Sharma et al. define a new primary atopic disorder caused by heterozygous gain-of-function variants in STAT6. This results in severe, early-onset allergies, and is seen in 16 patients from 10 families. Anti–IL-4Rα antibody and JAK inhibitor treatment were highly effective.
Abstract
STAT6 (signal transducer and activator of transcription 6) is a transcription factor that plays a central role in the pathophysiology of allergic inflammation. We have identified 16 patients from 10 families spanning three continents with a profound phenotype of early-life onset allergic immune dysregulation, widespread treatment-resistant atopic dermatitis, hypereosinophilia with esosinophilic gastrointestinal disease, asthma, elevated serum IgE, IgE-mediated food allergies, and anaphylaxis. The cases were either sporadic (seven kindreds) or followed an autosomal dominant inheritance pattern (three kindreds). All patients carried monoallelic rare variants in STAT6 and functional studies established their gain-of-function (GOF) phenotype with sustained STAT6 phosphorylation, increased STAT6 target gene expression, and TH2 skewing. Precision treatment with the anti–IL-4Rα antibody, dupilumab, was highly effective improving both clinical manifestations and immunological biomarkers. This study identifies heterozygous GOF variants in STAT6 as a novel autosomal dominant allergic disorder. We anticipate that our discovery of multiple kindreds with germline STAT6 GOF variants will facilitate the recognition of more affected individuals and the full definition of this new primary atopic disorder.
Introduction
Asthma and related atopic diseases, including atopic dermatitis, food allergy, allergic rhinitis, and eosinophilic gastrointestinal diseases, are estimated to affect ∼20% of the global population imposing immense health and economic burdens (Dierick et al., 2020). Identifying human single-gene defects that lead to severe allergic disease—so-called primary atopic disorders (PADs)—is a powerful strategy to define the cellular and molecular mechanisms that drive human allergic inflammation (Lyons and Milner, 2018; Vaseghi-Shanjani et al., 2021). Identifying new PADs accelerates the diagnosis and treatment of affected individuals and can uncover new molecular targets for preventing and treating common allergic disease.
Currently there are only a few known inborn errors of immunity (IEIs) underlying severe allergic disease (Milner, 2020). Indeed, most cases are of unknown etiology, particularly those that are isolated or sporadic. In this study, we describe a novel human PAD caused by germline heterozygous gain-of-function (GOF) variants in the gene STAT6 found in 16 individuals from 10 unrelated families spanning three continents. Signal transducer and activator of transcription 6 (STAT6) is the main transcription factor that mediates the biological effects of IL-4, a key cytokine necessary for type 2 differentiation of T cells, B cell survival, proliferation, and class switching to IgE (Goenka and Kaplan, 2011; Takeda et al., 1996; Villarino et al., 2020; Villarino et al., 2017), as well as that of IL-13, a cytokine linked to anaphylaxis (Gowthaman et al., 2019). Affected individuals experienced early-onset severe, sometimes fatal, multisystem allergic disease which was refractory to conventional treatments. Notably, precision therapeutics aimed at targeting exaggerated STAT6 signaling were beneficial in those who received them.
Results
Identification of 16 patients from 10 families with severe early-onset allergic disease heterozygous for rare damaging STAT6 variants
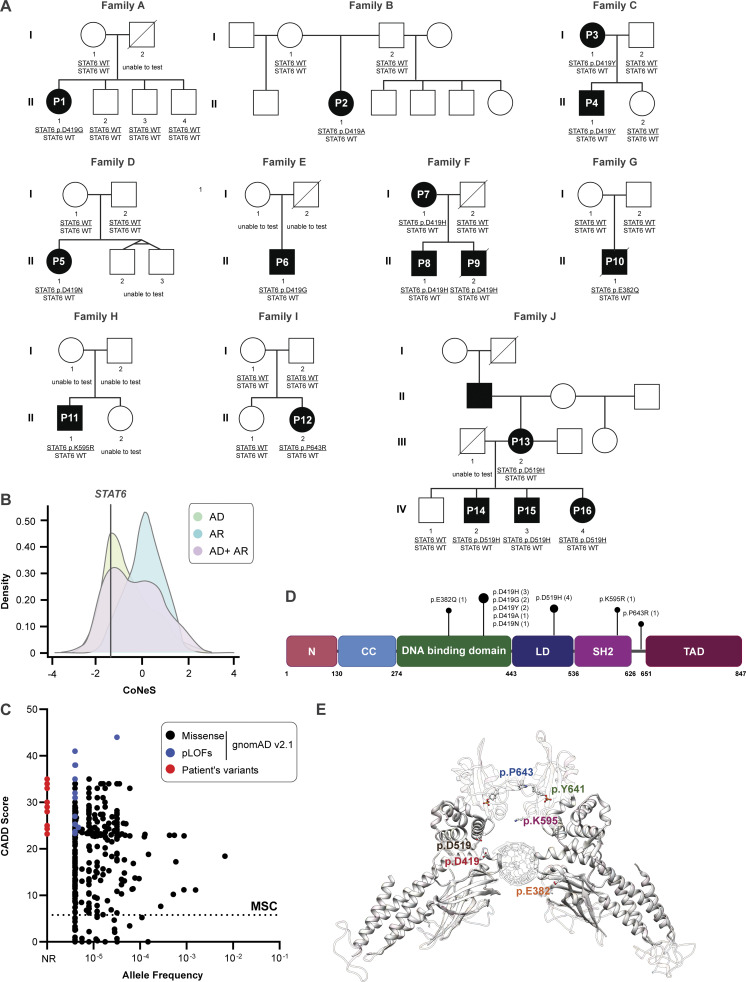
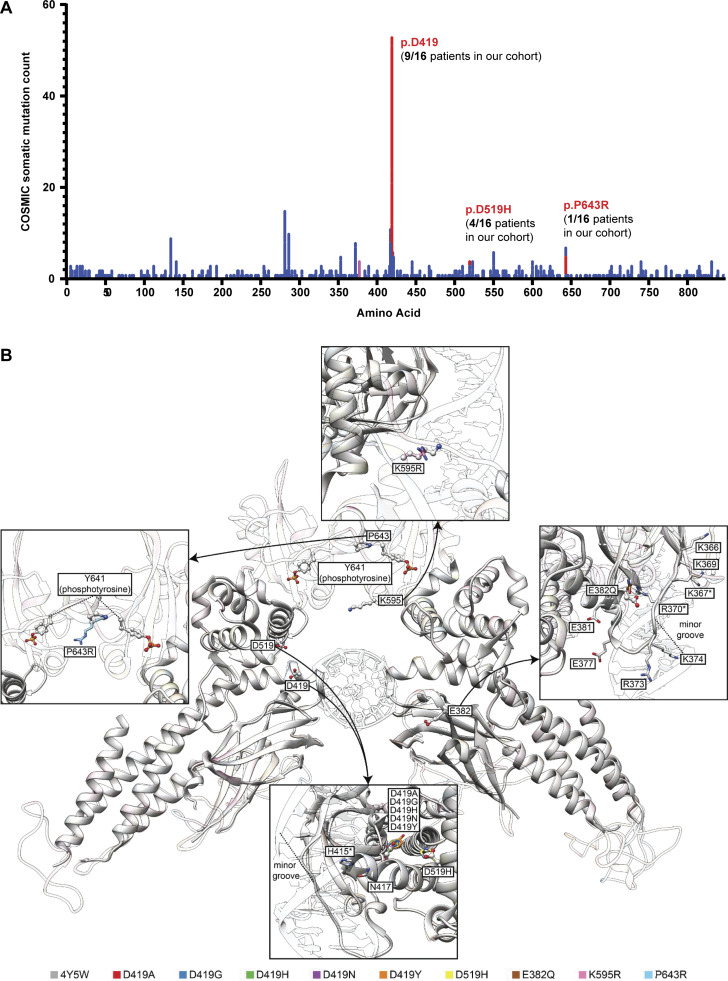
We studied 16 patients from 10 kindreds with severe early-onset allergic disease spanning three continents. Patients were identified by their expert clinicians as candidates for genetic assessment based on their extreme phenotype and, in some cases, their family history (see clinical narratives in Data S1; Tables S4 and S5; and Fig. 1 A). The patients were from diverse ethnicities, specifically European (Kindred D, F, and J), Middle Eastern (Kindred A and C), Hispanic (Kindred B), South Asian (Kindred H), East Asian (Kindred E), and Southeast Asian (Kindred Y). The cases were either sporadic (seven kindreds) or affected multiple individuals of either sex over different generations consistent with autosomal dominant (AD) inheritance (Kindreds C, F, and J). All patients carried monoallelic rare variants in STAT6 (NM_001178079.2). The consensus negative selection score of STAT6 reveals a negative selection score that overlaps with known AD IEIs (Rapaport et al., 2021), consistent with the AD inheritance pattern observed in Kindreds C, F, and J (Fig. 1 B). In addition, and also consistent with an AD disorder, by sequencing both healthy parents (when available) we established that the STAT6 mutation was de novo in Patient 2 (P2), P5, P10, and P12 (from Kindreds B, D, G, and I, respectively; Fig. 1 A). The disease was fully penetrant in the families studied, as all STAT6 variant carriers were affected. None of the variants have previously been reported in population databases (Fig. 1 C; i.e., gnomAD). All the variants were private to the studied kindreds, except the p.D419G variant which was common in Kindreds A and E. Pathogenicity prediction models identify all of these variants to be pathogenic, evidenced by high pathogenic CADD (Rentzsch et al., 2021), SIFT (Sim et al., 2012), and Polyphen-2 (Adzhubei et al., 2010) scores (Tables S4 and S5). Remarkably, nine patients from six kindreds carried a variant affecting amino acid D419. Importantly, variants leading to amino acid changes at p.D419, p.D519, and p.P643 can be found in the Catalogue of Somatic Mutations in Cancer (COSMIC) database as recurrent somatic variants in lymphoma with some experimental evidence for a GOF phenotype for variants at p.D419 (Yildiz et al., 2015; Zamò et al., 2018; Fig. S1 A). The reported variants lie in different protein domains of STAT6, including the DNA-binding domain (p.E382 and p.D419), the linker domain (p.D519), and the SH2 domain (p.K595), while p.P643 lies in close proximity to the p.Y641 phosphorylation site (Fig. 1, D–E). Although the variants were located within different domains of the STAT6 protein, modeling of STAT6 interacting with DNA reveals that all the identified variants (with the exception of p.P643) lie near the protein–DNA interface and result in amino acids changes leading to increased electro-positivity at physiological pH (Fig. 1 E). Notably, E382 and D419 are located in regions responsible for protein–DNA recognition (Li et al., 2016). Changes in these variants decrease the electro-negativity of the protein near the DNA-binding interface and are predicted to enhance STAT6 binding to DNA (Fig. 1 E and Fig. S1 B). In aggregate, these data suggest that the STAT6 germline monoallelic variants identified in the patients underlie severe allergic disease by a GOF mechanism.
Figure 1.
16 patients with severe allergic disease and STAT6 variants in different protein domains. (A) Family pedigree of the 16 patients from 10 different families. Filled symbols = affected individual; unfilled symbols = unaffected individual. (B) Consensus negative selection (CoNeS) score for STAT6 in relation to the score for known IEI genes reported with inheritance pattern of either AD, AR, or both (AD + AR). (C) Frequency and CADD score for missense (black) and predicted LOF (pLOF, blue) STAT6 variants reported in a public database and STAT6 variants reported in our patient cohort (red). The dotted line corresponds to the mutation significance cutoff (MSC). (D) Schematic illustrating the protein domains of STAT6. Amino acid location of the variants shown are highlighted, with the length of the bar corresponding to the number of patients reported with variants at that site. (E) Structural model of the DNA-STAT6 homodimer complex showing location of the different STAT6 variants in relation to the DNA-binding interface.
Figure S1.
Pathogenic STAT6 germline variants lie in different protein domains and are frequently identified as somatic variants. (A) Somatic mutation counts for different amino acid changed as reported by COSMIC for STAT6. Red highlighted changes are those germline variants also identified in our cohort that cause severe allergic disease. (B) Structural model of the DNA-STAT6 homodimer complex showing location of the different STAT6 variants in relation to the DNA-binding interface. Specifically, zoom-ins for variants at each location are shown in relation to previously described variants known to affect STAT6 function.
