative reaction. Numbers in parentheses indicate the number of isolates showing the reaction.
Group II included one rod-shaped isolate (PD9-1). It did not produce gas from glucose. It grew at pH 3 and 9, 15 and 45°C, and in 6 and 8% NaCl. It contained meso-DAP in its cell walls. It produced DL-lactic acid. It did not produce acid from aesculin. It did not hydrolyze arginine. It did not reduce nitrate. The representative isolate in this group showed 99.85% 16S rRNA gene sequence similarity (Table 2) to Lactiplantibacillus argentoratensis DSM 16365T (Fig. 2). Therefore, it was identified as Lactiplantibacillus argentoratensis and its phenotypic characteristics are shown in Table 3.
Group III included two rod-shaped isolates (PD10-1 and PD8-2). They produced gas from glucose. They grew at pH 3 and in 8% NaCl, but did not grow at pH 9. These isolates did not contain meso-DAP in their cell walls. They did not reduce nitrate. They produced DL-lactic acid. They hydrolyzed arginine. Neither of the isolates produced acid from D-cellobiose, D-galactose, lactose, D-mannose, D-mannitol, D-raffinose, L-rhamnose, salicin, D-sorbitol, D-trehalose, or aesculin. The representative isolate in this group showed 99.49% 16S rRNA gene sequence similarity (Table 2) to Limosilactobacillus fermentum CECT 562T (Fig. 2). Therefore, they were identified as Limosilactobacillus fermentum and their differential phenotypic characteristics are shown in Table 3.
Group IV included four rod-shaped isolates (PD12-2, PD7-1, PD6-3, and PD4-2). They did not produce gas from glucose. They grew at pH 3, at temperatures of 9, 15 and 45°C, and in 6 and 8% NaCl. They did not reduce nitrate. The isolates did not have meso-DAP in their cell walls. They produced DL-lactic acid. They did not produce acid from D-cellobiose, lactose, D-maltose, D-mannitol, D-melibiose, L-rhamnose, D-ribose, or D-trehalose. Variable acid production from D-xylose and aesculin was noted. They hydrolyzed arginine. The representative isolates in this group showed 99.63 to 99.71% 16S rRNA gene sequence similarity (Table 2) to Companilactobacillus pabuli NFFJ11T (Fig. 2). Therefore, they were identified as Companilactobacillus pabuli and their differential phenotypic characteristics are presented in Table 3.
Group V included five rod-shaped isolates (PD11-2, PD12-1, PD5-2, PD7-2, and PD10-2). They did not produce gas from glucose. They grew at pH 3 and 9, at temperatures of 15 and 45°C, and in 6% and 8% NaCl. They hydrolyzed arginine. They did not reduce nitrate. The isolates did not have meso-DAP in their cell walls. They produced L-lactic acid. They did not produce acid from L-arabinose, D-cellobiose, lactose, D-mannitol, D-melibiose, D-raffinose, L-rhamnose, D-ribose, D-sorbitol, D-trehalose, or D-xylose. Acid production from D-maltose, salicin, sucrose, and aesculin varied. The representative isolates in this group showed 99.77% to 99.85% 16S rRNA gene sequence similarity (Table 2) to Companilactobacillus farciminis KCTC 3681T (Fig. 2). Therefore, they were identified as Companilactobacillus farciminis and their differential phenotypic characteristics are shown in Table 3.
Group VI included six rod-shaped isolates (PD5-1, PD4-1, PD1-1, PD1-2, PD2-2, and PD2-1). They did not produce gas from glucose. They grew at pH 3 and 9, at temperatures of 15 and 45°C, and in 6 and 8% NaCl. They variably hydrolyzed arginine. The isolates did not have meso-DAP in their cell walls. They produced L-lactic acid. They did not reduce nitrate. None of the isolates produced acid from D-cellobiose, D-maltose, D-mannitol, D-melibiose, D-raffinose, L-rhamnose, D-ribose, D-sorbitol, D-trehalose, or D-xylose. Acid production from L-arabinose, D-galactose, lactose, and aesculin varied. Representative isolates showed 100% 16S rRNA gene sequence similarity (Table 2) to Companilactobacillus futsaii JCM 17355T (Fig. 2). Therefore, they were identified as Companilactobacillus futsaii and their differential phenotypic characteristics are shown in Table 3.
Group VII included one coccal isolate (PD3-2). It did not produce gas from glucose. It grew at pH 3 and 9, at temperatures of 15 and 45°C, and in 6 and 8% NaCl. It hydrolyzed arginine. The isolate did not have meso-DAP in its cell walls. Nitrate reduction was not observed. It produced L-lactic acid. The isolate did not produce acid from D-raffinose, L-rhamnose, D-sorbitol, sucrose, or D-xylose. The representative isolate PD3-2 showed 99.54% 16S rRNA gene sequence similarity (Table 2) to Enterococcus lactis BT159T (Fig. 2). Therefore, it was identified as En. Lactis and its differential phenotypic characteristics are shown in Table 3.
