This randomized clinical trial compares exemestane, 25 mg, given once daily, 3 times weekly, and once weekly for the treatment of estrogen receptor–positive breast cancer in postmenopausal women prior to surgery.
Key Points
Question
What is the noninferiority percentage change of serum estradiol with 2 exemestane alternative schedules (25 mg 3 times weekly or once weekly) compared with the standard dose of 25 mg once daily?
Findings
In this randomized clinical trial of 180 postmenopausal women with estrogen receptor–positive breast cancer, exemestane, 25 mg, given 3 times weekly was noninferior to a once-daily schedule in reducing circulating estradiol in compliant participants, whereas the once-weekly schedule was less effective. Adverse events were similar in all arms.
Meaning
Exemestane, 25 mg, given 3 times weekly in adherent patients was noninferior to the standard daily dose; this reduced schedule should be further studied in prevention studies and in women who do not tolerate the daily dose in the adjuvant setting.
Abstract
Importance
Successful therapeutic cancer prevention requires definition of the minimal effective dose. Aromatase inhibitors decrease breast cancer incidence in high-risk women, but use in prevention and compliance in adjuvant settings are hampered by adverse events.
Objective
To compare the noninferiority percentage change of estradiol in postmenopausal women with estrogen receptor–positive breast cancer given exemestane, 25 mg, 3 times weekly or once weekly vs a standard daily dose with a noninferiority margin of −6%.
Design, Setting, and Participants
This multicenter, presurgical, double-blind phase 2b randomized clinical trial evaluated 2 alternative dosing schedules of exemestane. Postmenopausal women with estrogen receptor–positive breast cancer who were candidates for breast surgery were screened from February 1, 2017, to August 31, 2019. Blood samples were collected at baseline and final visit; tissue biomarker changes were assessed from diagnostic biopsy and surgical specimen. Biomarkers were measured in different laboratories between April 2020 and December 2021.
Interventions
Exemestane, 25 mg, once daily, 3 times weekly, or once weekly for 4 to 6 weeks before surgery.
Main Outcomes and Measures
Serum estradiol concentrations were measured by solid-phase extraction followed by liquid chromatography–tandem mass spectrometry detection. Toxic effects were evaluated using the National Cancer Institute terminology criteria, and Ki-67 was assessed by immunohistochemistry.
Results
A total of 180 women were randomized into 1 of the 3 arms; median (IQR) age was 66 (60-71) years, 63 (60-69) years, and 65 (61-70) years in the once-daily, 3-times-weekly, and once-weekly arms, respectively. In the intention-to-treat population (n = 171), the least square mean percentage change of serum estradiol was −89%, −85%, and −60% for exemestane once daily (n = 55), 3 times weekly (n = 56), and once weekly (n = 60), respectively. The difference in estradiol percentage change between the once-daily and 3-times-weekly arms was −3.6% (P for noninferiority = .37), whereas in compliant participants (n = 153), it was 2.0% (97.5% lower confidence limit, −5.6%; P for noninferiority = .02). Among secondary end points, Ki-67 and progesterone receptor were reduced in all arms, with median absolute percentage changes of −7.5%, −5.0%, and −4.0% for Ki-67 in the once-daily, 3-times-weekly, and once-weekly arms, respectively (once daily vs 3 times weekly, P = .31; once daily vs once weekly, P = .06), and −17.0%, −9.0%, and −7.0% for progesterone receptor, respectively. Sex hormone–binding globulin and high-density lipoprotein cholesterol had a better profile among participants in the 3-times-weekly arm compared with once-daily arm. Adverse events were similar in all arms.
Conclusions and Relevance
In this randomized clinical trial, exemestane, 25 mg, given 3 times weekly in compliant patients was noninferior to the once-daily dosage in decreasing serum estradiol. This new schedule should be further studied in prevention studies and in women who do not tolerate the daily dose in the adjuvant setting.
Trial Registration
ClinicalTrials.gov Identifier: NCT02598557; EudraCT: 2015-005063-16
Introduction
Breast cancer incidence is increasing and remains the leading cause of cancer-related burden, even though mortality is decreasing.1 Modern prevention strategies are risk based and include personalized screening, lifestyle changes, selective estrogen receptor modulators,2 and aromatase inhibitors (AIs),3,4 specifically exemestane and anastrozole.5 Tamoxifen and, more recently, anastrozole have shown a long-lasting benefit after drug discontinuation2 with no new late adverse events (AEs).6
Exemestane is a steroidal AI, and its action results in an irreversible binding to the aromatase enzyme, causing permanent inactivation even when the drug is cleared.7 A phase 1 study of postmenopausal volunteers showed that a single dose of 5 mg was already effective,8 and 25 mg was considered the minimal dose with the maximal estradiol suppression. This effect was reached on day 3 and persisted up to 7 days.9
In the adjuvant-treatment setting, exemestane has shown greater efficacy than tamoxifen in high-risk premenopausal women in association with ovarian suppression, as well as in postmenopausal women.10,11,12,13 In the prevention setting, exemestane showed a 65% relative risk reduction in the annual incidence of invasive breast cancer relative to placebo.3
Adverse events play a prominent role in the low uptake of preventive therapy.14 Moreover, nonadherence to AIs is common in the adjuvant setting due to AEs, and it increases risk of breast cancer recurrence.15 However, the safety profile of existing drugs could be improved by searching for the minimal effective dose.16 For instance, we showed that low-dose tamoxifen 5 mg/d given for 3 years halved disease recurrence in women with previously diagnosed intraepithelial neoplasia.17
This presurgical phase 2b randomized clinical trial addressed 2 alternative exemestane dosing schedules compared with the standard dose in the percentage change reduction of serum estradiol level, a surrogate biomarker of efficacy.18 Furthermore, tissue biomarkers, including Ki-67, circulating sex hormones, lipids, and AEs, were evaluated.
