Summary
Understanding the glioblastoma (GBM) immune microenvironment and development of clinical treatment drugs rely on suitable preclinical GBM models. Here, we present a protocol to establish syngeneic orthotopic glioma mouse models. We also describe the steps to intracranially deliver immunotherapeutic peptides and monitor the treatment response. Finally, we show how to assess the tumor immune microenvironment with treatment outcomes.
For complete details on the use and execution of this protocol, please refer to Chen et al. (2021).1
Subject areas: Cancer, Health Sciences, Model Organisms, Neuroscience
Graphical abstract

Highlights
-
•
Establish syngeneic orthotopic glioma mouse models
-
•
Optimize the screw-guiding stereotactic surgery procedure in rodents
-
•
Intracranial administration of therapeutic drugs for a batch of mice
-
•
Immunological assessment of the brain tumor microenvironment in response to treatment
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Understanding the glioblastoma (GBM) immune microenvironment and development of clinical treatment drugs rely on suitable preclinical GBM models. Here, we present a protocol to establish syngeneic orthotopic glioma mouse models. We also describe the steps to intracranially deliver immunotherapeutic peptides and monitor the treatment response. Finally, we show how to assess the tumor immune microenvironment with treatment outcomes.
Before you begin
The protocol below describes the steps and timing on how to use murine glioma cell line QPP7 for establishing a batch of intracranial xenograft mice. Times that we listed here may either increase or decrease depending on cell lines and experimental skills. We have successfully used this protocol for other cell lines, including murine glioma cell line GL261 and GBM patient-derived neurosphere line TS543.1,2,3
Institutional permissions
Since this protocol describes work on experimental animals, appropriate approval or licenses for animal experiments from Institutional Animal Care and Use Committee (IACUC) should be obtained before performing experiments.
Animal experiments described in this protocol were approved by the IACUC of the University of Pittsburgh (#21049271) and conducted in accordance with NIH guidelines.
Stereotaxic frame, syringe pump, and syringes set-up
Timing: ∼1–2 months
The syringe pump and accessories including PHD Ultra Infusion only Pump (#703005) and PHD Ultra 6/10 Rack w/ Clamps (#703024A) were purchased from Harvard Apparatus. After purchasing the apparatus, the control panel (user interface part) and the syringe pump need to be separated. During the operation, an RS-485 cable is needed to connect the control panel and the syringe pump (Figure 1A). A lab-customized stereotaxic frame was made to hold the syringe pump, as shown in Figure 1B. The Hamilton syringe needle needs to be put on a sleeve (or cuff) to control the depth during the intracranial injection (Figure 1C). The lower end of the sleeve should be placed 5.5 mm away from the needle’s slender end. It will let the needle tip be 3.5 mm underneath the skull surface since the guide screw length is 2 mm. Set the pump with proper parameters and install the syringe on the stereotaxic frame, and ensure the whole intracranial injection system work smoothly before the formal experiments.
Note: The reason for taking one to two months in this step is because of purchasing the instrument and setting up the stereotaxic frame. The timing depends upon the availability of items from the vendor and the work schedules of the machine shop on campus. To make this customized frame, the syringe pump was first screwed into a metal holder in a vertical position. Then, the metal plate with the syringe pump was held by a copper pole. The height of the metal plate with the syringe pump can be adjusted by a screwdriver. Lastly, a steel plate (length 24” × width 18” × height 2.75”) was designed to hold the assembly including the copper pole, metal plate, and syringe pump (Figure 1B).
Alternatives: Instead of the lab-customized frame that is described in this protocol, any solid holders that can safely support the syringe pump vertically are alternatives. Commercial stereotaxic instruments for small animals can be obtained from companies such as KOPF or Stoelting. However, these frames can only operate a single mouse or a maximum of two mice simultaneously, and it takes about 30 min each time. Therefore, it is time-consuming and labor-intensive if experiments need more mice for stereotactic intracranial implantation/injection compared to the protocol that we described above, by which stereotactic surgery can be operated for 6–10 mice simultaneously.
Figure 1.
The assembly of the lab-customized stereotaxic frame, syringe pump, and syringes
(A) A lab-customized stereotaxic frame with a syringe pump connected to the user interface.
(B) The lab-customized frame can hold a syringe pump in a vertical position.
(C) A Hamilton syringe and needle with a sleeve.
Glioma cell culture preparation
Timing: ∼1–2 weeks
-
1.
Coat 6-well plates with fibronectin.
One day before seeding the cells, coat 6-well plates with the final concentration of 10 μg/mL of fibronectin in 1.5 mL DMEM/F12 medium for each well for about 12 h in a 37°C, 5% CO2 incubator.
Note: Make sure to coat the 6-well plates for at least 4 h; however, longer than 24 h of coating may lead to the cells failing to attach to the 6-well plates. Washing the coated wells with 1 mL of DPBS is optional because we did not notice that it affected the cell attachment without this washing step.
-
2.Thaw the QPP7 cells in a 37°C water bath and culture the cells with the NSC medium in 6-well plates at 37°C, 5% CO2 incubator.
-
a.Vacuum or remove the coating medium of 6-well plates immediately before seeding the cells. Rinse the plates with 1 mL DPBS for each well and discard. Seed 2×105 cells in each well and incubate the plates for about 12 h.Note: Always keep liquid in the coated well of the 6-well plates, and do not let them dry before seeding the cells.
-
b.Split the cells every two days for 2∼3 passages before the injection. This is normally a sub-culture, the same as described in step 3a. Apply 1 mL of pre-warmed DMEM/F12 medium each well to the 6-well plate and use a 1-mL pipettor to mix the cells to make them single cells.Note: it is recommended to let the cells grow for about one week to recover from liquid nitrogen. QPP7 cells should be passaged when they reach 80∼90% confluence. The cells used for injection should be in the log phage. When they are in this phase, it usually takes 1.5∼2 days to produce one generation. Also, note that the cells grow fast during the log phase; the medium easily changes its color to yellowish, and one needs to change the medium no more than two days.
-
c.Transfer the cells to a 15-mL conical tube and centrifuge at 400 × g for 5 min.
-
i.Discard the supernatant and resuspend the cells in a pre-warmed NSC medium.
-
ii.Put the cells back into new fibronectin-coated wells of a 6-well plate.
-
i.
-
a.
-
3.Prepare QPP7 cells for intracranial implantation. Each mouse will be injected with 1×105 living cells in 5 μL DPBS.
-
a.Remove the NSC medium by vacuum, and rinse the cells with 1 mL DPBS each well. Discard the DPBS with a vacuum, apply Accutase™, and incubate the 6-well plate in a 37°C incubator for 1 min. Inspect the plate and ensure the complete detachment of cells by gently tapping the side of the flask with the hand palm.