Unifying clinical features of the 16 patients with severe allergic disease
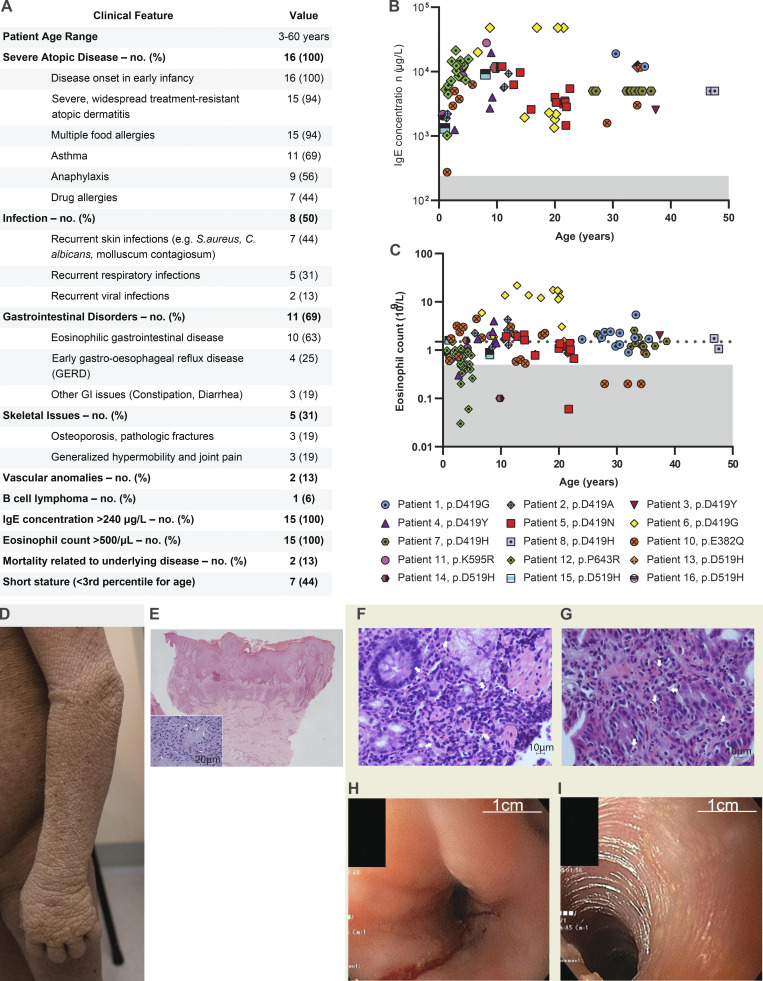
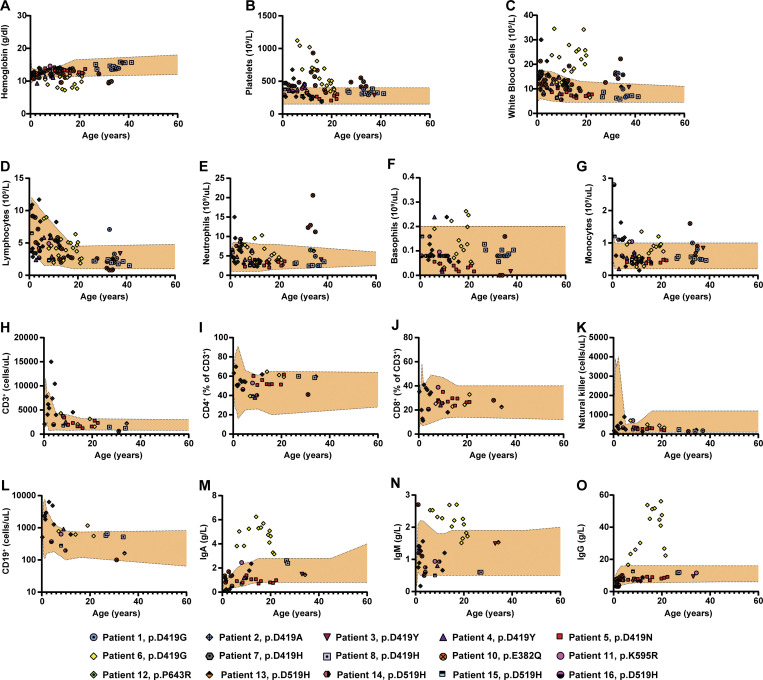
The patients in the cohort were aged from 3 to 60 yr. Full clinical narratives are provided in Data S1, and their clinical features are summarized in Fig. 2 A. All had severe allergic disease which began in their first year of life. Severe, treatment-resistant atopic dermatitis (15/16) and food allergies (15/16) were the most common clinical manifestations, followed by asthma (11/16) and eosinophilic gastrointestinal disease (10/16) and severe episodes of anaphylaxis (9/16). Clinical laboratory testing was notable for eosinophilia and markedly elevated serum IgE levels (Fig. 2, B and C). Other aspects of the clinical laboratory and immunological work up were largely unremarkable, although clinical hallmarks of chronic systemic inflammation were present in some patients (i.e., elevations in white blood cell counts, platelets, and serum immunoglobulin levels). T, B, and natural killer (NK) cell numbers were all typically in the normal range (Fig. S2). Clinical biopsies confirmed the presence of eosinophils in the skin and gastrointestinal tract (Fig. 2, E–G). Endoscopic visualization of the esophagus revealed trachealization and furrowing consistent with eosinophilic esophagitis (Bolton et al., 2018; Fig. 2, H and I).
Figure 2.
Major clinical features of the 16 patients. (A) Tabulation and comparison of the clinical phenotype for 16 patients. Please note blood eosinophil and IgE values were only available for 15 patients. (B) IgE concentration in whole blood for 15 out of the 16 patients. Shaded area represents IgE < 240 µg/liter, which is the typical upper limit of normal. (C) Eosinophil count in whole blood for 15 out of the 16 patients. Shaded area represents counts <0.5 × 109/liter, which is the typical upper limit of normal. The horizontal broken line denotes an eosinophil count of 1.5 × 109/liter, since hypereosinophilic syndrome is traditionally defined as peripheral blood eosinophilia >1.5 × 109/liter persisting ≥6 mo. (D) Photograph of widespread and severe atopic disease. (E) Photomicrograph of the skin biopsy showing marked pseudoepitheliomatous hyperplasia with acanthosis, hyperkeratosis, and focal parakeratosis, suggestive of lichen simplex chronicus (H&E stain, original magnification 2×). Moderate chronic inflammation within the papillary dermis in which scattered eosinophils (white arrows) are conspicuous (inset, H&E stain; original magnification, 40×). (F) Photomicrograph of duodenal biopsy showing abundant eosinophils (white arrows) amongst lymphocytes (H&E stain; original magnification, 40×). (G) Photomicrograph of gastric antral biopsy showing abundant infiltrate of eosinophils (arrows) amongst lymphocytes and plasma cells (H&E stain; original magnification, 40×). (H and I) Endoscopic images showing (H) furrowing and (I) trachealization in the middle esophagus, suggestive of eosinophilic esophagitis.
Figure S2.
Complete blood counts and immunological workup of patients with pathogenic STAT6 variants. (A–G) Complete blood count for 15 out of the 16 patients and age-based references (orange-shaded area) for the following populations: (A) hemoglobin, (B) platelets, (C) white blood cells, (D) lymphocytes, (E) neutrophils, (F) basophils, and (G) monocytes. (H–L) Immunological workup for 15 out of the 16 patients showing age-based references (orange-shaded area) and populations quantification for: (H) CD3+ T cells, (I) CD4+ CD3+ T cells, (J) CD8+ CD3+ T cells, (K) NK cells, and (L) CD19+ B cells. (M–O) Immunoglobulin concentrations for 15 out of the 16 patients showing age-based references (orange-shaded area): (M) IgA, (N) IgM, and (O) IgA.
In addition to atopic disease, half of the patients presented with recurrent skin, respiratory, and viral infections, although there were no deep-seated or fatal infections. Short stature (less than third percentile for age) was a common feature (7/16), and 5/16 had other skeletal issues such as pathologic fractures and generalized hypermobility. Finally, two of the patients died from their disease; one from anaphylaxis at the age of 20 yr and another from a cerebral aneurysm at age 35 yr. These clinical presentations highlight the severity of the multi-system disease found in this patient cohort.
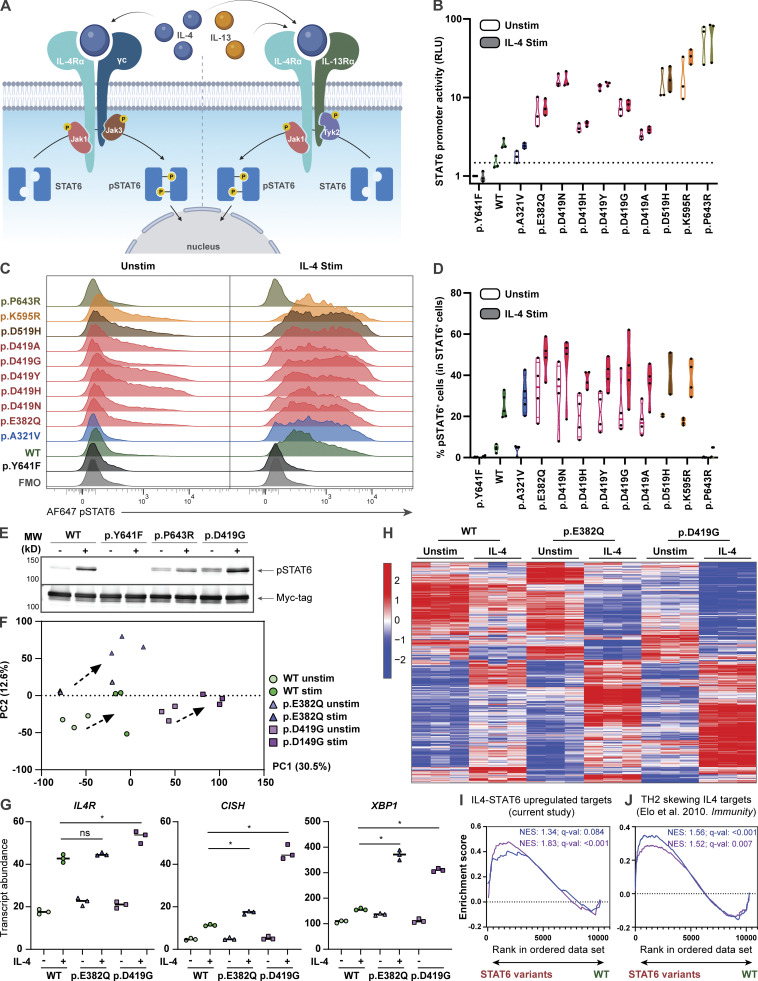
Functional analysis of the STAT6 variants confirms their GOF activity
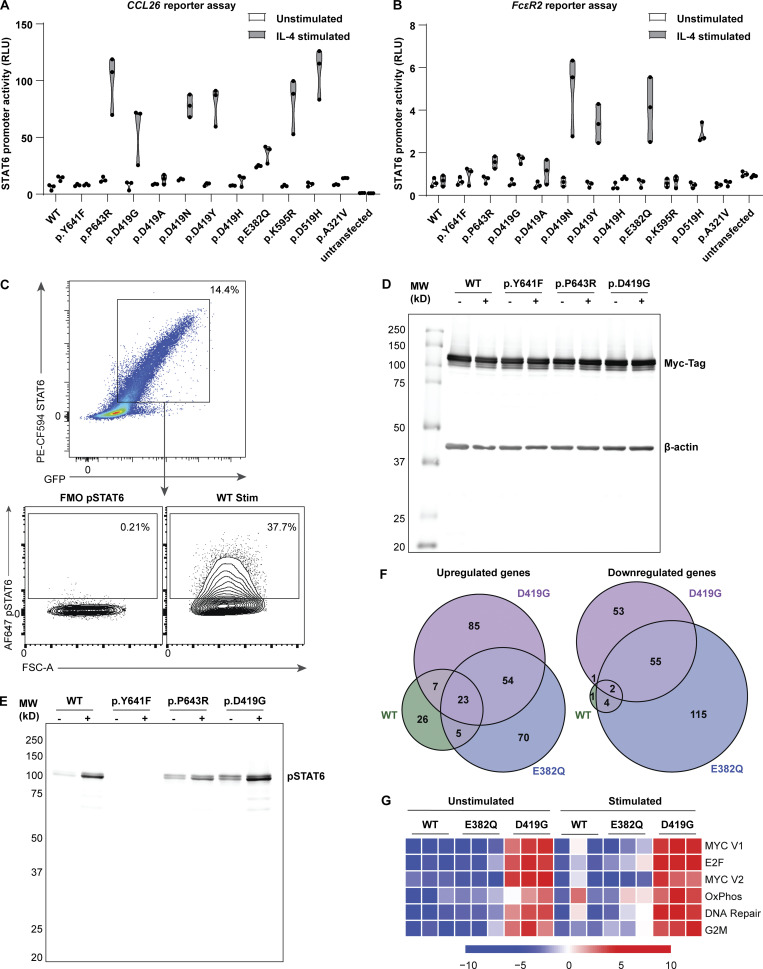
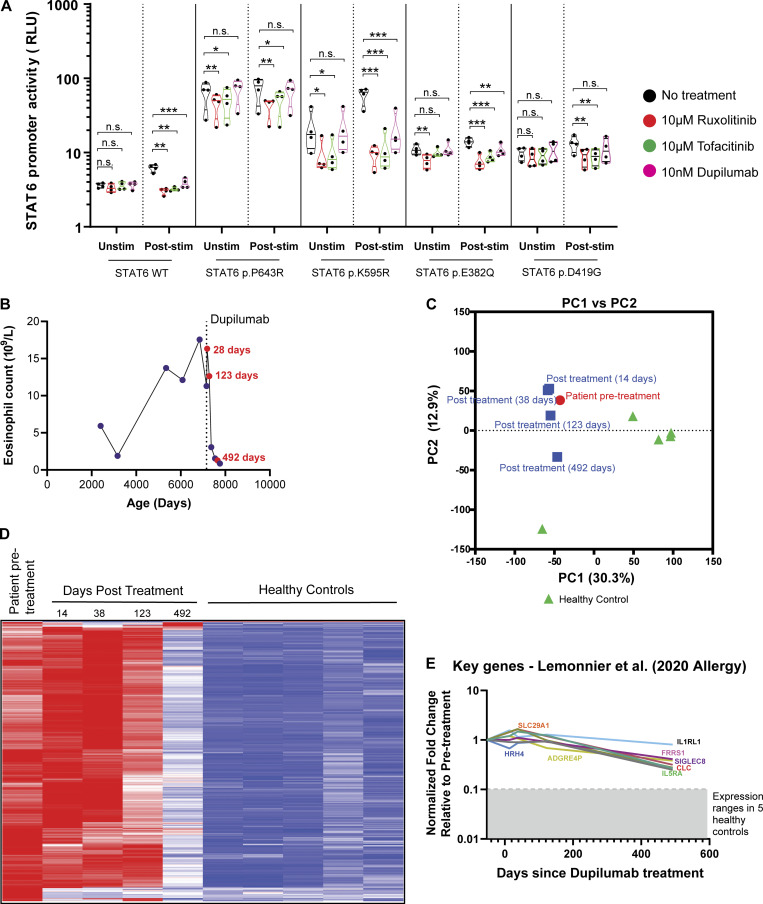
To assess the functional impact of the STAT6 variants, we selected HEK293 cells as our model system, as these cells lack endogenous STAT6 but express other components of the IL-4R pathway (Fig. 3 A; Mikita et al., 1996). HEK293 cells were transfected with each of the 10 patient STAT6 variants along with three different controls: WT STAT6, a predicted damaging STAT6 population variant found in the gnomAD healthy population database (p.A321V), and a biochemically inactive STAT6 variant (p.Y641F; Wick and Berton, 2000). To investigate STAT6 transcription factor activity, we conducted luciferase assays with three different reporter plasmids containing STAT6 binding sites (Li et al., 2016). While there were some difference related to the specific combinations of reporter plasmids and patient variants, there was evidence of GOF activity with all STAT6 patient variants resulting in higher promoter activity at baseline and after stimulation compared to the controls (Fig. 3 B and Fig. S3, A and B). The phosphorylation status of STAT6 variants was also quantified by flow cytometry and was confirmed to be higher at baseline and after stimulation compared to WT (Fig. 3, C and D; and Fig. S3 C). STAT6 phosphorylation was not detectable by flow cytometry for the p.P643R variant; however, increased baseline phosphorylation was confirmed by immunoblotting (Fig. 3 E and Fig. S3, D and E). This may point to a conformational change in tertiary structure of STAT6 near the phosphorylation site p.Y641 for this variant that rendered it inaccessible to the flow cytometry antibody.