Methods
Study Design
The study design was described in detail in a recent publication.19 Briefly, this was an international, presurgical, 3-arm, double-blind, noninferiority phase 2b trial (eFigure 1 in Supplement 1 shows participants by center). Main inclusion criteria were postmenopausal patients with (1) confirmed estrogen receptor–positive breast cancer stage cT0 to cT2 or cN0 to cN1 and (2) blood tests within their laboratory normal limits or with alterations with no clinical relevance. Participants were excluded if their body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) was less than 18.5 and if they had previous breast cancer treatment, uncontrolled illness, recent diagnoses of other cancers, or severe osteoporosis. Women were randomized (1:1:1) to exemestane, 25 mg, once daily, 3 times weekly, or once weekly for 4 to 6 weeks prior to surgery, stratified by center and BMI less than 25 vs 25 or higher. To maintain double blinding, weekly blister packs were manufactured containing 7 active pills for the once-daily arm, 3 active pills and 4 placebos for the 3-times-weekly arm, and 1 active pill and 6 placebos for the once-weekly arm. Pills were numbered from 1 to 7 in each blister pack. The final visit, scheduled on the day of surgery or the day before, included assessment for toxic effects, concomitant medications, blood collection, and compliance/review of pill diary. Participants continued the intervention until the night before surgery. Surgery was performed ideally on day 29 or alternatively on day 36 or 43 to maintain the same treatment schedule in each arm. Toxic effects were evaluated by Medical Dictionary for Regulatory Activities Terminology categories using the National Cancer Institute terminology criteria (Common Terminology Criteria for Adverse Events, version 4.0.3). Menopausal symptoms were assessed by a self-administered questionnaire (Menopause-Specific Quality of Life Questionnaire20 [Mapi Research Trust]) comparing pretreatment and posttreatment answers.
The protocol (Supplement 2) was approved by the National Cancer Institute Central Institutional Review Board and the local Italian institutional review boards, as well as the Regional Committees for Medical and Health Research Ethics in Western Norway. All participants provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Biomarkers Assessment
Morning fasting blood samples were collected at baseline and final visit (S-Monovette [Sarstedt]). After clotting, the tubes were centrifuged at 2000 times gravity for 10 minutes at room temperature. Serum was aliquoted (1 mL) in barcoded, labeled, plastic microtubes and stored at −80 °C until assayed.
Serum estradiol (the primary end point) and estrone concentrations were measured by solid-phase extraction (SPE) followed by liquid chromatography–tandem mass spectrometry (LC-MS/MS) detection, with lower limit of quantification of 1.0 pg/mL for serum estradiol (to convert to pmol/L, multiply by 3.671) and 5 pg/mL for estrone (to convert to pmol/L, multiply by 3.698) (API 5000 [Syneos Health]). Total serum estrone and estrone sulfate were measured using a liquid-liquid extraction (LLE) and analyzed via LC-MS/MS, with 50 and 25 pg/mL lower limit of quantification, respectively (API 4000 [Syneos Health]).
Due to the overall low estradiol concentrations in postmenopausal women treated with AIs, we also analyzed serum estradiol and estrone levels as secondary end points with an ultrasensitive LC-MS/MS method, using automated LLE without derivatization (lower limit of quantification was 0.8 pmol/L, corresponding to 0.22 pg/mL for serum estradiol, and 0.2 pmol/L, corresponding to 0.05 pg/mL, for estrone) at Haukeland University Hospital in Norway (QTRAP 6500+ [SCIEX]). Serum androstenedione and testosterone were also analyzed using a LC-MS/MS method (API 5500 [SCIEX]).21 Sex hormone–binding globulin (SHBG) serum levels were measured by a chemiluminescent immunoassay (IDS-iSYS Multi-Discipline Automated System [Immunodiagnostic Systems Limited]), with a lower limit of detection of 0.03 μg/mL (to convert to nmol/L, multiply by 8.896). Lipid profiles (total cholesterol, high-density lipoprotein [HDL], low-density lipoprotein, and triglycerides) were determined locally at baseline and final visit.
Pretreatment and posttreatment measurements were centralized at the pathology division of the European Institute of Oncology to minimize the variability among centers. The Ki-67 was assessed by immunohistochemistry according to recommendations22 using the Mib-1 monoclonal antibody with automated immunostainer (DakoCytomation [Agilent]).23 Immunohistochemistry was used to determine the expression of estrogen receptor and progesterone receptor (PgR) using pharmDx (Agilent) and human epidermal growth factor receptor 2 using HercepTest (Agilent).
Statistical Analysis
The primary objective of this study was to assess if the reduction in serum estradiol (measured by SPE) with the 2 lower-dosing schedules was noninferior to the standard dosage on the percentage change and absolute change of serum estradiol from baseline to posttreatment and compare differences between each experimental arm and the standard dose. Only the primary end point analysis was noninferiority.
Noninferiority P values with estimates of the difference between once-daily vs 3-times-weekly schedules and once-daily vs once-weekly schedules in the percentage change in time and corresponding 1-sided 97.5% CIs were provided with the −6% noninferiority limit, which was based on expert opinion. The primary analysis was intention to treat (ITT). We also conducted a planned per-protocol analysis of compliant participants (defined as participants who received ≥80% of the active scheduled pills and underwent blood testing as per protocol schedule). A per-protocol analysis of compliant participants was also conducted for Ki-67.
Given the expected relative reduction in estradiol of at least 80% with exemestane, 25 mg, once daily, we assumed a noninferiority difference of −6% from baseline in percentage change of estradiol after treatment with 25 mg 3 times weekly or 25 mg once weekly, using a 1-sided, 2-sample t test. A total sample size of 162 participants had 80% power to detect a noninferiority of −6% in the mean percentage change of the lower-dose regimens compared with the standard dose. The true difference between the mean percentage changes was assumed to be 0%. The data were drawn from populations with common SDs of 11%. Assuming a 10% dropout rate, 180 participants had to be randomized. The significance level of the test for the main end point was set at P = .025 to account for multiple comparisons. For secondary end points, 2-tailed P < .05 was considered statistically significant. Statistical analyses were performed using SAS, version 9.4 (SAS Institute). Additional information on statistical analyses is available in eMethods in Supplement 1.