Based on the results of phenotypic characteristics, the hierarchical cluster analysis, and 16S rRNA gene sequences, LAB belonged to the genus Companilactobacillus, followed by Lactiplantibacillus, Limosilactobacillus, and Enterococcus; they were identified as Companilactobacillus futsaii, Companilactobacillus farciminis, Companilactobacillus pabuli, Lb. pentosus, Lactiplantibacillus argentoratensis, Limosilactobacillus fermentum, and En. lactis. Microbial distribution is influenced by several factors, such as the type of fish, other ingredients, the source of fishes and ingredients, the fermentation time, and processing conditions. Traditional fish fermentation depends on spontaneous fermentation started by naturally occurring microorganisms, primarily LAB, which are present in the ingredients, in the processing facilities, and in the surrounding environment as natural starters (Valyasevi and Rolle, 2002; Visessanguan et al., 2004; Ngasotter et al., 2020). Fermented fish were collected from different provinces in Thailand, including the central part, Bangkok and Nonthaburi; the northeastern part, Ubon Ratchathani, Surin, and Chaiyaphum; and the northern part, Nakhonsawan, Chiangmai, and Chiangrai. Isolates of Lb. farciminis was obtained from pla-ra, pla-chom, kung-chom, and hoi-dong, which contained high concentrations of NaCl (>8%), isolates of Lactobacillus sp. were only found in pla-ra and pla-chom, and isolates of Lb. pentosus and Lb. plantarum were detected in pla-chom and kung-chom, which contained less NaCl (<8%) (Tanasupawat et al., 1998). Lb. brevis, Lb. casei, Lb. curvatus, Lb. farciminis, Lb. pentosus, Lb. plantarum, Lc. lactis, and Leuconostoc spp. were detected in som-fak, pla-ra, and pla-chom collected in the Lopburi and Bangkok provinces (Paludan-Müller et al., 1999; Ngasotter et al., 2020). LAB primarily contribute to sensorial properties via acidification (Huang et al., 2021). In addition, LAB metabolism prevents the growth of pathogenic and spoilage microflora and contributes to color stabilization and texture improvements (Fadda et al., 2010). Lactobacillus isolates are often found in fermented fish products (Syafitri et al., 2022). Enterococcus spp. may also be isolated and contribute to the development of organoleptic profiles (Comi et al., 2005).
Therefore, several isolates of Lb. pentosus (Group I) and Companilactobacillus farciminis (=Lb. farciminis, Group V) were isolated from the Nakhon Si Thammarat, Satul, and Songkhla provinces, whereas Companilactobacillus pabuli (Group IV) was isolated from the Nakhon Si Thammarat and Satul provinces. However, Companilactobacillus futsaii (Group VI) and En. lactis (VII) were only found in the Nakhon Si Thammarat province. Lactiplantibacillus argentoratensis (Group II) and Limosilactobacillus fermentum (=Lb. fermentum, Group III) were isolated from the Songkhla province. Since each province has specific raw materials and fermentative procedures, the distribution and viability of LAB differed, as shown in Table 1.
BSH activity
BSH activity is associated with reductions in cholesterol and is also recognized as an additional criterion for the selection of probiotics (Miremadi et al., 2014). By deconjugating bile salts, BSH activity enhances bacterial growth and colonization in the gut (Begley et al., 2006). Among 3 isolates, PD3-1 and PD9-2 exhibited BSH activity by developing opaque white colonies, whereas PD3-2 created halos around colonies (Table 2). The formation of opaque white colonies or bile acid precipitates around colonies is considered to reflect BSH activity (Dashkevicz and Feighner, 1989; Jayashree et al., 2014). Therefore, BSH-producing isolates were selected to examine probiotic characteristics. These BSH-positive isolates were identified as Lb. pentosus PD3-1 and PD9-2 (100% similarity) and En. lactis PD3-2 (99.54% similarity). BSH activity by probiotic LAB contributes to reductions in serum cholesterol levels and increases resistance to bile salts (Noriega et al., 2006). Based on the screening, the findings are consistent with previous studies (Abushelaibi et al., 2017; Liu et al., 2017), which showed that the hypocholesterolemic effects of LAB reduced serum cholesterol levels in an in vivo model and LAB isolated from food products exhibited BSH activity. The present results demonstrated that BSH-producing isolates were also present in non-human sources.
