Abstract
Despite the established role of low-density lipoprotein cholesterol (LDL-C) as a major risk factor for cardiovascular disease (CVD), and the persistence of CVD as the leading cause of morbidity and mortality in the United States, national quality assurance metrics no longer include LDL-C measurement as a required performance metric. This clinical perspective reviews the history of LDL-C as a quality and performance metric and the events that led to its replacement. It also presents patient, healthcare provider, and health system rationales for re-establishing LDL-C measurement as a performance measure to improve cholesterol control in high-risk groups and to stem the rising tide of CVD morbidity and mortality, cardiovascular care disparities, and related healthcare costs.
Keywords: Cardiovascular disease, LDL cholesterol, Risk factors, Blood pressure, Lipoprotein modifying, Statins
1. Introduction
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in the United States (U.S.), with most of the burden due to atherosclerotic CVD (ASCVD). Despite a steady decline in the age-adjusted mortality rate from CVD in the U.S. over the last four decades, a flattening of the curve and a slight increase in the death rate from CVD has been observed since 2015 [1,2] with more striking increases noted in 2020 [3]. Increases in ASCVD events in younger and middle-aged individuals have been particularly alarming.
Numerous epidemiological studies and clinical trials have established that levels of serum low-density lipoprotein cholesterol (LDL-C), blood glucose, and blood pressure (BP) are the principal causative metabolic and hemodynamic risk factors for ASCVD and are correlated with CVD mortality [4], [5], [6], [7], [8], [9]. Lifestyle and medication management of these three prevalent and modifiable risk factors along with smoking cessation play a major role in the prevention of CVD morbidity and mortality based on numerous randomized controlled trials (RCTs) and their meta-analyses [5,[10], [11], [12], [13], [14], [15]]. In the case of LDL-C, these analyses have shown that reductions in the relative risk of ASCVD events are proportional to the degree of LDL-C lowering [9], while reductions in the absolute risk of events are proportional to the level of LDL-C achieved with statin and nonstatin therapy [16].
Improving control of CVD risk factors is a major goal of quality assurance groups, e.g., the National Center for Quality Assurance (NCQA) and governmental and commercial payers, to optimize outcomes from CVD, and is essential for value-based care delivery and payment. National quality measures adopted by health systems help guide and incentivize optimal clinician management of hypertension, diabetes mellitus (DM), and LDL-C in high-risk individuals. However, current quality metrics for these three principal ASCVD risk factors are markedly different. For hypertension and DM, the focus is on intermediate outcome measures that target both measurement and control, i.e., measurement of BP and hemoglobin A1C, respectively, and reporting of the percentage of patients who achieve specific levels of control, e.g., hemoglobin A1C <8%. A similar strategy was adopted for LDL-C in the past; however, as discussed below, in 2015 the LDL-C performance measure was changed from an outcomes measure (percentage of patients with ASCVD or DM who have LDL-C <100 mg/dL) to a process measure (percentage of patients prescribed or taking a statin). Thus, no current incentives exist for clinicians to measure LDL-C levels, despite being recommended as a Class IA recommendation in recent guidelines [17]. The impact of this change has recently been analyzed, and accumulating data suggest that statin use has not substantively improved in patients with established ASCVD. For example, NCQA data show only a 2% absolute increase in statin use between 2016 and 2020 in patients with ASCVD insured by commercial healthcare plans, and only a 3–5% absolute increase among Medicaid or Medicare beneficiaries [18]. Also, recent claims data from a large commercial health plan on more than 600,000 individuals with documented ASCVD found that statins were used in only half of patients, and high-intensity statins were used in less than one-quarter [2]. Similar gaps in statin use have been reported in the last 5 years among 49,447 patients in the American College of Cardiology (ACC) Pinnacle Registry [19] and in 105,329 patients in a large Medicare claims data set [20]. The potential impact of these treatment gaps on health outcomes cannot be ignored. Data have associated gaps in lipid treatment with increased rates of CVD morbidity and mortality, and higher health costs [21,22]. The fact that lipid treatment outcomes are also worse among women, racial and ethnic minority groups, and individuals with lower socioeconomic status suggests that these gaps also worsen CVD health inequities [23], [24], [25], [26], [27]. Despite the enormous morbidity, mortality, and economic burden of CVD with recent adverse trends since 2020 [3], preliminary recent suggestions for a universal foundation of quality metrics for chronic conditions mention blood pressure and hemoglobin A1C but entirely leave out cholesterol measurement or management [28].
This clinical perspective reviews the history of LDL-C as a quality and performance metric and the events that led to its replacement. It also presents patient, healthcare provider, and health system rationales for re-establishing LDL-C measurement as a performance measure to improve cholesterol control in high-risk groups and to stem the rising tide of CVD morbidity and mortality, cardiovascular care disparities, and related healthcare costs.
2. What is the history of LDL-C as a quality and performance measure?
Evidence-based lipid treatment guidelines from the National Cholesterol Education Program and its Adult Treatment Panel (NCEP-ATP), and more recently from the ACC/American Heart Association (AHA), are regularly updated to incorporate evolving data. For more than two decades, lipid guidelines have also informed quality and performance reporting recommendations by the NCQA, federal and commercial payers, and professional organizations. The NCQA's principal tool for quality measurement, the Healthcare Effectiveness Data and Information Set (HEDIS), evaluates quality measures across six domains, including effectiveness of care. Although several other quality programs are now used for value-based reimbursement (i.e., pay for performance), HEDIS has been the principal source of quality measures for lipid lowering for commercial, Medicare, and Medicaid payer plans.
The 2001 NCEP-ATP III guideline established LDL-C as the principal target of therapy, and recommended a “treat-to-goal” approach matched to the level of risk [29]. The subsequent decision by the NCQA to establish a specific LDL-C threshold as a performance measure for ASCVD patients in managed care organizations was a major departure from prior HEDIS metrics, which had emphasized process measures over intermediate outcomes measures. However, although the 2001 guideline goal for LDL-C achievement in secondary prevention was <100 mg/dL, the NCQA set its new HEDIS performance metric as the percentage of secondary prevention patients who achieved an LDL-C level <130 mg/dL [30]. This variance highlighted the difference between a clinical goal for individual patients (≤100 mg/dL) versus a performance measure for evaluating the care of a population of patients (<130 mg/dL) and underscored the fact that clinical goals and performance measures need not be identical. It also likely reflected consideration by the NCQA and its Cardiovascular Measurement Advisory panel (of experts from cardiovascular professional and quality assurance organizations and governmental payer groups) that achievement of LDL-C ≤100 mg/dL was challenging with the agents available at that time and that health system resources needed for quality reporting and improvement across large populations of patients can be substantial. HEDIS eventually updated the performance measure for lipid control in patients with ASCVD in 2010, establishing LDL-C ≤100 mg/dL as the metric.