Figure 3.
STAT6 variants lead to increased STAT6 activity in HEK293 cells and Jurkat T cells. (A) Schematic illustrating classical IL-4–mediated STAT6 activation, dimerization, and phosphorylation. (B) Luciferase assay of STAT6 activity on a plasmid containing a 4× STAT6 binding site (TTCCCAAGAA; the underlined bases represent two half-sites for STAT6-specific binding) for WT-, different STAT6 variant–transfected HEK293 cells before and after stimulation with IL-4 (0.02 ng/ml for 4 h); n = 3. (C) Phospho-STAT6 (Y641) expression in WT- and STAT6 variant–transfected HEK293 cells before and after treatment with IL-4 (10 ng/ml for 30 min). Gating strategy for pSTAT6+ cells can be found in Fig. S3 C. (D) Quantification of C; n = 4. (E) Immunoblot in HEK293 cells transfected with WT-, inactive- (p.Y641F), p.P643R-, and p.D419G-STAT6 variants for pSTAT6, and Myc-tag before and after treatment with IL-4 (10 ng/ml for 30 min); n = 3. Full-length immunoblot for this can be found in Fig. S3, D and E. (F) Principal component analysis (PCA) comparing unstimulated and stimulated (100 ng/ml IL-4 for 4 h) WT (green), p.E382Q (blue), and p.D419G (purple) STAT6-transduced Jurkat T cells. Individual symbols represent technical replicates of one transduced pool for each genotype. PC1 and PC2 contribution is shown in brackets. (G) Normalized counts comparing stimulated WT (green) vs. p.E382Q (blue) or p.D419G (purple), for IL4R, CISH, and XBP1. (H) Heatmap representation of normalized counts of a transcription set defined as IL-4 targets in transduced Jurkat T cells. (I and J) Asterisk indicates adjusted P value <0.05. GSEA plots for (I) curated STAT6 target genes comparing WT vs. either p.E382Q (blue) or p.D419G (purple) at baseline, or (J) IL-4-TH2 targets genes comparing WT vs. either p.E382Q (blue) or p.D419G (purple) after stimulation with IL-4. Normalized enrichment score and adjusted P value are shown. Source data are available for this figure: SourceData F3.
Figure S3.
In vitro assays demonstrate that STAT6 variants lead to increased STAT6 activity. (A and B) Luciferase assay of STAT6 activity on a plasmid containing (A) CCL26 promoter and (B) FcεR2 promoter for WT-, different STAT6-variant transfected HEK293 cells before and after stimulation with IL-4 (100 ng/ml for 40 h), n = 3. (C) Gating strategy for determining % positive HEK293 pSTAT6 cells: dot plot for fluoresence minus one (FMO) is presented and was used for establishing pSTAT6+ cells. (D and E) Full-length immunoblots of the cropped immunoblots shown in Fig. 3 E, showing HEK293 cells transfected with WT-, inactive- (p.Y641F), p.P643R-, and p.D419G- STAT6 variants for (D) Myc-tag and β-actin, as well as (E) pSTAT6 before and after treatment with IL-4 (10 ng/ml for 30 min). (F) Significantly upregulated (i) and downregulated (ii) genes upon IL-4 treatment in WT (green), p.E382Q (blue), and p.D419G (purple) in Jurkat cells as shown through Venn diagram. (G) Sample level enrichment analyses of significantly enriched immune pathways from MSigDB Hallmark in unstimulated and IL-4–stimulated samples, comparing WT vs. either p.E382Q or p.D419G. Heatmap is normalized across the rows and shown as relative expression.
We next evaluated if the increased promoter activity leads to global transcriptomic changes. As transcriptomic studies on HEK293 cells after IL-4 stimulation have been challenging to interpret (Yildiz et al., 2015), we stably expressed p.E382Q and p.D419G STAT6 by lentiviral transduction in Jurkat T cells, which express endogenous STAT6 and serve as a model of heterozygosity (Kim et al., 2012). Here, we observed that WT-, p.E382Q-, and p.D419G-transduced cells clustered separately from each other both at baseline and after stimulation with IL-4 (Fig. 3 F). Differential gene expression analysis revealed significantly increased transcript abundance of known STAT6 target genes, including IL4R (Goenka and Kaplan, 2011), CISH (Yildiz et al., 2015), and XBP1 (Bettigole et al., 2015) in p.E382Q and p.D419G transduced cells when compared to WT transduced control (Fig. 3 G). Interestingly, p.E382Q and p.D419G had 67 and 80 uniquely increased hits, which did not overlap with WT nor with each other (Fig. 3 H, Fig. S3 F, and Table S6). This suggests that the altered activity of both p.E382Q and p.D419G is not restricted to enhanced activity of known STAT6 targets alone. Further investigation through gene set enrichment analyses (GSEA) showed increased enrichment in IL-4-STAT6 target genes at baseline (Fig. 3 I and Table S7), increased enrichment in T helper 2 (TH2) drivers after stimulation (Elo et al., 2010; Fig. 3 J and Table S7), and increased enrichment in proliferation pathways for p.D419G consistent with its known oncogenic activity (Ritz et al., 2009; Tate et al., 2019; Yildiz et al., 2015; Fig. S3 G).
Patients with GOF STAT6 variants have slower STAT6 dephosphorylation kinetics after IL-4 stimulation and an enhanced TH2 signature
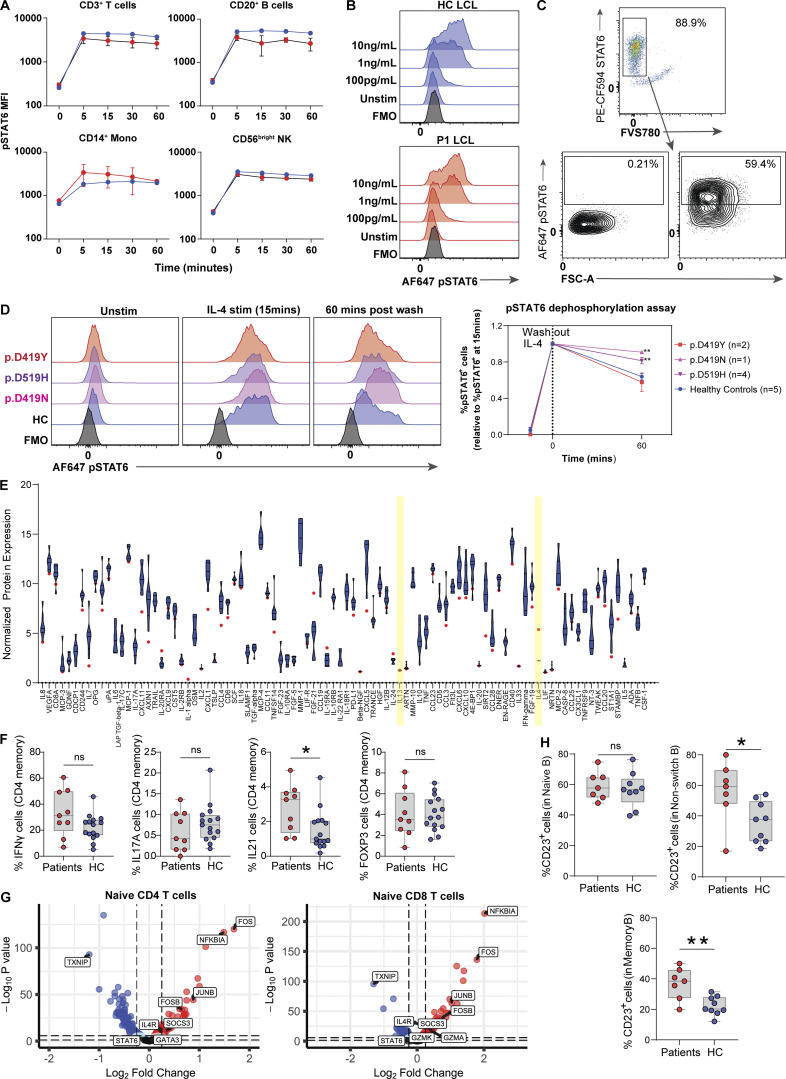
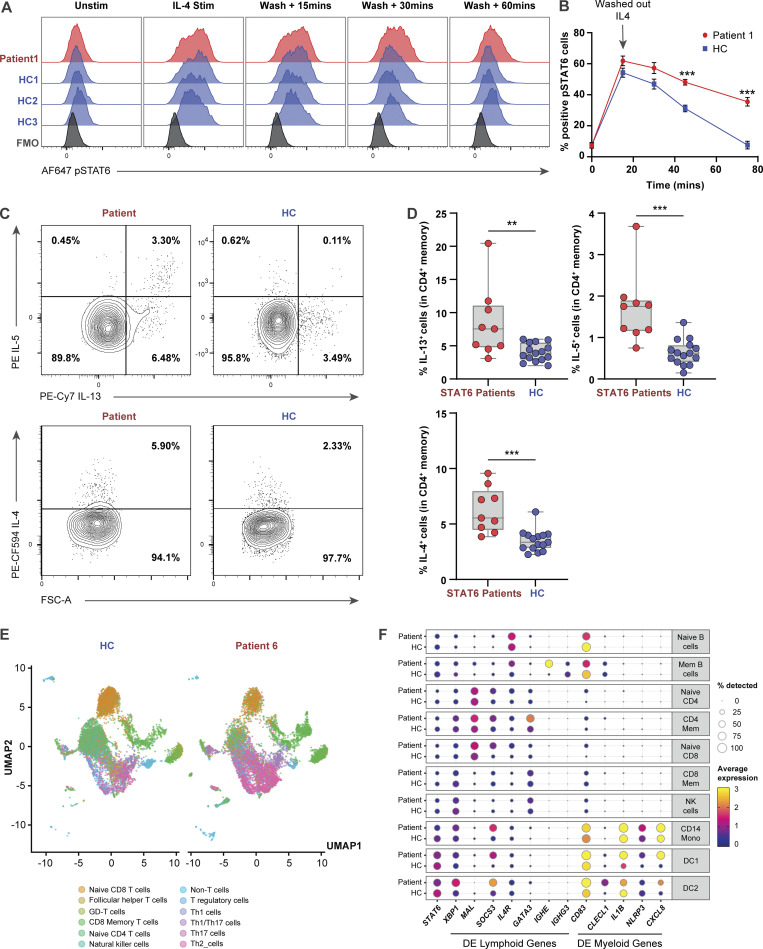
To further investigate the role of STAT6 GOF variants in primary cells, STAT6 phosphorylation was quantified in patient samples. Unexpectedly, we found no differences in the percentage of phospho-STAT6 positive cells between patients and healthy controls after IL-4 stimulation over a 60 min time course nor after stimulation with different doses of IL-4 (Fig. S4, A and B). However, differences emerged when we monitored the kinetics of STAT6 dephosphorylation after stimulation (Fig. 4, A and B; and Fig. S4 C). Specifically, washing out of IL-4 led to slower dephosphorylation kinetics of STAT6 in most patient cells compared to healthy controls (Fig. 4, A and B; and Fig. S4 D), supporting a GOF mechanism in patient lymphocytes. We did note that one of our kindreds did not display delayed dephosphorylation (Fig. S4 D), suggesting that this might not be the only GOF mechanism. Indeed, increased STAT6 activity without phosphorylation has previously been reported in follicular lymphoma research studying the p.D419 mutational hotspot (Yildiz et al., 2015).
Figure S4.