Cholesterol assimilation
Hypercholesterolemia is a cause of cardiovascular disease (CVD), the leading cause of mortality (Labarthe and Dunbar, 2012). Therefore, reductions in serum cholesterol levels are vital for the prevention of CVD. In the present study, the percentage of cholesterol assimilation by all isolates ranged between 21.40 and 54.07% (Table 2). The percentage of cholesterol assimilation by only one isolate was higher than 50%, namely, Companilactobacillus pabuli isolate PD6-3 at 54.07%.
The amount of cholesterol assimilated markedly differed among isolates. The ability to assimilate cholesterol in the present study was consistent with previous studies (Miremadi et al., 2014; Tomaro-Duchesneau et al., 2014; Shehata et al., 2016), which reported the cholesterol assimilation ability of LAB and variations in this ability. Cholesterol assimilation and BSH activity are cholesterol removal mechanisms and desirable characteristics for probiotics (Ishimwe et al., 2015). Since LAB probiotics consume cholesterol for metabolism, luminal cholesterol levels accessible for absorption are reduced (Bordoni et al., 2013).
Acid and bile tolerance
One of the essential properties of probiotic bacteria is acid and bile tolerance, which influences their capacity to exist in the acidic stomach and small intestine and, thus, their ability to accomplish their functional activity as a probiotic (Tannock, 2004; Ruiz et al., 2013). The effects of acidic and bile conditions on the survival of selected LAB are shown in Table 4. Based on BSH-positive activity, all BSH-positive isolates were selected to assess acid and bile tolerance. Under acidic conditions, the selected isolates tolerated pH 2 and 3. The viability of all isolates was significantly lower than that of the MRS control (24.20–38.39% reduction). This result is consistent with previous findings (Hassanzadazar et al., 2012), showing that acidic pH environments limit metabolism by and decrease the growth and viability of LAB. Resistance at pH 3 was previously established as a criterion for the acid tolerance of probiotics (Liong and Shah, 2005).
Table 4.
Survival of selected isolates after an incubation at various pH and % bile for 3 h.
| Isolate no. | Number of bacteria (logCFU mL–1) | % Reductionb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRSa | pH 2 | pH 3 | 0.3% Bile | 0.8% Bile | pH 2 | pH 3 | 0.3% Bile | 0.8% Bile | ||
| PD3-2 | 8.18±0.09 | 5.52±0.07* | 6.15±0.07* | 9.22±0.04* | 9.11±0.03* | 32.52 | 24.82 | +12.71 | +11.37 | |
| PD3-1 | 8.18±0.15 | 5.80±0.18* | 6.02±0.21* | 8.77±0.07* | 8.52±0.07* | 29.10 | 26.41 | +7.21 | +4.16 | |
| PD9-2 | 9.17±0.08 | 5.65±0.16* | 6.95±0.31* | 7.62±0.15* | 7.52±0.07* | 38.39 | 24.20 | 16.90 | 17.99 | |
Data are expressed as the mean±SD.
*P<0.05, significantly different from the negative control.
a MRS was used as a negative control.
b Percent reduction in the bacterial number relative to the negative control; +, indicates enhanced bacterial viability.
In bile conditions, all isolates survived in the presence of different percentages of bile salts with varying degrees of viability (Table 4). The viability of isolates significantly differed from that of the MRS control. The viability of isolate PD9-2 decreased in the presence of 0.3 and 0.8% bile salts (16.90 and 17.99% reductions, respectively). Conversely, the viabilities of En. lactis PD3-2 and Lb. pentosus PD3-1 increased (+4.16-12.71% reduction) with significant differences from the MRS control. This result is in accordance with previous findings (Thamacharoensuk et al., 2017), which demonstrated that high pH environments and bile salts supported the viability of some LAB isolates, but also limited the viability of other LAB isolates. Therefore, LAB in the present study survived and propagated under acidic and bile conditions, and bile salts may enhance the viability of some LAB.
Due to their resistance to acidic conditions and bile salts, these isolates may survive in the stomach and intestines or even compete with other bacterial groups and colonize the gastrointestinal tract (GIT), indicating good probiotic potential.