The 2013 ACC/AHA cholesterol treatment guideline update, the first by an ACC/AHA expert panel, recommended a paradigm shift away from a treat-to-goal strategy to treatment intensity using fixed doses of moderate- or high-intensity statins in four “statin benefit groups.” These groups included those with ASCVD, DM, LDL-C ≥190 mg/dL (a group enriched with patients with familial hypercholesterolemia [FH]), or a calculated 10-year ASCVD risk of ≥7.5% based on sex- and race-specific pooled cohort risk equations [31]. This shift reflected the use of fixed doses of statins in most RCTs, and meta-analyses [11] that showed that reductions in the relative risk of ASCVD events were proportional to the magnitude of LDL-C lowering. Statin doses to achieve desired LDL-C reductions of ≥50% for patients with either ASCVD or LDL-C ≥190 mg/dL, or 30–49% for persons with DM or 10-year ASCVD risk of ≥7.5%, were recommended, the latter after shared decision-making. A repeat lipid panel 4–12 weeks after initiation of statin therapy was recommended as a Class IA recommendation (should be performed) to assess adherence to, and adequacy of, lipid-lowering therapy, with reassessment every 3–12 months as clinically indicated to monitor ongoing efficacy and adherence to both lifestyle and medication therapy. Based on the quality of the evidence, monitoring of lipid levels was established as a Class IA recommendation. However, because the 2013 expert panel did not find sufficient a priori evidence for the use of specific LDL-C (or non–high-density lipoprotein cholesterol [non-HDL-C]) targets in statin trials, they concluded that adherence to statin intensity was the best indicator of the anticipated therapeutic response, and that LDL-C levels and percent LDL-C reduction should only be used to assess adherence and response to therapy.
3. What impacts on clinical practice resulted from removal of LDL-C as a treatment target?
Removal of the LDL-C treatment goal in the 2013 ACC/AHA cholesterol treatment guideline led to widespread and unanticipated impacts on clinical practice, patient expectations, managed care organizations, accountable care organizations (ACOs), federal public health agencies (including the Million Hearts Initiative), commercial and governmental payers, quality assurance organizations (NCQA), and professional clinical practice guidelines. Almost immediately, the elimination of LDL-C treatment goals and the classification of certain statins as "high intensity" led the medical community to assume incorrectly that on-treatment LDL-C measurement and monitoring were unnecessary, despite the fact that LDL-C measurement was a Class IA recommendation in the 2013 guideline. The subsequent elimination of LDL-C measurement and specific LDL-C goals was widespread, and affected most guidelines related to ASCVD over the ensuing decade, as shown in Table 1 (see also Supplemental Table 1) [32], [33], [34].
Table 1.
Current National Committee for Quality Assurance and Center for Medicare-Medicaid Services lipid performance measures.
| Patient population | Performance measure | |
|---|---|---|
| NCQA-HEDIS (2022) [40] | CMS (2022) [41] | |
| ASCVD | Percentage of males 21–75 years of age and females 40–75 years of age with clinical ASCVD during the measurement year who were prescribed ≥1 high-intensity or moderate-intensity statin during the measurement year | Percentage of patients who were previously diagnosed with or currently have an active diagnosis of clinical ASCVD who were prescribed or were on statin therapy during the measurement period |
| Percentage of males 21–75 years of age and females 40–75 years of age with clinical ASCVD who were on high-intensity or moderate-intensity statin for ≥80% of the treatment period | ||
| DM | Percentage of patients 40–75 years of age with DM and without clinical ASCVD who were prescribed a statin of any intensity during the measurement year | Percentage of patients 40–75 years of age with a diagnosis of diabetes who were prescribed or were on statin therapy during the measurement period |
| Percentage of patients 40–75 years of age with DM and without clinical ASCVD who were on a statin of any intensity for ≥80% of the treatment period | ||
| LDL-C ≥ 190 mg/dL or FH | Percentage of patients ≥21 years of age who have ever had a fasting or direct LDL-C level ≥190 mg/dL or were previously diagnosed with or currently have an active diagnosis of FH or pure hypercholesterolemia who were prescribed or were on statin therapy during the measurement period | |
ASCVD, atherosclerotic cardiovascular disease; CMS, Center for Medicare-Medicaid Services; DM, diabetes mellitus; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; NCQA-HEDIS, National Committee for Quality Assurance Healthcare Effectiveness Data and Information Set.
4. What new evidence supports the re-establishment of LDL-C measurement or targets as quality measures?
In 2018, the AHA/ACC/Multisociety cholesterol treatment guideline [17] was updated to reflect new evidence from RCTs of nonstatin lipid-lowering therapy with either ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies (alirocumab and evolocumab), all of which were shown to significantly lower LDL-C and non-HDL-C and the risk of ASCVD events when added to background statin therapy in high-risk groups [35], [36], [37]. Importantly, the 2018 AHA/ACC/Multisociety guideline continued to recommend measurement of lipids as a Class 1A recommendation 4–12 weeks after LDL-C–lowering medication or dose adjustment and every 3–12 months thereafter to monitor adherence. Also, in contrast with the 2013 ACC/AHA guideline, the 2018 AHA/ACC/virgule Multisociety guideline introduced an LDL-C value of ≥70 mg/dL and a non-HDL-C value of ≥100 mg/dL in patients with ASCVD as a threshold for intensification of lipid treatment with initiation of nonstatin therapy if needed, necessitating LDL-C measurement to determine whether LDL-C remains above 70 mg/dL on maximally tolerated statin. Therefore, LDL-C measurements are required to assess adherence to therapy, response to therapy, and need for intensification of therapy. Further, the more recent recommendation in the 2022 Expert Consensus Decision Pathway on nonstatin therapies [38] to consider additional lipid-lowering agents for an LDL-C threshold of ≥55 mg/dL (or non-HDL-C ≥85 mg/dL) in ASCVD patients at very high risk (about 50% of ASCVD patients) is additional justification for LDL-C measurement to determine need for treatment intensification. Finally, newer data that individual responses to high-intensity statin therapy are highly variable [39] represent an additional rationale for re-establishing LDL-C measurement as a quality metric.