-
b.Apply 1 mL of pre-warmed DMEM/F12 medium each well to the 6-well plate. Use a 1-mL pipettor to mix the cells several times and ensure they dissociated as single cells. Transfer the cells to a 15-mL conical tube. Use a yellow P200 tip to take 50 μL of cells to a 1.5 mL centrifuge tube and mix with the same volume of trypan blue.
-
c.Use a yellow P200 tip to take 10 μL of staining cells to a hemocytometer and count the cells under a microscope.Note: It is recommended that the cell number counted in the hemocytometer is 100∼200 since this range has the most reliable and accurate. Do not use automatic equipment to count cells since it is not accurate enough to count cells for intracranial injection. It is recommended to use the cells with > 90% of cell viability.
-
d.Add additional DMEM/F12 medium to the 15-mL conical tube to make the total volume of liquid 10 mL. Centrifuge the tube at 300 × g for 5 min to pellet the cells.
-
e.Carefully discard the supernatant and do not disturb the cell pellet. It is OK if it leaves some aqueous residue.
-
f.Add 1 mL of DPBS to the 15-mL conical tube and gently resuspend the cell pellet with a pipettor, and transfer all the cells to a 1.5 mL centrifuge tube. Centrifuge the cells at 400 × g for 5 min.
-
g.Carefully remove the supernatant and do not disturb the cell pellet. Use P200 and then white fine P10 tips to remove any residual liquid.
-
h.Add the calculated volume of DPBS to the cell pellet to make the cell density 1×105 cells/5 μL, gently mix the cells, and put them on ice. The cells are ready for intracranial implantation.
 CRITICAL: Although we tested the cells in DPBS on ice to sustain more than 90% survival for 4 h, preparing the fresh cells every 2 h is recommended if a large number of mice need the intracranial injection.
CRITICAL: Although we tested the cells in DPBS on ice to sustain more than 90% survival for 4 h, preparing the fresh cells every 2 h is recommended if a large number of mice need the intracranial injection.
-
a.
Key resources table
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Flow: Rat anti-mouse CD45-PerCP-Cy5.5 (Dilution: 1:80) | BioLegend | Cat# 103132; RRID: AB_893340 |
| Flow: Rat anti-mouse CD3-APC (Dilution: 1:40) | BioLegend | Cat# 100236; RRID: AB_2561456 |
| Flow: Rat anti-mouse CD4-APC-Cy7 (Dilution: 1:20) | BioLegend | Cat# 100414; RRID: AB_312699 |
| Flow: Rat anti-mouse CD8a-BV711 (Dilution: 1:20) | BioLegend | Cat# 100747; RRID: AB_11219594 |
| Flow: Rat anti-mouse CD25-BV650 (Dilution: 1:20) | BioLegend | Cat# 102037; RRID: AB_11125760 |
| Flow: Rat anti-mouse NK1.1-PE (Dilution: 1:160) | BioLegend | Cat# 108708; RRID: AB_313395 |
| Flow: Rat anti-mouse CD127-PE-Dazzle 594 (Dilution: 1:80) | BioLegend | Cat# 135032; RRID: AB_2564217 |
| Flow: Rat anti-mouse PD-1-PE (Dilution: 1:20) | BioLegend | Cat# 135206; RRID: AB_1877231 |
| Flow: Rat anti-mouse PD-L1-BV421 (Dilution: 1:20) | BioLegend | Cat# 124315; RRID: AB_10897097 |
| Flow: Rat anti-mouse CTLA-4-PE-Dazzle 594 (Dilution: 1:40) | BioLegend | Cat# 106318; RRID: AB_2564496 |
| Flow: Rat anti-mouse CD14-PE (Dilution: 1:20) | BioLegend | Cat# 123310; RRID: AB_940584 |
| Flow: Rat anti-mouse Ly-6G-BV421 (Dilution: 1:20) | BioLegend | Cat# 127627; RRID: AB_10897944 |
| Flow: Rat anti-mouse Ly-6C-BV711 (Dilution: 1:40) | BioLegend | Cat# 128037; RRID: AB_2562630 |
| Flow: Rat anti-mouse MHC II-APC-Cy7 (Dilution: 1:80) | BioLegend | Cat# 107628; RRID: AB_2069377 |
| Flow: Rat anti-mouse CD11b-PE-Cy7 (Dilution: 1:80) | BioLegend | Cat# 101216; RRID: AB_312799 |
| Flow: Rat anti-mouse F4/80-APC (Dilution: 1:80) | BioLegend | Cat# 123116; RRID: AB_893481 |
| Flow: Rat anti-mouse CD11b-PE (Dilution: 1:80) | BD Biosciences | Cat# 553311; RRID: AB_394775 |
| TruStain fcX™ anti-mouse CD16/32 (Dilution: 1:50) | BioLegend | Cat #101320; RRID: AB_1574975 |
| Live/Dead cell stain kit | Invitrogen | Cat# L34961 |
| Chemicals, peptides, and recombinant proteins | ||
| Human Recombinant EGF | Shenandoah | Cat# 100-26 |
| Human Recombinant FGF | Shenandoah | Cat# 100-146 |
| ReNcell® NSC Maintenance Media | Millipore | Cat# SCM005 |
| Accutase | Sigma | Cat# SCR005 |
| Collagenase type IV | Worthington Biochemical Corporation | Cat# LS004209 |
| Soybean trypsin inhibitor | Worthington Biochemical Corporation | Cat# LS003587 |
| Deoxyribonuclease I | Worthington Biochemical Corporation | Cat# LS002007 |
| ACK lysing buffer | Gibco | Cat# A10492-01 |
| Fetal bovine serum | Sigma-Aldrich | Cat# F0926 |
| Dulbecco’s phosphate buffered saline (DPBS) | Lonza | Cat# 17-512F |
| Bovine serum albumin (BSA) | Sigma-Aldrich | Cat# A7030 |
| Fibronectin, human plasma | Sigma-Aldrich | Cat# 341635 |
| BAMBANKER Cell Freezing Medium | Wako | Cat# CS-02-001 |
| Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) | Gibco | Cat# 11330057 |
| Penicillin-Streptomycin (100X) | Gibco | Cat# 15140122 |
| Invitrogen™ Trypan Blue Stain (0.4%) | Fisher Scientific | Cat #T10282 |
| True-Nuclear™ Transcription Factor Buffer Set | BioLegend | Cat #424401 |
| Fixation buffer | BioLegend | Cat #420801 |
| Cyto-Last™ Buffer | BioLegend | Cat #422501 |
| Paraformaldehyde, 32% w/v aq. soln., methanol free | Fisher Scientific | Cat# AA47377-9M |
| Ketamine | Covetrus | Cat# VINB-KET0-7021 |
| Xylazine | Vet One | Cat # Vet-Rx-MW 510650 |
| Ethanol | Fisher Scientific | Cat#011068005 |
| Meloxicam | Fisher Scientific | Cat# AC459550050 |
