Abstract
BACKGROUND
Quadriceps weakness is a known risk factor for the onset of knee osteoarthritis (OA). In addition to muscle weakness, increased passive stiffness of the quadriceps may affect knee biomechanics and hence contribute to the pathogenesis of knee OA. However, the association between quadriceps stiffness and the risk of knee OA development has not been prospectively investigated.
AIM
The aim of this study was to investigate how baseline quadriceps passive stiffness predicts the incidence of clinical knee OA at the 12-month follow-up.
DESIGN
Prospective cohort study.
SETTING
University laboratory.
POPULATION
Community-dwelling adults aged 60-80 years were recruited. We excluded participants with: 1) knee pain or known arthritis; 2) knee injury; 3) knee or hip joint replacement, 4) cognitive impairment; or 5) neurological conditions.
METHODS
At baseline, passive stiffness of the three superficial quadriceps muscle heads (rectus femoris [RF], vastus lateralis [VL], and vastus medialis oblique [VMO]) was evaluated using shear-wave ultrasound elastography. Knee muscle (quadriceps and hamstrings) strength was tested using a Cybex dynamometer. Knee OA was defined based on clinical criteria 12 months after baseline measurements. Generalized estimating equations were used to examine the associations of quadriceps stiffness and knee muscle strength with the risk of knee OA, controlling for age, sex, Body Mass Index, comorbidities, and activity level.
RESULTS
The analyses included 158 knees (58.2% females, age: 65.6±4.1 years). Twenty-eight knees (17.7%) were classified as having clinical OA at 12 months. Compared with the lowest stiffness tertiles, the highest stiffness tertiles of the RF (relative risk =5.31, 95% CI: 1.34-21.0), VMO (4.15, 1.04-16.6), and total superficial quadriceps (6.35, 1.48-27.3) at baseline were significantly associated with a higher risk of knee OA at the follow-up. The highest strength tertile of quadriceps has a trend of association with a lower risk of knee OA than the lowest tertile (0.18, 0.03-1.25, P=0.083).
CONCLUSIONS
Greater passive stiffness of the quadriceps at baseline was associated with a higher risk of clinical knee OA incidence at the 12-month follow-up.
CLINICAL REHABILITATION IMPACT
Interventions for reducing the passive stiffness of the quadriceps should be included in preventative training programs for older adults.
Key words: Quadriceps muscle; Muscle strength; Osteoarthritis, knee
Knee osteoarthritis (OA) is considered to be a whole-organ disease that develops slowly along a continuum from early clinical vulnerability to established OA.1 Alterations in gait mechanics and neuromuscular function have been linked to the initiation and progression of knee OA.2, 3 The loading response phase, which immediately follows the initial contact of the heel with the ground, induces an abrupt, large loading impact on the lower limb joints.4 During this critical phase, the quadriceps muscle functions eccentrically to control knee flexion excursion and thus attenuate the impact of knee loading.2, 4 Reduced quadriceps strength5-8 and increased co-contraction of muscles spanning the knee joint9 have been studied and shown to be significant predictors of the onset5-7 and progression of knee OA.8, 9 Conceptually, passive muscular tension may also influence knee flexion excursion and dynamic joint stiffness10, 11 and thus may increase the risk of knee OA.
Intersegmental movement is achieved by contractions of skeletal muscles and deformations of the periarticular structures, which generate resistance to joint movements and produce passive joint moments.12 Whittington et al. (2008) reported that substantial passive moments may be present during normal gait and that stretching of the articular muscles may influence these passive moments.13 The quadriceps muscle spans the knee joint via the muscle-tendon complex system. An increase in passive stiffness of the quadriceps, characterised by reduced compliance,14 may contribute to a high level of dynamic knee stiffness.10, 11 Dynamic knee joint stiffness, a biomechanical interaction between external knee flexion moments and knee flexion motion, is a mechanical factor associated with patellofemoral cartilage damage11 and the severity of tibiofemoral knee OA.15, 16 Age-related increases in passive stiffness of the quadriceps muscle have been reported.17 As the prevalence and incidence of knee OA in the elderly are especially high.18 It is thus important to explore whether passive stiffness of the quadriceps muscle is one of the muscle properties associated with knee OA in the older population.
Passive joint moments have been measured in vivo for joints that are passively moved automatically.13 More recently, ultrasound shear wave elastography has been used to measure in vivo muscle shear modulus (an index of stiffness).19 This technique allows passive stiffness to be measured for individual muscle heads, with good test-retest reliability.19, 20 The individual heads of quadriceps muscles have different attachment sites and anatomical features.21-24 Therefore, these individual muscle heads may induce different impacts on the knee via their connections with peri-articular ligaments and tendons. Fibres of the vastis medialis oblique (VMO) mesh with the medial patellofemoral ligament.22 The vastis lateralis (VL) connects with the lateral retinaculum, and the rectus femoris (RF) continues as the patellar tendon23 to reinforce the passive tension of the peri-articular structures. In addition, all quadriceps muscle heads terminate as a common aponeurosis, merging into the anterior third of the knee capsule, which is also known as the retinacular layer.24 Increased stiffness25 and altered length patterns26 of ligamentous structures in the knee may increase the force required for knee motion. An increase in the passive stiffness of quadriceps muscle heads may also affect normal knee kinematics and passive knee moments through their connections with peri-articular structures. These effects may be related to the development of knee OA.
The aim of this study was to determine whether passive stiffness of the quadriceps is associated with the incidence of knee OA in community-dwelling older adults and to explore whether this association is muscle-head-specific. We hypothesized that increased passive stiffness of the quadriceps muscle at baseline would be associated with a higher risk of developing clinical knee OA at the 12-month follow-up, and that this association may be significant for each muscle head.
Materials and methods
Study sample
This was a prospective cohort study. Participants were recruited from local communities from November 2018 to October 2019. Potential participants were included if they were: 1) aged 60-80 years; and 2) able to walk for 10 meters without using an assistive device. Potential participants were excluded if they had: 1) knee pain or known arthritis; 2) a history of knee injury; 3) knee or hip joint replacement; 4) cognitive impairment; or 5) a neurological condition (e.g., stroke or Parkinson’s disease). The study was approved by the human subject’s ethics sub-committee of the administering institution (ID no.: HSEARS20180110001). All eligible participants provided their written informed consent prior to data collection. The study was conducted at the Sports Training and Rehabilitation Laboratory (STAR LAB) of the administering institution.