5. What is the current status of the NCQA-HEDIS lipid measure in high risk groups ?
Lipid-related quality measures recommended by NCQA-HEDIS, the Center for Medicare-Medicaid Services (CMS), and other payers have not changed since the 2018 guideline was released [40,41] (see Table 1). Moreover, the NCQA's recently published 2023 Health Plan Rating measures (including HEDIS, the Consumer Assessment of Healthcare Providers and Systems, and the Health Outcomes Survey) continue to use the nearly decade-old measure of statin use only, without any consideration for measurement of lipids to assess efficacy, adherence, or the need for additional guideline-recommended nonstatin therapies [42]. As noted above, data from contemporary registries have now revealed gaps in statin use, raising questions about the usefulness of this metric to improve lipid-lowering medication adherence and clinical outcomes. Finally, despite an increasing focus on disease prevention in high-risk patients, the HEDIS measure for effective cardiovascular care has not been expanded to patients with LDL-C ≥190 mg/dL, i.e., suspected FH, an under-recognized and undertreated group of more than 1 million in the U.S. with an estimated 20-fold higher risk of ASCVD when untreated who have now been earmarked for NIH funding to improve their detection and treatment gaps.
6. What are the benefits of reinstituting lipid panel measurement as a performance measure?
6.1. Benefits of lipid monitoring at the patient level
As noted above, at the patient level, lipid monitoring is essential for assessing the individual response to a particular lipid-lowering pharmacotherapy. In adherent individuals, varying responses to lipid-lowering therapies may reflect differences in pharmacogenetics, interacting medications that alter statin metabolism, occult elevation of lipoprotein(a), dietary practices, and/or other clinical factors. Variation in statin efficacy was highlighted in an analysis of the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), which used a fixed dose of a high-intensity statin (rosuvastatin 20 mg daily) in intermediate-risk primary prevention patients whose adherence was high in the setting of the trial. Investigators found that 46.3% of rosuvastatin-allocated participants had an LDL-C reduction of ≥50%, 42.8% had a reduction of >0% to <50%, and 10.8% had no reduction or an increase in LDL-C compared with baseline [39]. Not surprisingly, the percent reduction in LDL-C achieved was directly associated with the risk of a first ASCVD event in a stepwise fashion, with incidence rates for the primary endpoint of 11.2, 9.2, 6.7, and 4.8 per 1000 person-years for those in the placebo arm and treatment arm with no LDL-C reduction, LDL-C reduction of <50%, and LDL-C reduction of ≥50%, respectively. These data confirm that prescription of a high-intensity statin does not guarantee a robust lipid-lowering response or preclude the need to measure the response to ensure ongoing efficacy. A similar practice is used when measuring BP after initiating an antihypertensive medication, or hemoglobin A1C after adding or intensifying antihyperglycemic agents.
Nonadherence to statin therapy may be a more important reason to incentivize regular lipid monitoring by clinicians. Primary nonadherence may occur in up to 50% of patients in the first year after statin initiation [43], [44], [45] and, as noted above, is associated with higher event rates and health costs in patients with ASCVD [46,47]. The reasons for medication nonadherence are complex; adherence is now viewed as a “set of behaviors” driven by a complex interplay of “individual, social, and environmental” factors that health teams and systems must work to optimize [48]. However, data indicate that LDL-C measurement is not only essential for monitoring statin adherence but may also improve adherence. Among 813,887 patients with ASCVD cared for in the Veterans Affairs (VA) health system, performance of at least one lipid panel in statin users was associated with a 5% absolute increase in medication adherence (defined as proportion of days covered of ≥0.8),and a 9% absolute increase in adherence among new statin users [49]. Similarly, among 19,604 patients with a recent ASCVD event treated in the Kaiser Permanente Northern California region, adherence to statin therapy (defined as a continuous medication gap of ≤20%) was significantly higher in patients with a postevent LDL-C measurement vs. those without (80.2% vs. 75.9%; odds ratio 1.38, 95% confidence interval 1.28–1.49, P <0.001) [50]. A large meta-analysis of 67 studies that assessed the relationship between statin adherence and clinical variables showed similar findings. Among 147 variables studied, 6 were significantly associated with statin nonadherence, only 2 of which were actionable: lack of performance of a lipid panel, and patient co-pays [45]. Among these two, performance of a lipid panel remains most actionable for a clinician and care team.
Several behavior theory constructs explain why lipid monitoring is an effective intervention for improving statin adherence in clinical practice. Goal setting and follow-up are evidence-based behavioral strategies associated with improved adherence to healthy lifestyles and have also been associated with improved medication adherence [51,52]. As noted [49], lipid monitoring also facilitates discussion of the patient's concern beliefs (e.g., the safety and side effects of taking statins) and necessity beliefs (e.g., the importance of statins for cardiovascular risk reduction), while self-monitoring of lipid results may reinforce adherence self-efficacy (i.e., the patient's confidence that he/she can adhere to statin treatment). Favorably influencing these beliefs and behaviors through performance of a lipid panel and subsequent discussion of the results may, in turn, reduce intentional medication nonadherence (i.e., missing doses to suit one's needs) and unintentional nonadherence (i.e., forgetting to take a medication). Patient-level health information technology tools associated with improved lipid outcomes are those that provide connectivity to the health system, e.g., telemedicine or secure text messaging [53]. Therefore, in the current era, lipid monitoring with telehealth follow-up may be a useful strategy for improving lipid treatment outcomes.
