Abstract
Background:
The human milk–fed preterm infant is at risk for growth failure, micronutrient deficiencies, and neurocognitive delay. Although protective and better tolerated than formula, human milk alone cannot meet the high nutrient requirements of this population, and fortification is necessary. Clinicians use assumptions of preterm human-milk composition to determine the type and quantity of fortification.
Objectives:
The objectives of this review were to identify evidence of macronutrient and micronutrient concentration in preterm human milk and to identify knowledge gaps regarding composition.
Methods:
PubMed and the Cumulative Index to Nursing and Allied Health Literature were used to identify original articles published between January 1950 and December 2019.
Results:
Twenty-seven articles were found containing original data on macronutrients and micronutrients. Most (67%) of the studies published after 2011 measured the macronutrients and included gestational ages from 28 to 36 weeks. Milk collection methods, experimental design, and analytical methods varied between studies. There are 15 countries represented in this review; all of the American studies (n = 7) were published from 1980 to 1984.
Conclusions:
African American women, or women delivering before 28 weeks’ gestation are not represented in the literature. Accurate and targeted human-milk fortification depends on comprehensive, complete, and representative human-milk nutrient data. We have aggregated all available preterm human-milk macronutrient and micronutrient data and reported trends associated with lactation stage and gestational age. This report can aid in the design of feeding plans that are appropriate for the gestational age of the preterm infant and the lactation stage of the breastmilk.
Keywords: human milk, human-milk fortification, macronutrients, micronutrients, premature infant
Introduction
Human milk–based diets have emerged as the nutrition foundation to support preterm infant growth. Although this emergence is based on sound evidence supporting human milk’s protective properties against prematurity-related diseases, including necrotizing enterocolitis, equally compelling evidence demonstrates that preterm milk composition is lacking in key nutrients to support physiologic growth of premature infants.1–4 Several factors contribute to this nutrition shortfall. First, the concentration of many nutrients is diminished over time as the volume of human-milk production increases. Second, replenishing or maintaining available nutrients to support physiological growth must be supplied in a relatively small volume of human milk. Finally, some nutrients are poorly expressed in human milk. The convergence of these physiological realities likely contributes to growth deceleration or failure that is characteristic of preterm infants provided an unsupplemented human-milk diet. Furthermore, the extrapolation of data from term human milk and the lack of appreciation for the dramatic differences in preterm human-milk composition over time are impediments to the design of feeding plans that meet the needs of the nutritionally vulnerable premature infant. One important intervention to support nutrient delivery within a limited volume of human milk is the widely practiced addition of human or bovine fortifiers to expressed human milk. Unfortunately, the composition of these fortifiers and the volume of the supplement is largely based on the assumed density of each nutrient in expressed human milk, which varies over time.5–7 To support accurate supplementation of expressed human milk, accurate and comprehensive assessment of preterm human-milk composition over time is required. In this review, we will (1) aggregate and summarize available evidence to provide a clear representation of macronutrient and micronutrient composition in human milk over time and (2) identify gaps and opportunities for further clarification of human milk composition.
Methods
PubMed and Cumulative Index to Nursing and Allied Health Literature (CINAHL)were used to identify original articles published between January 1950 and December 2019. The following keywords were used in different combinations: breastmilk, human milk, preterm, prematurity, composition, nutrients, protein, fat, carbohydrate, copper, phosphorus, sodium, potassium, chloride, zinc, magnesium, calcium, vitamin D, and electrolytes. Reference lists were reviewed, and the results were cross-checked with search-engine results. Review articles, books, and editorials were excluded. The inclusion/exclusion criteria were as follows:
the original articles published from January 1950 to December 2019;
at least 1 of the following nutrients was reported: fat, energy, carbohydrate, copper, phosphorus, sodium, potassium, chloride, zinc, magnesium, calcium, or vitamin D;
the milk expression was a complete expression; articles reporting composition of fore-milk or hind-milk only were excluded;
the mothers delivered at ≤36 weeks’ gestation;
the milk was expressed between day 1 and day 30 post partum;
the article was available in English;
the unit of measure and mean for each nutrient was reported and the method of data collection was clear; and
the milk pools were no more than a 3-day collection.
Data Extraction
Data points extracted from each report were converted to milligrams per deciliter (mg/dL), except for copper, which was converted to micrograms per deciliter. All decimals were rounded to the nearest 10th place.