| Ophthalmic ointment | Fisher Scientific | Cat# NC0490117 |
| Sucrose, Rnase free | Fisher Scientific | Cat# AC419760010 |
| Critical commercial assays | ||
| MyCoAlert PLUS Mycoplasma Detection Kit | Lonza | Cat# LT07-710 |
| Mycoalert assay control 10 tests | Lonza | Cat# lt07-518 |
| Experimental models: Cell lines | ||
| QPP7 GSC | Laboratory of Dr. Jian Hu (MDACC)4 | N/A |
| GL261 | Laboratory of Dr. Gary Kohanbash (UPMC of Pittsburgh) | RRID: CVCL_Y003 |
| Patient-derived GSC lines (TS543, TS576, TS603) | Laboratory of Dr. Cameron W. Brennan (MSKCC) | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: ICR SCID female (4∼6 weeks) | Taconic Biosciences | ICRSC-F |
| Mouse: C57BL/6 female (4∼6 weeks) | The Jackson Laboratory | Strain #:000664 |
| Mouse: C57BL/6 male (4∼6 weeks) | The Jackson Laboratory | Strain #:000664 |
| Software and algorithms | ||
| FlowJo_v10.7.1 | FlowJo | https://www.flowjo.com; RRID: SCR_008520 |
| GraphPad Prism | GraphPad Software | https://www.graphpad.com; RRID: SCR_002798 |
| ITK-SNAP (v.3.8.0) | Yushkevich et al. | http://www.itksnap.org; RRID: SCR_002010 |
| Other | ||
| Cell culture treated flasks | Fisher Scientific | Cat# 10-126-28 |
| 6-well plates | Fisher Scientific | Cat# 07-200-83 |
| Small animal ear tags | National Band & Tag Co. | Cat# 1005-1 |
| Applicator ear tag | National Band & Tag Co. | Cat# 1005s1 |
| Cincinnati Surgical Lance Sterile Single Use Carbon Blades, Size #21 | Fisher Scientific | Cat# 19-200-273 |
| High Precision #4 Style Scalpel Handle | Fisher Scientific | Cat# 12-000-164 |
| Sterile alcohol prep pads | Fisher Scientific | Cat# 22-363-750 |
| Non-sterile wound clips | Fisher Scientific | Cat# NC9247848 |
| Betadine solution | Fisher Scientific | Cat# NC0158124 |
| Surgical suture | Fisher Scientific | Cat# NC9066865 |
| Invitrogen™ Countess™ Cell Counting Chamber Slides | Fisher Scientific | Cat# C10228 |
| Basix™ polypropylene 15 mL centrifuge conical tubes | Fisher Scientific | Cat# 14-955-237 |
| 70 μm sterile cell strainers | Fisher Scientific | Cat# 50-105-0135 |
| 96-well round bottom polypropylene plate | USA Scientific | Cat# 1830-9600 |
| MTC Bio 25 mL Reservoir | Fisher Scientific | Cat# 50-112-7244 |
| Seal-Rite 1.5 mL graduated microcentrifuge tube, natural, polypropylene | USA Scientific | Cat# 1615-5500 |
| Fisherbrand™ Basix™ Polystyrene Serological Pipets, 5 mL | Fisher Scientific | Cat# 14-955-233 |
| Fisherbrand™ Basix™ Polystyrene Serological Pipets, 10 mL | Fisher Scientific | Cat# 14-955-234 |
| 1 mL syringe | Fisher Scientific | Cat# 14-823-434 |
| Disposable hypodermic needles 26G ×1/2 IN | Fisher Scientific | Cat# 14-840-83 |
| Hamilton™ Gastight Microliter™ Syringes: RN Termination, No Needle Included | Fisher Scientific | Cat# 14-824-662 |
| 26-gauge, Small Hub RN Needle; custom length, point style & angle; 6/package | Hamilton Robotics | Cat# 7804-03 |
| Guide Screw C212SDN | P1-Tech | Cat# 81C212SDNXXC |
| Round-bottom polystyrene test tubes with cell strainer snap cap | Fisher Scientific | Cat# 08-771-23 |
| Aluminum foil | Fisher Scientific | Cat# 01-213-105 |
| Plastic wrap | Fisher Scientific | Cat# 22305654 |
Materials and equipment
NSC (Neural stem cell) cell culture medium
To prepare the NSC cell culture medium, add 5 mL of 100× Penicillin-Streptomycin, EGF, and FGF (with a final concentration of 20 ng/mL and 10 ng/mL, respectively) in 500 mL of ReNcell® NSC Maintenance Media, sterilize with 0.22 μm filter and store at 4°C for up to 3 months.
Anesthesia mix (KX cocktail for mice)
| Reagent | Final concentration | Amount |
|---|---|---|
| Ketamine (100 mg/mL) | 17.5 mg/mL | 1.75 mL |
| Xylazine (100 mg/mL) | 2.5 mg/mL | 0.25 mL |
| ddH2O | N/A | 8 mL |
| Total | N/A | 10 mL |
Store the KX cocktail at 4°C, and avoid light for up to 3 months.
Note: Given that Ketamine is regulated under the controlled substances act by the Drug Enforcement Administration (DEA), a DEA license is required for purchasing and using this drug before conducting animal experiments with survival surgery.
Pain killer (meloxicam solution)
To make the stock solution, dissolve 5 mg of meloxicam powder in 1 mL DMSO. Store the stock solution at 25°C, and avoid light. To make the injectable Meloxicam solution, dilute 10× of the stock solution with sterile DPBS (pH 7.4). Store the Meloxicam solution at 25°C in the dark for up to a month.
Note: Dilute the Meloxicam stock with DPBS (pH 7.4) rather than ddH2O since the latter results in precipitates. It is recommended to make the fresh Meloxicam injectable solution each time.
Alternatives: Meloxicam SR (sustained-release meloxicam), as a better alternative, can be used instead of the Meloxicam solution made in the lab.
Collagenase IV cocktail
| Reagent | Final concentration | Amount |
|---|---|---|
| Collagenase type IV (32 mg/mL) | 3.2 mg/mL | 1 mL |
| Deoxyribonuclease (10 mg/mL) | 1 mg/mL | 1 mL |
| Soybean trypsin inhibitor | 2 mg/mL | 20 mg |
| DPBS | N/A | 8 mL |
| Total | N/A | 10 mL |
Store the Collagenase IV cocktail at 4°C for up to 3 months.
FACS buffer
Prepare the FACS Buffer by dissolving 1 g of Bovine Serum Albumin (BSA) in 100 mL of DPBS. Sterilize the solution with a 0.22 μm filter. Store the FACS Buffer at 4°C for up to 2 months.
Note: Do not add sodium azide to the FACS Buffer. 2 mM of EDTA could be included in the DPBS to prevent cells from aggregating.