6.2. Benefits of lipid monitoring at the clinician level
Clinician-level barriers are believed to contribute significantly to gaps in chronic care management. Therapeutic inertia, defined as failure to initiate or intensify therapy when clinically indicated, is a key clinician barrier that is common in clinical practice [54] and well documented in dyslipidemia management [55]. However, studies have shown that the practice of ordering lipid panels may reduce therapeutic inertia related to statin initiation, statin intensification, and addition of nonstatin therapy. Among 1,061,753 patients with ASCVD cared for in the VA health system [49], those with 1 or more lipid panels were more likely to have medication intensification (defined as the initiation of, or increase in the dose of, a statin and/or the addition of ezetimibe therapy) compared with those with no lipid panels during the study period (9.3% vs 5.4%, respectively; P <0.001). Also, in 287,636 ASCVD patients not on a statin at the index date, those with 1 or more lipid panels were more likely to have a statin initiated compared with those with none (21.5% vs 8.7%; P <0.001). In the multivariable-adjusted analyses, performance of lipid panels was independently associated with treatment intensification in a dose-dependent manner. Similarly, among 19,604 patients hospitalized with an ASCVD event in the Kaiser Permanente Northern California region in 2016–2017, LDL-C testing postevent was associated with significantly higher rates of intensification of the lipid-lowering regimen (16.1% vs 10.7%; odds ratio 1.51, 95% confidence interval 1.29–1.76, P <0.001) [50]. Also, among 12,332 patients treated at an urban medical center, the odds of lipid treatment intensification or additional LDL-C–lowering therapy increased with the number of lipid panels performed compared with no follow-up lipid monitoring [56]. Treatment intensification rates were 6.6%, 9.2%, 12.2%, and 22.4% among patients with 0, 1, 2, and >2 lipid panels, respectively. Adjusted odds (95% confidence intervals) of treatment intensification in fully adjusted models were 1.51 (1.16–1.97), 2.09 (1.61–2.71), and 4.37 (3.37–5.67) among those with 1, 2, or >2 lipid panels, respectively, compared with those with no lipid panels. These data suggest that lipid treatment monitoring may overcome clinical inertia and improve clinician management of hyperlipidemia in high-risk patients with either intensification of statin therapy or initiation of combination therapy as routinely done for treatment of BP and DM. Clinician-level health information technology interventions that have been shown to facilitate lipid monitoring and improve outcomes include programmable electronic health record (EHR)–based alerts, computerized decision support, and computerized physician order entry [53].
6.3. Benefits of lipid monitoring for health systems
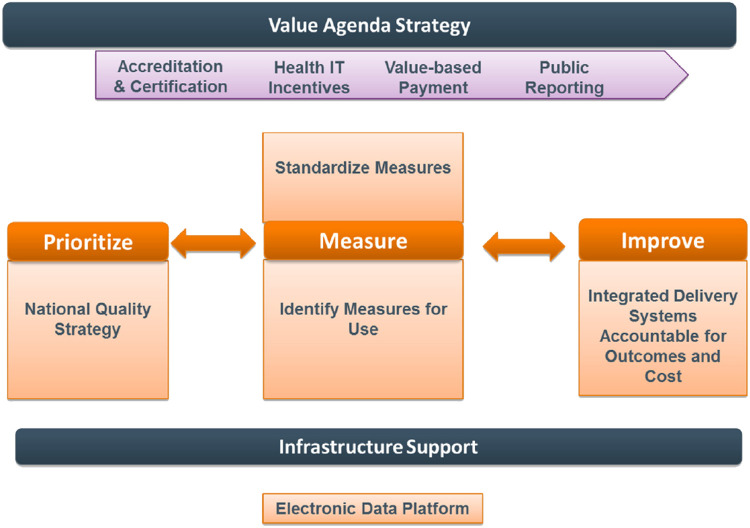
Health care in the U.S. is rapidly transitioning to value-based care delivery and payment models that reward the achievement of higher quality outcomes at lower costs. The value-based agenda incentivizes clinicians and quality leaders to be accountable for entire panels and practices of patients, and for ACOs to adopt population health management strategies. Like disease management, population health management coordinates care for patients with chronic diseases but intervenes to improve outcomes across the continuum of risk, including in those at lower risk, and engages in continuous performance reporting and improvement. Thus, the foundation of population health management is defining, measuring, and improving quality measures and outcomes (Fig. 1) [57]. Choosing measures that are most closely aligned with quality outcomes (e.g., LDL-C measurement or control vs. statin use) is fundamental to value-based care delivery and population health management within health systems. Broader, multilevel system approaches that use centralized care teams and clinical information systems are usually needed for population health management aimed at improving performance and outcomes. By reducing therapeutic inertia, increasing evidence-based statin prescribing, and increasing statin adherence, appropriate lipid measurement has the potential to improve LDL-C control, reduce ASCVD events, and avoid unnecessary initiation of nonstatin therapies when the actual need is to intensify statin therapy or improve statin adherence.
Fig. 1.
Strategy for Value-Based Care. Value-based care models reward the achievement of higher quality outcomes at lower costs. The value-based agenda incentivizes clinicians and quality leaders to be accountable for entire panels and practices of patients, and for accountable care organizations to adopt population health management strategies. IT, information technology. Reprinted from Cohen JD et al. J Clin Lipidol 2013;7:573–609.
7. What are the benefits of LDL-C measurement and control as performance metrics?
7.1. Lessons from early ACOs
Improving LDL-C control with the aim of optimizing ASCVD outcomes has traditionally been undertaken in the U.S. in large integrated health systems, e.g., Kaiser Permanente, Geisinger, and the VA systems, in which prepaid revenue streams drive a culture of accountable care and continuous quality improvement. In the Kaiser Permanente Northern California region, disease management of hyperlipidemia in patients with coronary artery disease and DM was initiated in the late 1980s using a clinical information system separate from the EHR, decision support via paper-based practice guidelines with LDL-C goals, and centralized lipid care or case management. Examples of the latter were intensive goal-directed lipid case management by trained RNs in almost all patients in the 6 months after an acute coronary event as part of the system's home-based cardiac rehabilitation program, which led to consistently high LDL-C goal attainment, followed by enrollment (if needed) in a cholesterol management program [58].
In 2005, Kaiser Northern California transitioned to a population management strategy for all individuals with elevated ASCVD risk, including those with coronary artery disease, DM, symptomatic peripheral artery disease, aortic aneurysm, and prior ischemic cerebrovascular accident/transient ischemic attack. All are now managed in a large registry called Prevent Heart Attacks and Strokes Everyday (PHASE) utilizing the system's EPIC-based EHR (Health Connect), other databases, and central care management with primary care clinician involvement. Pharmacotherapies (aspirin, statins, angiotensin-converting enzyme inhibitors, and beta-blockers post myocardial infarction), lifestyle therapies, and quality measures (LDL-C, BP, and hemoglobin A1C control) were protocolized. LDL-C control to <100 mg/dL across the registry rose from 50% to 63% by 2008 [58]. During 2008–2014, among 24,000 Kaiser Permanente Northern California post–myocardial infarction patients in the registry, achievement of LDL-C <100 mg/dL was reported to be >90% and was independently associated with lower mortality [59].