Results
Participant Characteristics
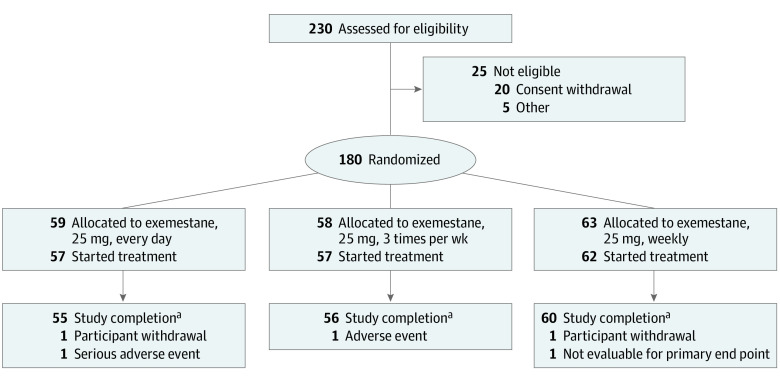
From February 1, 2017, to August 31, 2019, a total of 230 women were screened, and 180 women were randomized into 1 of the 3 arms (Figure 1); median (IQR) age was 66 (60-71) years, 63 (60-69) years, and 65 (61-70) years in the once-daily, 3-times-weekly, and once-weekly arms, respectively. Four participants did not start the treatment, and 4 dropped out (2 for personal reasons, 1 for AEs, and 1 for a severe AE unrelated to study treatment). The final evaluable participants for the primary end point included 55, 56, and 60 receiving exemestane, 25 mg, once daily, 3 times weekly, and once weekly, respectively. Study participants were stratified by center and BMI. eTable 1 in Supplement 1 shows the baseline participant and cancer characteristics. Drug intake was high, as 153 (89%) participants took at least 80% of the pills without difference among arms and between compliant and noncompliant participants (eTable 2 in Supplement 1). Forty-seven, 52, and 54 participants underwent blood testing as per protocol schedule in the once-daily, 3-times-weekly, and once-weekly arms, respectively. Eighty-eight, 44, and 16 participants had surgery exactly after 4, 5, or 6 weeks, respectively.
Figure 1. CONSORT Diagram.
aProvided blood sample for primary end point.
Circulating Biomarkers
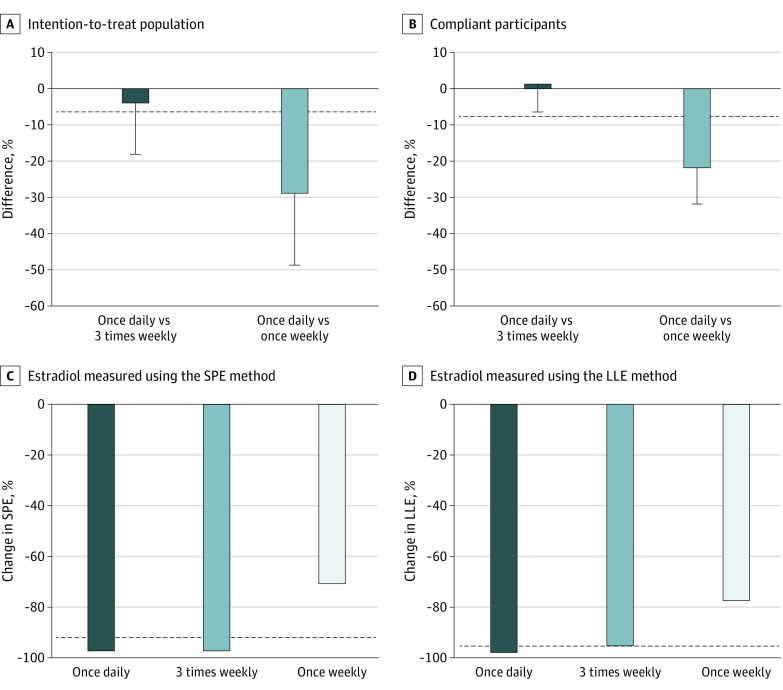
In the ITT population, the reduction in serum estradiol (SPE method) by the mean percentage change of serum estradiol was −89%, −85%, and −60% among the once-daily, 3-times-weekly, and once-weekly arms, respectively; in the compliant participants (n = 153), it was −91%, −92%, and −69%, respectively (Table 1). In the ITT population, the difference in estradiol percentage change between once-daily and 3-times-weekly arms was −3.6% (P for noninferiority = .37; Figure 2A), whereas in compliant participants it was 2.0% (97.5% lower confidence limit, −5.6%; P for noninferiority = .02; Table 1 and Figure 2A and B), indicating that the 3-times-weekly dosage was noninferior to the once-daily dosage among compliant participants. A secondary analysis using a more sensitive method (LLE) for estradiol showed similar estradiol reduction, with −96%, −91%, and −72% among the once-daily, 3-times-weekly, and once-weekly arms, respectively, in the ITT analysis and –96%, −92%, and −74%, respectively, in the compliant participants, but did not reach the noninferiority margin (Table 1). The median percentage change in estradiol in each arm showed no difference between the once-daily and 3-times-weekly dosages, using either SPE or LLE (Figure 2C and D). Finally, the percentage of participants with estradiol suppression below detection limit was 69.0%, 65.4%, and 17.2% by the SPE method and 77.7%, 47.2%, and 3.4% by LLE in the once-daily, 3-times-weekly, and once-weekly arms, respectively (once daily vs 3 times weekly, P = .78; eFigure 2 in Supplement 1).