Demographics
At baseline, age, sex, and Body Mass Index (BMI) of all eligible participants were collected. Known comorbidities including hypertension, diabetes, cardiopulmonary disease, and dyslipidemia were also documented. Each participant was categorized as having a sedentary or active lifestyle based on the type and duration of physical exercises recommended by the U.S. Department of Health and Human Services.27 The Montreal Cognitive Assessment, with a cut-off score of 22, was used to screen for the presence of mild cognitive impairment.28
Baseline measurements
Passive stiffness of the quadriceps
Muscle shear modulus, an index of muscle stiffness,19 of the three superficial muscle heads of the quadriceps – the RF, VL, and VMO – of both limbs were assessed using shear-wave elastography with an Aixplorer ultrasound scanner (Aixplorer Version 4.2; Supersonic Imagine, Aix-en-Provence, France), coupled with a linear ultrasound probe (4–15 MHz, Super Liner 15-4; Supersonic Imagine) following a previously established protocol.20
Measurements were conducted at the STAR LAB with the room temperature controlled at 25 °C. Participants were in the supine position with their knees at 60° flexion and their hips maintained in a neutral position using an adjustable brace. Such measurement position was chosen to assess the passive stiffness of individual quadriceps muscle heads beyond their slack angles, and to observe the possible distinctive effects of individual muscle heads, as the distinctive passive stiffness can be seen between the bi-articular (RF) and mono-articular (VL and VMO) muscle heads when the knee was flexed beyond 54° with hips in neutral position.20 The measurement sites were halfway between the anterior superior iliac spine (ASIS) and the patella superior border for the RF; distally 1/3 of the distance between the ASIS and the patella lateral border for the VL; and distally 1/5 of the distance between the ASIS and the patella medial border for the VMO.20 The transducer was positioned perpendicularly and aligned to the shortening direction of the lateral component of the RF fascicles and the muscle fiber directions for the VL and VMO.20, 29 Ultrasound gel was applied to the skin, and minimum pressure from the ultrasound probe was controlled to avoid compressing the underlying muscle.30 A 11-second video (1 sample/second with a spatial resolution of 1 × 1 mm) was captured when the tested muscle was at rest (monitored using ultrasound images).
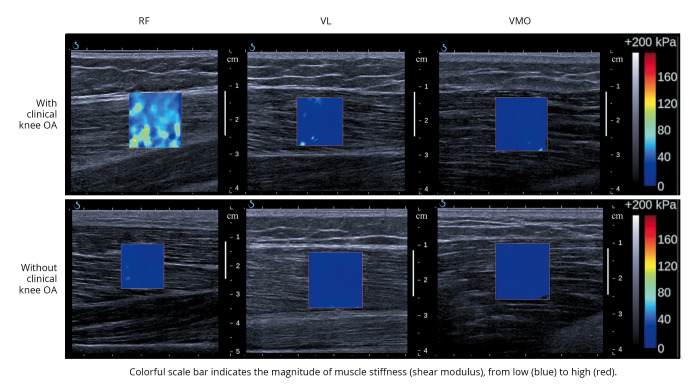
Each video was then transformed into 11 frames, with the middle five frames averaged for analysis. The region of interest (a two-dimensional map of the muscle shear modulus) was delineated according to the muscle thickness beneath the marked site (Figure 1).
Figure 1.
—Shear-wave elastography ultrasound images of the rectus femoris (RF), vastus lateralis (VL), and vastus medialis oblique (VMO) muscles from subjects with and without clinical knee osteoarthritis (OA).
The shear modulus values (in kPa) from each muscle head were computed using the MATLAB program (The MathWorks, Inc, Natick, MA, USA). The shear modulus of the total superficial quadriceps was calculated as the summation of the RF, VL, and VMO shear moduli. All measurements were conducted by a single examiner (LZP). The intra-rater reliability values for 20 healthy older adults (50% females) of a similar age were 0.94, 0.89, and 0.94 for the RF, VL, and VMO, respectively (unpublished results).
Quadriceps and hamstrings strength
The isokinetic peak torques of the quadriceps and hamstrings of both limbs were assessed at a constant angular velocity of 60°/second using a Cybex isokinetic dynamometer (Cybex Co., Ronkonkoma, NY, USA).7 Participants were in a seated position and were instructed to move their knee concentrically to full extension (0° of knee flexion) and to maximum knee flexion with maximal effort, and each for five times. A warm-up trial with 50% maximal voluntary effort was performed, and verbal encouragement was given during the tests. The peak torque values of the middle three trials for knee extension (quadriceps) and flexion (hamstrings) were averaged and normalized to body mass (Nm/kg). The test-retest reliability values for 20 healthy older adults (50% females) of a similar age and body mass were 0.90 and 0.91 for the quadriceps and hamstrings, respectively (unpublished results).
Clinical knee OA
All participants were invited for a follow-up visit at the STAR LAB, 12 months after their first visit. A clinical examination was conducted by a physiotherapist (HXP) with 8 years of postqualification experience. Clinical examinations of the knee joint were used to assess bony enlargement, warmth, bony tenderness, and crepitus based on a standardized protocol.31 Clinical knee OA was defined based on the American College of Rheumatology (ACR) clinical criteria. As all participants were older than 50 years, they were regarded as having clinical knee OA if they had pain in the knee and any two of the following items: 1) morning stiffness for <30 minutes; 2) crepitus; 3) bony tenderness; 4) bony enlargement, or 5) no palpable warmth of the synovium.32 Pain intensity (numeric rating scale), related activities, and the region of knee pain were also documented. The assessor and all participants were blinded to the baseline measurements.