Step-by-step method details
Install an implantable guide screw into the mouse skull
Timing: 20 min/mouse
This step describes how to anesthetize mice and install an implantable guide screw into the mouse skull.
Note: For more details about an implantable guide screw system, please refer to the reference by Lal et al.5
-
1.
Prepare a heating pad to place mice after anesthesia.
-
2.
Weigh the mice and anesthetize them via intraperitoneal (i.p.) injection with the dose of 100 μL/20 g bodyweight of anesthesia mix.
Note: The dose applied would lead to anesthetizing mice for 40∼60 min. C57BL/6 mice of age 4∼6 weeks are usually around 20 g of body weight, and it is OK to apply 100 μL of anesthesia mix when the mouse body weight is 20 ± 1 g.
-
3.
Place the anesthetized mice on the heating pad, and apply ophthalmic ointment to both eyes of the mice.
Note: Ophthalmic ointment should be applied to mice for anesthesia longer than 5 min. Missing this step may result in mice being blind since the mouse's eyes remain open under anesthesia.
-
4.Locate the point of the skull to install a guide screw.
-
a.Take an anesthetized mouse to put on an operating table, and let the operational light focus on the mouse head.
-
b.Sterilize the mouse head with an alcohol swab and betadine.
-
c.Use a Lance blade to make an incision in the center of the mouse skull vertically between the ears and reveal the midlines.
-
d.Find the bregma of the skull (Figure 2).Note: It may have blood when making the incision, making it hard to find the bregma. Use a cotton swab to remove the blood and let the skull dry for a while, making the bregma easier to find.
-
e.Use a ruler to locate 2.5 mm to the right and 1.5 mm up, and make a point marker using a fine-tip Sharpie pen.
-
a.
-
5.Install the guide screw into the skull.
-
a.Drill a hole on the point marker with a small hand-controlled drill that is 1 mm in diameter.
-
b.Place a guide screw in the hole and rotate it until the guide screw is tight in the skull.
-
a.
CRITICAL: It is crucial to make a proper size of the hole in the skull. A large hole leads to loosening an installed guide screw in the mouse skull. A 21-gauge needle or forceps with a similar diameter can be used to hold the guide screw and to gently squeeze into the hole (Methods video S1).
Figure 2.

The position of the guide screw on the skull
The guide screw locates 2.5 mm on the right and 1.5 mm up from the bregma on the skull.
-
6.
Seal the incision by using a surgical suture.
Alternatives: A clip can seal the incision with an autoclip (stapler); however, the clip needs to be removed by a stapler remover one week later.
-
7.
Conduct intraperitoneal injection (IP) injection of pain killer Meloxicam solution with 5 mg/kg dose (i.e., inject 200 μL of 0.5 mg/mL meloxicam solution into a mouse with a body weight of 20 g).
-
8.Place the mouse back on the heating pad until it wakes up.
-
a.Check the breathing of the mouse.
-
b.Reflex mouse routinely.
-
a.
The movie illustrates the procedure of preparation and manipulation of how to install a guide screw into the mouse skull and conduct intracranial implantation of cells into the mouse cortex using the lab-customized stereotaxic frame with the syringe pump for 10 mice at one time.
Intracranial implantation of QPP7 cells into mice
Timing: ∼1–2 h/10 mice
This step describes how to perform intracranial implantation of tumor cell lines (e.g., QPP7 cells) into mice.
-
9.
Anesthetize the mice as described in steps #1-#3.
-
10.Draw xenograft cells into Hamilton syringes.
-
a.Wash each syringe 10 times in each solution in the following order: 100% ethanol, 70% ethanol, and sterile DPBS.
 CRITICAL: It is important not to introduce bubbles into the syringe when drawing the cells. Gently and slowly wash the syringe with 100% ethanol, which will eliminate bubbles in the syringe.
CRITICAL: It is important not to introduce bubbles into the syringe when drawing the cells. Gently and slowly wash the syringe with 100% ethanol, which will eliminate bubbles in the syringe. -
b.Take the cells from the ice and gently flick the tube to mix the cells.
-
c.Prepare 10 syringes by gently withdrawing about 7 μL of cells in solution into each one.
-
d.Put the syringes on the injector frame and make the plunger underneath the driver of the infusion pump.
-
e.Control the pump to push the driver, ensure each syringe has a little liquid coming out, and all the plungers touch the driver well.
-
a.
-
11.Intracranial implantation of tumor cells into 10 mice using the screw-guide stereotactic frame (Figure 3 and Methods video S1).
-
a.Take an anesthetized mouse to put on an operating table, and let the operating light focus on the mouse head.
-
b.Sterilize the mouse head with an alcohol swab and betadine.
-
c.Use a Lance blade to make a small incision to expose the guide screw.
-
i.Move the mouse under the rack/clamp on the stereotactic frame.
-
ii.Make the needle of the Hamilton syringe into the guide screw hole.
-
iii.Make the plastic sleeve on the needle contact the skull tightly using forceps.Alternatives: Experienced researchers can avoid creating an additional incision by following the steps.
-
iv.Use a Hamilton syringe needle with a plastic sleeve (or cuff) to find the guide screw under the head skin and pierce it.
-
v.Put the needle of a syringe with pre-loaded tumor cells into the guide screw.
-
vi.Carefully move the mouse together with the syringe and assembly into the stereotactic frame.
-
i.
-
d.Put a small piece of cotton under the mouse head to stabilize the mouse syringe set-up if needed.Note: Do not put too much cotton under the mouse's head since it may block the mouse's breathing. Ensure the syringe plunger touches the injector pump driver well.
-
e.Repeat steps a-d until all the mice are set up in the stereotactic frame.Note: The number of mice for intracranial implantation depends upon the channel number of the injector. However, it is recommended not to perform more than 12 mice each time.
-
f.Intracranial injection of 5 μL tumor cells by setting the pump with a speed of 0.5 μL/min.
-
g.After the intracranial injection is finished, leave the needle in the guide screw for 10 min.
 CRITICAL: It is important to leave the needle in the mouse brain for a while to let the cells stabilize in the brain and prevent them from coming out. Gently press the mouse head to make the needle move out a little every three min.
CRITICAL: It is important to leave the needle in the mouse brain for a while to let the cells stabilize in the brain and prevent them from coming out. Gently press the mouse head to make the needle move out a little every three min. -
h.Wash each syringe 10 times with sterile DPBS. If no additional intracranial injection is performed, wash 10 times in each solution in the following order: 70% ethanol and 100% ethanol.Note: Immediately wash the syringe after each intracranial injection to prevent blocking in the needle.
-
a.
-
12.
Apply pain killer (Meloxicam solution) to mice and wait for them to wake up, as described in steps #7 - #8.
Figure 3.