Measure of STAT6 activity in patient primary lymphocytes. (A) 1-h time course to measure phosphorylation of STAT6 in different populations of lymphocytes from five patients (red) and one healthy control (blue) after stimulation with IL-4 (10 ng/ml). (B) Dose response in LCLs of patient one (red) vs. one healthy control (blue) after stimulation of cells with various doses of IL-4 15 min. (C) Gating strategy to determine % pSTAT6 positive cells in LCLs: dot plot for FMO is presented and was used for establishing pSTAT6+ cells. (D) Histograms showing phosphorylation of STAT6 in healthy control (blue) and patients with genotype p.D419Y (red, n = 2), p.D519H (purple, n = 4), p.D419N (pink, n = 1), and healthy controls (blue, n = 5) in T cell blasts that were stimulated with IL-4 (10 ng/ml) for 15 min, washed with PBS, and subsequently incubated in IL-4–free media for 60 min. Quantification of pSTAT6+ cells is presented and normalized to max stimulation (noted at 15 min). Two-way ANOVA followed by Šídák’s multiple comparisons was conducted. **, P < 0.01. (E) Readout of 92 biomarkers for P5 using throughput Olink proteomics. Eight healthy control distribution are shown as a violin plot in blue. The patient is shown as a red circle. Key cytokines, IL-4 and IL-13, are highlighted in yellow. (F) T helper cell distribution for nine patients (red) and 15 age-matched healthy controls (blue) each. (G) Transcriptomic comparison of naive CD4+ and naive CD8+ T cells between P6 and one healthy control measured through scRNAseq. Red genes are enriched in patient; blue genes are enriched in healthy control. The two dotted lines are the P value and adjusted P value respectively. (H) Quantification of % CD23 positive cells in naive, non-class switched memory, and class-switched memory B cells between patients (red, n = 7) and healthy controls (blue, n = 9) after stimulation with IL-4 (10 ng/ml) for 20 h. Unpaired t test. *, P < 0.05; **, P < 0.01.
Figure 4.
Primary lymphocytes of STAT6 GOF patients display higher STAT6 activity and TH2 skewing. (A) Histograms showing phosphorylation of STAT6 in healthy control (blue) and patient (red) LCLs that were stimulated with IL-4 (10 ng/ml) for 15 min, washed with PBS and subsequently incubated in IL-4–free media for 15, 30, and 60 min. Gating strategy for pSTAT6+ cells can be found in Fig. S4 C. (B) Quantification of pSTAT6+ cells from three separate experiments done in A, multiple unpaired t test corrected for multiple comparison using the Benjamini–Hochberg method. ***, P < 0.001. (C) Frequency of IL5+, IL13+, and IL4+ cells in memory CD4+ T cells of one representative patient, along with one representative healthy control. (D) Quantification of C showing IL5+, IL13+, and IL4+ cells in patients along with 15 age-matched healthy controls. **, P < 0.01; ***, P < 0.001. (E) Uniform manifold approximation and projection (UMAP) visualization of scRNAseq done on enriched T cells comparing one patient with one age-matched healthy control. (F) Dot plot showing expression of selected differentially expressed genes (adjusted P value < 0.05) observed in scRNAseq between patient and healthy control and associated with T cells, B cells, monocytes, or dendritic cells.
Given that the STAT6 axis is critical for the differentiation of TH2 cells (Kaplan et al., 1996), a subset of CD4+ helper T cells that is a major contributor to the pathogenesis of allergic disease, we next investigated TH2 signatures in these patients. Patients showed skewing towards TH2 pathway activity compared to healthy controls based on higher levels of the TH2 cytokines IL-5, IL-13, and IL-4 as measured by flow cytometry (Fig. 4, C and D), or through transcriptomic signature by single-cell RNA sequencing (scRNAseq; Fig. 4 E). High throughput proteomics also validated the increased IL-4 expression, but not high IL-13 expression (Fig. S4 E). Differences in proportions of other subsets of helper T cells were restricted to higher IL-21+ cells in patient memory CD4+ T cells (Fig. S4 F). scRNAseq showed that patient B cells expressed high IGHE and low IGHG3 (Fig. 4 F), reflecting patterns opposite of those seen in STAT6 knockout mice (Shimoda et al., 1996; Sulczewski et al., 2021), and CD4+ T cells express high GATA3. scRNAseq further demonstrated that IL4R, a gene encoding a key receptor that mediates STAT6 activation, was upregulated in all B and T cell subsets (Fig. 4 F and Fig. S4 G).
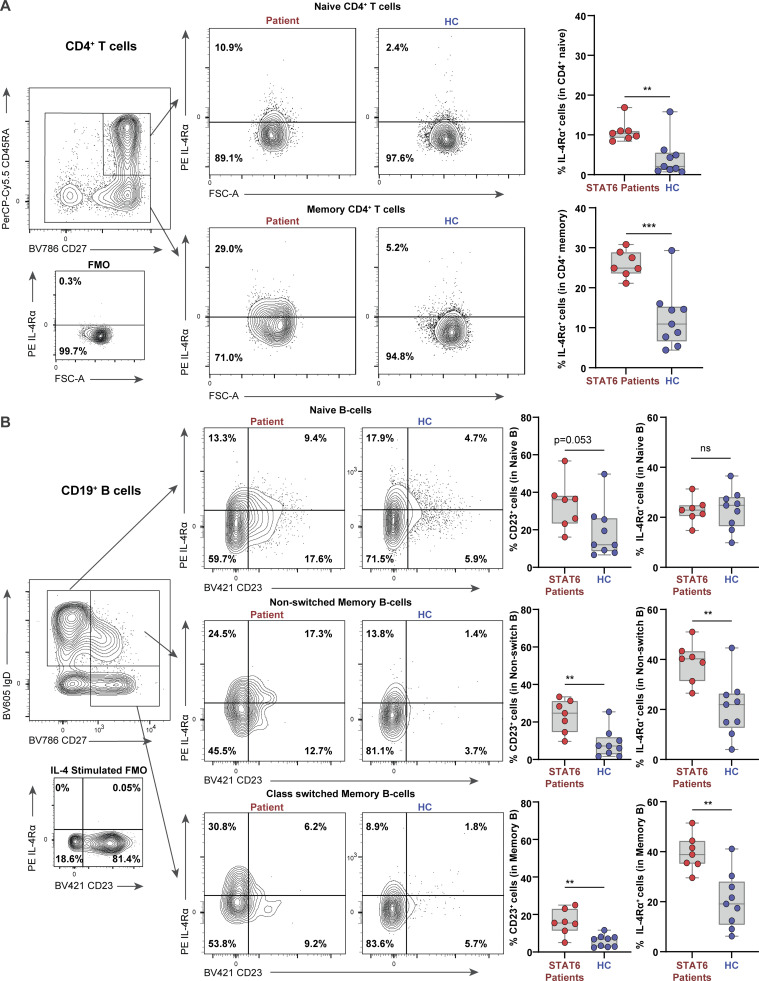
Using flow cytometry, we confirmed that IL-4Rα expression was significantly higher on both naive and memory CD4+ T cells of seven patients from three different kindreds compared to nine healthy controls (Fig. 5 A). IL-4Rα expression was also found to be higher in non-class switched memory and class switched memory B cells of unstimulated patient primary cells (Fig. 5 B). These findings suggest higher baseline activity of STAT6 in patient cells driving IL-4Rα expression similar to that seen in our Jurkat model (Fig. 3, G–J; and Tables S6 and S7). Finally, we measured the expression of CD23 (the low-affinity IgE receptor, FcεRII) which is known to be upregulated by STAT6 (Fig. S3 B; Kneitz et al., 2000) and we found higher expression of CD23 on all subsets of patient B cells at baseline (Fig. 5 B) and following stimulation with IL-4 (Fig. S4 H). Taken together, these experiments conducted using primary patient cells further confirm the STAT6 GOF phenotype.
Figure 5.
Primary lymphocytes of STAT6 GOF patients display high expression of STAT6 target genes. (A) Expression of IL4Rα in naive and memory CD4+ cells is quantified as % positive cells in primary patient cells (n = 7, red) and healthy controls (n = 9, blue). Gating strategy for naive and memory and CD4+ is presented along with a dot plot for a fluoresence minus one (FMO) IL-4Rα sample to display IL-4Rα+ gating, as well as a representative dot plot for a patient and healthy control. (B) Expression of CD23 and IL4Ra in naive, non-class switched memory and memory B cells is quantified as % positive cells in primary patient cells (n = 7, red) and healthy controls (n = 9, blue). Gating strategy for B cell subsets is presented along with a dot plot for an FMO IL-4Rα sample to display IL-4Rα+ gating, as well as a representative dot plot for a patient and healthy control. Unpaired t test. **, P < 0.01; ***, P < 0.001.
JAK inhibitors and IL-4Rα monoclonal antibody can be used as potential therapeutics for patients with STAT6 GOF variants
Due to the severity of the multi-system allergic disease experienced by the patients in our cohort, various treatment approaches were used over the years. Corticosteroids, administered topically and systemically, were the mainstay of treatment for most patients. Unfortunately, even high doses of corticosteroids were unable to control the allergic inflammation and they were responsible for many side-effects including cataracts and osteoporosis. Other treatments used in this cohort that proved ineffective included topical tacrolimus, oral methotrexate, and mepolizumab (an anti–IL-5 monoclonal antibody).
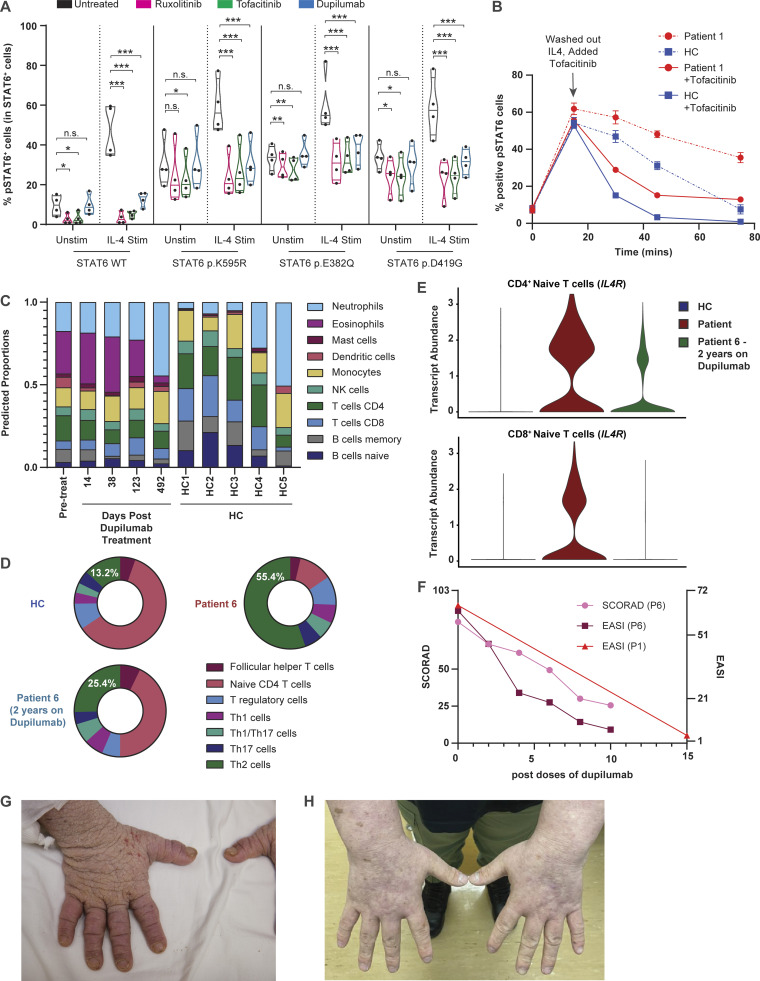
Having demonstrated that heterozygous STAT6 variants lead to higher STAT6 activity and TH2 immunological skewing, we tested in vitro whether targeting the STAT6 pathway could be clinically beneficial. Based on our findings of higher phosphorylation of STAT6, and higher expression of IL-4Rα, we selected the JAK inhibitors, ruxolitinib and tofacitinib, and the anti–IL-4Rα antibody, dupilumab, as drugs to test in vitro as they are all used clinically for treatment of allergic disease (Bacharier et al., 2021; Beck et al., 2014; Bissonnette et al., 2016; Kim et al., 2020). We demonstrated that both ruxolitinib and tofacitinib effectively decreased the patient-specific enhanced STAT6 phosphorylation/activation in HEK293 cells at baseline and after IL-4 stimulation, whereas dupilumab inhibited IL-4 mediated increase in STAT6 activity (Fig. 6 A and Fig. S5 A). We further confirmed in patient primary cells that tofacitinib accelerated STAT6 dephosphorylation following IL-4 stimulation (Fig. 6 B). These data suggest the potential clinical benefit of directly targeting the IL-4/STAT6 pathway in patients with STAT6 GOF variants.
Figure 6.
JAK inhibitors and IL-4Rα antibody can be used as potential therapeutics for patients with GOF STAT6 variants. (A) Quantification of phospho-STAT6 expression in transfected HEK293 cells left untreated (black) pre-treated with ruxolitinib (10 μM, 1 h; pink), tofacitinib (10 μM, 1 h; green), or dupilumab (10 nM, 1 h; blue), before and after stimulation with IL-4 (10 ng/ml, 30 min). Individual points represent separate transfections for each genotype (n = 4). Gating strategy for pSTAT6+ cells can be found in Fig. S3 C. One-way ANOVA and Tukey’s post-hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Quantification of pSTAT6+ cells in patient (red) and healthy control (blue) LCLs stimulated with IL-4 (10 ng/ml for 15 min), washed and incubated in tofacitinib (10 μM) for 15, 30, and 60 min. Dotted translucent lines are indicative of no tofacitinib treatment (Fig. 4 B); n = 1. Gating strategy for pSTAT6+ cells can be found in Fig. S4 C. (C) Cell type proportion gene signature as determined by the software Cibersort, in a patient undergoing dupilumab treatment for 2 yr and five healthy controls. Cell labels are listed on the right. (D) Donut plot showing frequencies of CD4+ T helper subsets in one patient, an age-matched healthy control (Fig. 4 E), and a 2-yr post-dupilumab treatment patient sample as measured by scRNAseq on enriched T cells. Frequency of TH2 cells is quantified in the donut plots of the different samples. (E) Violin plots showing expression of IL4R in the patient (red), healthy control (blue), and a 2-yr post-dupilumab sample (green). (F) Eczema scoring, EASI and SCORAD, for two patients after treatment with multiple doses of dupilumab. (G and H) Photographs of hands showing (G) the severity of atopic dermatitis before and (H) the improvement after dupilumab treatment.
Figure S5.