Collaborative pharmacist medication management within health systems, a growing model in the U.S., also has been utilized to maintain LDL-C control and quality in the Kaiser Permanente system. In the Colorado region, pharmacists used the clinical information system to establish a Clinical Pharmacy Cardiac Risk Service to monitor LDL-C goal attainment in all patients with ASCVD, followed by patient outreach until goals were achieved. Over a 4-year period, lipid screening rose from 67% to 97%, while LDL-C control to <100 mg/dL tripled from 25% to 73% [60]. At long-term follow-up, the relative risk of death had declined by 89% among program enrollees who were within 90 days of a cardiac event, and by 76% in others [61].
7.2. Lessons from emerging ACOs
Today, the widespread use of programmable EHRs, team care, and digital health technologies has the potential to transform population management of hyperlipidemia and ASCVD risk in health systems transitioning to ACO models of care. Researchers from Mass General Brigham, an academic institution recently certified as an ACO, have reported preliminary results from a disease/population health management program that uses digital health technology to remotely deliver protocolized management of hyperlipidemia (and hypertension) to individuals with ASCVD, DM, LDL-C ≥190 mg/dL, and other high-risk conditions. The program uses unlicensed "navigators" and pharmacists in collaborative practice agreements, supported by specialists, to perform outreach and initiate and titrate lipid and BP medications in patients with uncontrolled LDL-C and/or BP identified through EHR-based screening or referrals from primary care providers. Tracking is via a custom-built software program external to the EHR that requires no in-person visits. Over 3 years, 10,803 patients were enrolled in the program, with a 25% increase at the onset of the COVID-19 pandemic. In this remote, navigator-led, pharmacist-supported system-level program, 94% of patients achieved their LDL-C goal; notably, similar LDL-C reductions and LDL-C levels were achieved among Black and Hispanic patients, compared with White patients [62].
8. What are the potential economic impacts from population health management to improve LDL-C management?
As noted, higher health costs in high-risk individuals who are nonadherent to statin therapy are well documented. A recent analysis expanded this to include those on lipid therapy who do not achieve lipid targets. Investigators from the Italian healthcare system documented significantly higher mean total healthcare costs in patients treated with lipid-lowering drugs who did not achieve LDL-C targets compared with those who did, with costs proportional to the distance from the LDL-C target and driven primarily by increased hospitalizations [63]. A full economic analysis of the costs of suboptimal LDL-C control in individuals with ASCVD vs. the health system resources needed for population and disease management approaches to improve lipid control is beyond the scope of this paper.
9. What is the impact of LDL-C monitoring and LDL-C control in reducing healthcare disparities in ASCVD?
Large disparities in CVD treatment have been recognized as major barriers to health equity, a problem laid bare by the COVID-19 pandemic. It is well established that guideline-directed medical therapy should be applied to all patients, regardless of race/ethnicity, sex/gender, socioeconomic status, or geography. Unfortunately, large disparities in lipid treatment, including those based on race/ethnicity and sex/gender, have been documented in the U.S. [[23], [24], [25],64]. Contemporary lipid-lowering agents—statins, PCSK9 inhibitors, and ezetimibe—have unequivocal safety and efficacy by sex and race [65]. Nonetheless, all are underutilized in women and various racial/ethnic populations as compared with White adults [66].These disparities exist across the spectrum of CVD risk. In the Cascade Screening for Awareness and Detection of Familial Hypercholesterolemia (CASCADE-FH) registry, women were 40% less likely than men to achieve LDL-C goals and 40% less likely than men to receive any statin [25]. Asian and Black adults were 40–50% less likely to achieve LDL-C goals [25]. Although lipid-lowering therapies are essential for the primary and secondary prevention of ASCVD, statin undertreatment persists in women, minority adults, and younger patients [2,23,64,66,67]. Therefore, another important rationale for re-establishing LDL-C as a performance measure is to improve equity in cardiovascular care for historically undertreated populations by identifying those groups for whom treatment initiation, further treatment intensification, or efforts to improve medication adherence are needed.
10. Conclusion
Achievement of LDL-C levels <100 mg/dL in individuals with ASCVD or equivalent risk has been associated with improvements in ASCVD event rates and mortality, making it a Class IA recommendation and an established quality measure in the well-respected HEDIS tool in the past. The transition by the NCQA to a HEDIS process measure focused on statin use in 2015 reflected new data in support of higher-intensity statin treatment but did not incentivize LDL-C monitoring and/or improvement. Many data now support the re-establishment of LDL-C testing in high-risk subsets as a performance measure, especially in patients with established ASCVD:
-
•
Recent data from the NCQA and independent surveys show minimal improvement in statin use in individuals with ASCVD in recent years
-
•
Significant heterogeneity in LDL-C response from statin therapy
-
•
New evidence-based guidelines that support LDL-C monitoring to assess efficacy and adherence to statin therapy and assess the need for add-on therapies (e.g., if certain LDL-C thresholds are not met on statin therapy alone)
-
•
New clinical trial evidence with nonstatin therapies that supports the benefits of additional LDL-C lowering in high-risk patients already on maximal statin therapy
-
•
Advances in the use of advanced data analytics in the EHR that allow health systems and providers not only to monitor LDL-C levels but also to improve care quality and outcomes
Because of the time required to develop and implement new quality measures by NCQA-HEDIS and CMS and the urgency to reinstate measurement of LDL-C, consideration should also be given to restoration of at least one of the prior LDL-C–related quality measures for individuals with established ASCVD. The ACC/AHA Task Force on Performance Measures has identified 11 attributes of a good performance measure across 4 domains (Table 2), and LDL-C measurement easily meets these criteria. The ACC/AHA expects its performance measures to be based on Class I guideline recommendations [68]. Class I recommendations, such as LDL-C measurement, by definition meet the evidence-based attribute as well as the three validity attributes (face, content, and construct). Many prior studies have established that LDL-C measures are reliable, and the ACC/AHA guidelines describe the actions that clinicians may take based on LDL-C measurements. Thus, the totality of evidence, and established criteria for performance measures, favor re-establishment of an LDL-C measurement to improve population-wide lipid control, cardiovascular morbidity and mortality, and health equity.
Table 2.