Intracranial implantation using the lab-customized screw-guide stereotaxic surgery platform
Set-up of the intracranial implantation of 10 mice via the screw-guide stereotaxic frame with a syringe pump. The tumor cell line QPP7 of 1×105 cells in 5 μL DPBS were intracranially injected into each anesthetized C57BL/6 mouse.
Intracranial delivery of the peptide for the treatment
Timing: ∼1–2 h/10 mice
This step describes how to treat mouse glioblastoma by directly delivering the peptide to brain tumors using an implantable guide screw system.
-
13.
Prepare anesthetized mice and syringes with 20 μM peptides dissolved in sterilized DPBS as described in steps #9-#10.
-
14.
Intracranial injection of 5 μL peptides as described in step #11.
-
15.
Apply pain killer (Meloxicam solution) to mice and wait for them to wake up, as described in steps #7 - #8.
Examine tumor size with magnetic resonance imaging (MRI)
Timing: 20 min/mouse
This step describes how to examine tumor growth and the size of xenograft tumors by MRI.
-
16.Anaesthetize mice by inhalation with isoflurane.
-
a.Put a mouse in a clear plexiglass anesthesia induction box that allowed unimpeded visual monitoring of the animal.
-
b.Set 3% isoflurane mixed with oxygen in the induction box for a few min until the mouse achieves the anesthesia plane.
-
a.
Note: The depth of anesthesia of the mouse can be checked by toe reflex and respiration rate.
-
17.Acquire an MRI imaging of each mouse.
-
a.Transfer the mouse to the animal bed for imaging. Monitor the mouse’s respiration by using a pneumatic sensor between the animal bed and the mouse’s abdomen, and maintain the animal’s temperature at 37°C.
-
b.Put a nose cone with 1∼2% isoflurane in oxygen to maintain anesthesia.
-
c.Scan the mouse with an MRI spectrometer with proper parameters.
-
a.
-
18.
Place the mouse back in the animal cage and monitor the mouse until it wakes up.
Note: The time to check the tumor by MRI after intracranial implantation varies in different glioblastoma lines. For QPP7 cells, it usually takes about one month to form a tumor in the mouse brain; hence, it is recommended to start MRI in the third week after the intracranial implantation.
-
19.Determine the tumor size by MRI images.
-
a.Open the MRI image in ITK-SNAP (v.3.8.0).
-
b.Go through each layer and find the largest tumor layer. In the software (e.g., Mac OS), go to Tools > Image Contrast > Contrast Adjustment. In the pop-up window, drag the circle in the “index into the color map” to make the tumor has good contrast with its around the tissue in the image.Note: We usually get 11∼13 scanning image layers, and 5∼8 layers often have the biggest tumor intersection.
-
c.Go back to the main window of ITK-SNAP, choose the brush in the Main Toolbar on the left, and choose the proper size in the Brush Size drop-box.Note: Beginning with a bush size of 5 is a good start.
-
d.Use the brush to paint and cover the tumor in each layer that has the tumor.Note: Try to cover the tumor part with brush painting accurately. Do not over-painting or leave some part of the tumor unpainted since the software will determine the tumor size based on the painting of each layer.
-
e.Once finishing painting the tumor in each layer, go to Segmentation > Volumes and Statistics. The software will calculate the tumor size.
-
a.
Sample collection after peptide treatment
Timing: ∼15–20 min/mouse
This step describes how to collect mouse serum, spleen, and brain.
Note: When the mouse appears with phenomena of brain tumor (e.g., hunchback, irritability, trembling, and apastia), cease the animal for collecting samples.
-
20.Cease the mouse by CO2.
-
a.Place the mouse with the cage or directly into a clear plexiglass box that connects to a CO2 tank or pipeline.
-
b.Turn on the valve of CO2 to cease the mouse.
-
c.Once the mouse stops breathing, remove it from the plexiglass box.
-
d.Stabilize the mouse as its abdomen upwards by pinning its four claws on a polyform.
-
a.
-
21.Collect blood from mouse heart (Cardiac blood collection) (Figure 4).
-
a.Spray the skin with 70% ethanol to disinfect, and use a scissor to dissect the skin along the ventral midline.
-
b.Extend the dissection and gently open the skin without entering the peritoneal cavity.
-
c.Use the needle with a 1 mL syringe to puncture the thoracic cavity a little right of the ventral midline.
-
d.Withdraw the syringe plunger a little and slightly move the needle until the mouse blood enters the syringe.
-
e.Hold and stabilize the syringe, and withdraw the plunger until no more blood can be picked up.
-
f.Push the plunger to let the blood into an EDTA-coated 1.5-mL centrifuge tube.Note: The mouse must be anesthetized or euthanized immediately prior to blood collection. The blood can be set in the 1.5-mL centrifuge tube at 25°C for about 30 min until all other samples are collected. Do not disturb the tube.
-
g.Centrifuge the tube at 2000× g for 20 min at 4°C.
-
h.Transfer the upper layer of the serum to a clean 1.5-mL centrifuge tube and store at −80°C.
-
a.
-
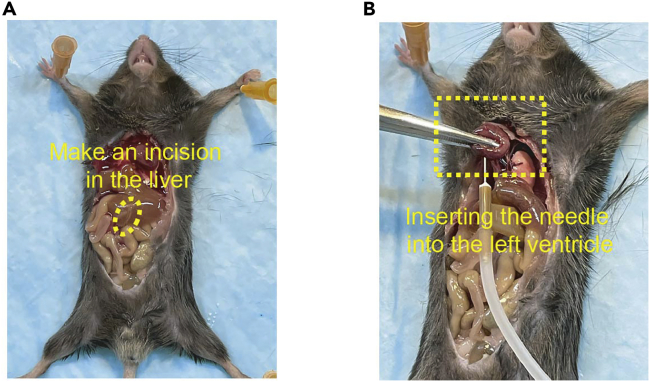
22.Perfuse the mouse (Figure 5).
-
a.Use a scissor to open the peritoneal and thoracic cavities.
-
b.Find the liver in the peritoneal cavity, and use a scissor to make a cut in the liver.
-
c.Find the heart in the thoracic cavity and gently hold it in place with forceps.
-
d.Take a DPBS-filled 30 mL syringe with an infusion needle attached.Alternatives: If the samples will be only used for immunohistochemistry, it is recommended to use 4% of PFA instead of DPBS to perform perfusion.
-
e.Insert the needle into the left side of the heart.
-
f.Push the syringe plunger to perfuse the heart with 20 mL of DPBS.Note: If perfusion is successful, the DPBS will come out from the incision in the liver, and it will gradually turn light brown. If the liver remains dark red, try perfusing again with additional DPBS.
-
a.
-
23.Collect spleen.
-
a.Reflect the liver craniodorsally and find the spleen on the right side of the abdomen, inferior to the stomach.
-
b.Remove the spleen with forceps and scissors.
-
c.Place the spleen in DPBS in one well of a 6-well plate at 25°C.
-
a.