STAT6 activity can be therapeutically targeted and can resolve clinical disease severity. (A) Quantification of luciferase assay in HEK293 transfected cells pre-treated with ruxolitinib (10 μM, 1 h), tofacitinib (10 μM, 1 h), or dupilumab (10 nM, 1 h), before and after stimulation with IL-4 (0.02 ng/ml, 4 h). n = 4. One-way ANOVA and Tukey’s post-hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Eosinophil counts before and following initiation of treatment with dupilumab are presented. Dots in red corresponds to transcriptomic data from this patient presented in Fig. 6 C. (C) PCA comparing whole blood bulk RNAseq of P6 before treatment with dupilumab and four time points after treatment, alongside five healthy controls. (D) Heatmap signatures of differentially expressed genes comparing pre-treatment patient samples against five healthy controls. Genes are row normalized. (E) Key genes, previously described to be biomarkers for allergic disease (Lemonnier et al., 2020) in whole blood RNA are presented for the patient samples. Gray shaded area is the range for the expression of these genes in five healthy controls.
Once their STAT6 GOF variant was identified, three of the patients were started on dupilumab and all showed significant clinical improvement. P6, who has been on treatment with dupilumab for over 2 yr, serves as an illustrative example. Mirroring peripheral blood eosinophil counts (Fig. S5 B), repeated whole blood RNAseq showed global transcriptomic changes that were suggestive of mildly increased eosinophilic and allergic gene signatures after 38 d, followed by a shift of the transcriptome towards healthy controls after 123 and 492 d, respectively (Fig. 6 C and Fig. S5, C–E). scRNAseq confirmed a decrease in TH2 gene signatures 2 yr following initiation of dupilumab, accompanied by a decrease in the expression of IL-4R on both naive CD4+ and CD8+ T cells (Fig. 6, D–E). Clinically, these changes were accompanied by dramatic increase in growth velocity, improved skin condition as quantified by SCORAD (SCORing atopic dermatitis) and EASI (eczema area and severity index) scores, and the ability to wean from oral corticosteroids (Fig. 6, F–H). Similarly, P1 experienced remarkable clinical benefits with dupilumab with improved skin inflammation (Fig. 6 D), resolution of pruritus, and the ability to discontinue oral daily high dose corticosteroids. In addition to resolution of skin inflammation with dupilumab, P2 was able to discontinue swallowed budesonide without a flare in the severity of her eosinophilic esophagitis. Our preclinical data also suggested that JAK inhibitors may be beneficial (Fig. 6, A and B), and P4 had received tofacitinib (5 mg/d) for 2 mo at the time this manuscript was finalized. His initial response to tofacitinib was encouraging with less dysphagia, less esophageal food impaction, improved endoscopic appearance of the esophagus, and a marked reduction in the number of intraepithelial eosinophils.
Discussion
We present a combination of clinical, genetic, molecular, and transcriptional evidence of a new human disorder caused by germline AD GOF STAT6 variants in 16 patients with life-long severe allergic disease. These variants led to sustained STAT6 phosphorylation, increased STAT6 target gene expression, and TH2 skewed transcriptional profile. Importantly, we demonstrate in three patients that dupilumab treatment is a highly effective targeted therapeutic option, improving both clinical manifestations of disease and immunological biomarkers.
Although the full phenotype(s) of individuals with GOF STAT6 variants will only be uncovered through the identification of additional affected individuals, we propose to classify human germline AD GOF STAT6 syndrome as a PAD (Lyons and Milner, 2018; Milner, 2020; Vaseghi-Shanjani et al., 2021). Based on our study, possible clinical “red flags” for this new disorder include: (i) early life onset; (ii) peripheral blood eosinophilia; (iii) elevated serum IgE; (iii) widespread, treatment-resistant atopic dermatitis; (iv) multiple food and drug allergies; (v) severe (and even fatal) anaphylaxis; (vi) recurrent skin and respiratory infections; (vii) eosinophilic gastrointestinal disorder, including eosinophilic esophagitis; (viii) asthma; (ix) allergic rhinoconjunctivitis; (x) short stature; and possibly (xi) vascular malformations of the brain.
STAT6 is intimately linked to the biology of allergic inflammation. The central and most studied role of STAT6 is in mediating the biological effects of IL-4, a cytokine necessary for TH2 differentiation, B cell survival, proliferation, and class switching to IgE (Elo et al., 2010; Takeda et al., 1996), as well as in driving M2 macrophage polarization (Ginhoux et al., 2016). In T cells, STAT6 activation induces the expression of GATA3, the master regulator of TH2 differentiation, which in turn enhances expression of IL-4, IL-5, and IL-13, cytokines necessary for promoting allergic responses by activating mast cells and eosinophils (Sloka et al., 2011). The presence of greater TH2 cell populations, or TH2 cells producing copious amounts of IL-4/IL-5/IL-13, could be a driver of the observed allergic phenotype presented in our patients. Elevated IgE in partnership with mast cells is important for both acute and chronic manifestations of allergic disorders and can be an additional driver of the allergic diathesis (Galli and Tsai, 2012). STAT6 hyperactivation in airway epithelial cells and resident dendritic cells can further create an environment favoring asthma and chronic lung disease, as this would induce production of chemokines that promote TH2 cells and eosinophil recruitment (Matsukura et al., 2001; Medoff et al., 2009). Population genetics provide further support for the central role that STAT6 plays in the development of human allergic disease. Multiple independent genome-wide association studies (GWAS) have found that polymorphisms in STAT6 associate with many allergic conditions (Table 1). Our study expands this appreciation of the role of STAT6 in human disease by establishing that heterozygous GOF variants cause a monogenic form of severe allergic disease.
Table 1.
Number of published GWAS studies linking polymorphisms (SNPs) in STAT6 to common allergic diseases in the population
| Phenotype | Number of published associations | References |
|---|---|---|
| Asthmaa | 14 | Daya et al., 2019; Demenais et al., 2018; Ferreira et al., 2019; Han et al., 2020; Johansson et al., 2019; Olafsdottir et al., 2020; Pickrell et al., 2016; Pividori et al., 2019; Sakaue et al., 2021; Shrine et al., 2019; Valette et al., 2021; Zhu et al., 2020; Zhu et al., 2018; Zhu et al., 2019 |
| Eosinophil count | 7 | Astle et al., 2016; Chen et al., 2020; Höglund et al., 2022; Kachuri et al., 2021; Kichaev et al., 2019; Sakaue et al., 2021; Vuckovic et al., 2020 |
| Allergic disease | 3 | Ferreira et al., 2017; Ferreira et al., 2020; Zhu et al., 2018 |
| Atopic dermatitis/eczema | 3 | Johansson et al., 2019; Kichaev et al., 2019; Tanaka et al., 2021 |
| Serum IgE level | 2 | Daya et al., 2021; Granada et al., 2012 |
| Allergic sensitization | 2 | Bønnelykke et al., 2013; Waage et al., 2018 |
| Allergic rhinitis | 1 | Johansson et al., 2019 |
| Eosinophilic gastrointestinal disorders | 1 | Sleiman et al., 2014 |
Significant genome-wide associations (P < 5 × 10−8) between STAT6 SNPs and all relevant allergic traits were obtained through the National Human Genome Research Institute–European Bioinformatics Institute GWAS Catalog (https://www.ebi.ac.uk/gwas/).
Includes asthma, childhood-onset asthma, adult-onset asthma, and atopic asthma.
The fatal cerebral aneurysm in P10 (p.E382Q) was not clinically anticipated, but it is possible that the STAT6 GOF variant also increased the risk of developing cerebral aneurysms. Indeed, P1 (p.D419G) also had multiple rare anatomical variants in the arteries of the Circle of Willis. Intracranial aneurysms have been reported in patients with both STAT3 loss-of-function (LOF) and STAT1 GOF (Chandesris et al., 2012; Dadak et al., 2017; Toubiana et al., 2016). Increased activation of other STAT family members, including STAT2, STAT3, and STAT5 have also been observed in human abdominal aortic aneurysms (STAT6 was not evaluated), although it is not clear whether enhanced STAT phosphorylation contributes to aneurysms or is the result of inflammation caused by aneurysms (Liao et al., 2012). As more individuals with STAT6 GOF variants are identified, the possibility of cerebral vascular anomalies warrants investigation.
It is noteworthy that the oldest patient in this cohort, P7 (p.D419H), experienced recurrent B cell lymphoma—follicular lymphoma at 49 yr and diffuse large B cell lymphoma at age 60 yr. Activating somatic mutations in STAT6 are well documented in B cell lymphoma with amino acid D419 being a particular mutational hotspot (Ritz et al., 2009; Tate et al., 2019; Yildiz et al., 2015). The patient’s p.D419H variant has been reported multiple times as a somatic mutation in COSMIC, as have other variants found in our patient cohort (i.e., p.D419, p.D519, and p.P643). More patients will need to be identified and followed to fully define the phenotype caused by germline STAT6 GOF variants, but it is biologically plausible that these patients may be at higher risk of developing B cell malignancies warranting enhanced clinical vigilance.
A GOF STAT6 model (designated STAT6VT) has previously been described in vitro (Daniel et al., 2000) and has been used to study chronic atopic dermatitis in mouse models (Bruns et al., 2003; DaSilva-Arnold et al., 2018). STAT6VT has the substitution of two amino acid residues, at positions 547 and 548, in the SH2 domain resulting in a STAT6 mutant that is constitutively active in an IL-4–independent manner and is unresponsive to IL-4 stimulation (Daniel et al., 2000). The humans we identified with STAT6 GOF variants and STAT6VT mice share a number of key features of the allergic diathesis, including elevated serum IgE and chronic atopic dermatitis. Very recently, a report was published describing a father and his two sons with severe allergic disease who were all heterozygous for the GOF STAT6 variant p.E377K (Suratannon et al., 2022). This new family shares many of the features we report in our cohort of 10 families (Fig. 2 A), further emphasizing that patients with early onset severe allergic disease should be assessed for underlying monogenic gene defects, including STAT6.
There is now a growing list of human single gene defects that cause the classic hyper-IgE phenotypic triad of eczema, recurrent skin and lung infections, and elevated serum IgE (Freeman and Milner, 2020; Vaseghi-Shanjani et al., 2021; Zhang et al., 2018). AD hyper-IgE syndrome caused by dominant negative variants in STAT3 (i.e., Job’s syndrome or STAT3 LOF) is the best characterized of these conditions, but this list of disorders also includes other AD (IL6ST; Beziat et al., 2020) and autosomal recessive (AR; ZNF341 [Béziat et al., 2018], IL6ST [Shahin et al., 2019]) disorders (Bergerson and Freeman, 2019; Minegishi, 2021). Notably, the patients we identified with STAT6 GOF variants did exhibit some of the extra-immunological features typical of STAT3 LOF, specifically hyperextensible joints, pathologic fractures, and vascular anomalies (Bergerson and Freeman, 2019).
Beyond defining the phenotype of STAT6 GOF, we also present laboratory and clinical evidence supporting the effectiveness of dupilumab and tofacitinib treatment in these patients. It was notable that the three patients (P1, P2, and P6) who received dupilumab have experienced dramatic improved atopic dermatitis and could be weaned off systemic corticosteroids. P6, who had short stature and delayed bone age before starting the biologic agent, experienced rapid height and weight gain following initiation of dupilumab. In addition to the documented clinical benefits of dupilumab therapy in patients with STAT6 GOF, we also present early data suggesting that the JAK inhibitors, ruxolitinib or tocafitinib, may be effective in this patient population.
While this study has many strengths, notably the extreme allergic phenotype of the 16 patients combined with in-depth functional assessment of their STAT6 variants; because of the global nature of our cohort, the study does have limitations. First, patients were identified by their local expert clinicians as candidates for genetic assessment based on their extreme allergic phenotype and, in some cases, their family history. As a result, we do not have prospectively defined inclusion criteria. Second, the global nature of the cohort and variation in local access to medications such as dupilumab limited our ability to run the same assays on pre-treatment primary cells from all patients. Despite these limitations, our study does identify GOF variants in STAT6 as a novel monogenic allergic disorder. We also present clinical and single cell evidence of the effectiveness of dupilumab in STAT6 GOF patients. We anticipate that this discovery will facilitate the recognition and targeted treatment of more affected individuals and, with time, a full definition of the human genotype-phenotype relationship caused by germline human STAT6 GOF variants will emerge, including understanding the risk of lymphoma.
Based on our findings reported in this study, we suggest that heterozygous GOF variants in STAT6 be added to the list of AD causes of the hyper-IgE phenotype. While each of the conditions known to cause a hyper-IgE phenotype has some specific clinical features (e.g., viral skin infections are a defining feature of DOCK8 deficiency; Biggs et al., 2017; Chu et al., 2012), there is considerable clinical overlap and clinically approved testing of these pathways is rarely available. Therefore, we recommend genetic testing as the most efficient initial diagnostic approach to patients who experience severe allergic disease beginning very early in life. Finally, we demonstrate that dupilumab and JAK inhibition may be an effective targeted treatment options for patients with GOF STAT6 variants.
Materials and methods
Ethical considerations
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All study participants and/or their parents/guardians provided written informed consent. Research study protocols were approved by local institutions, specifically: The University of British Columbia Clinical Research Ethics Board (H09-01228, H15-00641), University College London Research Ethics Committee (04/Q0501/119, 06/Q0508/16), University of Hong Kong Institutional Review Board (UW 08-301), National Institutes of Health Institutional Review Board (NCT01164241), Children’s Hospital of Philadelphia Institutional Review Board (19-016617), Children’s Hospital Bambino Gesù Ethical Committee (1702_OPBG_2018).