Evidence that LDL-C measurement fulfills performance measure attributes.
| ACC/AHA Task Force on Performance Measures Attributes for Performance Measures [68] | Does LDL-C Measurement Meet This Attribute? | |
|---|---|---|
| Characteristic | Description | |
| 1. Evidence Based | ||
| High-impact area that is useful in improving patient outcomes | a. For structural measures, the structure should be closely linked to a meaningful process of care that in turn is linked to a meaningful patient outcome. | Not applicable |
| b. For process measures, the scientific basis for the measure is well established and the process should be closely linked to a meaningful patient outcome. | Yes, ACC/AHA guidelines clearly outline the evidence for improvement in outcomes meaningful to patients with lowering high LDL-C levels. Measurement of lipids is a Class I recommendation. | |
| c. For outcome measures, the outcome should be clinically meaningful. If appropriate, performance measures based on outcomes should adjust for relevant clinical characteristics by using appropriate methodology and high-quality data sources. | Not applicable | |
| 2. Measure Selection | ||
| Measure definition | a. The patient group to whom the measure applies (denominator) and for whom conformance is achieved is clearly defined and clinically meaningful. | This patient group for measurement can be clearly defined as in the past. |
| Measure exceptions and exclusions | b. Exceptions and exclusions are supported by evidence. | Exceptions and exclusions can be easily defined. |
| Reliability | c. The measure is reproducible across organizations and delivery settings. | It is highly likely that LDL-C measurement rates can be reproduced in all settings using electronic health records. |
| Face validity | d. The measure appears to assess what it is intended to assess. | The measure clearly measures what is intended. |
| Content validity | e. The measure captures most meaningful aspects of care. | LDL-C measurement is the primary method of determining appropriateness and effectiveness of LDL-C treatment. |
| Construct validity | f. The measure correlates well with other measures of the same aspect of care. | LDL-C measurement will have some correlation with drug prescriptions and adherence for drugs to lower LDL-C, which are known to improve care in appropriate individuals. |
| 3. Measure Feasibility | ||
| Reasonable effort and cost | a. Data required for the measure can be obtained with reasonable effort and cost. | The cost of measuring data using the electronic health record is small compared with other measurements. |
| Reasonable period | b. Data required for the measure can be obtained within the period allowed for data collection. | The data from laboratory records and pharmacy prescription records are readily available in a timely manner. |
| 4. Accountability | ||
| Actionable | a. Those held accountable can affect the care process or outcome. | Those doing poorly on the measure can be held accountable for their care and have clear paths to improving care through guideline-directed changes in medical therapy. |
| Unintended consequences avoided | b. The likelihood of negative unintended consequences with the measure is low. | An unintended consequence of the measure may be increased prescription rates among inappropriate patients. However, the probability of poor outcomes related to inappropriate use is exceedingly low based on the favorable safety profile of LDL-C lowering treatments. Restricting the measure to those who are high risk will reduce the probability of unintended consequences. |
ACC/AHA, American College of Cardiology/American Heart Association; LDL-C, low-density lipoprotein cholesterol.
Funding
None
Disclosures
Salim S. Virani: Grant support: Department of Veterans Affairs, National Institutes of Health, World Heart Federation, Tahir and Jooma Family; Honorarium (American College of Cardiology in my role as associate editor for innovations, acc.org)
Karen Aspry: Grant/research support: Amgen, Esperion, Novartis, Ionis; Speaker: National Lipid Association
Dave L. Dixon: Grant/research support: Boehringer Ingelheim
Keith C. Ferdinand: Consulting fee: Amgen, Novartis
Paul A. Heidenreich: None
Elizabeth J. Jackson: Advisory boards: Regeneron, Novartis, Esperion; Speaker for Esperion
Terry A. Jacobson: Consultant for Astra Zeneca, Esperion, Novartis, Amgen, and Regeneron
Janice L. McAlister: Advisor and speaker's bureau for Amgen; speaker's bureau for Esperion.
David R. Neff: None
Martha Gulati: Speaker: Siemans; Advisory board: Novartis, Bayer
Christie M. Ballantyne: Grant/research support (through his institution): Abbott Diagnostic, Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, Novo Nordisk, Regeneron, and Roche Diagnostic; Consultant: 89Bio, Abbott Diagnostics, Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, Astra Zeneca, Denka Seiken, Esperion, Genentech, Gilead, Illumina, Matinas BioPharma Inc, Merck, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, and Roche Diagnostic.
CRediT authorship contribution statement
Salim S. Virani: Writing – original draft, Writing – review & editing. Karen Aspry: Writing – original draft, Writing – review & editing. Dave L. Dixon: Writing – original draft, Writing – review & editing. Keith C. Ferdinand: Writing – original draft, Writing – review & editing. Paul A. Heidenreich: Writing – original draft, Writing – review & editing. Elizabeth J. Jackson: Writing – original draft, Writing – review & editing. Terry A. Jacobson: Writing – original draft, Writing – review & editing. Janice L. McAlister: Writing – original draft, Writing – review & editing. David R. Neff: Writing – original draft, Writing – review & editing. Martha Gulati: Writing – original draft, Writing – review & editing. Christie M. Ballantyne: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Salim S. Virani reports a relationship with Department of Veterans Affairs, National Institutes of Health, World Heart Federation, Tahir and Jooma Family that includes: funding grants. Salim S. Virani reports a relationship with American College of Cardiology in his role as associate editor for innovations, acc.org that includes honorarium. Karen Aspry reports a relationship with Amgen, Esperion, Novartis, Ionis that includes: funding grants. Karen Aspry reports a relationship with National Lipid Association that includes: speaking and lecture fees. Dave L. Dixon reports a relationship with Boehringer Ingelheim Corp USA that includes: funding grants. Keith C. Ferdinand reports a relationship with Amgen, Novartis that includes: consulting or advisory. Elizabeth J. Jackson reports a relationship with Regeneron, Novartis that includes: consulting or advisory. Elizabeth J. Jackson reports a relationship with Esperion Therapeutics Inc that includes: consulting or advisory and speaking and lecture fees. Terry A. Jacobson reports a relationship with Astra Zeneca, Esperion, Novartis, Amgen, Regeneron that includes: consulting or advisory. Janice L. McAlister reports a relationship with Amgen Inc that includes: consulting or advisory and speaking and lecture fees. Janice L. McAlister reports a relationship with Esperion Therapeutics Inc that includes: speaking and lecture fees. Martha Gulati reports a relationship with Siemans that includes: speaking and lecture fees. Martha Gulati reports a relationship with Novartis, Bayer that includes: consulting or advisory. Christie M. Ballantyne reports a relationship with Abbott Diagnostic, Amgen, Arrowhead, Esperion, Merck, Novartis, Novo Nordisk, Regeneron, Roche Diagnostic that includes: consulting or advisory and funding grants. Christie M. Ballantyne reports a relationship with Akcea, Ionis that includes: funding grants. Christie M. Ballantyne reports a relationship with 89Bio, Alnylam Pharmaceuticals, Althera, Amarin, Astra Zeneca, Denka Seiken, Genentech, Gilead, Illumina, Matinas BioPharma Inc, New Amsterdam, Pfizer that includes: consulting or advisory.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100472.