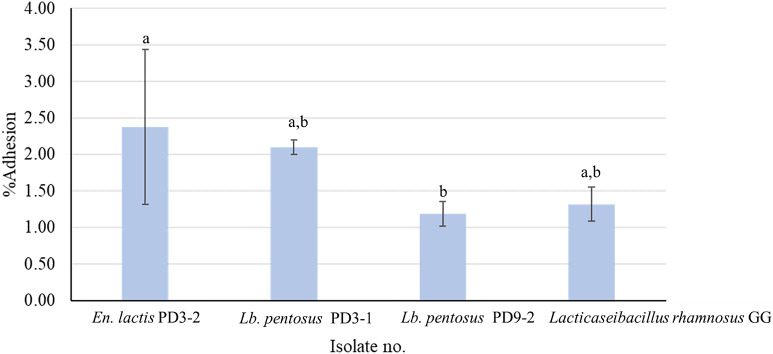
Adhesion
One of the essential properties of probiotics is their capacity to adhere to target sites for colonization in the digestive tract. Based on BSH-positive activity and acid and bile tolerance, three isolates were selected to assess in vitro adhesion properties: Lb. pentosus PD3-1 and PD9-2, and En. lactis PD3-2. The adhesion abilities of the selected isolates are shown in Fig. 4. The percentages of En. lactis PD3-2, Lb. pentosus PD3-1, Lb. pentosus PD9-2, and Lb. rhamnosus GG adhering to cells were 2.38±1.06, 2.10±0.10, 1.19±0.17, and 1.32±0.23%, respectively. The adhesion abilities of the selected isolates did not significantly different from that of Lb. rhamnosus GG. The adhesion ability of LAB in the present study was consistent with that in previous studies (Duary et al., 2011; García-Cayuela et al., 2014; Thamacharoensuk et al., 2017), which showed that the adhesion abilities of LAB to human intestinal epithelium cells, such as Caco-2 cells, were dependent on the strain and bacterial cell-surface composition.
Fig. 4.
Percentage of selected isolates adhering to Caco-2 cell lines. Selected isolates were enumerated by bacterial cultures and interpreted as percent adherence relative to the control. Data represent the mean±SD. Different letters indicate a significant difference (P<0.05).
The present results indicated the strain-specific adhesion abilities of Lacticaseibacillus and Enterococcus isolates to Caco-2 cells, which varied within the same species (Duary et al., 2011).
In summary, the selected isolates (PD3-1, PD9-2, and PD3-2) from fermented fish (pla-paeng-daeng) samples demonstrated a superior ability to Lb. rhamnosus GG (one of the most widely marketed and researched probiotic isolates [Stage et al., 2020]) to adhere to Caco-2 cells in vitro. These isolates exhibited excellent capabilities and, thus, have potential as candidate probiotics. Further in vivo studies are needed to assess their health-promoting effects because they have the ability to colonize the gut.
Immunomodulatory effects of LAB
The present results revealed that the immunomodulatory effects of the selected isolates (based on BSH-positive activity) significantly differed from those of the control (Table 5).
Table 5.
Immunomodulatory effects of selected isolates.
| Species/isolate no. | IL-12 (ng mL –1 ) | IFN-γ (ng mL –1 ) | hBD-2 (relative value) | NO (μM) |
|---|---|---|---|---|
| En. lactis PD3-2 | 57.45±7.22* | 53.88±13.80* | 1.96±0.10* | 8.30±0.09** |
| Lactiplantibacillus pentosus PD3-1 | 7.72±2.85* | 32.91±5.79* | 2.06±0.27* | 18.07±0.25** |
| Lb. pentosus PD9-2 | 15.38±4.93* | 33.95±7.93* | 2.45±0.25* | 19.13±0.20** |
| PBS (no stimulation) | 29.52±5.87 | 43.23±12.72 | 1.00±0.00 | Not detected |
| LPS (positive control) | Not determined | 32.47±0.14 | ||
Data are expressed as the mean±SD.
*P<0.05, significantly different from PBS (no stimulation) within each column; **P<0.05, significantly different from LPS (positive control).
En. lactis PD3-2 was the strongest inducer of IL-12 production (57.45±7.22 ng mL–1), whereas Lb. pentosus PD3-1 suppressed its production (7.72±2.85 ng mL–1). The capacity of LAB to induce IL-12 in the present study was consistent with previous findings (Iwabuchi et al., 2012; Chen et al., 2013; Thamacharoensuk et al., 2017), which showed that dead and viable LAB cells both modulated IL-12 levels.
Regarding IFN-γ, En. lactis PD3-2 was the strongest inducer of IFN-γ production (53.88±13.80 ng mL–1), whereas Lb. pentosus PD3-1 suppressed its production (32.91±5.79 ng mL–1). These results are consistent with previous findings (Ou et al., 2011; Yamane et al., 2018), showing that LAB cells function as immunomodulatory agents that may suppress and stimulate the production of IFN-γ.
Concerning hBD-2 production, in vitro results revealed that all of the selected isolates up-regulated the expression of hBD-2. These results are in accordance with previous studies (Schlee et al., 2008; Kobatake and Kabuki, 2019), which reported that LAB up-regulated the expression of hBD-2 in order to prevent infection. Therefore, the present results suggest that various non-pathogenic probiotic bacteria, including LAB, promote innate immunity through the induction of defensin. Furthermore, the probiotic activation of defensins may be an appealing novel approach to strengthen innate immunity (Schlee et al., 2008).