Table 1. Least Square (LS) Mean Percentage Change in Estradiol in Each Study Arm Receiving Exemestane, 25 mg, by Regimen.
| Arm | LS mean change (95% CI), %a | Contrast | Difference in LS mean, % | 97.5% Confidence limit, % | P for noninferiorityb |
|---|---|---|---|---|---|
| Intention to treat | |||||
| Solid-phase extraction | |||||
| QD | −89 (−95 to −83) | QD vs TIW | −3.6 | −17.8 | .37 |
| TIW | −85 (−98 to −73) | QD vs QW | −28.8 | −48.7 | .98 |
| QW | −60 (−78 to −42) | NA | NA | NA | NA |
| Liquid-liquid extraction | |||||
| QD | −96 (−97 to −95) | QD vs TIW | −4.9 | −8.7 | .28 |
| TIW | −91 (−95 to −88) | QD vs QW | −23.9 | −29.2 | >.99 |
| QW | −72 (−77 to −67) | NA | NA | NA | NA |
| Compliant | |||||
| Solid-phase extraction | |||||
| QD | −91 (−98 to −84) | QD vs TIW | 2.0 | −5.6 | .02 |
| TIW | −92 (−96 to −89) | QD vs QW | −21.5 | −31.4 | >.99 |
| QW | −69 (−76 to −62) | NA | NA | NA | NA |
| Liquid-liquid extraction | |||||
| QD | −96 (−97 to −95) | QD vs TIW | −3.8 | −7.4 | .11 |
| TIW | −92 (−95 to −89) | QD vs QW | −22.2 | −27.3 | >.99 |
| QW | −74 (−78 to −69) | NA | NA | NA | NA |
Abbreviation: NA, not applicable; QD, once daily; QW, once weekly; TIW, 3 times weekly.
LS mean percentage changes are from a generalized linear model, and 95% CIs were obtained with bootstrap.
1-Sided noninferiority t test.
Figure 2. Circulating Estradiol in Each Study Arm Receiving Exemestane, 25 mg, by Regimen.
Contrasts between arms of least square mean percentage changes of estradiol using the solid-phase extraction (SPE) method and 1-sided 97.5% confidence limit in the intention-to-treat population (A) and in compliant participants (B). C, Median percentage change of serum estradiol measured using the SPE method by study arm. D, Median percentage change of serum estradiol measured using a liquid-liquid extraction (LLE) method by study arm. In all panels, the horizontal dashed line represents the −6% noninferiority margin.
Table 2 summarizes median absolute changes adjusted for baseline and percentage changes for other hormones and lipids analyzed. In this secondary end point analysis, there were no statistical differences for estrone, total estrone, and estrone sulfate between the once-daily and the 3-times-daily arms, whereas the lower dose was less effective. No major changes were observed for androstenedione or testosterone with any dose, whereas for SHBG, a dose response was noted, with an absolute change of −12.8, −7.0, and −2.3 nmol/L for exemestane once daily, 3 times weekly, and once weekly, respectively. Regarding the lipid profile, there was an expected HDL cholesterol reduction with the once-daily dosage vs only a marginal effect with the 2 lower-dose regimens (−10, −4, and 1 mg/dL with once daily, 3 times weekly, and once weekly, respectively).
Table 2. Pretreatment, Posttreatment, Percentage Change, and Absolute Change of Circulating Hormone and Lipid Levels by Study Arm.
| Biomarker | Median (IQR) | P value change, adjusteda | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exemestane, 25 mg, QD (n = 55) | Exemestane, 25 mg, TIW (n = 56) | Exemestane, 25 mg, QW (n = 60) | ||||||||||||
| Pretreatment | Posttreatment | % Change | Absolute change | Pretreatment | Posttreatment | % Change | Absolute change | Pretreatment | Posttreatment | % Change | Absolute change | QD vs TIW | QD vs QW | |
| Estrone, pmol/L | ||||||||||||||
| Solid-phase extraction | 104.3 (73.8 to 141.0) | 7.0 (1.3 to 13.4) | −93 (−98 to −88) | −95.5 (−126.8 to −65.9) | 99.4 (69.4 to 137.6) | 9.1 (4.6 to 16.1) | −89 (−96 to −83) | −80.0 (−119.7 to −60.4) | 92.2 (66.7 to 122.8) | 26.1 (18.5 to 43.1) | −73 (−81 to −52) | −61.7 (−92.2 to −36.4) | .42 | .004 |
| Liquid-liquid extraction | 101.0 (69.6 to 143.0) | 1.6 (0.90 to 2.9) | −99 (−99 to −98) | −97.6 (−135.8 to −67.1) | 96.2 (72.1 to 147.5) | 4.3 (2.5 to 6.6) | −96 (−97 to −93) | −88.4 (−125.1 to −67.2) | 93.4 (69.5 to 132.0) | 21.8 (12.8 to 34.2) | −78 (−85 to −62) | −65.2 (−103.3 to −43.9) | .29 | .004 |
| Total | 294.7 (182.6 to 451.4) | 12.5 (12.5 to 12.5) | −95 (−97 to −91) | −267.4 (−437.3 to −167.6) | 302.2 (160.0 to 429.9) | 12.5 (12.5 to 12.5) | −94 (−96 to −90) | −286.5 (−397. 8 to 145.6) | 246.1 (164.0 to 362.1) | 61.9 (32.3 to 92.6) | −76 (−85 to −63) | −179.6 (−273.4 to −117.1) | .74 | .01 |
