Abstract
Background:
Patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may have an increased risk of acute cardiovascular events in the convalescent period.
Aims:
To determine whether patients with SARS-CoV-2 infection have an increased risk of cardiovascular events during the convalescent period.
Methods:
We analyzed 10,691 hospitalized adult pneumonia patients with SARS-CoV-2 infection and contemporary matched controls of pneumonia patients without SARS-CoV-2 infection. The risk of new cardiovascular events following >30 days pneumonia admission (convalescent period) was ascertained using Cox proportional hazards regression analysis to adjust for potential confounders.
Results:
Among 10,691 pneumonia patients with SARS-CoV-2 infection, 697 patients (5.8%; 95% CI, 5.4–6.2%) developed new cardiovascular events (median time interval of 218 days post pneumonia admission; interquartile range Q1 = 117 days, Q3 = 313 days). The risk of new cardiovascular events was not significantly higher among pneumonia patients with SARS-CoV-2 infection compared with those with pneumonia without SARS-CoV-2 infection (hazard ratio (HR), 0.90, 95% CI, 0.80–1.02) after adjustment for potential confounders. In addition, no significant difference in the rate of a new ischemic stroke (HR, 0.84; 95% CI, 0.70–1.02) or ischemic heart disease (HR, 1.00; 95% CI, 0.87–1.15) was observed between the pneumonia patients with and without SARS-CoV-2 infection.
Conclusion:
Our study suggests that new cardiovascular events rate in the convalescent period among pneumonia patients with SARS-CoV-2 infection was not significantly higher than the rate seen with other pneumonias.
Keywords: Severe acute respiratory syndrome coronavirus 2, coronavirus disease, cardiovascular events, pneumonia, stroke
Introduction
An increased risk for acute ischemic stroke (AIS) and myocardial infarction (MI) may be seen in the convalescent period of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection since the systemic elevation of inflammatory mediators and endothelial dysfunction may persists for months after the infection.1,2 An increased risk for cardiovascular events has been reported in the convalescent period with other respiratory tract infections.3,4 One study5 reported that the risk of AIS in the convalescent period among men with asymptomatic SARS-CoV-2 infection was two folds higher compared with the estimated rate in the age-, sex-, and ethnicity-matched cohort from general population. We performed the study using a large cohort representative of the United States to analyze the occurrence of new cardiovascular events in the convalescent period after SARS-CoV-2 infection due to implications for preventive strategies and the long-term disability6,7 among approximately 222 million survivors worldwide.8
Methods
Patients
We analyzed the Cerner Real-World Data (Cerner Real World Data Q3 2021 file) extracted from the electronic medical records of 110 health care facilities9,10 from 1 January 2020 to 1 October 2021.
Selection of patients
We selected patients diagnosed with pneumonia during a hospitalization lasting more than 24 h based on International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) primary diagnosis codes J12-J18. The hospital admission with pneumonia diagnosis was designated as the reference encounter. Furthermore, patients had (1) at least two encounters within 2 years prior to their reference encounter and (2) at least two encounters more than 30 days after the reference encounter admission.
Motivations for selection criteria
Only patients with pneumonia were included in this analysis to ensure that observed effects were due to SARS-CoV-2 infection and not generic respiratory illness. The requirement of ⩾2 encounters in the preceding 2 years was intended to ensure that patients had up to date prior medical history. The requirement for ⩾2 subsequent encounters following the reference encounter was intended to ensure patients had follow-up data.
Identification of patients with SARS-CoV-2 infection
Patients with a positive laboratory test for SARS-CoV-2 were identified based on Logical Observation Identifiers Names and Codes (LOINC®) 41458-1, 94309-2, 94500-6, 94533-7, 94534-5, and 94646-7.
Identification of controls
Each SARS-CoV-2-infected patient was matched with a pneumonia patient without SARS-CoV-2 infection (confirmed by a negative laboratory test) using age, gender, race/ethnicity, and reference encounter admission date. This resulted in two cohorts with nearly identical distributions of demographics and hospitalization dates.