Regarding NO production, NO boosts a host’s defenses against infections and tumor cells. The results of the NO assay revealed that all of the selected isolates induced NO production at levels that significantly differed from those of the control (Table 4). Lb. pentosus PD9-2 induced the highest level of NO production (19.13±0.20 μM), followed by Lb. pentosus PD3-1 (18.07±0.25 μM) and En. lactis PD3-2 (8.30±0.09 μM). LAB-induced NO production levels in the present study are consistent with those in previous studies (Korhonen et al., 2001; Kmonickova et al., 2012; Surayot et al., 2014), showing that cell components of LAB stimulated various NO production levels and important factors were the type of cell component (i.e., carbohydrates, lipids, and proteins) and the strain.
In the present study, non-viable cells of LAB still exerted immunomodulatory effects; therefore, the advantages of dead/dormant cells of probiotics include a reduced risk of probiotic sepsis and drug resistance as well as a longer shelf-life because storage is not necessary to maintain the viability of probiotics (Shripada et al., 2020; Zendeboodi et al., 2020). The present study revealed that bacterial isolates have various functional properties even if they are of the same species (Kang et al., 2021a). Therefore, these isolates may stimulate immunity and protect against invading pathogens (Kato et al., 1999; Kang et al., 2021b).
Conclusions
This is the first study on the distribution and characteristics of LAB in pla-paeng-daeng, which included Companilactobacillus, Lactiplantibacillus, Limosilactobacillus, and Enterococcus species. Two Lb. pentosus isolates and one En. lactis isolate exhibited BSH activity by forming opaque white colonies and halos around the colonies, respectively. They not only tolerated, but grew under acidic (pH 2 and 3) and bile salt (0.3 and 0.8%) conditions. They also adhered well to Caco-2 cells. Furthermore, BSH-producing isolates exerted immunostimulatory effects. En. lactis PD3-2 strongly induced the production of IL-12 and IFN-γ. Lb. pentosus PD9-2 appeared to have enhanced the secretion of hBD-2 and production of NO. The role of LAB in the prevention of hypercholesterolemia and immunomodulation is currently being investigated. Therefore, these isolates have potential as probiotics because they reduce cholesterol, exert immunomodulatory effects, show adhesion abilities, and tolerate acid and bile conditions, all of which are essential probiotic characteristics. Further clinical studies are required.
Citation
Kingkaew, E., Konno, H., Hosaka, Y., Phongsopitanun, W., and Tanasupawat, S. (2023) Characterization of Lactic Acid Bacteria from Fermented Fish (pla-paeng-daeng) and Their Cholesterol-lowering and Immunomodulatory Effects. Microbes Environ 38: ME22044.
https://doi.org/10.1264/jsme2.ME22044
Fig. 1.
Fermented fish (Pla-paeng-daeng)
Acknowledgements
This research was supported by the Thailand Research Fund and the National Research Council of Thailand for the 2017 Royal Golden Jubilee Ph.D. Program as a scholarship to E. K. (PHD/0226/2560) and the Grant for International Research Integration: Research Pyramid, Ratchadaphiseksomphot Endowment Fund (CUGRP-61-01-33-01), Chulalongkorn University. We thank the Pharmaceutical Research Instrument Center, Faculty of Pharmaceutical Sciences, Chulalongkorn University for providing research facilities.
References
- Abushelaibi, A., Al-Mahadin, S., El-Tarabily, K., Shah, N.P., and Ayyash, M. (2017) Characterization of potential probiotic lactic acid bacteria isolated from camel milk. LWT-Food Sci Technol 79: 316–325. [Google Scholar]
- Albano, C., Morandi, S., Silvetti, T., Casiraghi, M.C., Manini, F., and Brasca, M. (2018) Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J Dairy Sci 101: 10807–10818. [DOI] [PubMed] [Google Scholar]
- Angmo, K., Kumari, A., and Bhalla, T.C. (2016) Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT-Food Sci Technol 66: 428–435. [Google Scholar]
- Baek, K.-S., Hong, Y.D., Kim, Y., Sung, N.Y., Yang, S., Lee, K.M., et al. (2015) Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res 39: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley, M., Hill, C., and Gahan, C.G. (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72: 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni, A., Amaretti, A., Leonardi, A., Boschetti, E., Danesi, F., Matteuzzi, D., et al. (2013) Cholesterol-lowering probiotics: in vitro selection and in vivo testing of bifidobacteria. Appl Microbiol Biotechnol 97: 8273–8281. [DOI] [PubMed] [Google Scholar]