| Estrone sulfate, pmol/L | 581.9 (363.6 to 918.9) | 13.8 (13.8 to 35.7) | −96 (−98 to −94) | −568.1 (−853.7 to −349.8) | 625.8 (324.7 to 955.6) | 13.8 (13.8 to 55.2) | −95 (−97 to −92) | −546.6 (−775.4 to −301.4) | 452.3 (315.1 to 710.6) | 124.8 (55.7 to 193.9) | −75 (−83 to −64) | −322.7 (−518.8 to −222.3) | .39 | .15 |
| Androstenedione, nmol/L | 1.5 (1.0 to 1.9) | 1.4 (1.10 to 1.90) | 0 (−18 to 18) | 0.00 (−0.30 to 0.20) | 1.7 (1.1 to 2.5) | 1.8 (1.1 to 2.6) | 11 (−20 to 30) | 0.20 (−0.20 to 0.50) | 1.4 (1.1 to 2.0) | 1.6 (1.1 to 2.0) | 11 (−14 to 43) | 0.10 (−0.20 to 0.60) | .21 | .46 |
| Testosterone, nmol/L | 0.60 (0.40 to 0.90) | 0.45 (0.30 to 0.80) | −19 (−33 to 0) | −0.10 (−0.20 to 0) | 0.70 (0.40 to 1.1) | 0.50 (0.30 to 0.90) | 0 (−25 to 0) | 0.00 (−0.20 to 0.00) | 0.60 (0.40 to 0.90) | 0.60 (0.40 to 0.80) | 0 (−20 to 18) | 0.0 (−0.10 to 0.10) | .72 | .30 |
| SHBG, nmol/L | 53.4 (39.1 to 64.0) | 36.0 (27.6 to 50.3) | −29 (−36 to −17) | −12.8 (−20.7 to −8.7) | 47.9 (36.6 to 65.1) | 40.0 (30.3 to 56.7) | −15 (−23 to −7) | −7.0 (−11.4 to −2.9) | 50.1 (31.9 to 67.8) | 45.8 (26.4 to 60.9) | −6 (−17 to 1) | −2.3 (−6.0 to 0.40) | .01 | .004 |
| Cholesterol, mg/dL | ||||||||||||||
| HDL | 62 (55 to 72) | 56 (46 to 63) | −14 (−21 to −3) | −10 (−13 to −2) | 59 (48 to 71) | 57 (46 to 65) | −5 (−13 to 4) | −4 (−9 to 2) | 57 (50 to 67) | 57 (50 to 69) | −1 (−9 to 4) | 1 (−22 to 14) | .05 | .06 |
| Total | 205 (181 to 233) | 201 (175 to 222) | −6 (−17 to 3) | −11 (−35 to 7) | 208 (182 to 226) | 195 (173 to 210) | −6 (−11 to 1) | −11 (24 to 2) | 220 (200 to 247) | 220 (195 to 245) | 0 (−8 to 6) | −1 (−5 to 2) | .50 | .05 |
Abbreviations: HDL, high-density lipoprotein; QD, once daily; QW, once weekly; SHBG, sex hormone–binding globulin; TIW, 3 times weekly.
SI conversion factor: To convert HDL to mmol/L, multiply by 0.0259.
P values from analysis of covariance models on absolute changes in time, adjusted for baseline values, age, and body mass index. All P values were also adjusted for multiple testing.
Tissue Biomarkers
Cell proliferation measured by the Ki-67 labeling index and PgR expression were analyzed pretreatment and posttreatment (Table 3). The median Ki-67 percentage absolute change adjusted for baseline was −7.5%, −5.0%, and −4.0% in the once-daily, 3-times-weekly, and once-weekly arms, respectively, showing no statistically significant differences among arms (once daily vs 3 times weekly, P = .31; once daily vs once weekly, P = .06). As exploratory analysis, Ki-67 expression was also analyzed in compliant participants and normal adjacent tissue. Due to the very low expression of Ki-67 in normal tissue, no modulation was observed (Table 3). In adherent participants (eFigure 3 in Supplement 1), the reduction of Ki-67 was similar to the ITT analysis. Additionally, PgR expression was modulated by exemestane, with a median absolute change of −17.0% in the once-daily arm, −9.0% in the 3-times-weekly arm, and −7.0% in the once-weekly arm (once daily vs 3 times weekly, P = .44; once daily vs once weekly, P = .06; Table 3).
Table 3. Median Absolute Change in Ki-67 and Progesterone Receptor (PgR) Expression After 4 to 6 Weeks of Treatment by Study Arm.
| Compliant participants | Median (IQR), % | P value, adjusteda | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exemestane, 25 mg, QD (n = 52) | Exemestane, 25 mg, TIW (n = 53) | Exemestane, 25 mg, QW (n = 55) | |||||||||
| Biopsy | Surgery | Absolute change | Biopsy | Surgery | Absolute change | Biopsy | Surgery | Absolute change | QD vs TIW | QD vs QW | |
| Ki-67 | 13.0 (7 to 17) | 4.5 (2 to 8) | −7.5 (−11 to −3) | 13.0 (7 to 20) | 5.0 (2 to 12) | −5.0 (−10 to −1) | 12.0 (6 to 19) | 6.0 (3 to 13) | −4.0 (−8 to −1) | .31 | .06 |
| PgR | 65.0 (10 to 95) | 4.0 (0 to 40) | −17.0 (−67 to −4) | 70.0 (10 to 99) | 5.0 (0 to 70) | −9.0 (−50 to 0) | 70.0 (8 to 95) | 25.0 (0 to 80) | −7.0 (−25 to 0) | .44 | .06 |
| Adjacent tissue | Exemestane, 25 mg, QD (n = 28) | Exemestane, 25 mg, TIW (n = 22) | Exemestane, 25 mg, QW (n = 27) | QD vs TIW | QD vs QW | ||||||
| Ki-67 | 1.0 (1 to 2) | 1.5 (1 to 2) | 0.0 (0 to 1) | 1.0 (0 to 3) | 1.0 (1 to 2) | 0.0 (−0.5 to 0.5) | 1.0 (1 to 2) | 2.0 (1 to 3) | 0.0 (−1 to 1) | .30 | .88 |
Abbreviations: QD, once daily; QW, once weekly; TIW, 3 times weekly.