Alternatives: If the sample is perfused with 4% PFA, place the sample in a 15-mL conical tube with 4% PFA for about 12 h at 25°C, then switch to 20% sucrose for another day at 4°C before embedding into paraffin or OCT block.
-
24.Collect brain.
-
a.Decapitate the mouse using scissors.
-
b.Gently pull and remove the mouse head skin and fur with scissors.
-
c.Keep the scissors toward the outside of the skull and carefully cut up the brainstem along the midline of the skull.Note: Try not to cut the brain when using scissors to cut the skull.
-
d.Blunt dissection of the skull by using hemostatic forceps.
-
e.Gently insert the hemostatic forceps underneath the brain and remove the brain from the skull. Compared to the normal mouse brain, the brain with tumors shows hemorrhage on the cortical surface and swelling (Figure 6).
-
f.Place the brain in DPBS in one well of a 6-well plate at 25°C.Alternatives: If the sample is perfused with 4% PFA, place the sample in a 15-mL conical tube with 4% PFA for about 12 h at 25°C, then switch to 20% sucrose for another day at 4°C before embedding into paraffin or OCT block.
-
a.
Figure 4.

Cardiac blood collection
The mouse was euthanized with CO2. The blood was collected immediately using a 1-mL syringe with a 26G ×1/2 inches needle.
Figure 5.
Perfusion of the mouse
(A) Creation of a perfusion solution outlet by cutting the mouse liver.
(B) Conduction of the perfusion by a hypodermic needle filled with DPBS.
Figure 6.
Comparison of normal mouse brain vs. brain with tumors
(A) A normal mouse brain.
(B) A mouse brain with tumors. The tumors grew in the cerebral cortex of the brain and emerged in the olfactory bulbs; the brain shows hemorrhage on the cortical surface and swelling.
Mouse brain and spleen processing and staining for flow cytometry analyses
Timing: ∼8–10 h
This step describes how to isolate the mouse brain and spleen and then analyze the cell populations by flow cytometry.
-
25.Process brain.
-
a.Place the brain on the lid of the 6-well plate.
-
b.Use a carbon blade and scissors to isolate the tumor region of the brain.
-
c.Transfer the tumor back to the 6-well plate well containing DPBS, and use scissors to cut the tumor into 1 mm slices.Note: Do not mash up or mince the tumor since this will reduce cell viability.
-
d.Use a P1000 pipettor with a slightly cut tip to transfer the tumor tissue into a 15-mL conical tube. Rinse the well with DPBS and transfer all residues of tumor tissue to the same 15-mL conical tube.
-
e.Centrifuge the tube at 400 × g for 5 min at 25°C, and discard the supernatant.
-
f.Add 2 mL of Collagenase IV cocktail to the tumor tissue, and use a P1000 pipettor with a slightly cut tip to pipette the tumor tissue slurry up and down 15∼30 times.Note: Slowly pipette up and down the tumor slurry to ensure no tissue gets stuck in the pipette tip and then suck it up into the barrel.
-
g.Incubate the tumor tissues in a Biometra OV5 Duo-Therm Hybridization Oven Incubator by rotating the conical tube bottom-to-top for 15 min at 37°C.Note: One can start to process the spleen by following step #24 while the brain tumor sample is incubating.
-
h.Check for tumor dissociation by gently pipetting up and down 30 times with a P1000 with a slightly cut tip and then 20∼30 times with a P1000 uncut tip.Note: Usually, 15 min of incubation with Collagenase IV cocktail followed by pipetting up and down is enough for the tumor tissues to be dissociated into single cells. If tissue dissociation remains incomplete, repeat step g one or two times with additional 15-min incubation until the tumor fragments can fit through an uncut P1000 tip.
-
i.Add 8 mL of ice-cold DPBS into the conical tube to make the total volume 10 mL, and gently flip the tube to mix the tumor cells.
-
j.Filter the suspension through a 70-μm cell strainer into a new 15-mL conical tube, and rinse the strainer twice with 1 mL of ice-cold DPBS each time.
-
k.Centrifuge the cell suspension at 400 × g for 5 min at 4°C, and discard the supernatant.
-
l.Lyse the red blood cells by resuspending the cell pellet with 2 mL of ACK Lysing Buffer.Note: Place the conical tube at 25°C for 1∼2 min, and swirl the tube several times every 30 s.
-
m.Add 8 mL of ice-cold DPBS into the conical tube and gently flip the tube to mix the tumor cells to quench the lysis.
-
n.Aliquot 10 μL of the suspension into a 1.5-mL centrifuge tube for cell counting.Note: It is recommended to use auto-counting equipment to count cells at this step if multiple samples are processed.
-
o.Centrifuge the cell suspension at 400 × g for 5 min at 4°C, discard the supernatant and remove any liquid residue.
 Pause Point: The cells can be placed into Bambanker Cell Freezing Medium and stored in liquid nitrogen for up to 2 months before staining.
Pause Point: The cells can be placed into Bambanker Cell Freezing Medium and stored in liquid nitrogen for up to 2 months before staining. -
p.Resuspend tumor cells in the appropriate volume of ice-cold FACS Buffer and plate 2∼3 × 106 tumor cells in each well of a 96-well round bottom polypropylene plate on ice. Bring the final volume in each well to 100 μL with ice-cold FACS buffer.
-
a.
-
26.Process spleen.
-
a.Place a 40-μm cell strainer in the 6-well plate well containing DPBS at 25°C, and transfer the spleen to the strainer.
-
b.Use the end of a 1-mL syringe plunger to macerate the spleen. Once the majority of the spleen tissue has filtered through the cell strainer, rinse the end of the syringe plunger and strainer with DPBS. Discard the strainer.
-
c.Transfer the dissociated spleen cells to a 15-mL conical tube. Rinse the 6-well plate well with DPBS and transfer it to the same conical tube. Add additional DPBS to make the volume up to 10 mL in total.
-
d.Centrifuge the spleen cells at 400 × g for 5 min at 4°C. Discard the supernatant and remove any liquid residue.
-
e.Lyse the red blood cells by following step #25 l-m.
-
f.Centrifuge the cell suspension at 400 × g for 5 min at 4°C, and discard the supernatant.Note: Spleen contains more red cells than the brain, and it may need to repeat this step one more time to get rid of all red cells.
-
g.Aliquot 10 μL of the suspension into a 1.5-mL centrifuge tube for cell counting.Note: It is recommended to use auto-counting equipment to count cells at this step if multiple samples are processed.
-
h.Centrifuge the cell suspension at 400 × g for 5 min at 4°C, discard the supernatant and remove any liquid residue.
 Pause Point: The cells can be placed into Bambanker Cell Freezing Medium and stored in liquid nitrogen for up to 2 months before staining.