Identification of new cardiovascular events
We used the ICD-10-CM primary diagnosis codes I63 for AIS, I61 for intracerebral hemorrhage (ICH), I60 for subarachnoid hemorrhage (SAH), I21, I22, and I25.2 for acute MI, and I20.0 for unstable angina to identify the patients diagnosed with new cardiovascular events. Convalescent period was defined as > 30 days from the admission date of the reference encounter consistent with previous studies11–13 and the 4-week time frame for medical observation and isolation after initial diagnosis recommended by European Centre for Disease Prevention and Control.14
Data ascertained
Factors known or suspected to affect the risk of new cardiovascular events as identified in previous studies15–20 were extracted for each patient at any encounter using ICD-10-CM codes (see supplementary file). Any occurrence of septic shock, respiratory failure, dyspnea, chest pain, or intubation/mechanical ventilation during the reference encounter was ascertained as surrogate markers for severity of pneumonia.21–23 Outcomes (routine, non-routine, or death) were assessed by evaluating discharge status from hospitalizations more than 30 days after the reference encounter admission.
Statistical analysis
We performed Cox proportional hazards regression analysis including all cases and controls to identify the independent effect of SARS-CoV-2 infection and included age, gender, race, hypertension, diabetes mellitus, hyperlipidemia, nicotine dependence/tobacco use, alcohol use or abuse, atrial fibrillation, congestive heart failure, previous acute MI, previous AIS, previous ICH, previous SAH, and previous unstable angina; and septic shock, respiratory failure, dyspnea, chest pain, and intubation/ mechanical ventilation (during reference encounter) in the multivariate model.21–23 The Kaplan–Meier curve was used to provide visual representation of the probability of a new cardiovascular event over time. All the hypothesis tests were two-sided, with p < 0.05 considered statistically significant, and all the analyses were done using R (version 3.6.1).
Results
Overall rates of new cardiovascular events in the convalescent period
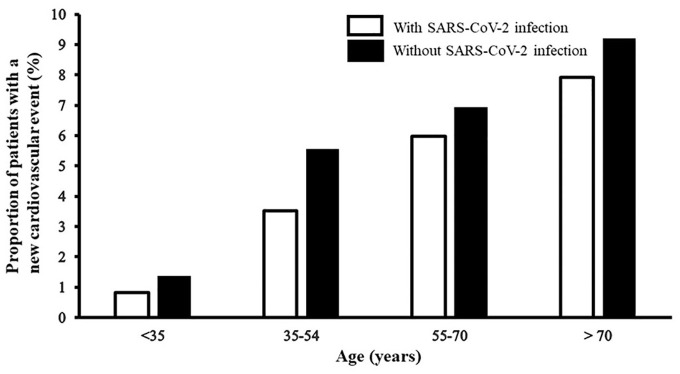
Among 10,691 patients with pneumonia and SARS-CoV-2 infection, 697 patients (5.8%; 95% CI, 5.4–6.2%) developed new cardiovascular events (median time interval of 218 days post reference encounter admission; interquartile range Q1 = 117 days, Q3 = 313 days). Among 10,691 patients with pneumonia but without SARS-CoV-2 infection, 756 (7.1%, 95% CI, 6.6–7.6%) developed new cardiovascular events (median time interval of 242 days post reference encounter admission; interquartile range Q1 = 131 days, Q3 = 343 days). The probability of a new cardiovascular event over time in pneumonia patients with and without SARS-CoV-2 infection is presented in Figure 1 (Kaplan–Meier curve). The rate of new cardiovascular events was lower among patients with SARS-CoV-2 infection and increased in age strata of older age in both groups (see Figure 2).
Figure 1.
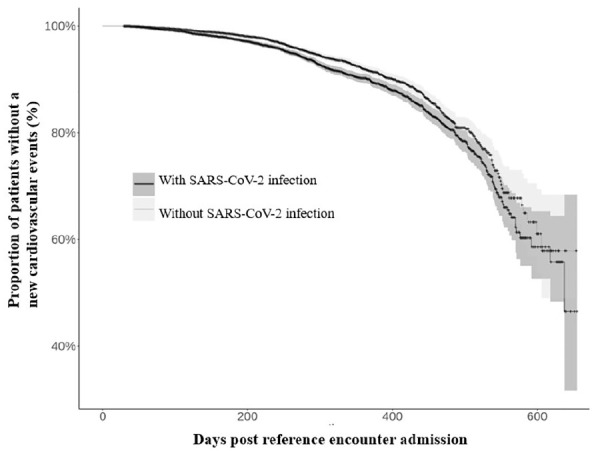
Kaplan–Meier curve demonstrating the probability of a cardiovascular event over time in pneumonia patients with and without SARS-CoV-2 infection. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Figure 2.