Adjusted for baseline levels, age, and body mass index. All P values were also adjusted for multiple testing.
Adverse Events
Overall, treatment was well tolerated, with a total of 358 AEs (255 [71%] were grade 1). No statistically significant differences were detected among the 3 arms. eTable 3 in Supplement 1 summarizes AEs occurring in 5% or more of participants. Women’s perception of menopausal symptoms showed no clinically relevant differences among arms (eTable 4 in Supplement 1).
Discussion
The uptake of breast cancer preventive therapy is low despite strong evidence for efficacy with selective estrogen receptor modulators and AIs, primarily because of the fear of AEs.14,24,25 Compliance to AIs is also hampered by AEs in the adjuvant setting, and this decreases efficacy.15 The risk-benefit ratio of existing drugs could be improved by dose de-escalation studies searching for the minimal effective dose.16 The previous published phase 3 trial of low-dose tamoxifen to treat breast intraepithelial neoplasia recurrence showed retained efficacy and fewer toxic effects compared with placebo.17
For these reasons, we wanted to explore 2 alternative exemestane schedules in reducing estradiol levels, a risk biomarker,18 and a measure of AI potency.26,27 In earlier studies,8,9 exemestane showed a prolonged effect despite its short half-life, but the minimal effective dose was not assessed. Johannessen et al28 showed that the maximal estradiol and estrone suppression can already be reached with 10 mg/d, which may be the equivalent of 25 mg 3 times weekly.29
In the current study, exemestane, 25 mg, 3 times weekly was noninferior to the standard daily dosing in the compliant participants, representing 89% of the whole population, with a −92% mean decrease of estradiol in the 3-times-weekly arm and −91% in the once-daily arm using the per-protocol SPE method. However, in the ITT analysis, we did not show a noninferiority effect below 6% of 3-times-weekly arm. The different findings between compliant and noncompliant participants are not due to baseline characteristics, but are probably due to the lower variability in estradiol in the former group. The present findings also indicate that the median percentage change of estrone, total estrone, and estrone sulfate showed no differences between once-daily and 3-times-weekly dosing, whereas a dose-response modulation was noted for SHBG, with the daily dose significantly reducing this protective biomarker.30 The weekly dose was significantly less effective compared with the daily dose on most biomarkers but still attained a mean of 69% decrease in estradiol in compliant participants, which may be sufficient for a preventive activity. Testosterone and androstenedione were not modulated in this study, in line with some studies,28,31 whereas other studies showed an increase in testosterone by exemestane.32,33 Interestingly, HDL cholesterol was significantly reduced in the daily dose compared with the once-weekly and the 3-times-weekly arms.
Estradiol was chosen as the primary end point because it is a direct marker of drug potency, although there is no clear threshold of clinical efficacy and phase 3 trials have shown no different efficacy among the 3 AIs,27 despite different estradiol suppression potency.26,34 The −6% noninferiority margin may be too restrictive considering the large intra-individual and interindividual variability of estradiol suppression. In noninferiority trials using end point biomarkers, the noninferiority margin is usually not fixed in advance and depends on the reference intervention estimate.35 The median percentage change in the 3-times-weekly dose regimen was similar to the daily dose even with the most sensitive LLE assay, suggesting that in many individuals 3-times-weekly dosing is capable of suppressing estradiol to the same level of daily dosing. On the other hand, estradiol suppression below detection limit by LLE showed a stepwise dose response in the 3 dose levels.
Ki-67 is an established surrogate biomarker in presurgical studies,22,36,37 based on which a low dose of tamoxifen was selected38 for a successful phase 3 trial.17 In the present study, the median change in Ki-67 with the 3-times-weekly dose regimen was not significantly different than the daily dose, and this may have important clinical implications in terms of efficacy. Indeed, an absolute reduction of 3% in Ki-67, after 4 weeks of tamoxifen, had an effect on disease-free (a relative 15% reduction) and overall survival (18% reduction).37 In the current study, the median (IQR) absolute change of Ki-67 was −5% (−10% to −1%) with the 3-times-weekly regimen and −7.5% (−11% to −3%) with the daily dose (P = .31). In a previous study of 6-week exposure to exemestane, 25 mg/d, the median (IQR) absolute change was −10% (−18% to −5%).33 In normal tissue, the lack of Ki-67 modulation is probably due to the very low Ki-67 expression and the limited number of available samples at baseline—2 reasons that prevent any meaningful conclusion.
The effect of exemestane on the downregulation of PgR showed no statistically significant difference between the once-daily and 3-times-weekly arms. The PgR reduction was identified in this presurgical study and in different neoadjuvant studies of exemestane.39,40,41 Because PgR expression is modulated by estrogens,42 its downregulation by AIs can be considered an indicator of effective estrogen inhibition.
Of note, all dosages showed a similar magnitude of AEs. However, the short treatment duration likely prevents reliable conclusions regarding AEs limiting daily activities. A study of 6 to 12 months could better address this issue before a prevention trial of the 3-times-weekly schedule is launched.
Limitations
This study has some limitations, in particular the tight −6% noninferiority margin of estradiol, which is not a validated clinical threshold, and the choice of the percentage change vs the absolute change, which increased the biomarker variability. Also, the primary end point measure was circulating estradiol measured with a SPE method, which proved to be less sensitive than the LLE method. However, even if the overall results did not show noninferiority activity in the 3-times-weekly schedule among all participants, a marked and consistent activity is shown throughout the secondary end points, and there is no evidence that subtle differences in the extent of estradiol suppression correspond to a greater clinical benefit.34,43 Another limitation due to the short treatment exposure is that the toxic effect evaluation is not representative of longer treatment. For example, AI-related musculoskeletal symptoms arose after a median time of 1.6 months, and the referral average time was 3.7 months in one study.44 The quality of life in the MAP.3 study45 showed that the majority of symptoms were already present at 6 months but still increased following 1 year of treatment.