Identification of STAT6 variant via next-generation sequencing
Based on local availability, research or clinical next-generation sequencing of the genomic DNA was performed using either whole exome or a targeted panel approach (as described previously; Béziat et al., 2021; Campbell et al., 2022; Chovanec et al., 2022; Hebert et al., 2022; Murrell et al., 2022; Tarailo-Graovac et al., 2016). After bioinformatic analysis, de novo and inherited STAT6 variants that were predicted to be damaging and that segregated with disease were identified in each family (Tables S4 and S5).
Generation and expression of STAT6 variant plasmids
STAT6 variants described in this study were generated through site-directed mutagenesis (SDM) for transfection purposes. Expression of WT STAT6 or STAT6 variants were induced transiently in HEK293 cells using lipofectamine, or stably in Jurkat T cells using a lenti-viral approach. See supplemental methods at the end of the PDF for details.
Luciferase reporter assays
Luciferase reporter plasmids encoding a 4× STAT6 binding site (TTCCCAAGAA; the underlined bases represent the two half-sites for STAT6-specific binding), encoding the promoter site for CCL26, and encoding the promoter site for FCER2 were used to assess WT and variant STAT6 promoter activity (Li et al., 2016; Yildiz et al., 2015). See supplemental methods at the end of the PDF for details.
Flow cytometry
(a) Phospho-flow cytometry: STAT6 phosphorylation was determined via phospho-flow cytometry for STAT6-transfected HEK293 cells, peripheral blood mononuclear cells (PBMCs), T cell blasts, and EBV-transformed lymphoblastoid B cell lines (LCLs). (b) Intracellular cytokine staining: Intracellular cytokine staining was conducted on nine patient PBMCs, alongside 15 healthy controls, to study CD4+ T helper subsets as previously described (Sharma et al., 2022). (c) CD23 and IL-4Rα expression was studied on seven patient PBMCs and nine healthy control PBMCs. See supplemental methods at the end of the PDF for details.
Immunoblotting
Immunoblotting was conducted as previously described (Sharma et al., 2022) to assess the phosphorylation status of p.P643R STAT6 variant, as phosphorylation of this variant could not be detected via flow cytometry, using an antibody against the tyrosine 641 phosphorylation site. See supplemental methods at the end of the PDF for details.
RNAseq
(a) Jurkat cells: To model transcriptomics changes caused by STAT6 variants, Jurkat T cells were transduced with either c.1144G>C, p.E382Q, c.1256A>G, p.D419G, or WT STAT6. The cells were either left unstimulated or stimulated with 100 ng/ml of IL-4 for 4 h and subsequently used for RNA extraction and sequencing. (b) Whole blood: Bulk RNAseq was done on 10 samples: one patient sample before dupilumab treatment initiation, four patient samples after dupilumab treatment initiation, and five healthy controls. (c) scRNAseq: Performed on PBMCs and enriched T cells from the patient sample before and 2 yr after dupilumab treatment, along with one age-matched healthy control. See supplemental methods at the end of the PDF for details.
Histology
Formalin-fixed, paraffin-embedded gastric, duodenal, and skin tissue were sectioned and subjected to H&E staining.
Structural modeling
The effects of the STAT6 variants on the protein function and structure were analyzed using three-dimensional models. SWISS-MODEL (Waterhouse et al., 2018) was used to model the variants based on a template structure of the human STAT6 transcription factor solved as a homodimer and in complex with DNA (PDB: 4Y5W, resolution: 3.1 Å, chains A, C, M, and N; Li et al., 2016). Structures were visualized with UCSF Chimera (Pettersen et al., 2004).
Online supplemental material
Clinical narratives for each patient are presented as Data S1. Fig. S1 is a detailed structural model showing the DNA and STAT6 variant interface. Fig. S2 shows complete blood count and the immunological workup of all the patients. Fig. S3 shows additional in vitro data confirming the GOF nature of the STAT6 variants. Further workup of the primary patient cells is shown in Fig. S4. Additional IL4Rα antibody and JAK inhibitor treatment data of cells and patients is presented in Fig. S5. Table S1 lists primers used for site-directed mutagenesis. Table S2 lists antibodies used for phospho-flow on different immune subsets. Table S3 lists antibodies used for TH phenotyping in patient PBMCs. Pathogenicity prediction of the variants are presented in Tables S4 and S5. Tables S6 and S7 are gene lists from GSEA analysis shown in Fig. 3. Supplemental methods are listed at the end of the PDF.
Supplementary Material
lists primers used for site-directed mutagenesis.
lists antibodies used for phospho-flow on different immune subsets.
lists antibodies used for TH phenotyping in patient PBMCs.
shows variant annotation and pathogenicity prediction of the variants reported within the DNA-binding domain of STAT6 for 10 patients.
shows variant annotation and pathogenicity prediction of the variants reported outside of the DNA-binding domain of STAT6 for six patients.
lists genes upregulated (gray) and downregulated (red) in transduced WT-, p.E382Q-, and p.D419G-transduced Jurkats that meet the cutoff of fold-change (FC) > 1.25 and adjusted P value <0.05.
lists genes in the leading edge driving the enrichment of two pathways (IL-4/STAT6 pathway and TH2 pathway) between transduced WT- vs. p.E382Q- and WT- vs. p.D419G- Jurkats
provides clinical narratives for each patient.
contains original blots for Fig. 3.
Acknowledgments
We thank the National Institute for Health and Care Research (NIHR) BioResource volunteers for their participation, and gratefully acknowledge NIHR BioResource centers, National Health Service Trusts, and staff for their contribution. A list of NIHR BioResource Rare Diseases Consortium members is available at the end of the PDF. We would also like to acknowledge the Biomedical Research Centre Sequencing Core for their assistance with RNAseq and processing. We acknowledge the extended clinical care team at the National Institute of Allergy and Infectious Diseases who helped in the care and evaluation of Patient 11. We also thank the extended clinical team at the Department of Paediatrics and Adolescent Medicine, Queen Mary Hospital, Hong Kong, China for providing expert care and support. We thank the Genomics Core of the Centre for PanorOmic Sciences of the University of Hong Kong for their professional performance of bulk and scRNAseq. Finally, we thank the patients and their families for their trust and support.
This work was supported by grants from the Canadian Institutes of Health Research (PJT 178054; S.E. Turvey), Genome British Columbia (SIP007; S.E. Turvey), and BC Children’s Hospital Foundation. S.E. Turvey holds a Tier 1 Canada Research Chair in Pediatric Precision Health and the Aubrey J. Tingle Professor of Pediatric Immunology. M. Sharma was supported by a CIHR Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award and University of British Columbia Four Year Doctoral Fellowship (4YF). H.Y. Lu is supported by a Canada Graduate Scholarship, 4YF, Killam Doctoral Scholarship, Friedman Award for Scholars in Health, and a BC Children’s Hospital Research Institute Graduate Studentship. M. Vaseghi-Shanjani is funded by the Vanier Canada Graduate Scholarship and 4YF. The work by J. Heimall was supported by the Elizabeth Paige Lavin Endowed Chair fund. This project has also been funded in part with federal funds from the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the French National Research Agency (ANR) under the “Investments for the Future” program (ANR-10-IAHU-01), ANR CARMIL2 (ANR-21-CE15-0034), Instituts Thématiques Multiorganismes (ITMO) Cancer of Aviesan, and Institut National du Cancer (INCa) within the framework of the 2021–2030 Cancer Control Strategy (on funds administered by the Institut National de la Santé et de la Recherche Médicale), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (EQU201903007798), the Square Foundation, Institut National de la Santé et de la Recherche Médicale, and Paris University Cité. This work was also supported by Children’s Hospital Bambino Gesù, where L. Pacillo and B. Rivalta were supported by 4-yr doctoral scholarships. C. Cifaldi and C. Cancrini were supported by the Italian Ministry of Health; C. Cifaldi was supported with a 5x1000 Children’s Hospital post-doctoral scholarship, and C. Cancrini holds a Development of Innovative Diagnostic and Therapeutic Approaches for PID grant (Programma di rete, NET-2011-02350069) and Ricerca Corrente. Additionally, the optimization of the Olink platform was supported by the PENTA Foundation, funded through an independent grant by ViiV Healthcare UK, named EPIICAL. Y.L. Lau is supported by the Society for the Relief of Disabled Children, Jeffrey Modell Foundation, Doris Zimmern Endowed Professorship in Community Child Health, and Chung Ko Lee and Cheung Yuen Kan Education and Research Fund. D. Leung is supported by the Croucher Foundation. J.S.D. Rosa Duque is supported by a donation in memory of Dr. Ton Lung Quong and Reverend Marion Quong. Z. Liu, R. Liang, and X. Yang are supported by the Edward and Yolanda Wong Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions: Conceptualization, methodology, formal analysis, investigation, visualization, writing—original draft preparation, writing—review and editing: M. Sharma, D. Leung, M. Momenilandi, L.C.W. Jones, L. Pacillo, A.E. James, J.R. Murrell, S. Delafontaine, J. Maimaris, M. Vaseghi-Shanjani, K.L. Del Bel, and H.Y. Lu. Investigation, resources, writing—review and editing: G.T. Chua, S. Di Cesare, O. Fornes, Z. Liu, G. Di Matteo, M.P. Fu, D. Amodio, I.Y.S. Tam, G.S.W. Chan, A.A. Sharma, J. Dalmann, R. van der Lee, G. Blanchard-Rohner, S. Lin, Q. Philippot, P.A. Richmond, J.J. Lee, A. Matthews, M. Seear, A.K. Turvey, R.L. Philips, T.F. Brown-Whitehorn, C.J. Gray, K. Izumi, J.R. Treat, K.H. Wood, J. Lack, A. Khleborodova, J.E. Niemela, X. Yang, R. Liang, L. Kui, C.S.M. Wong, G.W.K. Poon, A. Hoischen, C.I. van der Made, J. Yang, K.W. Chan, J.S.D. Rosa Duque, P.P.W. Lee, M.H.K. Ho, B.H.Y. Chung, H.T.M. Le, W. Yang, P. Rohani, A. Fouladvand, H. Rokni-Zadeh, M. Changi-Ashtiani, M. Miryounesi, A. Puel, M. Shahrooei, A. Finocchi, P. Rossi, B. Rivalta, C. Cifaldi, A. Novelli, C. Passarelli, S. Arasi, D. Bullens, K. Sauer, T. Claeys, C.M. Biggs, E.C. Morris, S.D. Rosenzweig, J.J. O’Shea, W.W. Wasserman, H.M. Bedford, C.D.M. van Karnebeek, and P. Palma. Conceptualization, methodology, investigation, validation, visualization, resources, funding acquisition, project administration, supervision, writing—original draft preparation, writing—review and editing: S.O. Burns, I. Meyts, J.-L. Casanova, J.J. Lyons, N. Parvaneh, A.T.V. Nguyen, C. Cancrini, J. Heimall, H. Ahmed, M.L. McKinnon, Y.L. Lau, V. Beziat, and S.E. Turvey.