- Chen, C.Y., Tsen, H.Y., Lin, C.L., Lin, C.K., Chuang, L.T., Chen, C.S., and Chiang, Y.C. (2013) Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. J Med Microbiol 62: 1657–1664. [DOI] [PubMed] [Google Scholar]
- Comi, G., Urso, R., Iacumin, L., Rantsiou, K., Cattaneo, P., Cantoni, C., and Cocolin, L. (2005) Characterisation of naturally fermented sausages produced in the North East of Italy. Meat Sci 69: 381–392. [DOI] [PubMed] [Google Scholar]
- Dashkevicz, M.P., and Feighner, S.D. (1989) Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol 55: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Man, J.C., Rogosa, D., and Sharpe, M.E. (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23: 130–135. [Google Scholar]
- De Vries, M.C., Vaughan, E.E., Kleerebezem, M., and de Vos, W.M. (2006) Lactobacillus plantarum—survival, functional and potential probiotic properties in the human intestinal tract. Int Dairy J 16: 1018–1028. [Google Scholar]
- Duary, R.K., Rajput, Y.S., Batish, V.K., and Grover, S. (2011) Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells. Indian J Med Res 134: 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda, S., López, C., and Vignolo, G. (2010) Role of lactic acid bacteria during meat conditioning and fermentation: Peptides generated as sensorial and hygienic biomarkers. Meat Sci 86: 66–79. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J. (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- García-Cayuela, T., Korany, A.M., Bustos, I., de Cadiñanos, L.P.G., Requena, T., Peláez, C., and Martínez-Cuesta, M.C. (2014) Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res Int 57: 44–50. [Google Scholar]
- Han, Q., Kong, B.H., Chen, Q., Sun, F.D., and Zhang, H. (2017) In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J Funct Foods 32: 391–400. [Google Scholar]
- Hassanzadazar, H., Ehsani, A., Mardani, K., and Hesari, J. (2012) Investigation of antibacterial, acid and bile tolerance properties of lactobacilli isolated from Koozeh cheese. Vet Res Forum 3: 181–185. [PMC free article] [PubMed] [Google Scholar]
- Hosaka, Y., Itoh, K., Matsutani, S., Kawate, S., Miura, A., Mizoura, Y., et al. (2021) Fermented food Tempeh induces interleukin 12 and enhances macrophage phagocytosis. J Food Biochem 45: e13958. [DOI] [PubMed] [Google Scholar]
- Huang, Z., Shen, Y., Huang, X., Qiao, M., He, R.K., and Song, L. (2021) Microbial diversity of representative traditional fermented sausages in different regions of China. J Appl Microbiol 130: 133–141. [DOI] [PubMed] [Google Scholar]
- Ishimwe, N., Daliri, E.B., Lee, B.H., Fang, F., and Du, G. (2015) The perspective on cholesterol‐lowering mechanisms of probiotics. Mol Nutr Food Res 59: 94–105. [DOI] [PubMed] [Google Scholar]
- Iwabuchi, N., Yonezawa, S., Odamaki, T., Yaeshima, T., Iwatsuki, K., and Xiao, J.-Z. (2012) Immunomodulating and anti-infective effects of a novel strain of Lactobacillus paracasei that strongly induces interleukin-12. FEMS Immunol Med Microbiol 66: 230–239. [DOI] [PubMed] [Google Scholar]
- Jayashree, S., Pooja, S., Pushpanathan, M., Rajendhran, J., and Gunasekaran, P. (2014) Identification and characterization of bile salt hydrolase genes from the genome of Lactobacillus fermentum MTCC 8711. Appl Biochem Biotechnol 174: 855–866. [DOI] [PubMed] [Google Scholar]
- Kang, C.-H., Kim, J.-S., Kim, H., Park, H.M., and Paek, N.-S. (2021a) Heat-killed lactic acid bacteria inhibit nitric oxide production via inducible nitric oxide synthase and cyclooxygenase-2 in RAW 264.7 cells. Probiotics Antimicrob Proteins 13: 1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, C.-H., Kim, J.-S., Park, H.M., Kim, S., and Paek, N.-S. (2021b) Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech 11: 217–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, I., Tanaka, K., and Yokokura, T. (1999) Lactic acid bacterium potently induces the production of interleukin-12 and interferon-γ by mouse splenocytes. Int J Immunopharmacol 21: 121–131. [DOI] [PubMed] [Google Scholar]
- Kmonickova, E., Kverka, M., Tlaskalová-Hogenová, H., Kostecka, P., and Zídek, Z. (2012) Stimulation of nitric oxide, cytokine and prostaglandin production by low-molecular weight fractions of probiotic Lactobacillus casei lysate. Neuro Endocrinol Lett 33: 166–172. [PubMed] [Google Scholar]