Conclusions
In this randomized clinical trial, the present data indicate that in the ITT analysis, the reduction of exemestane by the 2 lower dosages was not noninferior to the standard dosage in decreasing serum estradiol. However, for compliant participants representing 90% of the study population, 3-times-weekly dosing was shown to be noninferior to once-daily dosing. Similar decreases between the 2 groups were also observed for estrone, total estrone level, Ki-67, and PgR expression. Moreover, SHBG and HDL cholesterol had a more favorable profile. These data support the use of a 3-times-weekly schedule for further studies of exemestane in breast cancer prevention.
eMethods
eTable 1. Participant and tumor characteristics (median, IQR) by study arm
eTable 2. Participant and tumor characteristics (median, IQR) by study arm and treatment adherence
eTable 3. Adverse Events (all grades) occurring in ≥5% of participants by study arm
eTable 4. Quality of Life: pre-treatment, post-treatment and absolute change in MenQoL mean score (SD) by study arm
eFigure 1. Number (%) of randomized participants by center
eFigure 2. Comparison in circulating estradiol suppression by study arm
eFigure 3. Median absolute change of Ki-67 by study arm in compliant participants (P-values for QD vs TIW = 0.3173 and QD vs QW=0.0480)
Trial Protocol
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Bonanni B, et al. ; SERM Chemoprevention of Breast Cancer Overview Group . Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827-1834. doi: 10.1016/S0140-6736(13)60140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss PE, Ingle JN, Alés-Martínez JE, et al. ; NCIC CTG MAP.3 Study Investigators . Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381-2391. doi: 10.1056/NEJMoa1103507 [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Sestak I, Forbes JF, et al. ; IBIS-II investigators . Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041-1048. doi: 10.1016/S0140-6736(13)62292-8 [DOI] [PubMed] [Google Scholar]
- 5.Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37(33):3152-3165. doi: 10.1200/JCO.19.01472 [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Sestak I, Forbes JF, et al. ; IBIS-II investigators . Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395(10218):117-122. doi: 10.1016/S0140-6736(19)32955-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzdar AU, Robertson JFR, Eiermann W, Nabholtz JM. An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane. Cancer. 2002;95(9):2006-2016. doi: 10.1002/cncr.10908 [DOI] [PubMed] [Google Scholar]
- 8.Zilembo N, Noberasco C, Bajetta E, et al. Endocrinological and clinical evaluation of exemestane, a new steroidal aromatase inhibitor. Br J Cancer. 1995;72(4):1007-1012. doi: 10.1038/bjc.1995.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans TR, Di Salle E, Ornati G, et al. Phase I and endocrine study of exemestane (FCE 24304), a new aromatase inhibitor, in postmenopausal women. Cancer Res. 1992;52(21):5933-5939. [PubMed] [Google Scholar]
- 10.Francis PA, Pagani O, Fleming GF, et al. ; SOFT and TEXT Investigators and the International Breast Cancer Study Group . Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122-137. doi: 10.1056/NEJMoa1803164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—a randomized controlled phase III trial. J Clin Oncol. 2013;31(11):1398-1404. doi: 10.1200/JCO.2012.44.7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derks MGM, Blok EJ, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in women with postmenopausal early breast cancer (TEAM): 10-year follow-up of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(9):1211-1220. doi: 10.1016/S1470-2045(17)30419-9 [DOI] [PubMed] [Google Scholar]
- 13.Morden JP, Alvarez I, Bertelli G, et al. Long-term follow-up of the intergroup exemestane study. J Clin Oncol. 2017;35(22):2507-2514. doi: 10.1200/JCO.2016.70.5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noonan S, Pasa A, Fontana V, et al. A survey among breast cancer specialists on the low uptake of therapeutic prevention with tamoxifen or raloxifene. Cancer Prev Res (Phila). 2018;11(1):38-43. doi: 10.1158/1940-6207.CAPR-17-0162 [DOI] [PubMed] [Google Scholar]
- 15.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529-537. doi: 10.1007/s10549-010-1132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah M, Rahman A, Theoret MR, Pazdur R. The drug-dosing conundrum in oncology—when less is more. N Engl J Med. 2021;385(16):1445-1447. doi: 10.1056/NEJMp2109826 [DOI] [PubMed] [Google Scholar]
- 17.DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37(19):1629-1637. doi: 10.1200/JCO.18.01779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA; Multiple Outcomes of Raloxifene Evaluation (MORE) Trial . Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287(2):216-220. doi: 10.1001/jama.287.2.216 [DOI] [PubMed] [Google Scholar]
- 19.Guerrieri-Gonzaga A, Serrano D, Thomas P, et al. Alternative dosing of exemestane in postmenopausal women with ER-positive breast cancer: design and methods of a randomized presurgical trial. Contemp Clin Trials. 2021;107:106498. doi: 10.1016/j.cct.2021.106498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Dole KB, DeVellis RF, Brown RD, Funk ML, Gaynes BN, Williams RE. Evaluation of the Menopause-Specific Quality of Life Questionnaire: a factor-analytic approach. Menopause. 2012;19(2):211-215. doi: 10.1097/gme.0b013e31822817f9 [DOI] [PubMed] [Google Scholar]
- 21.Methlie P, Hustad SS, Kellmann R, et al. Multisteroid LC-MS/MS assay for glucocorticoids and androgens, and its application in Addison’s disease. Endocr Connect. 2013;2(3):125-136. doi: 10.1530/EC-13-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowsett M, Smith IE, Ebbs SR, et al. ; IMPACT Trialists Group . Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99(2):167-170. doi: 10.1093/jnci/djk020 [DOI] [PubMed] [Google Scholar]