Appendix. Supplementary materials
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Nelson A.J., Haynes K., Shambhu S., et al. High-intensity statin use among patients with atherosclerosis in the U.S. J Am Coll Cardiol. 2022;79:1802–1813. doi: 10.1016/j.jacc.2022.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2023 update: a report from the American heart association. Circulation. 2023 doi: 10.1161/CIR.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 4.Neaton J.D., Blackburn H., Jacobs D., et al. Serum cholesterol level and mortality findings for men screened in the multiple risk factor intervention trial. Arch Intern Med. 1992;152:1490–1500. [PubMed] [Google Scholar]
- 5.Cholesterol treatment trialists' (CTT) collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 7.Rapsomaniki E., Timmis A., George J., et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratton I.M., Adler A.I., Neil H.A., et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd-Jones D.M., Hong Y., Labarthe D., et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 11.Cholesterol treatment trialists' (CTT) collaboration. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 12.Bundy J.D., Li C., Stuchlik P., et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775–781. doi: 10.1001/jamacardio.2017.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blood Pressure Lowering Treatment Trialists Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 14.Blood Pressure Lowering Treatment Trialists Collaboration Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 15.Gaede P., Vedel P., Larsen N., Jensen G.V., Parving H.H., Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 16.Ference B.A., Cannon C.P., Landmesser U., Luscher T.F., Catapano A.L., Ray K.K. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the cholesterol treatment trialists collaboration. Eur Heart J. 2018;39:2540–2545. doi: 10.1093/eurheartj/ehx450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy S.M., Stone N.J., Bailey A.L., et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Quality Assurance. Statin therapy for patients with cardiovascular disease and diabetes (SPC/SPD). https://www.ncqa.org/hedis/measures/statin-therapy-for-patients-with-cardiovascular-disease-and-diabetes/. Accessed 21 November 2022.
- 19.Virani S.S., Kennedy K.F., Akeroyd J.M., et al. Variation in lipid-lowering therapy use in patients with low-density lipoprotein cholesterol ≥190 mg/dl: insights from the national cardiovascular data registry−practice innovation and clinical excellence registry. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serban M.C., Colantonio L.D., Manthripragada A.D., et al. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69:1386–1395. doi: 10.1016/j.jacc.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Bui A., Kwon J., Kim J., Lucas A. Overcoming barriers to statin adherence. US Pharm. 2019;44:19–22. [Google Scholar]
- 22.Graham J.H., Sanchez R.J., Saseen J.J., Mallya U.G., Panaccio M.P., Evans M.A. Clinical and economic consequences of statin intolerance in the United States: results from an integrated health system. J Clin Lipidol. 2017;11 doi: 10.1016/j.jacl.2016.10.003. 70-79 e1. [DOI] [PubMed] [Google Scholar]
- 23.Suero-Abreu G.A., Karatasakis A., Rashid S., et al. Factors associated with disparities in appropriate statin therapy in an outpatient inner city population. Healthcare (Basel) 2020;8 doi: 10.3390/healthcare8040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metser G., Bradley C., Moise N., Liyanage-Don N., Kronish I., Ye S. Gaps and disparities in primary prevention statin prescription during outpatient care. Am J Cardiol. 2021;161:36–41. doi: 10.1016/j.amjcard.2021.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amrock S.M., Duell P.B., Knickelbine T., et al. Health disparities among adult patients with a phenotypic diagnosis of familial hypercholesterolemia in the CASCADE-FH patient registry. Atherosclerosis. 2017;267:19–26. doi: 10.1016/j.atherosclerosis.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Ohm J., Skoglund P.H., Habel H., et al. Association of socioeconomic status with risk factor target achievements and use of secondary prevention after myocardial infarction. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hero C., Karlsson S.A., Franzen S., et al. Impact of socioeconomic factors and gender on refill adherence and persistence to lipid-lowering therapy in type 1 diabetes. Diabetes Ther. 2021;12:2371–2386. doi: 10.1007/s13300-021-01115-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs D.B., Schreiber M., Seshamani M., Tsai D., Fowler E., Fleisher L.A. Aligning quality measures across CMS—The universal foundation. N Engl J Med. 2023 doi: 10.1056/NEJMp2215539. [DOI] [PubMed] [Google Scholar]
- 29.Expert Panel on Detection Evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Lee T.H., Cleeman J.I., Grundy S.M., et al. Clinical goals and performance measures for cholesterol management in secondary prevention of coronary heart disease. JAMA. 2000;283:94–98. doi: 10.1001/jama.283.1.94. [DOI] [PubMed] [Google Scholar]
- 31.Stone N.J., Robinson J.G., Lichtenstein A.H., et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Kernan W.N., Ovbiagele B., Black H.R., et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 33.Drozda J.P., Jr., Ferguson T.B., Jr., Jneid H., et al. 2015 ACC/AHA focused update of secondary prevention lipid performance measures: a report of the American college of cardiology/American heart association task force on performance measures. J Am Coll Cardiol. 2016;67:558–587. doi: 10.1016/j.jacc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Jneid H., Addison D., Bhatt D.L., et al. 2017 AHA/ACC clinical performance and quality measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American college of cardiology/American heart association task force on performance measures. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/HCQ.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 35.Cannon C.P., Blazing M.A., Giugliano R.P., et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 36.Sabatine M.S., Giugliano R.P., Keech A.C., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz G.G., Steg P.G., Szarek M., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd-Jones D.M., Morris P.B., Ballantyne C.M., et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;80:1366–1418. doi: 10.1016/j.jacc.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Ridker P.M., Mora S., Rose L., JUPITER Trial Study Group Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016;37:1373–1379. doi: 10.1093/eurheartj/ehw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Committee for Quality Assurance. HEDIS MY 2022: measures and descriptions. https://www.ncqa.org/wp-content/uploads/2021/12/HEDIS-MY-2022-Measure-Descriptions.pdf. Accessed 27 October 2022.
- 41.Centers for Medicare & Medicaid Services. 2022 CMS web interface. PREV-13: statin therapy for the prevention and treatment of cardiovascular disease. https://qpp.cms.gov/docs/QPP_quality_measure_specifications/Web-Interface-Measures/2022_Measure_PREV13_CMSWebInterface_v6.0.pdf. Accessed 27 October 2022.