Statistical analysis
Data analyses were performed using SPSS (Version 23.0, IBM Corp., Armonk, NY, USA). The normal distribution of each variable was assessed using the Shapiro-Wilk test. The mean±standard deviation and median (interquartile range) were reported for normally and non-normally distributed data, respectively. Associations between baseline muscle properties (passive stiffness of the RF, VL, VMO, and total superficial quadriceps [RF + VL + VMO]; quadriceps and hamstrings muscle strength) and potential covariates were examined using logistic (for the dichotomous variables: sex and activity level), ordinal (for the ordinal variable: comorbidity), or linear (for the continuous variables: age and BMI) regression. The relationships between potential covariates and knee OA were also determined using logistic regression analysis.
The tertiles of each baseline measurement were computed to define the cut-off values for higher or lower muscle stiffness and strength in this cohort. Participants were grouped into three categories based on the lowest, middle, and highest tertiles.7 Logistic regression was used to examine the potential associations between baseline muscle properties (the independent variables: quadriceps stiffness and knee muscle strength) and the incidence of clinical knee OA (the dependent variable). We used generalized estimating equations to control for between-knee correlations within each participant. Factors (age, sex, BMI, comorbidities, and activity level) having significant correlations with baseline muscle properties or with clinical knee OA were entered as covariates.
The relative risk (RR) with 95% confidence intervals (95% CIs) and corresponding P values are reported for each model. Statistical significance was set at P<0.05 (two-tailed).
Results
Two hundred and twenty-five older adults were screened. The baseline measurements of 162 eligible older adults were conducted from November 2018 to October 2019. At the 12-month follow-up visit, 83 participants were lost or had dropped out. Seventy of these participants were reluctant to leave home because of the coronavirus disease 2019 (COVID-19) pandemic, five dropped out due to loss of contact, and eight dropped out due to loss of interest. Further analyses were performed on 158 knees from 79 participants (58.2% females, age: 65.6±4.1 years; Figure 2). No significant differences in demographic data or baseline measurements were found between the participants who completed the study and those who dropped out (Table I).
Figure 2.
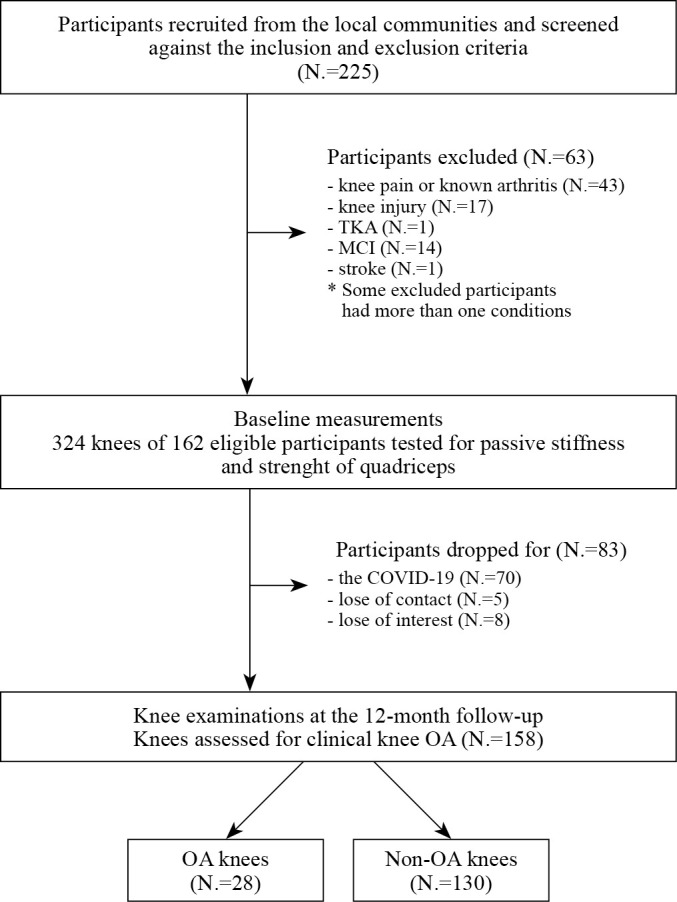
—Participant inclusion diagram. TKA: total knee arthroplasty; MCI: mild cognitive impairment; OA: osteoarthritis.
Table I. — Baseline characteristics of the 162 participants.
| All (324 case knees from 162 participants) |
Analyzed (158 case knees from 79 participants) |
Dropped (166 case knees from 83 participants) |
P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 65.8±4.0 | 65.6±4.1 | 66.0±3.9 | 0.471 |
| Gender (females: males) | 99: 63 | 46: 33 | 53: 30 | 0.463 |
| BMI (kg/m2) | 23.2±3.4 | 23.4±3.5 | 23.1±3.3 | 0.556 |
| Activity level (N. of sedentary) | 83 | 40 | 43 | 0.881 |
| † Number of comorbidities (N. of participants) | 0: 92 1: 44 >1: 26 |
0: 44 1: 21 >1: 14 |
0: 48 1: 23 >1: 12 |
0.700 |
| Passive stiffness (kPa) | ||||
| Total superficial quadriceps | 20.7 (6.5) | 20.4 (5.9) | 20.9 (7.1) | 0.355 |
| Rectus femoris | 10.5 (4.7) | 10.4 (4.5) | 10.6 (5.2) | 0.557 |
| Vastus lateralis | 5.4 (2.0) | 5.3 (1.9) | 5.5 (2.2) | 0.556 |
| Vastus medialis oblique | 4.4 (1.2) | 4.5 (1.3) | 4.4 (1.2) | 0.510 |
| Strength (Nm/kg) | ||||
| Quadriceps | 1.28±0.36 | 1.27±0.36 | 1.28±0.36 | 0.765 |
| Hamstrings | 0.61±0.20 | 0.63±0.21 | 0.59±0.18 | 0.080 |
Values are mean±SD, numbers or median (interquartile range) unless other indicates. †Including hypertension, diabetes, cardiopulmonary disease, and dyslipidemia.