Pause Point: The cells can be placed into Bambanker Cell Freezing Medium and stored in liquid nitrogen for up to 2 months before staining. -
i.Resuspend tumor cells in the appropriate volume of ice-cold FACS Buffer and plate 1 × 105 tumor cells in each well of a 96-well round bottom polypropylene plate on ice. Bring the final volume in each well to 100 μL with ice-cold FACS buffer.
-
a.
-
27.Incubate cells with Fc block.
-
a.Incubate cells with TruStain fcXTM at 1.0 μg per 2∼3 × 106 brain tumor cells (i.e., 2 μL of 0.5 μg/μL TruStain fcXTM stock per well) and 1.0 μg per 1 × 105 spleen cells in 100 μL for 10 min on ice.
-
b.Add the correct volume of antibody panel cocktail to its corresponding well on the plate. Mix thoroughly by gently pipetting up and down 3 times.
-
a.
Note: Do not introduce bubbles when mixing the cells after adding the antibody cocktail.
-
28.Extracellular staining with antibody cocktail.
-
a.Add the correct volume of antibody panel cocktail to its corresponding well on the plate. Mix thoroughly by gently pipetting up and down 3 times.
-
b.Incubate the plate at 4°C for 30 min, and avoid light by covering the plate with aluminum foil.
-
c.Centrifuge the plate at 400 × g for 5 min at 4°C, and discard the supernatants.
-
d.Wash the cell pellets twice with 200 μL of ice-cold FACS Buffer per well.
-
a.
Note: If only extracellular staining is performed, directly go to step #28.
-
29.Intracellular staining with antibody cocktail.
-
a.Prepare fresh 1× True Nuclear Fix Concentrate by diluting True Nuclear 4× Fix Concentrate with True Nuclear Fix Diluent.
-
b.Add 200 μL of True Nuclear 1× Fix Concentrate to each well. Mix thoroughly by gently pipetting up and down 3∼5 times with a multi-channel P200 pipettor.
-
c.Incubate at 25°C for 1 h and avoid light.
-
d.Centrifuge the plate at 400 × g for 5 min at 25°C, and discard the supernatants.
 Pause Point: Cells resuspended with 200 μL/well of Cyto-Last Buffer can be stored at 4°C for 12∼18 h protected from light. Use plastic wrap to cover the plate to avoid buffer evaporation.
Pause Point: Cells resuspended with 200 μL/well of Cyto-Last Buffer can be stored at 4°C for 12∼18 h protected from light. Use plastic wrap to cover the plate to avoid buffer evaporation. -
e.Prepare fresh True Nuclear 1× Perm Buffer by diluting True Nuclear 10× Perm Buffer with distilled water.
-
f.Resuspend the fixed cell pellets in 200 μL of True Nuclear 1× Perm Buffer.
-
g.Centrifuge the plate at 400 × g for 5 min at 25°C, and discard the supernatants.
-
h.Repeat steps f-g one more time.
-
i.Resuspend the cell pellets in 100 μL of True Nuclear 1× Perm Buffer.
-
j.Add the correct volume of antibody panel cocktail to its corresponding well on the plate. Mix thoroughly by gently pipetting up and down 3 times.Note: Do not introduce bubbles when mixing the cells after adding the antibody cocktail.
-
k.Incubate the plate at 25°C for 30 min, and avoid light by covering the plate with aluminum foil.
-
l.Centrifuge the plate at 400 × g for 5 min at 25°C, and discard the supernatants.
-
m.Add 200 μL of True Nuclear 1× Perm Buffer to each well and mix gently and thoroughly.
-
n.Centrifuge the plate at 400 × g for 5 min at 25°C, and discard the supernatants.
-
o.Repeat steps m-n one more time.
-
a.
-
30.Fix the cells (optional).
 Pause Point: This step is for extracellular staining only. This step is unnecessary if the cytometry analysis is performed on the same day.
Pause Point: This step is for extracellular staining only. This step is unnecessary if the cytometry analysis is performed on the same day.-
a.Resuspend the cell pellets with 200 μL of Fixation Buffer.
-
b.Incubate the plate for 20 min at 25°C and avoid light.
-
c.Centrifuge the plate at 400 × g for 5 min at 4°C, and discard the supernatants.
-
a.
-
31.
Resuspend the cell pellets in 200 μL of Cyto-Last Buffer.
Pause Point: Fixed cells can be stored at 4°C and avoid light for up to 3 days before going to cytometry analysis.
-
32.
Transfer cells to flow cytometry tubes and store samples at 4°C with plastic wrap to protect them from buffer evaporation in the dark until ready to analyze by flow cytometry.
Expected outcomes
Depending upon the cell line and the number of cells used, tumors are usually established in days and weeks. For using 1×105 cells, the QPP7 cell line usually forms a tumor in one month, while the GL261 cell line takes two to three weeks to develop tumors. When the peptides are administered via intracranial injection or the implanted cells are genetically modified, times to establish xenografted tumors in mouse brains need to be adjusted by researchers on a case-by-case basis. To date, we have not been notified of any obvious effects on mouse behaviors using this protocol (Methods video S2). Tumor growth was monitored by MRI, and mouse survival was obtained according to mouse lapse time. By following the step of processing samples, each tumor mass was processed to isolate 3∼15 million cells, of which the majority of the cells were tumor cells and a small fraction of immune cells. The obtained cells were enough for flow cytometry analyses. The representative examples showed that the treatment of the immunotherapeutic peptide (GMP) resulted in decreasing tumor size in the measurement using the MRI scan (Figure 7A) and increasing mouse survival (Figure 7B). Flow cytometry analysis revealed that GMP treatment increased M1-like tumor-associated macrophages (TAMs) but decreased M2-like TAMs (Figure 7C), and significantly enhanced CD8+ cell infiltration and their exhaustion in the glioma (Figure 7D).
Figure 7.
Evaluation of the immunotherapeutic peptide treatment in the syngeneic glioma mouse models
(A) Representative MRI images from mice bearing glioma derived from murine glioma cell line GL261 overexpressing CHI3L1 (GL261-CHI3L1) cells after the treatment of the control peptide (SCP) and the immunotherapeutic peptide (GMP), respectively.
(B) Kaplan-Meier tumor-free survival analysis of mice bearing GL261-CHI3L1 tumors treated with indicated peptides.
(C) The percentage of M1/M2-like TAMs in the tumors derived from the mice with intracranial implantation of GL261-CHI3L1 cells after SCP and GMP treatment.
(D) Flow cytometry analysis showed the frequency of CD8+ T cells with expression of PD-1 and CTLA-4 in tumors derived from the mice with intracranial implantation of GL261-CHI3L1 cells after the treatment with GMP versus SCP. Each dot represents 1 mouse; data are presented as the mean ± SEM. P values were calculated using a 1-tailed, unpaired t test. Adapted with modifications and permission from.1
Left: a mouse with an installed guide screw; right: a mouse without a guide screw in the brain skull.