Search Results
A total of 2595 records (1683 duplicates) were identified in the initial search. After removing duplicates, 885 articles failed to meet the inclusion criteria (Figure 1). A total of 27 articles representing data from 15 different countries were included for review (Table 1). From the 27 included reports, 9 reports were from North American (USA and Canada) populations, 9 reports were from Europe (Italy, France, Denmark, the Netherlands, and Turkey), 6 reports were from Asia (India, Korea, and Taiwan), and 3 reports were from Africa (Nigeria, Kenya, and Egypt). Most reports were published between 1980 and 1989 (52%, n = 14), followed by 9 (33%) reports published between 2011 and 2019. Only 4 reports included in this review were published between 1990 and 2010. For studies included in this review, the gestational age at delivery ranged from 28 to 34 completed weeks with no data available from gestations below 28 completed weeks.
Figure 1.
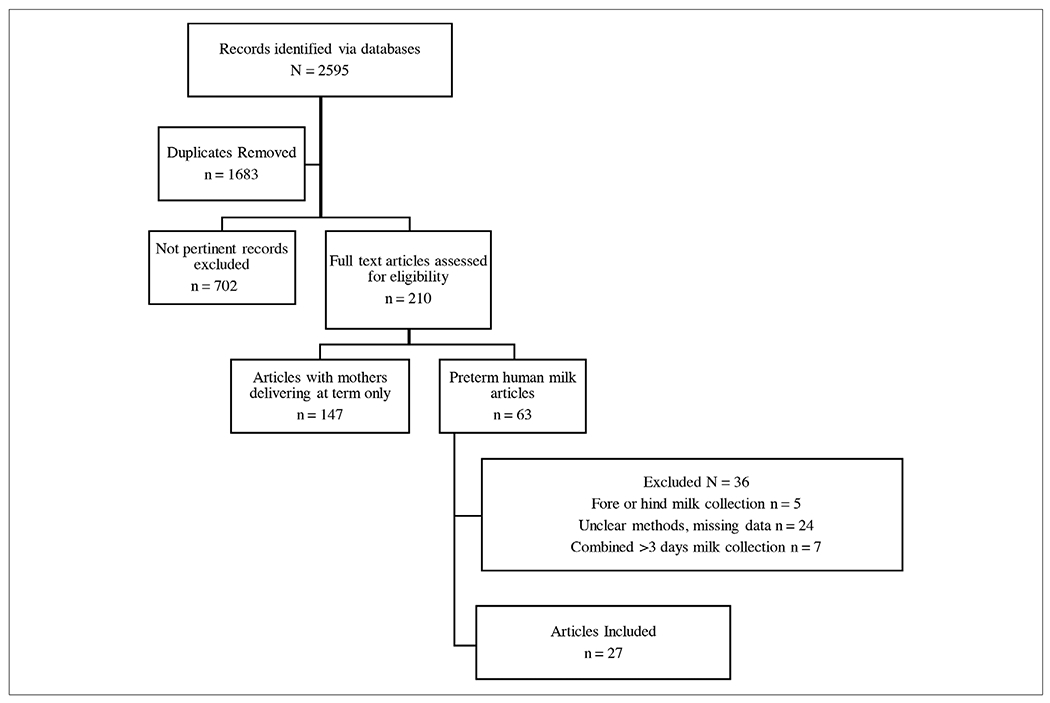
The initial search identified 2595 articles. A total of 1683 duplicate articles were removed. From the remaining articles 702 articles were excluded because they did not measure the nutrients of interest or they were not original articles. The remaining 210 full-text articles were reveiewed and 147 were excluded because the breastmilk analyzed was from mothers delivering at term. The remaining 63 articles were examined, and an additional 36 were excluded because the methods of collection did not meet inclusion criteria. In total, 27 articles met inclusion criteria.
Table 1.
Summary of Studies and Nutrients Measured by Each.