New onset cardiovascular events in the convalescent period according to age groups among pneumonia patients. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Comparison of risk factors in patients with pneumonia
There was a significantly higher rate of hypertension, diabetes mellitus, and hyperlipidemia among pneumonia patients with SARS-CoV-2 infection compared with those without SARS-CoV-2 infection (see Table 1). However, the rates of other cardiovascular risk factors were significantly lower among patients with SARS-CoV-2 infection. The proportion of patients who had septic shock or chest pain during reference hospitalization was lower among patients with SARS-CoV-2 infection. The proportion of patients who had dyspnea or respiratory failure during reference hospitalization was higher among patients with SARS-CoV-2 infection. There was no different in the proportion of patients who required intubation/mechanical ventilation during reference hospitalization between the two groups.
Table 1.
Demographic and clinical characteristics of pneumonia patients according to presence or absence of SARS-CoV-2 infection.
| Variables | Patients with SARS-CoV-2
infection (n = 10,470) |
Patients without SARS-CoV-2
infection (n = 10,470) |
p-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | |||
| <35 | 728 (6.8%) | 728 (6.8%) | 1.00 |
| 35–54 | 2333 (21.8%) | 2335 (21.8%) | 1.00 |
| 55–70 | 3857 (36.0%) | 3805 (35.6%) | 0.46 |
| >70 | 3771 (35.3%) | 3823 (35.8%) | 0.46 |
| Sex | |||
| Men | 4918 (46.0%) | 4918 (46.0%) | 1.00 |
| Women | 5773 (54.0%) | 5773 (54.0%) | 1.00 |
| Race/ethnicity | |||
| White | 7575 (70.9%) | 7575 (70.9%) | 1.00 |
| Black | 1266 (11.8%) | 1266 (11.8%) | 1.00 |
| Hispanic | 765 (7.2%) | 765 (7.2%) | 1.00 |
| Asian | 56 (0.5%) | 56 (0.5%) | 1.00 |
| Other | 1029 (9.6%) | 1029 (9.6%) | 1.00 |
| Cardiovascular risk factors/diseases | |||
| Hypertension | 8302 (77.7%) | 8127 (76.0%) | 0.004 |
| Diabetes mellitus | 5666 (53.0%) | 4655 (43.5%) | <0.001 |
| Hyperlipidemia | 6850 (64.0%) | 6268 (58.3%) | <0.001 |
| Nicotine dependence/tobacco use | 2027 (19.0%) | 4077 (38.1%) | <0.001 |
| Alcohol use or abuse | 661 (6.2%) | 1394 (13.0%) | <0.001 |
| Atrial fibrillation | 2199 (20.6%) | 2846 (26.6%) | <0.001 |
| Congestive heart failure | 2708 (25.3%) | 4083 (38.2%) | <0.001 |
| Previous acute myocardial infarction | 727 (6.8%) | 919 (8.9%) | <0.001 |
| Previous ischemic stroke | 500 (4.7%) | 601 (5.6%) | 0.002 |
| Previous intracerebral hemorrhage | 56 (0.5%) | 74 (0.7%) | 0.11 |
| Previous subarachnoid hemorrhage | 30 (0.3%) | 36 (0.3%) | 0.46 |
| Previous unstable angina | 110 (1.0%) | 122 (1.1%) | 0.43 |
| Events during reference hospitalization | |||
| Respiratory failure | 7219 (67.5%) | 4996 (46.7%) | <0.001 |
| Dyspnea | 3752 (35.1%) | 3405 (31.8%) | <0.001 |
| Chest pain | 970 (9.1%) | 1375 (12.9%) | <0.001 |
| Septic shock | 559 (5.2%) | 670 (6.3%) | 0.001 |
| Intubation/mechanical ventilation | 683 (6.4%) | 633 (5.9%) | 0.15 |
| Non-routine discharge (excluding death) | 8295 (77.6%) | 8656 (81.0%) | <0.0001 |
| Expired in hospital | 293 (2.7%) | 468 (4.4%) | <0.0001 |
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Risk of new onset cardiovascular events in multivariate analysis
The risk of new cardiovascular events in the convalescent period was not significantly different in pneumonia patients with SARS-CoV-2 infection compared with those without SARS-CoV-2 infection (hazard ratio (HR), 0.90; 95% CI, 0.80–1.02) after adjustment for age, gender, race/ethnicity, hypertension, diabetes mellitus, hyperlipidemia, nicotine dependence/tobacco use, alcohol use or abuse, atrial fibrillation, congestive heart failure, previous acute MI, previous AIS, previous ICH, previous SAH, and previous unstable angina; and respiratory failure, dyspnea, chest pain, septic shock and intubation/ mechanical ventilation (during reference encounter) (see Table 2). The risk of a new AIS (HR, 0.84; 95% CI, 0.70–1.02) or ischemic heart disease (HR, 1.00; 95% CI, 0.87–1.15) was not higher in patients with SARS-CoV-2 infection compared with those without SARS-CoV-2 infection after adjusting for the aforementioned confounders.