- Kobatake, E., and Kabuki, T. (2019) S-layer protein of Lactobacillus helveticus SBT2171 promotes human β-defensin 2 expression via TLR2–JNK signaling. Front Microbiol 10: 2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen, R., Korpela, R., Saxelin, M., Mäki, M., Kankaanranta, H., and Moilanen, E. (2001) Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation 25: 223–232. [DOI] [PubMed] [Google Scholar]
- Kumar, S., Stecher, G., and Tamura, K. (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarthe, D.R., and Dunbar, S.B. (2012) Global cardiovascular health promotion and disease prevention 2011 and beyond. Circulation 125: 2667–2676. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Lane, D.J. (1991) 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics. Stackebrandt, E., and Goodfellow, M. (eds). New York, NY: Wiley, pp. 115–175. [Google Scholar]
- Liong, M.T., and Shah, N.P. (2005) Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J Dairy Sci 88: 55–66. [DOI] [PubMed] [Google Scholar]
- Liu, Y.F., Zhao, F.C., Liu, J.Y., Wang, H.M., Han, X., Zhang, Y.X., and Yang, Z.Y. (2017) Selection of cholesterol-lowering lactic acid bacteria and its effects on rats fed with high-cholesterol diet. Curr Microbiol 74: 623–631. [DOI] [PubMed] [Google Scholar]
- Miremadi, F., Ayyash, M., Sherkat, F., and Stojanovska, L. (2014) Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic lactobacilli and bifidobacteria. J Funct Foods 9: 295–305. [Google Scholar]
- Moon, P.-D., Lee, J.S., Kim, H.-Y., Han, N.-R., Kang, I., Kim, H.-M., and Jeong, H.-J. (2019) Heat-treated Lactobacillus plantarum increases the immune responses through activation of natural killer cells and macrophages on in vivo and in vitro models. J Med Microbiol 68: 467–474. [DOI] [PubMed] [Google Scholar]
- Nakai, H., Hirose, Y., Murosaki, S., and Yoshikai, Y. (2019) Lactobacillus plantarum L‐137 upregulates hyaluronic acid production in epidermal cells and fibroblasts in mice. Microbiol Immunol 63: 367–378. [DOI] [PubMed] [Google Scholar]
- Nasrollahzadeh, A., Mokhtari, S., Khomeiri, M., and Saris, P. (2022) Mycotoxin detoxification of food by lactic acid bacteria. Int J Food Contam 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngasotter, S., Waikhom, D., Mukherjee, S., Devi, M.S., and Singh, A.S. (2020) Diversity of lactic acid bacteria (LAB) in fermented fish products: a review. Int J Curr Microbiol App Sci 9: 2238–2249. [Google Scholar]
- Noriega, L., Cuevas, I., Margolles, A., and Los Reyes-Gavilan, C.G.D. (2006) Deconjugation and bile salts hydrolase activity by Bifidobacterium strains with acquired resistance to bile. Int Dairy J 16: 850–855. [Google Scholar]
- Ohashi, Y., and Ushida, K. (2009) Health-beneficial effects of probiotics: Its mode of action. Anim Sci J (Richmond, Aust) 80: 361–371. [DOI] [PubMed] [Google Scholar]
- Ou, C.C., Lin, S.L., Tsai, J.J., and Lin, M.Y. (2011) Heat‐killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J Food Sci 76: M260–M267. [DOI] [PubMed] [Google Scholar]
- Paludan-Müller, C., Huss, H.H., and Gram, L. (1999) Characterization of lactic acid bacteria isolated from a Thai low-salt fermented fish product and the role of garlic as substrate for fermentation. Int J Food Microbiol 46: 219–229. [DOI] [PubMed] [Google Scholar]
- Phithakpol, B., Varanyanond, W., Reungmaneepaitoon, S., and Wood, H. (1995) The traditional fermented foods of Thailand. Kuala Lumpur: Asean Food Handling Bureau. [Google Scholar]
- Phuengjayaem, S., Phinkian, N., Tanasupawat, S., and Teeradakorn, S. (2017) Diversity and succinic acid production of lactic acid bacteria isolated from animals, soils and tree barks. Res J Microbiol 12: 177–186. [Google Scholar]
- Pithva, S., Shekh, S., Dave, J., and Vyas, B.R. (2014) Probiotic attributes of autochthonous Lactobacillus rhamnosus strains of human origin. Appl Biochem Biotechnol 173: 259–277. [DOI] [PubMed] [Google Scholar]
- Rodpai, R., Sanpool, O., Thanchomnang, T., Wangwiwatsin, A., Sadaow, L., Phupiewkham, W., et al. (2021) Investigating the microbiota of fermented fish products (Pla-ra) from different communities of northeastern Thailand. PLoS One 16: e0245227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel, L.L., and Morris, M.D. (1973) Determination of cholesterol using o-phthalaldehyde. J Lipid Res 14: 364–366. [PubMed] [Google Scholar]