| Reference | Year | EGA* | BMIa | Country | kcal | Fat | Pro | CHO | Ca | P | Na | K | Mg | Cl | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gross9 | 1980 | 31.4 | NA | U.S. | x | x | x | x | x | x | x | x | ||||
| Schanler24 | 1980 | NA | NA | U.S. | x | x | x | x | ||||||||
| Anderson10 | 1981 | 28.5 ± 0.5b | NA | Canada | x | x | x | |||||||||
| Atinmo44 | 1982 | 28-34 | NA | Nigeria | x | x | ||||||||||
| Guerrini53 | 1981 | 33.3 ± 2.2 | NA | Italy | x | |||||||||||
| Mendelson45 | 1982 | 28.7 ± 2.2 | NA | Canada | x | x | ||||||||||
| Lemons11 | 1982 | 33 | NA | U.S. | x | x | x | x | x | x | x | x | x | x | ||
| Anderson54 | 1983 | 31 ± 1.4 | NA | U.S. | x | x | x | x | ||||||||
| Moran46 | 1983 | 30.3 (27–32) | NA | U.S. | x | x | ||||||||||
| Butte23 | 1984 | 33.9 ± 2.3 | 20.9 | U.S. | x | x | x | x | x | x | x | x | ||||
| Ehrenkranz55 | 1984 | 29 ± 0.4 | NA | U.S. | x | |||||||||||
| Kumbhat32 | 1985 | 32.56 ± 2.62 | NA | India | x | x | x | x | ||||||||
| Jitta56 | 1986 | 30.3 ± 0.44 | NA | Kenya | x | x | x | x | ||||||||
| Darwish57 | 1989 | 28–36 | NA | Egypt | x | x | x | |||||||||
| Aquilo58 | 1996 | 28–36 | NA | Italy | x | x | ||||||||||
| Maas59 | 1998 | 25–29 | NA | The Netherlands | x | x | x | x | ||||||||
| Faerk60 | 2001 | 28 | NA | Denmark | x | x | x | x | ||||||||
| Narang21 | 2006 | <33 | NA | India | x | x | x | |||||||||
| Kim48 | 2012 | 31.5 ± 2.90 | 21.6 | Korea | x | x | ||||||||||
| Zachariassen13 | 2013 | <32 | NA | Denmark | x | x | x | x | ||||||||
| Dutta61 | 2014 | 31.4 ± 2.3 | 24.8 | India | x | x | x | x | x | x | x | |||||
| Hsu29 | 2014 | 29 ± 2.39 | 24.8 | Taiwan | x | x | x | x | x | x | ||||||
| Kreissl62 | 2016 | 23–34 | NA | Austria | x | x | x | x | ||||||||
| Mahajan19 | 2017 | <34 | NA | India | x | x | x | x | ||||||||
| Bulut12 | 2019 | ≤32 | NA | Turkey | x | x | x | x | ||||||||
| Hascoet63 | 2019 | 31 | 23.2 | France | x |
Ca, calcium; Cl, chloride; CHO, carbohydrate; Cu, copper; EGA, estimated gestational age; K, potassium; Mg, magnesium; NA, not available; Na, sodium; P, phosphorus; Pro, protein; Zn, zinc.
Maternal prepregnancy body mass index (BMI), kg/m2.
SEM.
Macronutrients
Energy
Fourteen reports measured energy density in preterm human milk (Table 1). The methodology, study design, and milk collection varied, with 7 studies reporting 24-hour pooled samples and 4 studies reporting a single morning sample, 1 study used a variety of methods, and another did not disclose the method of collection. Reporting the method and timing of milk collection is critical for interpreting these study findings because evening milk has a higher fat and energy concentration than morning milk, as is reflected in the wide range of energy density between 42.3 and 79 kcal/dL reported from these studies (Figure 2A and B).8 An aggregate of the study findings over time demonstrates that energy density (Figure 2A) increases sharply over the first 7–14 days, after which energy density is largely consistent. However, it is important to note that mature preterm human milk (eg, >14 days) appears to have a nearly 50% increase in energy density over early preterm human milk. In comparison with term human milk, conflicting reports have suggested that preterm milk is more energy dense or that energy density is equivalent with the differences likely due to the influence of timing and technique of sample collection.9–11 Like our own aggregate report on energy density in Figure 2A, the caloric density of term milk increases over time.
Figure 2.