Table 2.
Results of Cox proportional hazards regression analyses for new cardiovascular events in convalescent period.
| Cardiovascular events | Number | Number of the event | Event rate | Multivariate adjusted HR (95% CI)a |
|---|---|---|---|---|
| Any cardiovascular event | ||||
| Patients with SARS-CoV-2 infection | 10,691 | 618 | 5.8% | 0.90 (0.80–1.02) |
| Patients without SARS-CoV-2 infection | 10,691 | 756 | 7.1% | Reference |
| Ischemic heart disease or stroke event | ||||
| Patients with SARS-CoV-2 infection | 10,691 | 594 | 5.6% | 0.93 (0.82–1.04) |
| Patients without SARS-CoV-2 infection | 10,691 | 716 | 6.7% | Reference |
| Ischemic heart disease | ||||
| Patients with SARS-CoV-2 infection | 10,691 | 411 | 3.8% | 1.00 (0.87–1.15) |
| Patients without SARS-CoV-2 infection | 10,691 | 482 | 4.5% | Reference |
| Ischemic stroke | ||||
| Patients with SARS-CoV-2 infection | 10,691 | 219 | 2.0% | 0.84 (0.70–1.02) |
| Patients without SARS-CoV-2 infection | 10,691 | 268 | 2.5% | Reference |
| Intracerebral or subarachnoid hemorrhage | ||||
| Patients with SARS-CoV-2 infection | 10,691 | 29 | 0.3% | 0.42 (0.26–0.69) |
| Patients without SARS-CoV-2 infection | 10,691 | 56 | 0.5% | Reference |
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; HR: hazard ratio; CI: confidence interval.
See the “Methods” section for variables adjusted in multivariate model.
Discussion
New cardiovascular events in SARS-CoV-2 infection survivors
We did not identify an increased rate of new cardiovascular events among survivors of pneumonia associated with SARS-CoV-2 infection in the convalescent period compared with those without SARS-CoV-2 infection. The control population was derived from patients with pneumonia related to other infections which may have a higher risk of cardiovascular events compared with general population in the convalescent period.3,4 Our findings are different to a previous study by Taquet et al.24 who found that the risk of AIS was higher among patients with SARS-CoV-2 infection compared with patients admitted with influenza and those with other respiratory tract infections. However, Taquet et al.24 included occurrence of AIS as an outcome at any time between 1 and 180 days after the index event. Similarly, Tu et al.5 identified 18 men who developed AIS at any time between 1 and 130 days (median period of 55 days) after diagnosis of SARS-CoV-2 infection. The estimated annual incidence rate of AIS was 82.6 cases per 100,000 persons which was higher than the incidence rate of 38.2 cases per 100,000 people in the historical matched cohort (rate ratio, 2.16). Our study included occurrence of cardiovascular events after 30 days of index event to focus on occurrence of cardiovascular events in the convalescent phase and avoid the confounding effect of acute phase events. Xie et al.12 compared the 12-month cardiovascular event rates among patients with SARS-CoV-2 infection who survived the first 30 days and contemporary control group of the US Veterans Health Administration system users without SARS-CoV-2 infection (predominantly non-hospitalized patients). Patients with SARS-CoV-2 infection had an increased risk of stroke (HR = 1.52) and MI (HR = 1.63). The difference between the results of ours and Xie et al.12 analysis is perhaps due to a large proportion of non-hospitalized control patients in the study by Xie et al.12 (at low risk of cardiovascular events).