- 42.National Committee for Quality Assurance. 2023 Health plan ratings required HEDIS®, CAHPS® and HOS measures. https://www.ncqa.org/wp-content/uploads/2022/04/2023-HPR-List-of-Required-Performance-Measures_4.25.2022.pdf. Accessed.
- 43.Mahtta D., Ahmed S.T., Ramsey D.J., et al. Statin prescription rates, adherence, and associated clinical outcomes among women with PAD and ICVD. Cardiovasc Drugs Ther. 2020;34:745–754. doi: 10.1007/s10557-020-07057-y. [DOI] [PubMed] [Google Scholar]
- 44.Mahtta D., Ramsey D.J., Al Rifai M., et al. Evaluation of aspirin and statin therapy use and adherence in patients with premature atherosclerotic cardiovascular disease. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemstra M., Blackburn D., Crawley A., Fung R. Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can J Cardiol. 2012;28:574–580. doi: 10.1016/j.cjca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 46.May H.T., Knowlton K.U., Anderson J.L., Lappe D.L., Bair T.L., Muhlestein J.B. High-statin adherence over 5 years of follow-up is associated with improved cardiovascular outcomes in patients with atherosclerotic cardiovascular disease: results from the IMPRES study. Eur Heart J Qual Care Clin Outcomes. 2022;8:352–360. doi: 10.1093/ehjqcco/qcab024. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez F., Maron D.J., Knowles J.W., Virani S.S., Lin S., Heidenreich P.A. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:206–213. doi: 10.1001/jamacardio.2018.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steiner J.F. Rethinking adherence. Ann Intern Med. 2012;157:580–585. doi: 10.7326/0003-4819-157-8-201210160-00013. [DOI] [PubMed] [Google Scholar]
- 49.Jia X., Al Rifai M., Ramsey D.J., et al. Association between lipid testing and statin adherence in the veterans affairs health system. Am J Med. 2019;132:e693–e700. doi: 10.1016/j.amjmed.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Rana J.S., Virani S.S., Moffet H.H., et al. Association of low-density lipoprotein testing after an atherosclerotic cardiovascular event with subsequent statin adherence and intensification. Am J Med. 2022;135:603–606. doi: 10.1016/j.amjmed.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller N.H. Adherence behavior in the prevention and treatment of cardiovascular disease. J Cardiopulm Rehabil Prev. 2012;32:63–70. doi: 10.1097/HCR.0b013e318235c729. [DOI] [PubMed] [Google Scholar]
- 52.Bailey R.R. Goal setting and action planning for health behavior change. Am J Lifestyle Med. 2019;13:615–618. doi: 10.1177/1559827617729634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aspry K.E., Furman R., Karalis D.G., et al. Effect of health information technology interventions on lipid management in clinical practice: a systematic review of randomized controlled trials. J Clin Lipidol. 2013;7:546–560. doi: 10.1016/j.jacl.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Dixon D.L., Sharma G., Sandesara P.B., et al. Therapeutic inertia in cardiovascular disease prevention: time to move the bar. J Am Coll Cardiol. 2019;74:1728–1731. doi: 10.1016/j.jacc.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Virani S.S., Woodard L.D., Chitwood S.S., et al. Frequency and correlates of treatment intensification for elevated cholesterol levels in patients with cardiovascular disease. Am Heart J. 2011;162 doi: 10.1016/j.ahj.2011.07.013. 725-732 e1. [DOI] [PubMed] [Google Scholar]
- 56.Tran C., Vo V., Taylor P., Koehn D.A., Virani S.S., Dixon D.L. Adherence to lipid monitoring and its impact on treat intensification of LDL-C lowering therapies at an urban academic medical center. J Clin Lipidol. 2022;16:491–497. doi: 10.1016/j.jacl.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Cohen J.D., Aspry K.E., Brown A.S., et al. Use of health information technology (HIT) to improve statin adherence and low-density lipoprotein cholesterol goal attainment in high-risk patients: proceedings from a workshop. J Clin Lipidol. 2013;7:573–609. doi: 10.1016/j.jacl.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 58.McCarthy D., Mueller K., Wrenn J. Kaiser Permanente: bridging the quality divide with integrated practice, group accountability, and health information technology. The Commonwealth Fund publication 1278. 2009 [Google Scholar]
- 59.Solomon M.D., Leong T.K., Levin E., et al. Cumulative adherence to secondary prevention guidelines and mortality after acute myocardial infarction. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olson K.L., Rasmussen J., Sandhoff B.G., Merenich J.A. Clinical pharmacy cardiac risk service study group. Lipid management in patients with coronary artery disease by a clinical pharmacy service in a group model health maintenance organization. Arch Intern Med. 2005;165:49–54. doi: 10.1001/archinte.165.1.49. [DOI] [PubMed] [Google Scholar]
- 61.Sandhoff B.G., Kuca S., Rasmussen J., Merenich J.A. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12:4–11. doi: 10.7812/tpp/08-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blood A.J., Cannon C.P., Gordon W.J., et al. Results of a remotely delivered hypertension and lipid program in more than 10 000 patients across a diverse health care network. JAMA Cardiol. 2023;8:12–21. doi: 10.1001/jamacardio.2022.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Degli Esposti L., Borghi C., Galvani M., et al. The management of cholesterol level controlwith lipid-lowering drugs in Italian clinical practice: findings from the STREAM (Supporting with the real-world evidence the assessment of medicines and health technologies) study (abstract; P357) Eur Heart J. 2022;24:C192–C193. [Google Scholar]
- 64.Agarwala A., Bekele N., Deych E., et al. Racial disparities in modifiable risk factors and statin usage in black patients with familial hypercholesterolemia. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cholesterol treatment trialists' (CTT) collaboration. efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 66.Singh C., Valero D.J., Nisar J., et al. Statins versus proprotein convertase subtilisin/kexin type 9 inhibitors- are we doing better? A systematic review on treatment disparity. Cureus. 2020;12:e10965. doi: 10.7759/cureus.10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanna M.G., Wang T.Y., Xiang Q., et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spertus J.A., Bonow R.O., Chan P., et al. ACCF/AHA new insights into the methodology of performance measurement: a report of the American college of cardiology foundation/American heart association task force on performance measures. Circulation. 2010;122:2091–2106. doi: 10.1161/CIR.0b013e3181f7d78c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.