At the follow-up visit, 28 knees (17.7%) from 19 participants (10 unilateral and 9 bilateral) were classified as having clinical OA (73.7% females). Knee pain was mostly reported during walking up or down stairs (92.9%), walking up or down slopes (85.7%), and squatting (71.4%), followed by kneeling (64.3%), walking (39.3%), running (35.7%), and jumping (35.7%). The painful regions were most commonly the anterior (50.0%) and lateral (50.0%) regions of the knee, followed by the medial (42.9%) and posterior (32.1%) regions. Most of the participants’ knees had crepitus (92.9%) and morning stiffness for less than 30 minutes (82.1%). Bony tenderness was present in 25.0% of the participants, while bony enlargement (3.6%) was relatively uncommon (Supplementary Digital Material 1: Supplementary Table I). A higher BMI was significantly correlated with a higher risk of knee OA, while age, sex, activity level, and comorbidities were not significant factors predicting knee OA in this cohort. Descriptive data of the muscle properties in OA and non-OA knees are presented in Table II.
Table II. —Relative risk of knee osteoarthritis between different tertiles of quadriceps stiffness and knee muscle strength.
| Muscle properties | Tertiles | Range in each tertile | Number of knees (OA knees) | Crude RR (95% CI) |
P value | Adjusted † RR (95% CI) |
P value |
|---|---|---|---|---|---|---|---|
| Total superficial quadriceps shear modulus (kPa) |
Low (reference) | 5.6-18.6 | 54 (4) | 1.00 | - | 1.00 | - |
| Medium | 18.7-22.9 | 59 (10) | 2.76 (0.80, 9.45) | 0.107 | 2.96 (0.72, 12.2) | 0.132 | |
| High | 23.0-38.5 | 45 (14) | 5.92 (1.66, 21.1)* | 0.006* | 6.35 (1.48, 27.3)* | 0.013* | |
| RF Shear modulus (kPa) |
Low (reference) | 3.0-9.3 | 53 (4) | 1.00 | - | 1.00 | - |
| Medium | 9.4-12.0 | 49 (8) | 2.60 (0.74, 9.20) | 0.138 | 1.93 (0.48, 7.79) | 0.355 | |
| High | 12.1-19.8 | 56 (16) | 5.01 (1.47, 17.1)* | 0.010* | 5.31 (1.34, 21.0)* | 0.017* | |
| VL Shear modulus (kPa) |
Low (reference) | 1.5-4.6 | 46 (7) | 1.00 | - | 1.00 | - |
| Medium | 4.7-5.7 | 60 (10) | 1.26 (0.42, 3.78) | 0.683 | 1.52 (0.47, 4.88) | 0.484 | |
| High | 5.8-15.5 | 52 (11) | 1.63 (0.54, 4.98) | 0.390 | 1.92 (0.61, 6.01) | 0.265 | |
| VMO Shear modulus (kPa) |
Low (reference) | 1.1-4.0 | 51 (4) | 1.00 | - | 1.00 | - |
| Medium | 4.1-4.7 | 49 (9) | 3.00 (0.88, 10.2) | 0.080 | 3.91 (0.93, 16.4) | 0.062 | |
| High | 4.8-8.1 | 58 (15) | 4.41 (1.30, 15.0)* | 0.017* | 4.15 (1.04, 16.6)* | 0.044* | |
| Quadriceps strength (Nm/kg) |
Low (reference) | 0.50-1.11 | 56 (17) | 1.00 | - | 1.00 | - |
| Medium | 1.12-1.41 | 50 (7) | 0.37 (0.11, 1.22) | 0.103 | 0.37 (0.11, 1.22) | 0.102 | |
| High | 1.42-2.41 | 52 (4) | 0.19 (0.05, 0.77)* | 0.020* | 0.18 (0.03, 1.25) | 0.083 | |
| Hamstrings strength (Nm/kg) |
Low (reference) | 0.18-0.50 | 50 (14) | 1.00 | - | 1.00 | - |
| Medium | 0.51-0.68 | 50 (8) | 0.48 (0.16, 1.46) | 0.194 | 0.51 (0.16, 1.64) | 0.257 | |
| High | 0.69-1.16 | 58 (6) | 0.28 (0.07, 1.03) | 0.056 | 0.32 (0.08, 1.27) | 0.105 |
In OA knees (N.=28): the median (interquartile range) for the passive stiffness of total superficial quadriceps, RF, VL, and VMO were 23.0 (6.3), 12.8 (5.5), 5.4 (2.1), and 4.8 (1.3), respectively; the mean±SD for peak torques of quadriceps and hamstrings were 1.08±0.33 and 0.55±0.20, respectively. In non-OA knees (N.=130): the median (interquartile range) for the passive stiffness of total superficial quadriceps, RF, VL, and VMO were 19.6 (5.1), 10.1 (4.2), 5.3 (1.6), and 4.4 (1.4), respectively; the mean±SD for peak torques of quadriceps and hamstrings were 1.31±0.35 and 0.64±0.21, respectively. OA: osteoarthritis; RF: rectus femoris; VL: vastus lateralis; VMO: vastus medialis oblique; RR: relative risk; 95% CI: 95% confidence interval of RR. †Controlling for age, gender, body mass index, comorbidities, and activity level; *significant associations.
Logistic regression analyses revealed that, compared with the lowest stiffness tertiles, the highest stiffness tertiles for the RF (RR=5.01, 95% CI: 1.47-17.1, P=0.010), VMO (RR=4.41, 95% CI: 1.30-15.0, P=0.017), and total superficial quadriceps (RR=5.92, 95% CI: 1.66-21.1, P=0.006) at baseline were significantly associated with a higher risk of clinical knee OA at the follow-up (Table II; Figure 3).
Figure 3.