Interpretation and implications for treatment
An important aspect in interpretation and implications for treatment is that the rate of new cardiovascular events among survivors of pneumonia with SARS-CoV-2 infection in the convalescent period was not different from those without SARS-CoV-2 infection. The rate of new cardiovascular events maybe higher than patients without pneumonia12 and whether routine antithrombotic treatment may be of value in patients with SARS-CoV-2 infection and pneumonia in the convalescent period needs to be considered. The accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-4B Outpatient Thrombosis Prevention Trial25 analyzed 558 randomly assigned participants who received daily aspirin (81 mg), prophylactic-dose apixaban (5 mg), therapeutic-dose apixaban (10 mg), or matching placebo for 45 days. The primary composite end point (all-cause mortality, symptomatic venous or arterial thromboembolism, MI, stroke, or hospitalization for cardiovascular or pulmonary causes) occurred in one patient (0.7%), one patient (–0.7%), two patients (1.4%), and one patient (0.7%) in the aspirin group, 5 mg apixaban, 10 mg apixaban, and placebo groups, respectively. The absolute risk reductions compared with placebo for the primary outcome ranged from 0% to 1.4% (non-significant) in the various therapeutic groups. Our results support the National Institutes of Health COVID-19 treatment guidelines26 which recommend that anticoagulants and antiplatelet therapy should not be initiated for the prevention of venous thromboembolism or arterial thrombosis for non-hospitalized patients with SARS-CoV-2 infection, unless the patient has other indications for the therapy or is participating in a clinical trial (strong evidence based on expert opinion). Leentjens et al.27 also concluded that routine antithrombotic treatment is not recommended in patients with SARS-CoV-2 infection after discharge based on pathophysiology of SARS-CoV-2 infection-related coagulopathy and data from randomized controlled trials. Although the reference encounter occurred in many of the patients after vaccination for SARS-CoV-2 infection was available,28 the CERNER dataset did not have reliable information on vaccination status of the patients. A previous study12 did not demonstrate any modifying effect of vaccination status on relationship between SARS-CoV-2 infection and new cardiovascular events.
Limitations
We used ICD-10 codes which have a high positive predictive value to identify AIS from the principle discharge diagnosis.29 The ICD-10 codes have a high positive predictive value for AIS (82%),30 acute coronary syndrome (86.6%), and acute MI (100%).31 We acknowledge the effect of variability in hospitalization criteria over time and between institutions on our analysis is not known. Our ability to quantify the severity of the pneumonia observed in our cohort of hospitalized patients was limited due lack of data on physical examination, and laboratory, and radiographic findings21,23 which were not available.32 We were unable to provide in-depth information regarding the use of primary or secondary preventive strategies used in these patients and therefore are unable to comment upon the modifying effect of such strategies on our results. We acknowledge that using a definition of >30 days after reference admission for convalescent period is arbitrary but consistent with previous studies.11–14 Some studies have used a short time period of > 21 days from either positive test or admission for SARS-CoV-2 infection33,34 while others have used a longer period > 8 weeks from discharge to define convalescent period.35 The variance is attributed to whether clinical symptom resolution, transmissibility, or resolution of laboratory abnormalities is used to define resolution of the acute phase.
Conclusion
We did not identify any increased risk of new cardiovascular events in the convalescent period among pneumonia patients with SARS-CoV-2 infection compared with patients with pneumonia without SARS-CoV-2 infection. Our results do not support additional monitoring or use of antithrombotic medication among SARS-CoV-2 survivors in the convalescent period.
Supplemental Material
Supplemental material, sj-docx-1-wso-10.1177_17474930221114561 for New cardiovascular events in the convalescent period among survivors of SARS-CoV-2 infection by Adnan I Qureshi, William I Baskett, Wei Huang, Yasemin Akinci, M Fareed K Suri, S Hasan Naqvi, Brandi R French, Farhan Siddiq, Camilo R Gomez and Chi-Ren Shyu in International Journal of Stroke
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.I.Q. has received consultation fees from AstraZeneca. No other potential conflict of interest relevant to this article was reported.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (5T32LM012410 to W.I.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: Adnan I Qureshi  https://orcid.org/0000-0003-4962-540X
https://orcid.org/0000-0003-4962-540X
Yasemin Akinci  https://orcid.org/0000-0001-6984-033X
https://orcid.org/0000-0001-6984-033X
Chi-Ren Shyu  https://orcid.org/0000-0001-9197-9522
https://orcid.org/0000-0001-9197-9522
Supplemental material: Supplemental material for this article is available online.