References
- Adzhubei, I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., and Sunyaev S.R.. 2010. A method and server for predicting damaging missense mutations. Nat. Methods. 7:248–249. 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran, D., Looney A.P., Liu L., Wu E., Fong V., Hsu A., Chak S., Naikawadi R.P., Wolters P.J., Abate A.R., et al. 2019. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20:163–172. 10.1038/s41590-018-0276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle, W.J., Elding H., Jiang T., Allen D., Ruklisa D., Mann A.L., Mead D., Bouman H., Riveros-Mckay F., Kostadima M.A., et al. 2016. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell. 167:1415–1429.e19. 10.1016/j.cell.2016.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacharier, L.B., Maspero J.F., Katelaris C.H., Fiocchi A.G., Gagnon R., de Mir I., Jain N., Sher L.D., Mao X., and Liu D., et al. 2021. Dupilumab in children with uncontrolled moderate-to-severe asthma. N. Engl. J. Med. 385:2230–2240. 10.1056/NEJMoa2106567 [DOI] [PubMed] [Google Scholar]
- Beck, L.A., Thaçi D., Hamilton J.D., Graham N.M., Bieber T., Rocklin R., Ming J.E., Ren H., Kao R., Simpson E., et al. 2014. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 371:130–139. 10.1056/NEJMoa1314768 [DOI] [PubMed] [Google Scholar]
- Bergerson, J.R.E., and Freeman A.F.. 2019. An update on syndromes with a hyper-IgE phenotype. Immunol. Allergy Clin. North Am. 39:49–61. 10.1016/j.iac.2018.08.007 [DOI] [PubMed] [Google Scholar]
- Bettigole, S.E., Lis R., Adoro S., Lee A.H., Spencer L.A., Weller P.F., and Glimcher L.H.. 2015. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat. Immunol. 16:829–837. 10.1038/ni.3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béziat, V., Li J., Lin J.X., Ma C.S., Li P., Bousfiha A., Pellier I., Zoghi S., Baris S., Keles S., et al. 2018. A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci. Immunol. 3:3. 10.1126/sciimmunol.aat4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béziat, V., Rapaport F., Hu J., Titeux M., Bonnet des Claustres M., Bourgey M., Griffin H., Bandet É., Ma C.S., Sherkat R., et al. 2021. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell. 184:3812–3828.e30. 10.1016/j.cell.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat, V., Tavernier S.J., Chen Y.H., Ma C.S., Materna M., Laurence A., Staal J., Aschenbrenner D., Roels L., Worley L., et al. 2020. Dominant-negative mutations in human IL6ST underlie hyper-IgE syndrome. J. Exp. Med. 217:e20191804. 10.1084/jem.20191804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs, C.M., Keles S., and Chatila T.A.. 2017. DOCK8 deficiency: Insights into pathophysiology, clinical features and management. Clin. Immunol. 181:75–82. 10.1016/j.clim.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette, R., Papp K.A., Poulin Y., Gooderham M., Raman M., Mallbris L., Wang C., Purohit V., Mamolo C., Papacharalambous J., and Ports W.C.. 2016. Topical tofacitinib for atopic dermatitis: A phase IIa randomized trial. Br. J. Dermatol. 175:902–911. 10.1111/bjd.14871 [DOI] [PubMed] [Google Scholar]
- Bolton, S.M., Kagalwalla A.F., and Wechsler J.B.. 2018. Eosinophilic esophagitis in children: Endoscopic findings at diagnosis and post-intervention. Curr. Gastroenterol. Rep. 20:4. 10.1007/s11894-018-0607-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bønnelykke, K., Matheson M.C., Pers T.H., Granell R., Strachan D.P., Alves A.C., Linneberg A., Curtin J.A., Warrington N.M., and Standl M., et al. 2013. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nat. Genet. 45:902–906. 10.1038/ng.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns, H.A., Schindler U., and Kaplan M.H.. 2003. Expression of a constitutively active Stat6 in vivo alters lymphocyte homeostasis with distinct effects in T and B cells. J. Immunol. 170:3478–3487. 10.4049/jimmunol.170.7.3478 [DOI] [PubMed] [Google Scholar]
- Campbell, T.M., Liu Z., Zhang Q., Moncada-Velez M., Covill L.E., Zhang P., Alavi Darazam I., Bastard P., Bizien L., Bucciol G., et al. 2022. Respiratory viral infections in otherwise healthy humans with inherited IRF7 deficiency. J. Exp. Med. 219:e20220202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandesris, M.O., Azarine A., Ong K.T., Taleb S., Boutouyrie P., Mousseaux E., Romain M., Bozec E., Laurent S., Boddaert N., et al. 2012. Frequent and widespread vascular abnormalities in human signal transducer and activator of transcription 3 deficiency. Circ. Cardiovasc. Genet. 5:25–34. 10.1161/CIRCGENETICS.111.961235 [DOI] [PubMed] [Google Scholar]
- Chen, M.-H., Raffield L.M., Mousas A., Sakaue S., Huffman J.E., Moscati A., Trivedi B., Jiang T., Akbari P., and Vuckovic D., et al. 2020. Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global populations. Cell. 182:1198–1213.e14. 10.1016/j.cell.2020.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Lun A.T., and Smyth G.K.. 2016. From reads to genes to pathways: Differential expression analysis of RNA-seq experiments using rsubread and the edgeR quasi-likelihood pipeline. F1000 Res. 5:1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovanec, J., Tunc I., Hughes J., Halstead J., Mateja A., Liu Y., O’Connell M., Kim J., Park Y.H., Wang Q., et al. 2022. Genetically determining individualized clinical reference ranges for the biomarker tryptase can limit unnecessary procedures and unmask myeloid neoplasms. Blood Adv.:bloodadvances.2022007936. 10.1182/bloodadvances.2022007936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, E.Y., Freeman A.F., Jing H., Cowen E.W., Davis J., Su H.C., Holland S.M., and Turner M.L.. 2012. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch. Dermatol. 148:79–84. 10.1001/archdermatol.2011.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadak, M., Jacobs R., Skuljec J., Jirmo A.C., Yildiz Ö., Donnerstag F., Baerlecken N.T., Schmidt R.E., Lanfermann H., Skripuletz T., et al. 2017. Gain-of-function STAT1 mutations are associated with intracranial aneurysms. Clin. Immunol. 178:79–85. 10.1016/j.clim.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Daniel, C., Salvekar A., and Schindler U.. 2000. A gain-of-function mutation in STAT6. J. Biol. Chem. 275:14255–14259. 10.1074/jbc.C000129200 [DOI] [PubMed] [Google Scholar]
- DaSilva-Arnold, S.C., Thyagarajan A., Seymour L.J., Yi Q., Bradish J.R., Al-Hassani M., Zhou H., Perdue N.J., Nemeth V., Krbanjevic A., et al. 2018. Phenotyping acute and chronic atopic dermatitis-like lesions in Stat6VT mice identifies a role for IL-33 in disease pathogenesis. Arch. Dermatol. Res. 310:197–207. 10.1007/s00403-018-1807-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya, M., Cox C., Acevedo N., Boorgula M.P., Campbell M., Chavan S., Cho M.H., David G.L., Kachroo P., and Lasky-Su J., et al. 2021. Multiethnic genome-wide and HLA association study of total serum IgE level. J. Allergy Clin. Immunol. 148:1589–1595. 10.1016/j.jaci.2021.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya, M., Rafaels N., Brunetti T.M., Chavan S., Levin A.M., Shetty A., Gignoux C.R., Boorgula M.P., Wojcik G., and Campbell M., et al. 2019. Association study in African-admixed populations across the Americas recapitulates asthma risk loci in non-African populations. Nat. Commun. 10:880. 10.1038/s41467-019-08469-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenais, F., Margaritte-Jeannin P., Barnes K.C., Cookson W.O.C., Altmüller J., Ang W., Barr R.G., Beaty T.H., Becker A.B., and Beilby J., et al. 2018. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat. Genet. 50:42–53. 10.1038/s41588-017-0014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick, B.J.H., van der Molen T., Flokstra-de Blok B.M.J., Muraro A., Postma M.J., Kocks J.W.H., and van Boven J.F.M.. 2020. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharmacoecon. Outcomes Res. 20:437–453. 10.1080/14737167.2020.1819793 [DOI] [PubMed] [Google Scholar]
- Elo, L.L., Järvenpää H., Tuomela S., Raghav S., Ahlfors H., Laurila K., Gupta B., Lund R.J., Tahvanainen J., Hawkins R.D., et al. 2010. Genome-wide profiling of interleukin-4 and STAT6 transcription factor regulation of human Th2 cell programming. Immunity. 32:852–862. 10.1016/j.immuni.2010.06.011 [DOI] [PubMed] [Google Scholar]
- Ferreira, M.A., Vonk J.M., Baurecht H., Marenholz I., Tian C., Hoffman J.D., Helmer Q., Tillander A., Ullemar V., and van Dongen J., et al. 2017. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat. Genet. 49:1752–1757. 10.1038/ng.3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, M.A.R., Mathur R., Vonk J.M., Szwajda A., Brumpton B., Granell R., Brew B.K., Ullemar V., Lu Y., and Jiang Y., et al. 2019. Genetic architectures of childhood- and adult-onset asthma are partly distinct. Am. J. Hum. Genet. 104:665–684. 10.1016/j.ajhg.2019.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, M.A.R., Vonk J.M., Baurecht H., Marenholz I., Tian C., Hoffman J.D., Helmer Q., Tillander A., Ullemar V., and Lu Y., et al. 2020. Age-of-onset information helps identify 76 genetic variants associated with allergic disease. PLoS Genet. 16:e1008725. 10.1371/journal.pgen.1008725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, A.F., and Milner J.D.. 2020. The child with elevated IgE and infection susceptibility. Curr. Allergy Asthma Rep. 20:65. 10.1007/s11882-020-00964-y [DOI] [PubMed] [Google Scholar]
- Fung, S.Y., Lu H.Y., Sharma M., Sharma A.A., Saferali A., Jia A., Abraham L., Klein T., Gold M.R., Noterangelo L.D., et al. 2021. MALT1-Dependent cleavage of HOIL1 modulates canonical NF-κB signaling and inflammatory responsiveness. Front. Immunol. 12:749794. 10.3389/fimmu.2021.749794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, S.J., and Tsai M.. 2012. IgE and mast cells in allergic disease. Nat. Med. 18:693–704. 10.1038/nm.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux, F., Schultze J.L., Murray P.J., Ochando J., and Biswas S.K.. 2016. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 17:34–40. 10.1038/ni.3324 [DOI] [PubMed] [Google Scholar]
- Goenka, S., and Kaplan M.H.. 2011. Transcriptional regulation by STAT6. Immunol. Res. 50:87–96. 10.1007/s12026-011-8205-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowthaman, U., Chen J.S., Zhang B., Flynn W.F., Lu Y., Song W., Joseph J., Gertie J.A., Xu L., Collet M.A., et al. 2019. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. 365:365. 10.1126/science.aaw6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granada, M., Wilk J.B., Tuzova M., Strachan D.P., Weidinger S., Albrecht E., Gieger C., Heinrich J., Himes B.E., Hunninghake G.M., et al. 2012. A genome-wide association study of plasma total IgE concentrations in the Framingham Heart Study. J. Allergy Clin. Immunol. 129:840–845.e21. 10.1016/j.jaci.2011.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafemeister, C., and Satija R.. 2019. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20:296. 10.1186/s13059-019-1874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y., Jia Q., Jahani P.S., Hurrell B.P., Pan C., Huang P., Gukasyan J., Woodward N.C., Eskin E., Gilliland F.D., et al. 2020. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat. Commun. 11:1776. 10.1038/s41467-020-15649-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, A., Simons A., Schuurs-Hoeijmakers J.H.M., Koenen H.J.P.M., Zonneveld-Huijssoon E., Henriet S.S.V., Schatorjé E.J.H., Hoppenreijs E.P.A.H., Leenders E.K.S.M., Janssen E.J.M., et al. 2022. Trio-based whole exome sequencing in patients with suspected sporadic inborn errors of immunity: A retrospective cohort study. Elife. 11:e78469. 10.7554/eLife.78469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund, J., Hadizadeh F., Ek W.E., Karlsson T., and Johansson Å.. 2022. Gene-based variant analysis of whole-exome sequencing in relation to eosinophil count. Front. Immunol. 13:862255. 10.3389/fimmu.2022.862255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, Å., Rask-Andersen M., Karlsson T., and Ek W.E.. 2019. Genome-wide association analysis of 350 000 Caucasians from the UK Biobank identifies novel loci for asthma, hay fever and eczema. Hum. Mol. Genet. 28:4022–4041. 10.1093/hmg/ddz175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachuri, L., Jeon S., DeWan A.T., Metayer C., Ma X., Witte J.S., Chiang C.W.K., Wiemels J.L., and de Smith A.J.. 2021. Genetic determinants of blood-cell traits influence susceptibility to childhood acute lymphoblastic leukemia. Am. J. Hum. Genet. 108:1823–1835. 10.1016/j.ajhg.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, M.H., Schindler U., Smiley S.T., and Grusby M.J.. 1996. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 4:313–319. 10.1016/S1074-7613(00)80439-2 [DOI] [PubMed] [Google Scholar]
- Kichaev, G., Bhatia G., Loh P.-R., Gazal S., Burch K., Freund M.K., Schoech A., Pasaniuc B., and Price A.L.. 2019. Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet. 104:65–75. 10.1016/j.ajhg.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B.S., Howell M.D., Sun K., Papp K., Nasir A., Kuligowski M.E., Investigators I.S., and INCB 18424-206 Study Investigators . 2020. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J. Allergy Clin. Immunol. 145:572–582. 10.1016/j.jaci.2019.08.042 [DOI] [PubMed] [Google Scholar]
- Kim, Y., Kwon E.K., Jeon J.H., So I., Kim I.G., Choi M.S., Kim I.S., Choi J.K., Jung J.U., and Cho N.H.. 2012. Activation of the STAT6 transcription factor in Jurkat T-cells by the herpesvirus saimiri Tip protein. J. Gen. Virol. 93:330–340. 10.1099/vir.0.036087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneitz, C., Goller M., Seggewiss R., Yaman A., Serfling E., and Tony H.P.. 2000. STAT6 and the regulation of CD23 expression in B-chronic lymphocytic leukemia. Leuk. Res. 24:331–337. 10.1016/S0145-2126(99)00191-5 [DOI] [PubMed] [Google Scholar]
- Kulpa, D.A., Talla A., Brehm J.H., Ribeiro S.P., Yuan S., Bebin-Blackwell A.G., Miller M., Barnard R., Deeks S.G., Hazuda D., et al. 2019. Differentiation into an effector memory phenotype potentiates HIV-1 latency reversal in CD4+ T cells. J. Virol. 93:93. 10.1128/JVI.00969-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner, R.H., Zhang X.Y., and Reiser J.. 2009. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 4:495–505. 10.1038/nprot.2009.22 [DOI] [PubMed] [Google Scholar]