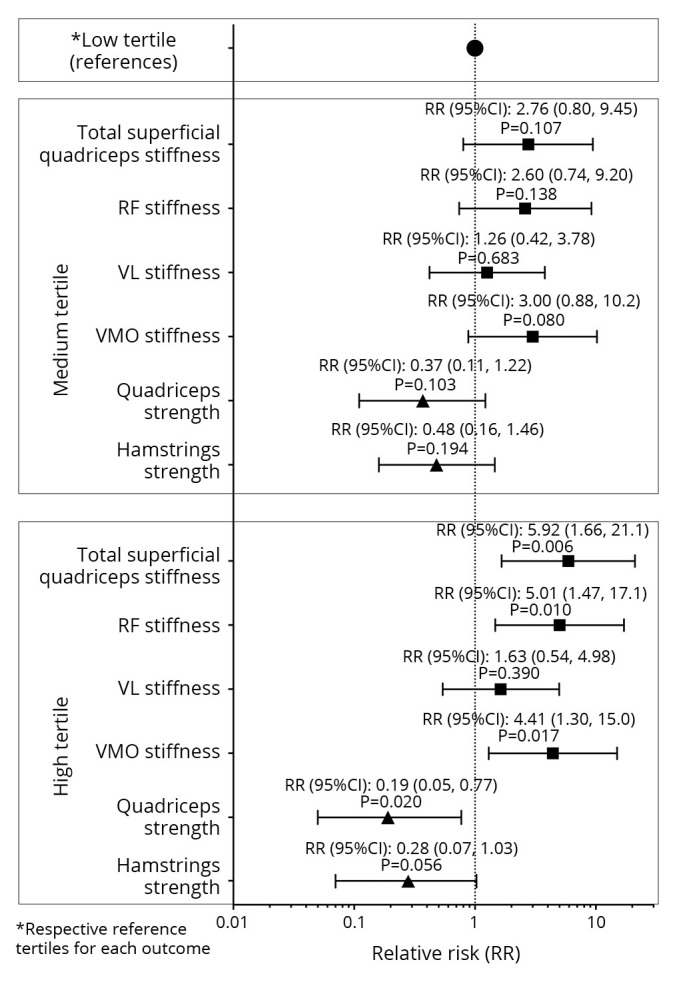
—Relative risk of knee osteoarthritis between different tertiles of quadriceps stiffness and knee muscle strength.
After controlling for covariates, similar results persisted for the RF (RR=5.31, 95% CI: 1.34-20.1, P=0.017), VMO (RR=4.15, 95% CI: 1.04-16.6, P=0.044), and total superficial quadriceps (RR=6.35, 95% CI: 1.48-27.3, P=0.013; Table II). Compared with the lowest quadriceps strength tertile, the highest tertile at baseline was significantly associated with a lower risk of clinical knee OA at the follow-up (RR=0.19, 95% CI: 0.05-0.77, P=0.020), and this trend persisted after adjusting for covariates (RR=0.18, 95% CI: 0.03-1.25, P=0.083). However, hamstrings strength at baseline was not significantly associated with the risk of clinical knee OA at the follow-up, with or without adjustment for covariates (P=0.056-0.105; Table II; Figure 3).
Discussion
Our study indicated that quadriceps muscle stiffness at baseline was associated with the incidence of knee OA at the 12-month follow-up. More specifically, an increase in the stiffness of quadriceps muscles, particularly the RF and VMO muscles, increased the likelihood of having clinical knee OA within 12 months. In addition, there was a trend towards greater quadriceps strength being associated with a lower risk of clinical knee OA.
Of the 158 knees from 79 older adults, 28 (17.7%) developed clinical OA. The annual incidence rate was similar to the previously reported incidence rates for clinical knee OA (11-27.5%),33 but was higher than the incidence rates reported for radiographic (2.0-3.6%), symptomatic (0.2-3.6%), and self-reported (2.3%) knee OA.7, 18 The radiographic criteria for knee OA are based on evidence of osteophyte formation or narrowing of the joint space (grade ≥2 in the Kellgren-Lawrence [K-L] classification system34), which may fail to identify the early stage of knee OA (defined as K-L grade 0 or 1, but with clinical signs and symptoms).35 Magnetic resonance images studies has identified tissue lesions in knees with K-L grades below 2.36, 37 In this study, the ACR clinical criteria32 were applied, based on which, 28 knees with clinical OA were identified. Most of the participants’ knees had pain while walking up or down stairs (92.9%), crepitus (92.9%), and morning stiffness for less than 30 minutes (82.1%), while clinical signs relating to structural damage were uncommon (i.e., bony enlargement: 3.6%). It was suggested that the onset of self-reported knee pain during stair climbing is an important criterion for the clinical definition of early knee OA.38 In addition, knee pain was more prevalent in the anterior (50.0%) and lateral (50.0%) regions than in the medial region (42.9%). This was different from individuals with radiographically established knee OA (K-L grade ≥2), for whom the most frequent pain zone was around the medial joint line (75%).39 As such, the 28 knees identified as having OA at 12 months were most likely at the early stage of the disease.
This was the first prospective study relating passive muscle properties to the incidence of clinical knee OA. We found that greater passive stiffness of the superficial quadriceps heads at baseline was associated with a higher risk of having clinical knee OA at the 12-month follow-up. The question remains why an increase in passive stiffness of the quadriceps would increase the risk of clinical knee OA. An increase in passive stiffness of the quadriceps may cause an overall increase in knee joint stiffness via its muscle-tendon complex system or its connection with the ligamentous system around the knee joint.22, 23 Increased passive stiffness of skeletal muscle generates a steeper length–tension relationship (reflecting reduced compliance)14 during joint motion. The initial loading phase during gait is the crucial time during which flexion of the knee modulates the rapid increase in ground reaction force.2, 4 High dynamic joint stiffness during the loading response phase, due to the increased resistance of the muscles or soft tissues around the knee during knee joint movement,10, 11 has been related to the risk of knee OA.11, 15, 16 However, further studies are required to assess the effects of increased quadriceps stiffness on gait mechanics.