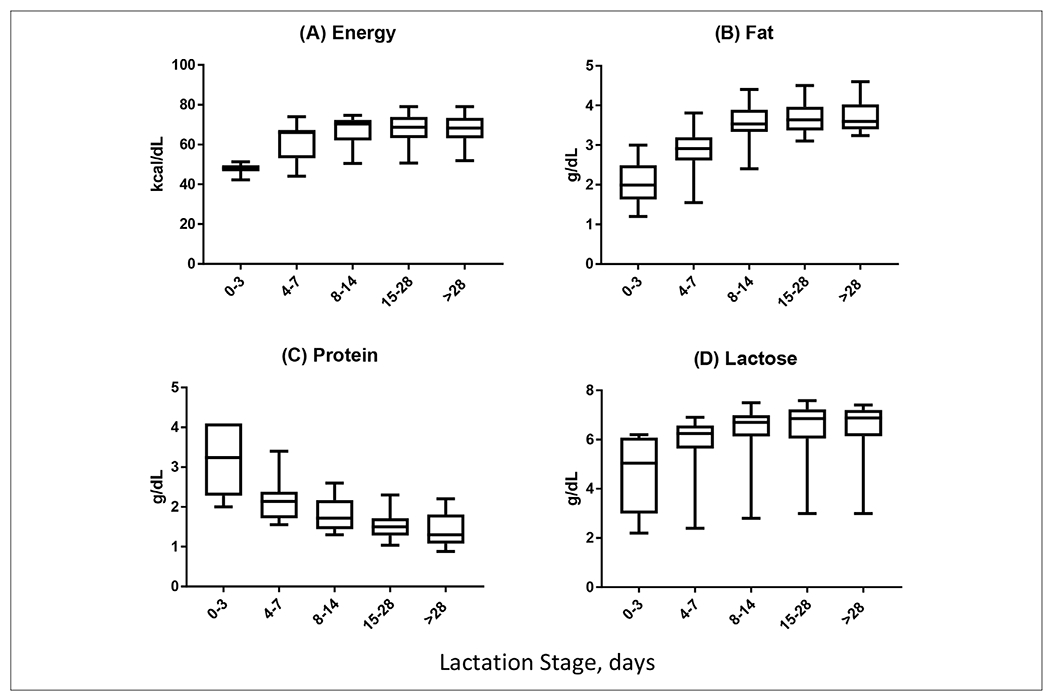
Aggregated results for macronutrients (energy, fat, protein, lactose) in premature human milk at lactation days 0–3, 4–7, 8–14, 15–28, and >28.
Maternal body mass index (BMI) is thought to influence energy density of human milk; however, this relationship is not direct and is clearly influenced by the composition of the human milk. Bulut et al found no correlation between maternal BMI and energy density but pointed to fat content in human milk as the major source of energy, whereas Zachariassen et al reported significantly higher energy content in mature milk in women with a BMI above 25 kg/m2 compared with women with a lower BMI.12,13 Unfortunately, the data do not provide clear evidence of a linear relationship between maternal BMI and energy density of human milk, and the BMI threshold used by Zachariassen et al is much lower than the mean BMI of American women.14 This lack of clarity has real implications for caregivers of preterm neonates because fortification strategies assume that the energy density of preterm milk approximates term human milk. Based on the assumption that caloric content is fixed at 20 calories per ounce (67 kcal/dL), the standard addition of fortifier (1 premeasured packet of fortifier per 25 mL of human milk) is anticipated to yield a fixed density of 24 calories per ounce (80 kcal/dL).5–7 These assumptions may be unsupported particularly when fortifying expressed human milk during the first days after preterm birth. Furthermore, the wide variation of energy density in mature preterm milk suggests that standard fortification may fall short of the expected 24 calories per ounce (80 kcal/dL) by up to 25%.5–7,15
Fat
Fat is a major energy source and contributes nearly 50% of the total caloric density of human milk. Early reports suggested that fat content in human milk is closely linked to prepregnancy BMI. Overweight and obese women in Eastern Europe and China had higher concentrations of fat and energy per volume of expressed human milk when compared with women considered to have a normal prepregnancy BMI.16,17 These studies are supported by a Danish cohort that demonstrated much higher fat (P = .02) and energy (P = .01) content in expressed human milk from women with a prepregnancy BMI > 25 kg/m2 when compared with women who had a prepregnancy BMI < 25 kg/m2.18
Individual published reports suggest that fat content in preterm human milk increases over time with some notable exceptions. Lemons et al provide a comprehensive assessment of macronutrients and micronutrients but failed to identify changes in fat content in preterm milk over time.11 In comparison with most studies that identify fat accretion in preterm human milk, Lemons et al did not collect or assess samples during the first 72 hours after birth, when fat content in human milk is low. Based on the aggregate data from 19 available reports of fat content in preterm human milk, fat content appears to increase nearly 2-fold over the first 2 weeks after birth and is relatively flat in mature preterm human milk (Figure 2B). These changes in fat content mirror the rise in caloric density across the first 2 weeks of life. Most importantly, standard fortification of preterm human milk would fall below recommended fat intake by up to 40%.5–7,15
Protein