Limitations
We modified and optimized the procedure of the stereotactic surgery using an implantable screw-guide system (maximum of 12 mice under intracranial implantation simultaneously) in this protocol, which can minimize the variation of tumor formation compared with the traditional stereotactic frame (one or two mice each time). However, the accuracy of the cell number injected is critical for the consistency of tumor latency, especially for those cell lines that are aggressive and fast-growing. Given the small volume with a larger number of cells (usually 1×105 cells in 2∼5 μL), a notable variation in tumor engraftment and formation could be observed, depending upon the accuracy of cell number counting.
Peptide treatment of mice via intracranial delivery can be used to evaluate the effect of the peptides for glioblastoma therapy. However, this method does not address the pharmacokinetic properties of the given peptides, nor their ability to pass through the blood-brain barrier. It also does not address the possible systemic toxicity or side-effect of the peptides.
Additionally, this protocol is time-consuming, and the number of brain tumor and spleen samples processed for flow cytometry analysis at once is dependent upon the researchers’ experience and experimental skills. When the sample number increases, it also increases the processing time and may reduce the viability of the cells, resulting in variation in the result. The researchers may conduct experiments separately if there are too many samples to deal with; however, it introduces batch-to-batch or run-to-run variations. Hence, the researchers need to consider the trade-off and decide which way is better to achieve experimental goals.
Troubleshooting
Problem 1
Needle clogging when performing an intracranial injection (step 11).
Potential solution
Before the first intracranial injection, rinse the needle and syringe 10 times in each solution in the order of 100% ethanol, 70% ethanol, and sterile DPBS. If the plunger still does not move smoothly when pushing the liquid in the syringe out, wash the syringe and needle more times with the mentioned solution at a faster speed. If the issue still exists, soak the needle and syringe in methanol and sonicate for 2 h, and then rinse the syringe as above.
If the clogging happens during the intracranial injection, it is recommended to exclude the mouse from the analysis. It is advised to rinse the needle with DPBS immediately after each injection. Wash the needle and syringe thoroughly with DPBS and ethanol immediately after the last injection.
Problem 2
Mouse waking up during installing a guide screw or intracranial injection (step 4 & step 11).
Potential solution
Make sure the mouse is deeply anesthetized before any manipulation by checking the toe reflex and respiration rate. If the mouse fails to reach the desired plane of anesthesia, IP injects an additional KX cocktail (We usually inject an additional 50 μL of KX cocktail for each mouse).
Problem 3
The tumor grows extracranially (step 11).
Potential solution
When using a guide screw for intracranial injection, extracranial tumor growth occurs rarely. However, it happens when cell suspension is transferred to the outside of the mouse skull, which is majorly caused by the injected cell suspension being pushed back and out through the guide screw hole. Ensure to keep the needle in the mouse brain for 10 min after injection, and slowly move the needle out of the hole gradually every 2∼3 min.
Problem 4
Inconsistent tumor formation in mice brain (step 11).
Potential solution
The accuracy of the implanted cell number is critical to forming consistent tumors among mice. Ensure to count cells accurately and try to use the same batch of counted cells for the same experiment. Moreover, inject the cells slowly and keep the rate at 0.5 μL/min or less. Another reason for inconsistent tumor formation is extracranial tumor growth that is caused by the injected cell suspension leaking out through the hole of the guide screw. Follow the solution described in problem 3.
Problem 5
Low viability of isolated cells (mouse brain and spleen processing and staining for flow cytometry analyses).
Potential solution
The processing time of samples is essential for cell viability. Immediately start to process the samples once the mouse brain and spleen are isolated. Over-cut or digestion of brain tumors also reduces cell viability. Reducing the time of digestion by Collagenase IV cocktail may be helpful to keep high cell viability. Also, the process temperature is vital, and avoiding a big deviation of temperature is required in each step.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Baoli Hu (baolihu@pitt.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was conducted in the Hu lab that is supported by the NIH/NCI 1R01CA259124, NIH/NINDS 1R21NS125218, and the Scientific Program Fund from the Children’s Hospital of Pittsburgh. A.C. is currently supported by the Central Public-interest Scientific Institution Basal Research Fund (grant no.110231160042015), the National Natural Science Foundation of China (grant no. 32273019), and the Natural Science Foundation of Gansu Province (grant no.22JR5RA028). We are grateful to past and present members of the Hu lab and the collaborators for their contributions to this work. The graphical abstract and diagrams were created using BioRender.com.
Author contributions
A.C. conducted the experiments; A.C. and B.H. analyzed the data; Y.C., H.Z., L.H.M., and G.K. helped with the protocol optimization; A.C. and B.H. wrote the paper; and B.H. supervised the project.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102049.
Contributor Information
Apeng Chen, Email: chenapeng@caas.cn.
Baoli Hu, Email: baolihu@pitt.edu.
Data and code availability
This protocol does not generate or analyze any datasets or codes.
References
- 1.Chen A., Jiang Y., Li Z., Wu L., Santiago U., Zou H., Cai C., Sharma V., Guan Y., McCarl L.H., et al. Chitinase-3-like-1 protein complexes modulate macrophage-mediated immune suppression in glioblastoma. J. Clin. Invest. 2021;131:e147552. doi: 10.1172/JCI147552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B., Wang Q., Wang Y.A., Hua S., Sauvé C.E.G., Ong D., Lan Z.D., Chang Q., Ho Y.W., Monasterio M.M., et al. Epigenetic activation of WNT5A drives glioblastoma stem cell differentiation and invasive growth. Cell. 2016;167:1281–1295.e18. doi: 10.1016/j.cell.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong D.S.T., Hu B., Ho Y.W., Sauvé C.E.G., Bristow C.A., Wang Q., Multani A.S., Chen P., Nezi L., Jiang S., et al. PAF promotes stemness and radioresistance of glioma stem cells. Proc. Natl. Acad. Sci. USA. 2017;114:E9086–E9095. doi: 10.1073/pnas.1708122114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shingu T., Ho A.L., Yuan L., Zhou X., Dai C., Zheng S., Wang Q., Zhong Y., Chang Q., Horner J.W., et al. Qki deficiency maintains stemness of glioma stem cells in suboptimal environment by downregulating endolysosomal degradation. Nat. Genet. 2017;49:75–86. doi: 10.1038/ng.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lal S., Lacroix M., Tofilon P., Fuller G.N., Sawaya R., Lang F.F. An implantable guide-screw system for brain tumor studies in small animals. J. Neurosurg. 2000;92:326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The movie illustrates the procedure of preparation and manipulation of how to install a guide screw into the mouse skull and conduct intracranial implantation of cells into the mouse cortex using the lab-customized stereotaxic frame with the syringe pump for 10 mice at one time.
Left: a mouse with an installed guide screw; right: a mouse without a guide screw in the brain skull.
Data Availability Statement
This protocol does not generate or analyze any datasets or codes.