Our findings indicate that the association between passive stiffness of the quadriceps and the incidence of clinical knee OA was specific to the RF and VMO muscles. During daily weight-bearing activities, eccentric contraction of the quadriceps is essential to de-load the knee joint during the loading phase.40 During this process, the bi-articular RF muscle head is subjected to more dynamic stretching than the other mono-articular quadriceps heads.41 In addition, Xu et al. (2019) found that the eccentric-exercise-induced increase in passive stiffness of the RF is significantly associated with a reduction in peak torque of the quadriceps.41 This may suggest that an increase in passive stiffness of the RF hampers the contraction of the quadriceps, thus affecting the efficiency of internal force production to counteract the external load on the knee. VMO muscle fibers mesh with the medial patellofemoral ligament (MPFL).22 An increase in passive stiffness of the VMO may reinforce the passive tension on the medial collateral ligament (MCL) via the VMO-MPFL complex and the medial knee retinacular fibres.22, 42 The length of the MCL increases by approximately 20% during knee flexion, suggesting that the normal length pattern of the MCL is important for knee kinematics.26 Increased VMO stiffness may increase dynamic knee stiffness through its effect on the medial collateral ligament and hence increase the likelihood of developing knee OA. Moreover, the increased passive stiffness of the knee extensor system may increase the risk of knee injuries (e.g., floating knee43), which may also affect the development of knee OA. Future studies are needed to explore therapeutic methods (e.g., physical agent modalities44 or nutraceutical therapy45) aiming to decrease the muscle-head-specific stiffness, which may be beneficial for the prevention of knee OA.
The VL connects with the lateral retinaculum.23 Conceptually, a stiffer VL may also increase the overall stiffness of the knee joint via its connection with lateral retinacular fibres. However, no significant association was observed between VL stiffness at baseline and the risk of clinical knee OA at the 12-month follow-up. The reason for this result may be that the VL has a larger cross-sectional area than the RF and VMO.46 For skeletal muscles, the slope of the positive linear relationship between muscle passive stiffness and passive force is largely determined by the anatomical cross-sectional area (i.e., a larger muscle anatomical cross-sectional area results in a smaller slope).47 The relative contribution of muscle passive stiffness and muscle cross-sectional area may have different effects on the overall stiffness of the knee and the incidence of knee OA over a 12-month period. The contribution of physiological and biomechanical mechanisms to the associations between muscle heads and the incidence of knee OA requires further investigation.
Our results echo those from previous reports that quadriceps weakness is a risk factor for knee OA in older adults at around 30-month follow-up time point.7 However, our effect size (RR=0.19) for quadriceps strength was approximately double the effect sizes reported in the previous study.7 There may be two possible reasons for this. First, previous studies have been based on radiographic knee OA, whereas we used clinical criteria to diagnose OA. Quadriceps weakness may have a larger effect on clinical symptoms than knee joint space narrowing.48 Second, if a true effect exists, the radiographic criteria (K/L grade ≥2) may have missed cases with early knee OA (K/L grade 0 or 1)35 and may have underestimated the effect of quadriceps strength on the incidence of knee OA. The clinical criteria that we used to define knee OA at 12-month follow-up in this study may represent early-stage knee OA. This signifies the importance of muscle strength in the prevention of the onset of knee OA. The possible mechanism whereby quadriceps strength affects the incidence of knee OA may be related to excessive knee joint loading caused by insufficient protection by the weak muscles.49 After adjusting for covariates, a trend remained for quadriceps strength (RR=0.18, 95% CI: 0.03-1.25, P=0.083). Previous prospective studies with larger sample sizes have found that significant associations between baseline quadriceps weakness and the incidence of knee OA at a later timepoint only exist in females, but not in males.5, 6 In this cohort, sex was also a significant cofounder. Thus, we conducted sensitivity analyses for each sex. Similar trends were found for females (RR=0.19, 95% CI: 0.03-1.06, P=0.058) and males (RR=0.13, 95% CI: 0.01-1.33, P=0.086). Because of the reduced study power (especially in the male group), we had difficulties in drawing a firm conclusion regarding the sex effect. Future studies with larger sample sizes are warranted to confirm the sex effect on the association between quadriceps strength and the early incidence of knee OA. Our results showed that hamstrings strength is not a significant predictor of the incidence of knee OA, which is consistent with a previous 31-month follow-up study.5
Limitations of the study
The present study has several limitations. First, only the three superficial muscle heads were measured to represent quadriceps stiffness. The deep head, vastus intermedius muscle, was not measured because its deep location beneath the superficial heads results in poor reliability for shear-wave elastography measurements.20 Second, our prior power analysis based on the previous study showed that 154 participants (308 knees) were needed.7 After considering the expected 20% dropouts, we were originally planning to recruit over 200 eligible participants (>400 knees). Unfortunately, our baseline tests were interrupted in January 2020 by the COVID-19. Moreover, due to the pandemic, this cohort had a high drop-out rate. However, the demographics and baseline measurements were similar between the participants who dropped out and those who completed the study. In addition, the study population involved relatively healthy, well-functioning older adults living in community settings, and the 12-month follow-up primarily identified early-stage knee OA; hence, no participant dropped out because of disease. Therefore, the risk of study bias is low. However, the reduced study power may have affected the examination of the effect of sex on such associations. As this was the first study to prospectively examine the association between passive quadriceps stiffness and the risk of knee OA. Future studies with larger sample size are expected to further confirm our findings. Third, there may be concerns about the relatively low specificity (69%) of the ACR clinical criteria to define knee OA.32 We acknowledge the high disagreement between knee OA cases defined by the ACR clinical criteria and those defined by the radiographic criteria.50 Therefore, our findings should be interpreted as the effects of baseline muscle properties on clinically defined knee OA.
Conclusions
Increased passive stiffness of the quadriceps at baseline is associated with a higher risk of developing clinical knee OA at the 12-month follow-up in older adults. Our findings suggest that good pliability and strength of the quadriceps may both be essential for the prevention of clinical knee OA.
Supplementary Digital Material 1
Supplementary Table I
Profiles of the knees classified as having clinical osteoarthritis at the 12-month follow-up.