Fourteen studies measured protein concentration and met inclusion criteria (Table 1), with most of the included studies showing a dramatic reduction of protein concentration over time (Figure 2C). Early preterm milk is enriched for protein, with several studies suggesting that protein content may be as high as 4.1 ± 2.1 g/dL.19 The high protein content in early preterm milk reflects the nutrition needs of the preterm infant wherein protein accretion in the fetus is ~2 g/kg/d body protein and protein stores diminish rapidly after birth in the absence of supplementation.20 Early introduction of human-milk feedings may prevent protein catabolism that begins soon after birth. However, as fat content increases over the first week to support higher-calorie human milk, protein content falls dramatically by as much as 60%.19,21 Despite the clear trend in protein content of preterm milk, there are wide variations in protein content between sample populations. The protein at days 4–7 range from 1.6 to 3.4 ± 0.5 g/dL, and day 28 values ranged from 0.9 ± 0.1 to 2.2 ± 0.6 g/dL. These significant variations could influence the actual protein intake vs perceived protein intake of preterm infants and contribute to poor growth. Using these reported numbers, human milk fortified with currently available human-milk fortifiers may fall below recommended protein intake by up to 25% and, in some cases, may exceed the recommended intake of protein by 69%.5–7,15,16
Carbohydrate
Fourteen articles measured carbohydrate and met the inclusion criteria with wide variation in carbohydrate content in preterm human milk reported for these cohorts (Table 1). Despite the wide variation from each cohort, carbohydrate content appears to remain relatively flat from birth until 30 days of life. Five papers reported lactose on days 0–3 ranging from 2.2 ± 0.7 to 6.2 ± 1.1 g/dL, with day 28 values ranging from 3 ± 0.9 to 7.4 ± 0.5 g/dL. All of the papers reported lactose of at least 5.4 g/dL between days 4 and 28, except Mahajan et al, which reported a nearly 2-fold reduction in carbohydrate content when compared with other cohorts that met the inclusion criteria.19 The reasons for the difference in lactose concentration in this report is not clear.
Micronutrients
Calcium
Calcium is a critical micronutrient for bone health and functions as a ubiquitous second messenger in cell-signaling pathways.22 The skeleton serves as a repository for calcium from which calcium can be mobilized by changes in parathyroid hormone and vitamin D to adjust circulating calcium concentrations. For the developing preterm infant, supporting adequate calcium intake and maintenance of calcium stores improves bone mineralization and prevents osteopenia of prematurity, improves vascular tone and myocardial function, and supports neural adaptation. The preterm infant is particularly susceptible to low plasma calcium and will sacrifice storage of calcium in the developing skeleton to meet calcium needs. The preterm infant’s calcium requirement far exceeds the needs of the term infant. A full-term infant with a birth weight of 3.5 kg requires 57 mg/kg/d calcium, whereas a 1-kg preterm infant requires 200 mg/kg/d. Therefore, human milk must provide a minimum of 135 mg/dL to meet these demands.15 We identified 6 reports that measured calcium in preterm human milk (Table 1). The concentration of calcium in preterm human milk was similar across the first 30 days after birth among all studies ranging from 21.3 to 35.9 mg/dL (Figure 3A). Interestingly, the limited number of reports did identify conflicting findings regarding the influence of prematurity on breast milk calcium concentration. Gross et al and Lemons et al found no difference in calcium content between term and preterm human milk.9–11 Conversely, Butte et al identified higher calcium content in term milk than in preterm milk, whereas Schanler et al showed that preterm human milk was enriched for calcium compared with term milk.23,24 These differences likely reflect small samples sizes and differences in testing methods, milk-sampling techniques, or maternal factors. An aggregate of the available data (Figure 3A) suggests that preterm human milk must be supplemented with at least 135 mg/dL of calcium to meet current recommendations for intake.15
Figure 3.