- 23.Viale G, Giobbie-Hurder A, Regan MM, et al. ; Breast International Group Trial 1-98 . Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26(34):5569-5575. doi: 10.1200/JCO.2008.17.0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi: 10.1093/annonc/mdv590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters EA, McNeel TS, Stevens WM, Freedman AN. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134(2):875-880. doi: 10.1007/s10549-012-2089-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon JM, Renshaw L, Young O, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol. 2008;26(10):1671-1676. doi: 10.1200/JCO.2007.13.9279 [DOI] [PubMed] [Google Scholar]
- 27.De Placido S, Gallo C, De Laurentiis M, et al. ; GIM Investigators . Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):474-485. doi: 10.1016/S1470-2045(18)30116-5 [DOI] [PubMed] [Google Scholar]
- 28.Johannessen DC, Engan T, Di Salle E, et al. Endocrine and clinical effects of exemestane (PNU 155971), a novel steroidal aromatase inhibitor, in postmenopausal breast cancer patients: a phase I study. Clin Cancer Res. 1997;3(7):1101-1108. [PubMed] [Google Scholar]
- 29.Bajetta E, Zilembo N, Noberasco C, et al. The minimal effective exemestane dose for endocrine activity in advanced breast cancer. Eur J Cancer. 1997;33(4):587-591. doi: 10.1016/S0959-8049(96)00494-7 [DOI] [PubMed] [Google Scholar]
- 30.Dimou NL, Papadimitriou N, Gill D, et al. Sex hormone binding globulin and risk of breast cancer: a Mendelian randomization study. Int J Epidemiol. 2019;48(3):807-816. doi: 10.1093/ije/dyz107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gatti-Mays ME, Venzon D, Galbo CE, et al. Exemestane use in postmenopausal women at high risk for invasive breast cancer: evaluating biomarkers of efficacy and safety. Cancer Prev Res (Phila). 2016;9(3):225-233. doi: 10.1158/1940-6207.CAPR-15-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadji P, Kauka A, Bauer T, Tams J, Hasenburg A, Kieback DG. Effects of exemestane and tamoxifen on hormone levels within the Tamoxifen Exemestane Adjuvant Multicentre (TEAM) trial: results of a German substudy. Climacteric. 2012;15(5):460-466. doi: 10.3109/13697137.2011.647839 [DOI] [PubMed] [Google Scholar]
- 33.Aristarco V, Serrano D, Gandini S, et al. A randomized, placebo-controlled, phase II, presurgical biomarker trial of celecoxib versus exemestane in postmenopausal breast cancer patients. Cancer Prev Res (Phila). 2016;9(5):349-356. doi: 10.1158/1940-6207.CAPR-15-0311 [DOI] [PubMed] [Google Scholar]
- 34.Robarge JD, Desta Z, Nguyen AT, et al. Effects of exemestane and letrozole therapy on plasma concentrations of estrogens in a randomized trial of postmenopausal women with breast cancer. Breast Cancer Res Treat. 2017;161(3):453-461. doi: 10.1007/s10549-016-4077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandie AB, Molinari N, Wanjoya A, Kouanfack C, Laurent C, Tchatchueng-Mbougua JB. Non-inferiority test for a continuous variable with a flexible margin in an active controlled trial: an application to the “Stratall ANRS 12110 / ESTHER” trial. Trials. 2022;23(1):202. doi: 10.1186/s13063-022-06118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100(19):1380-1388. doi: 10.1093/jnci/djn309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeCensi A, Guerrieri-Gonzaga A, Gandini S, et al. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol. 2011;22(3):582-587. doi: 10.1093/annonc/mdq427 [DOI] [PubMed] [Google Scholar]
- 38.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95(11):779-790. doi: 10.1093/jnci/95.11.779 [DOI] [PubMed] [Google Scholar]
- 39.Torrisi R, Bagnardi V, Pruneri G, et al. Antitumour and biological effects of letrozole and GnRH analogue as primary therapy in premenopausal women with ER and PgR positive locally advanced operable breast cancer. Br J Cancer. 2007;97(6):802-808. doi: 10.1038/sj.bjc.6603947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mlineritsch B, Tausch C, Singer C, et al. ; Austrian Breast, Colorectal Cancer Study Group (ABCSG) . Exemestane as primary systemic treatment for hormone receptor positive post-menopausal breast cancer patients: a phase II trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-17). Breast Cancer Res Treat. 2008;112(1):203-213. doi: 10.1007/s10549-007-9843-x [DOI] [PubMed] [Google Scholar]
- 41.Dowsett M, Ebbs SR, Dixon JM, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer–a study from the IMPACT trialists. J Clin Oncol. 2005;23(11):2477-2492. doi: 10.1200/JCO.2005.07.559 [DOI] [PubMed] [Google Scholar]
- 42.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721-7735. doi: 10.1200/JCO.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Folkerd EJ, Dixon JM, Renshaw L, A’Hern RP, Dowsett M. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012;30(24):2977-2980. doi: 10.1200/JCO.2012.42.0273 [DOI] [PubMed] [Google Scholar]
- 44.Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111(2):365-372. doi: 10.1007/s10549-007-9774-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maunsell E, Goss PE, Chlebowski RT, et al. Quality of life in MAP.3 (Mammary Prevention 3): a randomized, placebo-controlled trial evaluating exemestane for prevention of breast cancer. J Clin Oncol. 2014;32(14):1427-1436. doi: 10.1200/JCO.2013.51.2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Participant and tumor characteristics (median, IQR) by study arm
eTable 2. Participant and tumor characteristics (median, IQR) by study arm and treatment adherence
eTable 3. Adverse Events (all grades) occurring in ≥5% of participants by study arm
eTable 4. Quality of Life: pre-treatment, post-treatment and absolute change in MenQoL mean score (SD) by study arm
eFigure 1. Number (%) of randomized participants by center
eFigure 2. Comparison in circulating estradiol suppression by study arm
eFigure 3. Median absolute change of Ki-67 by study arm in compliant participants (P-values for QD vs TIW = 0.3173 and QD vs QW=0.0480)
Trial Protocol
Data Sharing Statement