References
- 1.Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol 2016;12:92–101. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26439406&dopt=Abstract 10.1038/nrrheum.2015.135 [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Mündermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol 2006;18:514–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16896293&dopt=Abstract 10.1097/01.bor.0000240365.16842.4e [DOI] [PubMed] [Google Scholar]
- 3.Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol 2011;7:57–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21119605&dopt=Abstract 10.1038/nrrheum.2010.195 [DOI] [PubMed] [Google Scholar]
- 4.Perry J, Burnfield J. Gait analysis: normal and pathological function. Thorofare: Slack Incorporated; 2010. [Google Scholar]
- 5.Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum 1998;41:1951–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9811049&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Thorlund JB, Felson DT, Segal NA, Nevitt MC, Niu J, Neogi T, et al. Effect of knee extensor strength on incident radiographic and symptomatic knee osteoarthritis in individuals with meniscal pathology: data from the multicenter osteoarthritis study. Arthritis Care Res (Hoboken) 2016;68:1640–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26991698&dopt=Abstract 10.1002/acr.22889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum 2009;61:1210–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19714608&dopt=Abstract 10.1002/art.24541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemnitz J, Wirth W, Eckstein F, Ruhdorfer A, Culvenor AG. Longitudinal change in thigh muscle strength prior to and concurrent with symptomatic and radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2017;25:1633–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28698106&dopt=Abstract 10.1016/j.joca.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 9.Hodges PW, van den Hoorn W, Wrigley TV, Hinman RS, Bowles KA, Cicuttini F, et al. Increased duration of co-contraction of medial knee muscles is associated with greater progression of knee osteoarthritis. Man Ther 2016;21:151–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26254263&dopt=Abstract 10.1016/j.math.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Davis RB, DeLuca PA. Gait characterization via dynamic joint stiffness. Gait Posture 1996;4:224–31. 10.1016/0966-6362(95)01045-9 [DOI] [Google Scholar]
- 11.Chang AH, Chmiel JS, Almagor O, Guermazi A, Prasad PV, Moisio KC, et al. Association of baseline knee sagittal dynamic joint stiffness during gait and 2-year patellofemoral cartilage damage worsening in knee osteoarthritis. Osteoarthritis Cartilage 2017;25:242–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27729289&dopt=Abstract 10.1016/j.joca.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axel K, Desailly E, Dumas R. Contribution of passive moments to inter-segmental moments during gait: A systematic review. J Biomech 2021;122:110450. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33930687&dopt=Abstract 10.1016/j.jbiomech.2021.110450 [DOI] [PubMed] [Google Scholar]
- 13.Whittington B, Silder A, Heiderscheit B, Thelen DG. The contribution of passive-elastic mechanisms to lower extremity joint kinetics during human walking. Gait Posture 2008;27:628–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17928228&dopt=Abstract 10.1016/j.gaitpost.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 1984;139:677–89. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6526719&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 15.Zeni JA, Jr, Higginson JS. Dynamic knee joint stiffness in subjects with a progressive increase in severity of knee osteoarthritis. Clin Biomech (Bristol, Avon) 2009;24:366–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19250725&dopt=Abstract 10.1016/j.clinbiomech.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon SJ, Hinman RS, Creaby MW, Kemp G, Crossley KM. Knee joint stiffness during walking in knee osteoarthritis. Arthritis Care Res (Hoboken) 2010;62:38–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20191489&dopt=Abstract 10.1002/acr.20012 [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Fu SN, Hug F. Age-related increase in muscle stiffness is muscle length dependent and associated with muscle force in senior females. BMC Musculoskelet Disord 2021;22:829. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34579696&dopt=Abstract 10.1186/s12891-021-04519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira D, Peleteiro B, Araújo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19:1270–85. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21907813&dopt=Abstract 10.1016/j.joca.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 19.Koo TK, Guo JY, Cohen JH, Parker KJ. Relationship between shear elastic modulus and passive muscle force: an ex-vivo study. J Biomech 2013;46:2053–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23769175&dopt=Abstract 10.1016/j.jbiomech.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Hug F, Fu SN. Stiffness of individual quadriceps muscle assessed using ultrasound shear wave elastography during passive stretching. J Sport Health Sci 2018;7:245–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30356470&dopt=Abstract 10.1016/j.jshs.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waligora AC, Johanson NA, Hirsch BE. Clinical anatomy of the quadriceps femoris and extensor apparatus of the knee. Clin Orthop Relat Res 2009;467:3297–306. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19690926&dopt=Abstract 10.1007/s11999-009-1052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc 2006;14:7–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16001289&dopt=Abstract 10.1007/s00167-005-0631-z [DOI] [PubMed] [Google Scholar]
- 23.Merican AM, Amis AA. Anatomy of the lateral retinaculum of the knee. J Bone Joint Surg Br 2008;90:527–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18378934&dopt=Abstract 10.1302/0301-620X.90B4.20085 [DOI] [PubMed] [Google Scholar]
- 24.Flandry F, Hommel G. Normal anatomy and biomechanics of the knee. Sports Med Arthrosc Rev 2011;19:82–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21540705&dopt=Abstract 10.1097/JSA.0b013e318210c0aa [DOI] [PubMed] [Google Scholar]
- 25.Takeda Y, Xerogeanes JW, Livesay GA, Fu FH, Woo SL. Biomechanical function of the human anterior cruciate ligament. Arthroscopy 1994;10:140–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8003139&dopt=Abstract 10.1016/S0749-8063(05)80081-7 [DOI] [PubMed] [Google Scholar]
- 26.Wang CJ, Walker PS, Wolf B. The effects of flexion and rotation on the length patterns of the ligaments of the knee. J Biomech 1973;6:587–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4757478&dopt=Abstract 10.1016/0021-9290(73)90016-X [DOI] [PubMed] [Google Scholar]
- 27.Physical Activity Guidelines for Americans. Physical Activity Guidelines Advisory Committee of U.S. Washington, DC: Department of Health and Human Services; 2008. p. 29-34. [Google Scholar]