- Ruiz, L., Margolles, A., and Sánchez, B. (2013) Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol 4: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou, N., and Nei, M. (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425. [DOI] [PubMed] [Google Scholar]
- Schlee, M., Harder, J., Köten, B., Stange, E.F., Wehkamp, J., and Fellermann, K. (2008) Probiotic lactobacilli and VSL# 3 induce enterocyte β-defensin 2. Clin Exp Immunol 151: 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata, M.G., El Sohaimy, S.A., El-Sahn, M.A., and Youssef, M.M. (2016) Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann Agric Sci 61: 65–75. [Google Scholar]
- Shripada, R., Gayatri, A.-J., and Sanjay, P. (2020) Chapter 5-Paraprobiotics. In Precision Medicine for Investigators, Practitioners and Providers. Faintuch, J., and Faintuch, S. (eds). Cambridge: Academic Press, pp. 39–49. [Google Scholar]
- Stage, M., Wichmann, A., Jørgensen, M., Vera-Jimenéz, N.I., Wielje, M., Nielsen, D.S., et al. (2020) Lactobacillus rhamnosus GG genomic and phenotypic stability in an industrial production process. Appl Environ Microbiol 86: e02780-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surayot, U., Wang, J., Seesuriyachan, P., Kuntiya, A., Tabarsa, M., Lee, Y., et al. (2014) Exopolysaccharides from lactic acid bacteria: structural analysis, molecular weight effect on immunomodulation. Int J Biol Macromol 68: 233–240. [DOI] [PubMed] [Google Scholar]
- Syafitri, Y., Kusumaningrum, H.D., and Dewanti-Hariyadi, R. (2022) Identification of microflora and lactic acid bacteria in pado, a fermented fish product prepared with dried Pangium edule seed and grated coconut. Food Sci Technol 42: e19921. [Google Scholar]
- Tanasupawat, S., and Komagata, K. (1995) Lactic acid bacteria in fermented foods in Thailand. World J Microbiol Biotechnol 11: 253–256. [DOI] [PubMed] [Google Scholar]
- Tanasupawat, S., Okada, S., and Komagata, K. (1998) Lactic acid bacteria found in fermented fish in Thailand. J Gen Appl Microbiol 44: 193–200. [DOI] [PubMed] [Google Scholar]
- Tannock, G.W. (2004) A special fondness for lactobacilli. Appl Environ Microbiol 70: 3189–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamacharoensuk, T., Taweechotipatr, M., Kajikawa, A., Okada, S., and Tanasupawat, S. (2017) Induction of cellular immunity interleukin-12, antiproliferative effect, and related probiotic properties of lactic acid bacteria isolated in Thailand. Ann Microbiol 67: 511–518. [Google Scholar]
- Tomaro-Duchesneau, C., Jones, M.L., Shah, D., Jain, P., Saha, S., and Prakash, S. (2014) Cholesterol assimilation by Lactobacillus probiotic bacteria: An in vitro investigation. BioMed Res Int 2014: 380316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76: 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyasevi, R., and Rolle, R.S. (2002) An overview of small-scale food fermentation technologies in developing countries with special reference to Thailand: scope for their improvement. Int J Food Microbiol 75: 231–239. [DOI] [PubMed] [Google Scholar]
- Visessanguan, W., Benjakul, S., Riebroy, S., and Thepkasikul, P. (2004) Changes in composition and functional properties of proteins and their contributions to nham characteristics. Meat Sci 66: 579–588. [DOI] [PubMed] [Google Scholar]
- Wang, J., Mendelsohn, R., Dinar, A., Huang, J., Rozelle, S., and Zhang, L. (2009) The impact of climate change on China's agriculture. Agric Econ 40: 323–337. [Google Scholar]
- Whitmire, J., and Merrell, D. (2012) Successful culture techniques for Helicobacter species: general culture techniques for Helicobacter pylori. Methods Mol Biol 921: 17–27. [DOI] [PubMed] [Google Scholar]
- Yamane, T., Sakamoto, T., Nakagaki, T., and Nakano, Y. (2018) Lactic acid bacteria from kefir increase cytotoxicity of natural killer cells to tumor cells. Foods 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Xing, R., Liu, S., Qin, Y., Li, K., Yu, H., and Li, P. (2018) Immunostimulatory effects of sulfated chitosans on RAW 264.7 mouse macrophages via the activation of PI3 K/Akt signaling pathway. Int J Biol Macromol 108: 1310–1321. [DOI] [PubMed] [Google Scholar]
- Yoon, S.-H., Ha, S.-M., Kwon, S., Lim, J., Kim, Y., Seo, H., and Chun, J. (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67: 1613–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendeboodi, F., Khorshidian, N., Mortazavian, A.M., and da Cruz, A.G. (2020) Probiotic: conceptualization from a new approach. Curr Opin Food Sci 32: 103–123. [Google Scholar]