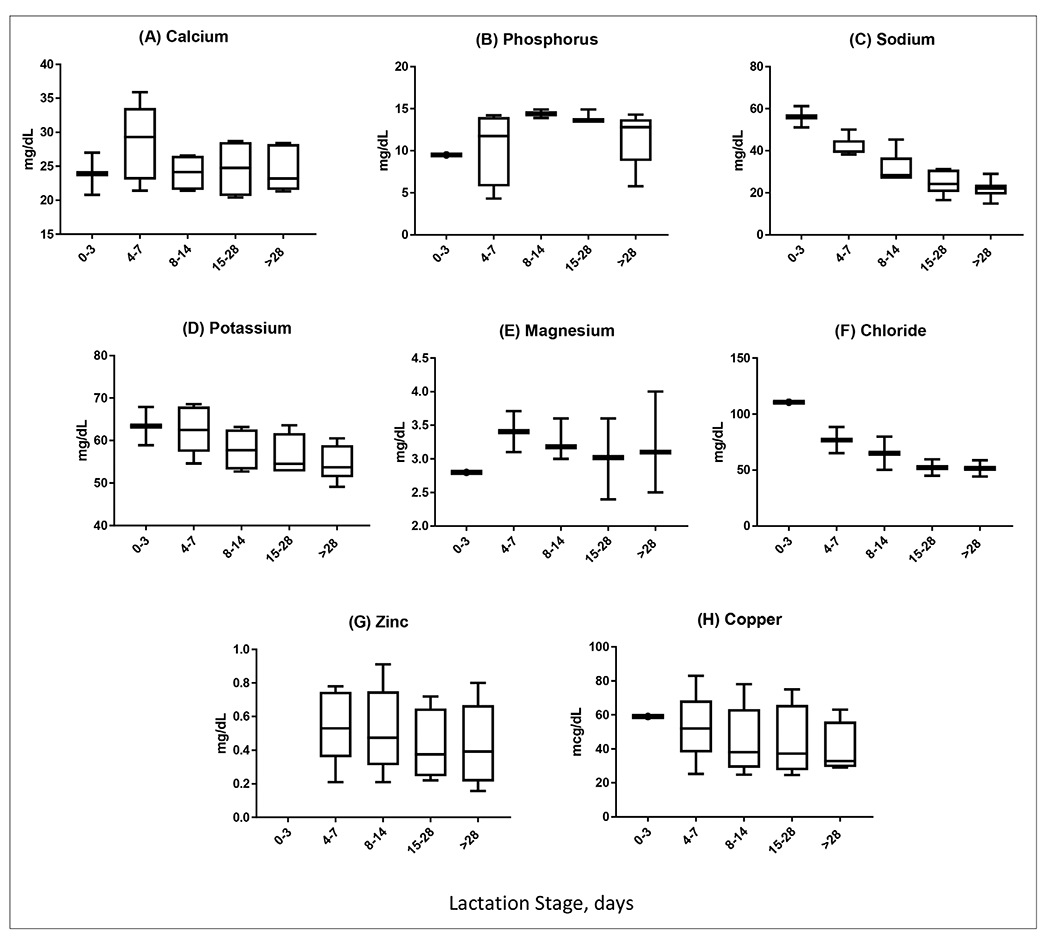
Aggregated results for micronutrients (calcium, phosphorus, sodium, potassium, magnesium, chloride, zinc, copper) in premature human milk at lactation days 0–3, 4–7, 8–14, 15–28, and >28.
Phosphorus
Fetal accretion of phosphorus occurs in the third trimester of pregnancy at a rate of 50 to 65 mg/d.25 Preterm infants born prior to the third trimester are at risk for developing hypophosphatemia shortly after birth.26,27 Relevant to preterm infants, hypophosphatemia is linked to risk for sepsis, prolonged ventilation, and bronchopulmonary dysplasia.27,28 Preterm human milk does not appear to reflect the preterm infant’s increased need for phosphorus because preterm human milk is reported to be less dense with phosphorus than term mother’s milk.11 We identified 5 reports that measured phosphorus in preterm human milk (Table 1). Values for phosphorus were similar in 4 of the 5 included studies, ranging from 10.1 to 14.9 ± 3.8 mg/dL, and remained constant from the first through the fourth week (Figure 3B). Variation due to ethnicity was suggested in a cohort from Taiwan that showed phosphorus levels in human milk were 75% lower than earlier reports from the United States of America.29
Electrolytes
Sodium
The management of electrolytes is one of the more challenging aspects of nutrition management of the preterm infant. The human body does not produce or release sodium endogenously and is entirely reliant on intake to meet sodium needs.30 This aspect of the sodium cycle is particularly important for preterm infants because sodium loss through urine and stool is inversely related to postmenstrual age. Increased losses or inadequate intake of sodium impairs longitudinal growth and weight gain in preterm infants.31 In addition, the frequent use of diuretics for diseases related to prematurity further depletes sodium stores and often results in hyponatremia. We identified 6 reports of sodium content in preterm human milk (Table 1). The aggregated data from the 6 studies demonstrate a clear linear trend of reduced sodium concentration in preterm human milk over the first 30 days after birth (Figure 3C). In fact, the aggregated data revealed that sodium concentration of early preterm human milk is nearly 3-fold higher than that in mature preterm human milk. The data comparing sodium concentration in preterm and term human milk are not clear, with an equal number of studies suggesting that sodium concentration in preterm human milk is either higher, lower, or identical to term human milk.9,11,23,32 These conflicting studies are likely the result of methodological differences as reflected in the wide variation in results (12.7 to 61.2 mg/dL). Most important, the aggregated data suggest that preterm human milk fortified with currently available human-milk fortifiers may fall below recommended sodium intake by up to 40%.5–7,15
Potassium
Potassium loss in preterm stools is twice as high as term infant sodium loss, and these losses may disrupt the metabolic and cellular functions critical to the developing preterm infant.30,31 Not surprisingly, consumption of large quantities of potassium (78–195 mg/kg/d) is necessary to maintain potassium equilibrium, but intake through unsupplemented breast milk feedings may fall well below the needs of the preterm infant.15 We identified 5 reports that measured potassium in preterm human milk (Table 1). Like sodium, potassium concentration in preterm human milk decreases linearly over the first 30 days after birth with an aggregate range from 49.1 to 68.6 ± 2 mg/dL (Figure 3D). Unlike reports of sodium concentration in preterm human milk, evidence suggests that preterm and term human milk maintain similar concentrations of potassium with the exception of the first week of life, when preterm milk samples are more potassium dense.24 Based on these reference values, standard fortification of human milk will meet the potassium needs of the preterm infant.