- 28.Lee JY, Cho SJ, Na DL, Kim SK, Youn JH, Kwon M, et al. ; Dong Woo Lee; Hong Jin Jeon; You Ra Lee; Maeng Je Cho. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol 2008;21:104–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18474719&dopt=Abstract 10.1177/0891988708316855 [DOI] [PubMed] [Google Scholar]
- 29.Ema R, Wakahara T, Mogi Y, Miyamoto N, Komatsu T, Kanehisa H, et al. In vivo measurement of human rectus femoris architecture by ultrasonography: validity and applicability. Clin Physiol Funct Imaging 2013;33:267–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23692615&dopt=Abstract 10.1111/cpf.12023 [DOI] [PubMed] [Google Scholar]
- 30.Alfuraih AM, O’Connor P, Hensor E, Tan AL, Emery P, Wakefield RJ. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: variables affecting reliability of SWE. J Clin Ultrasound 2018;46:108–15. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28990683&dopt=Abstract 10.1002/jcu.22534 [DOI] [PubMed] [Google Scholar]
- 31.Knoop J, Dekker J, Klein JP, van der Leeden M, van der Esch M, Reiding D, et al. Biomechanical factors and physical examination findings in osteoarthritis of the knee: associations with tissue abnormalities assessed by conventional radiography and high-resolution 3.0 Tesla magnetic resonance imaging. Arthritis Res Ther 2012;14:R212. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23039323&dopt=Abstract 10.1186/ar4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association . Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039–49. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3741515&dopt=Abstract 10.1002/art.1780290816 [DOI] [PubMed] [Google Scholar]
- 33.Damen J, van Rijn RM, Emans PJ, Hilberdink WK, Wesseling J, Oei EH, et al. Prevalence and development of hip and knee osteoarthritis according to American College of Rheumatology criteria in the CHECK cohort. Arthritis Res Ther 2019;21:4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30611305&dopt=Abstract 10.1186/s13075-018-1785-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=13498604&dopt=Abstract 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luyten FP, Bierma-Zeinstra S, Dell’Accio F, Kraus VB, Nakata K, Sekiya I, et al. Toward classification criteria for early osteoarthritis of the knee. Semin Arthritis Rheum 2018;47:457–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28917712&dopt=Abstract 10.1016/j.semarthrit.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 36.Mahmoudian A, Lohmander LS, Mobasheri A, Englund M, Luyten FP. Early-stage symptomatic osteoarthritis of the knee - time for action. Nat Rev Rheumatol 2021;17:621–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34465902&dopt=Abstract 10.1038/s41584-021-00673-4 [DOI] [PubMed] [Google Scholar]
- 37.Sharma L, Hochberg M, Nevitt M, Guermazi A, Roemer F, Crema MD, et al. Knee tissue lesions and prediction of incident knee osteoarthritis over 7 years in a cohort of persons at higher risk. Osteoarthritis Cartilage 2017;25:1068–75. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28232012&dopt=Abstract 10.1016/j.joca.2017.02.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hensor EM, Dube B, Kingsbury SR, Tennant A, Conaghan PG. Toward a clinical definition of early osteoarthritis: onset of patient-reported knee pain begins on stairs. Data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2015;67:40–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25074673&dopt=Abstract 10.1002/acr.22418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Ginckel A, Bennell KL, Campbell PK, Wrigley TV, Hunter DJ, Hinman RS. Location of knee pain in medial knee osteoarthritis: patterns and associations with self-reported clinical symptoms. Osteoarthritis Cartilage 2016;24:1135–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26836285&dopt=Abstract 10.1016/j.joca.2016.01.986 [DOI] [PubMed] [Google Scholar]
- 40.LaStayo PC, Woolf JM, Lewek MD, Snyder-Mackler L, Reich T, Lindstedt SL. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J Orthop Sports Phys Ther 2003;33:557–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14620785&dopt=Abstract 10.2519/jospt.2003.33.10.557 [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Fu SN, Zhou D, Huang C, Hug F. Relationship between pre-exercise muscle stiffness and muscle damage induced by eccentric exercise. Eur J Sport Sci 2019;19:508–16. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30362889&dopt=Abstract 10.1080/17461391.2018.1535625 [DOI] [PubMed] [Google Scholar]
- 42.Robinson JR, Sanchez-Ballester J, Bull AM, Thomas RW, Amis AA. The posteromedial corner revisited. An anatomical description of the passive restraining structures of the medial aspect of the human knee. J Bone Joint Surg Br 2004;86:674–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15274262&dopt=Abstract 10.1302/0301-620X.86B5.14853 [DOI] [PubMed] [Google Scholar]
- 43.Rollo G, Falzarano G, Ronga M, Bisaccia M, Grubor P, Erasmo R, et al. Challenges in the management of floating knee injuries: results of treatment and outcomes of 224 consecutive cases in 10 years. Injury 2019;50(Suppl 4):S30–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30910244&dopt=Abstract 10.1016/j.injury.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 44.Letizia Mauro G, Scaturro D, Gimigliano F, Paoletta M, Liguori S, Toro G, et al. Physical Agent Modalities in Early Osteoarthritis: A Scoping Review. Medicina (Kaunas) 2021;57:1165. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34833383&dopt=Abstract 10.3390/medicina57111165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ripani U, Manzarbeitia-Arroba P, Guijarro-Leo S, Urrutia-Graña J, De Masi-De Luca A, Vitamin C., Vitamin C. May Help to Reduce the Knee’s Arthritic Symptoms. Outcomes Assessment of Nutriceutical Therapy. Med Arh 2019;73:173–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31404121&dopt=Abstract 10.5455/medarh.2019.73.173-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol 2001;90:2070–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11356767&dopt=Abstract 10.1152/jappl.2001.90.6.2070 [DOI] [PubMed] [Google Scholar]
- 47.Koo TK, Hug F. Factors that influence muscle shear modulus during passive stretch. J Biomech 2015;48:3539–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26113291&dopt=Abstract 10.1016/j.jbiomech.2015.05.038 [DOI] [PubMed] [Google Scholar]
- 48.Culvenor AG, Ruhdorfer A, Juhl C, Eckstein F, Øiestad BE. Knee extensor strength and risk of structural, symptomatic, and functional decline in knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2017;69:649–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27563843&dopt=Abstract 10.1002/acr.23005 [DOI] [PubMed] [Google Scholar]
- 49.Bennell KL, Wrigley TV, Hunt MA, Lim BW, Hinman RS. Update on the role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am 2013;39:145–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23312414&dopt=Abstract 10.1016/j.rdc.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 50.Peat G, Thomas E, Duncan R, Wood L, Hay E, Croft P. Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Ann Rheum Dis 2006;65:1363–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16627539&dopt=Abstract 10.1136/ard.2006.051482 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Profiles of the knees classified as having clinical osteoarthritis at the 12-month follow-up.