- Lemonnier, N., Melén E., Jiang Y., Joly S., Ménard C., Aguilar D., Acosta-Perez E., Bergström A., Boutaoui N., Bustamante M., et al. 2020. A novel whole blood gene expression signature for asthma, dermatitis, and rhinitis multimorbidity in children and adolescents. Allergy. 75:3248–3260. 10.1111/all.14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Rodriguez J.P., Niu F., Pu M., Wang J., Hung L.W., Shao Q., Zhu Y., Ding W., Liu Y., et al. 2016. Structural basis for DNA recognition by STAT6. Proc. Natl. Acad. Sci. USA. 113:13015–13020. 10.1073/pnas.1611228113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, M., Xu J., Clair A.J., Ehrman B., Graham L.M., and Eagleton M.J.. 2012. Local and systemic alterations in signal transducers and activators of transcription (STAT) associated with human abdominal aortic aneurysms. J. Surg. Res. 176:321–328. 10.1016/j.jss.2011.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H.Y., Sharma M., Sharma A.A., Lacson A., Szpurko A., Luider J., Dharmani-Khan P., Shameli A., Bell P.A., Guilcher G.M.T., et al. 2021. Mechanistic understanding of the combined immunodeficiency in complete human CARD11 deficiency. J. Allergy Clin. Immunol. 148:1559–1574.e13. 10.1016/j.jaci.2021.04.006 [DOI] [PubMed] [Google Scholar]
- Lundberg, M., Eriksson A., Tran B., Assarsson E., and Fredriksson S.. 2011. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 39:e102. 10.1093/nar/gkr424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons, J.J., and Milner J.D.. 2018. Primary atopic disorders. J. Exp. Med. 215:1009–1022. 10.1084/jem.20172306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura, S., Stellato C., Georas S.N., Casolaro V., Plitt J.R., Miura K., Kurosawa S., Schindler U., and Schleimer R.P.. 2001. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 24:755–761. 10.1165/ajrcmb.24.6.4351 [DOI] [PubMed] [Google Scholar]
- Medoff, B.D., Seung E., Hong S., Thomas S.Y., Sandall B.P., Duffield J.S., Kuperman D.A., Erle D.J., and Luster A.D.. 2009. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J. Immunol. 182:623–635. 10.4049/jimmunol.182.1.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikita, T., Campbell D., Wu P., Williamson K., and Schindler U.. 1996. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol. Cell. Biol. 16:5811–5820. 10.1128/MCB.16.10.5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, J.D. 2020. Primary atopic disorders. Annu. Rev. Immunol. 38:785–808. 10.1146/annurev-immunol-042718-041553 [DOI] [PubMed] [Google Scholar]
- Minegishi, Y. 2021. Hyper-IgE syndrome, 2021 update. Allergol. Int. 70:407–414. 10.1016/j.alit.2021.07.007 [DOI] [PubMed] [Google Scholar]
- Monaco, G., Lee B., Xu W., Mustafah S., Hwang Y.Y., Carre C., Burdin N., Visan L., Ceccarelli M., Poidinger M., et al. 2019. RNA-seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep. 26:1627–1640 e1627. 10.1016/j.celrep.2019.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, J.R., Nesbitt A.M.I., Baker S.W., Pechter K.B., Balciuniene J., Zhao X., Denenberg E.H., DeChene E.T., Wu C., Jayaraman P., et al. 2022. Molecular diagnostic outcomes from 700 cases: What can we learn from a retrospective analysis of clinical exome sequencing? J. Mol. Diagn. 24:274–286. 10.1016/j.jmoldx.2021.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., Hoang C.D., Diehn M., and Alizadeh A.A.. 2015. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 12:453–457. 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novershtern, N., Subramanian A., Lawton L.N., Mak R.H., Haining W.N., McConkey M.E., Habib N., Yosef N., Chang C.Y., Shay T., et al. 2011. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 144:296–309. 10.1016/j.cell.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsdottir, T.A., Theodors F., Bjarnadottir K., Bjornsdottir U.S., Agustsdottir A.B., Stefansson O.A., Ivarsdottir E.V., Sigurdsson J.K., Benonisdottir S., Eyjolfsson G.I., et al. 2020. Eighty-eight variants highlight the role of T cell regulation and airway remodeling in asthma pathogenesis. Nat. Commun. 11:393. 10.1038/s41467-019-14144-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen, E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., and Ferrin T.E.. 2004. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 25:1605–1612. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- Pickrell, J.K., Berisa T., Liu J.Z., Ségurel L., Tung J.Y., and Hinds D.A.. 2016. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 48:709–717. 10.1038/ng.3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pividori, M., Schoettler N., Nicolae D.L., Ober C., and Im H.K.. 2019. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: Genome-wide and transcriptome-wide studies. Lancet Respir. Med. 7:509–522. 10.1016/S2213-2600(19)30055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport, F., Boisson B., Gregor A., Béziat V., Boisson-Dupuis S., Bustamante J., Jouanguy E., Puel A., Rosain J., Zhang Q., et al. 2021. Negative selection on human genes underlying inborn errors depends on disease outcome and both the mode and mechanism of inheritance. Proc. Natl. Acad. Sci. USA. 118:118. 10.1073/pnas.2001248118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch, P., Schubach M., Shendure J., and Kircher M.. 2021. CADD-Splice-improving genome-wide variant effect prediction using deep learning-derived splice scores. Genome Med. 13:31. 10.1186/s13073-021-00835-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., and Smyth G.K.. 2015. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43:e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz, O., Guiter C., Castellano F., Dorsch K., Melzner J., Jais J.P., Dubois G., Gaulard P., Möller P., and Leroy K.. 2009. Recurrent mutations of the STAT6 DNA binding domain in primary mediastinal B-cell lymphoma. Blood. 114:1236–1242. 10.1182/blood-2009-03-209759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, M.D., and Smyth G.K.. 2007. Moderated statistical tests for assessing differences in tag abundance. Bioinformatics. 23:2881–2887. 10.1093/bioinformatics/btm453 [DOI] [PubMed] [Google Scholar]
- Sakaue, S., Kanai M., Tanigawa Y., Karjalainen J., Kurki M., Koshiba S., Narita A., Konuma T., Yamamoto K., and Akiyama M., et al. 2021. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53:1415–1424. 10.1038/s41588-021-00931-x [DOI] [PubMed] [Google Scholar]
- Shahin, T., Aschenbrenner D., Cagdas D., Bal S.K., Conde C.D., Garncarz W., Medgyesi D., Schwerd T., Karaatmaca B., Cetinkaya P.G., et al. 2019. Selective loss of function variants in cause Hyper-IgE syndrome with distinct impairments of T-cell phenotype and function. Haematologica. 104:609–621. 10.3324/haematol.2018.194233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M., Fu M.P., Lu H.Y., Sharma A.A., Modi B.P., Michalski C., Lin S., Dalmann J., Salman A., Del Bel K.L., et al. 2022. Human complete NFAT1 deficiency causes a triad of joint contractures, osteochondromas, and B-cell malignancy. Blood. 140:1858–1874. 10.1182/blood.2022015674 [DOI] [PubMed] [Google Scholar]
- Shimoda, K., van Deursen J., Sangster M.Y., Sarawar S.R., Carson R.T., Tripp R.A., Chu C., Quelle F.W., Nosaka T., Vignali D.A., et al. 1996. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 380:630–633. 10.1038/380630a0 [DOI] [PubMed] [Google Scholar]
- Shrine, N., Portelli M.A., John C., Soler Artigas M., Bennett N., Hall R., Lewis J., Henry A.P., Billington C.K., Ahmad A., et al. 2019. Moderate-to-severe asthma in individuals of European ancestry: A genome-wide association study. Lancet Respir. Med. 7:20–34. 10.1016/S2213-2600(18)30389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim, N.L., Kumar P., Hu J., Henikoff S., Schneider G., and Ng P.C.. 2012. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 40:W452–W457. 10.1093/nar/gks539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman, P.M.A., Wang M.-L., Cianferoni A., Aceves S., Gonsalves N., Nadeau K., Bredenoord A.J., Furuta G.T., Spergel J.M., and Hakonarson H.. 2014. GWAS identifies four novel eosinophilic esophagitis loci. Nat. Commun. 5:5593. 10.1038/ncomms6593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloka, S., Silva C., Wang J., and Yong V.W.. 2011. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J. Neuroinflammation. 8:56. 10.1186/1742-2094-8-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulczewski, F.B., Martino L.A., da Silva Almeida B., Yamamoto M.M., Rosa D.S., and Boscardin S.B.. 2021. STAT6 signaling pathway controls germinal center responses promoted after antigen targeting to conventional type 2 dendritic cells. Curr. Res. Immunol. 2:120–131. 10.1016/j.crimmu.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratannon, N., Ittiwut C., Dik W.A., Ittiwut R., Meesilpavikkai K., Israsena N., Ingrungruanglert P., Dalm V.A.S.H., van Daele P.L.A., Sanpavat A., et al. 2022. A germline STAT6 gain-of-function variant is associated with early-onset allergies. J. Allergy Clin. Immunol. 151:565–571.e9 [DOI] [PubMed] [Google Scholar]
- Takeda, K., Tanaka T., Shi W., Matsumoto M., Minami M., Kashiwamura S., Nakanishi K., Yoshida N., Kishimoto T., and Akira S.. 1996. Essential role of Stat6 in IL-4 signalling. Nature. 380:627–630. 10.1038/380627a0 [DOI] [PubMed] [Google Scholar]
- Tanaka, N., Koido M., Suzuki A., Otomo N., Suetsugu H., Kochi Y., Tomizuka K., Momozawa Y., Kamatani Y., and Ikegawa S., et al. 2021. Eight novel susceptibility loci and putative causal variants in atopic dermatitis. J. Allergy Clin. Immunol. 148:1293–1306. 10.1016/j.jaci.2021.04.019 [DOI] [PubMed] [Google Scholar]
- Tarailo-Graovac, M., Shyr C., Ross C.J., Horvath G.A., Salvarinova R., Ye X.C., Zhang L.H., Bhavsar A.P., Lee J.J., Drögemöller B.I., et al. 2016. Exome sequencing and the management of neurometabolic disorders. N. Engl. J. Med. 374:2246–2255. 10.1056/NEJMoa1515792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate, J.G., Bamford S., Jubb H.C., Sondka Z., Beare D.M., Bindal N., Boutselakis H., Cole C.G., Creatore C., Dawson E., et al. 2019. COSMIC: The Catalogue of somatic mutations in cancer. Nucleic Acids Res. 47:D941–D947. 10.1093/nar/gky1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana, J., Okada S., Hiller J., Oleastro M., Lagos Gomez M., Aldave Becerra J.C., Ouachée-Chardin M., Fouyssac F., Girisha K.M., and Etzioni A., et al. 2016. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 127:3154–3164. 10.1182/blood-2015-11-679902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valette, K., Li Z., Bon-Baret V., Chignon A., Bérubé J.-C., Eslami A., Lamothe J., Gaudreault N., Joubert P., Obeidat M., et al. 2021. Prioritization of candidate causal genes for asthma in susceptibility loci derived from UK Biobank. Commun. Biol. 4:700. 10.1038/s42003-021-02227-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi-Shanjani, M., Smith K.L., Sara R.J., Modi B.P., Branch A., Sharma M., Lu H.Y., James E.L., Hildebrand K.J., Biggs C.M., and Turvey S.E.. 2021. Inborn errors of immunity manifesting as atopic disorders. J. Allergy Clin. Immunol. 148:1130–1139. 10.1016/j.jaci.2021.08.008 [DOI] [PubMed] [Google Scholar]
- Villarino, A.V., Gadina M., O'Shea J.J., and Kanno Y.. 2020. SnapShot: Jak-STAT signaling II. Cell. 181:1696–1696.e1691. 10.1016/j.cell.2020.04.052 [DOI] [PubMed] [Google Scholar]
- Villarino, A.V., Kanno Y., and O’Shea J.J.. 2017. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 18:374–384. 10.1038/ni.3691 [DOI] [PubMed] [Google Scholar]
- Vuckovic, D., Bao E.L., Akbari P., Lareau C.A., Mousas A., Jiang T., Chen M.-H., Raffield L.M., Tardaguila M., and Huffman J.E., et al. 2020. The polygenic and monogenic basis of blood traits and diseases. Cell. 182:1214–1231.e11. 10.1016/j.cell.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage, J., Standl M., Curtin J.A., Jessen L.E., Thorsen J., Tian C., Schoettler N., Flores C., Abdellaoui A., and Ahluwalia T.S., et al. 2018. Genome-wide association and HLA fine-mapping studies identify risk loci and genetic pathways underlying allergic rhinitis. Nat. Genet. 50:1072–1080. 10.1038/s41588-018-0157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L., et al. 2018. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46:W296–W303. 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick, K.R., and Berton M.T.. 2000. IL-4 induces serine phosphorylation of the STAT6 transactivation domain in B lymphocytes. Mol. Immunol. 37:641–652. 10.1016/S0161-5890(00)00088-2 [DOI] [PubMed] [Google Scholar]
- Yildiz, M., Li H., Bernard D., Amin N.A., Ouillette P., Jones S., Saiya-Cork K., Parkin B., Jacobi K., Shedden K., et al. 2015. Activating STAT6 mutations in follicular lymphoma. Blood. 125:668–679. 10.1182/blood-2014-06-582650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamò, A., Pischimarov J., Horn H., Ott G., Rosenwald A., and Leich E.. 2018. The exomic landscape of t(14;18)-negative diffuse follicular lymphoma with 1p36 deletion. Br. J. Haematol. 180:391–394. 10.1111/bjh.15041 [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Boisson B., Béziat V., Puel A., and Casanova J.L.. 2018. Human hyper-IgE syndrome: Singular or plural? Mamm. Genome. 29:603–617. 10.1007/s00335-018-9767-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Z., Guo Y., Shi H., Liu C.-L., Panganiban R.A., Chung W., O’Connor L.J., Himes B.E., Gazal S., Hasegawa K., et al. 2020. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J. Allergy Clin. Immunol. 145:537–549. 10.1016/j.jaci.2019.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Z., Lee P.H., Chaffin M.D., Chung W., Loh P.-R., Lu Q., Christiani D.C., and Liang L.. 2018. A genome-wide cross-trait analysis from UK Biobank highlights the shared genetic architecture of asthma and allergic diseases. Nat. Genet. 50:857–864. 10.1038/s41588-018-0121-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Z., Zhu X., Liu C.-L., Shi H., Shen S., Yang Y., Hasegawa K., Camargo C.A. Jr, and Liang L.. 2019. Shared genetics of asthma and mental health disorders: A large-scale genome-wide cross-trait analysis. Eur. Respir. J. 54:1901507. 10.1183/13993003.01507-2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lists primers used for site-directed mutagenesis.
lists antibodies used for phospho-flow on different immune subsets.
lists antibodies used for TH phenotyping in patient PBMCs.
shows variant annotation and pathogenicity prediction of the variants reported within the DNA-binding domain of STAT6 for 10 patients.
shows variant annotation and pathogenicity prediction of the variants reported outside of the DNA-binding domain of STAT6 for six patients.
lists genes upregulated (gray) and downregulated (red) in transduced WT-, p.E382Q-, and p.D419G-transduced Jurkats that meet the cutoff of fold-change (FC) > 1.25 and adjusted P value <0.05.
lists genes in the leading edge driving the enrichment of two pathways (IL-4/STAT6 pathway and TH2 pathway) between transduced WT- vs. p.E382Q- and WT- vs. p.D419G- Jurkats
provides clinical narratives for each patient.
contains original blots for Fig. 3.