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong SWX, Fong SW, Young BE, et al. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infect Dis 2021; 8: ofab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P.Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004; 351: 2611–2618. [DOI] [PubMed] [Google Scholar]
- 5.Tu TM, Seet CYH, Koh JS, et al. Acute ischemic stroke during the convalescent phase of asymptomatic COVID-2019 infection in men. JAMA Netw Open 2021; 4: e217498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal M.Cardiovascular disease and COVID-19. Diabetes Metab Syndr 2020; 14: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi AI, Abd-Allah F, Al-Senani F, et al. Management of acute ischemic stroke in patients with COVID-19 infection: report of an international panel. Int J Stroke 2020; 15: 540–554. [DOI] [PubMed] [Google Scholar]
- 8.Number of coronavirus (COVID-19) cases recoveries deaths worldwide as of October 26 2021, https://www.statista.com/statistics/1087466/covid19-cases-recoveries-deaths-worldwide/ (accessed 28 October 2021).
- 9.Cerner Corporation. Cerner provides access to de-identified patient data for COVID-19 research and vaccine development, https://www.cerner.com/newsroom/cerner-provides-access-to-de-identified-patient-data-for-covid-19-research-and-vaccine-development (accessed 16 September 2020).
- 10.Laird-Maddox M, Mitchell SB, Hoffman M.Integrating research data capture into the electronic health record workflow: real-world experience to advance innovation. Perspect Health Inf Manag 2014; 11: 1e. [PMC free article] [PubMed] [Google Scholar]
- 11.Neeland MR, Bannister S, Clifford V, et al. Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children. Nat Commun 2021; 12: 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y, Xu E, Bowe B, Al-Aly Z.Long-term cardiovascular outcomes of COVID-19. Nat Med 2022; 28: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H, Fu L, Jin Y, et al. Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection. J Clin Lab Anal 2020; 34: e23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention Control. Guidance on ending the isolation period for people with COVID-19, third update. Stockholm: ECDC, 2022. [Google Scholar]
- 15.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 74: e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richard E, Andrieu S, Solomon A, et al. Methodological challenges in designing dementia prevention trials—the European Dementia Prevention Initiative (EDPI). J Neurol Sci 2012; 322: 64–70. [DOI] [PubMed] [Google Scholar]
- 19.van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry 2005; 76: v2–v7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020; 395: 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243–250. [DOI] [PubMed] [Google Scholar]
- 22.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu G, Zhou S, Wang Y, et al. A prediction model of outcome of SARS-CoV-2 pneumonia based on laboratory findings. Sci Rep 2020; 10: 14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ.6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psych 2021; 8: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connors JM, Brooks MM, Sciurba FC, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA 2021; 326: 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antithrombotic therapy in patients with COVID-19, https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/ (accessed 25 October 2021).
- 27.Leentjens J, van Haaps TF, Wessels PF, Schutgens REG, Middeldorp S.COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol 2021; 8: e524–e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan I, Kosinska M, Palakollu M, et al. Current global guidelines for severe acute respiratory syndrome coronavirus 2 booster immunization. Healthcare Res J 2021; 2: 64–69. [Google Scholar]
- 29.Alhajji M, Kawsara A, Alkhouli M.Validation of acute ischemic stroke codes using the international classification of diseases tenth revision. Am J Cardiol 2020; 125: 1135. [DOI] [PubMed] [Google Scholar]
- 30.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA.Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS ONE 2015; 10: e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bezin J, Girodet PO, Rambelomanana S, et al. Choice of ICD-10 codes for the identification of acute coronary syndrome in the French hospitalization database. Fundam Clin Pharmacol 2015; 29: 586–591. [DOI] [PubMed] [Google Scholar]
- 32.Ammann EM, Schweizer ML, Robinson JG, et al. Chart validation of inpatient ICD-9-CM administrative diagnosis codes for acute myocardial infarction (AMI) among intravenous immune globulin (IGIV) users in the Sentinel Distributed Database. Pharmacoepidemiol Drug Saf 2018; 27: 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen K, Ren S, Heath K, et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2022; 376: e068414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021; 373: n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Yang L, Chen Y, Xu Z, Wang H, Zhang X.A longitudinal follow-up of COVID-19 patients in the convalescent phase showed recovery in radiological results, the dynamics of lymphocytes, and a decrease in the level of IgG antibody: a single-centre, observational study. J Thorac Dis 2021; 13: 2986–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-wso-10.1177_17474930221114561 for New cardiovascular events in the convalescent period among survivors of SARS-CoV-2 infection by Adnan I Qureshi, William I Baskett, Wei Huang, Yasemin Akinci, M Fareed K Suri, S Hasan Naqvi, Brandi R French, Farhan Siddiq, Camilo R Gomez and Chi-Ren Shyu in International Journal of Stroke