Magnesium
Magnesium is a cofactor for >300 enzymes and is required for energy production, glucose control, and protein synthesis. Magnesium plays a major role in the transport of active calcium and potassium ions across cell membranes, and insufficient intake of magnesium may lead to hypocalcemia and hypokalemia.33 Fetal accretion of magnesium occurs during the last 3 months of pregnancy at a rate of 3–5 mg/kg/d.34 Therefore, the preterm infant is born with low magnesium reserves and is reliant on increased magnesium intake (8–15 mg/kg/d).15 We identified 3 reports that measured magnesium in preterm human milk (Table 1). Relevant to the interpretation of these studies, the sample sizes were small, ranging from 8 to 25 with significant attrition of participants from the first to fourth weeks.9,11,23 The concentration of magnesium varied among the 3 papers, ranging from 2.4 ± 0.1 to 5.1 ± 1.8 mg/dL, but the mean value remained relatively flat from the first to the fourth week of life (Figure 3E). Based on the aggregated data, human milk fortified with currently available human-milk fortifiers may fall below recommended magnesium intake by up to 35%.15 An important recommendation for mothers who provide expressed milk may include increasing their own dietary intake of magnesium as maternal intake is directly associated with magnesium concentration in expressed human milk.35
Chloride
Adequate intake of electrolytes is necessary to support longitudinal growth and weight gain in preterm infants, and supplemental chloride is often needed to achieve appropriate growth.30,36 Chloride requirements for the preterm infant are reported to be as much as 200 mg/kg/d, meaning that the enteral diet must provide 80–125 mg of chloride per dL of intake to meet this need.15 We identified 2 reports that measured chloride in preterm human milk with only 1 study that measured chloride content in the first week of life (Table 1). Gross et al found that chloride levels were highest in colostrum and declined sharply over subsequent weeks.9 Both groups found that preterm milk had higher chloride content when compared with term human milk (P < .005 and P < .001, respectively), but Gross et al reported values that were 27% higher in preterm samples than those reported by Lemons et al11 (Figure 3F). Using these reported values, human milk fortified with currently available human-milk fortifiers may fall below recommended chloride intake by up to 17%.14
Vitamin D
No studies that measured vitamin D in the human milk of women delivering prematurely met the inclusion criteria.
Trace Elements
Zinc
Zinc deficiency is common in infancy and is particularly true in preterm infants provided a human diet.37–40 Stunted growth, skin rash or breakdown, increased risk for infections, and impaired neurodevelopment are consequences of inadequate zinc intake.41 Human-milk zinc does not appear to be influenced by maternal diet or intake of supplements, and little is known about the influence of other factors on human-milk zinc content.42,43 We identified 6 reports that measured zinc in preterm human milk (Table 1). Aggregate data from all 6 studies suggest that zinc concentration in preterm human milk is relatively flat from the first to the fourth week of life (Figure 3G); however, 4 of 6 studies showed a steady decline in zinc in human milk over the first 30 days after preterm birth.23,44–46 Thus, the aggregate data reflect a wide variation in reported measurements and may not reflect the trends in zinc content in preterm human milk.
Copper
Like many nutrients, copper is accumulated in the third trimester of pregnancy, making the preterm infant vulnerable to copper deficiency.47 Copper is an essential nutrient and its deficiency results in anemia, neutropenia, thrombocytopenia, and osteoporosis. We identified 5 reports that measured the copper concentration of preterm human milk (Table 1). All of the reports showed a decline in copper over time, with copper concentration in immature preterm human milk nearly 50% higher than in mature preterm human milk.48 Although the overall trends in copper content in preterm human milk are clear, the wide variation in reported copper concentration in preterm human milk is reflected in the aggregate data (Figure 3H). As with zinc, copper concentration in expressed human milk does not appear to correlate with maternal diet.49
Summary
The data in this review suggest that macronutrients and micronutrients vary considerably based on maternal factors, including gestational age at delivery, BMI, and lactation stage. Energy concentration increases slightly overtime, but protein drops dramatically from the first to the fourth week. There are extreme variations seen between studies for lactose, calcium, zinc, and copper, suggesting a strong influence of maternal factors on these nutrients. When fortifying human milk for the preterm infant, it may be prudent to consider the gestational age of the infant and the lactation stage of the breast milk and fortify accordingly.
Limitations
This review covers all currently available evidence for preterm human-milk nutrient composition. We have reported aggregated results and provided a concise representation of the data to assist in the development of feeding plans for preterm infants. However, a limitation of our report is that the included data may not represent the current American population, obese women, women delivering prior to the 28th week of pregnancy, or African American women.
Opportunities
The composition of preterm human milk is largely based on extrapolated data from term human milk and a limited number of studies with varied methodologies and cohort demographics. Relevant to the interpretation of our aggregate data, most well-designed studies are now 4 decades removed from the present population, with rising preterm birth rate, improving survival of extremely preterm neonates, increasing prevalence of maternal obesity, and higher exposure to human milk. A complete lack of data on human-milk composition associated with preterm birth prior to 28 weeks forces enteral feeding plans to conform to those that are more appropriate for older and heavier preterm infants. Furthermore, the aggregate reference values presented here provide a platform for several necessary future studies of preterm human milk. First, the descriptive studies of human-milk composition from extremely preterm births are urgently needed to understand the unique nutrition needs of this population. Second, the use of human and bovine human-milk fortifiers often underestimates the nutrition needs of the preterm infant. The one-size-fits-all approach to fortifiers lacks precision for a population at high risk for extrauterine growth failure. Designing gestational age–specific fortifiers requires a complete understanding of their nutrition needs and composition of enteral intake. Human-milk analyzers have been suggested as a means to address the nutrient disparity in human-milk samples to promote better weight gain through targeted fortification.50–52 Human-milk analyzers are expensive and may not be feasible outside of the research setting in which daily or weekly analysis of breast milk would be cumbersome and time consuming. In addition, currently available milk analyzers measure protein, fat, and lactose, but not micronutrients. Finally, published detail about the content of preterm donor or mother’s own milk will allow care teams to tailor feeding plans and improve the composition of preterm formulas and supplements without requiring bedside milk analysis. The aggregated data for macronutrient and micronutrient composition in preterm human milk presented in this review are a platform for these opportunities to be achieved.
Acknowledgment
A. Gates would like to thank Jatinder Bhatia, MD, FAAP, former Chief of the Division of Neonatology, Department of Pediatrics at Augusta University for his continued support and mentorship.
Footnotes
Conflicts of interest: A. Gates is associated with Mead Johnson Nutrition Speaker’s Bureau and Advisory Board and has received a grant from Medolac Laboratories. T. Marin is associated with Mead Johnson Nutrition Speaker’s Bureau. G. De Leo and B. K. Stansfield have nothing to disclose.
Statement of Authorship
A. Gates wrote the study protocol, performed the literature search, selected the articles for inclusion, and drafted the initial draft of the article. T. Marin, G. De Leo, and B. K. Stansfield assisted with study design and oversight. All authors reviewed and approved the final draft of the article.
References
- 1.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–567. [DOI] [PubMed] [Google Scholar]
- 2.Cristofalo E, Schanler R, Blanco C, Sullivan S, Abrams S. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr 2013;163(6):1592–1595. [DOI] [PubMed] [Google Scholar]
- 3.Ghandehari H, Lee ML, Rechtman DJ. An exclusive human milk-based diet in extremely premature infants reduces the probability of remaining on total parenteral nutrition: a reanalysis of the data. BMC Res Notes. 2012;5(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. 2010;3(126):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Human Milk Fortification. Pediatric Nutrition Product Guide. Columbus: Abbott Nutrition; 2011:72. [Google Scholar]
- 6.Enfamil Human Milk Fortifier Acidified Liquid. Pediatric Products Handbook. Evansville: Mead Johnson Nutrition; 2012:90. [Google Scholar]
- 7.Prolact+4 H2MF Nutrition Information. Prolact+4 H2MF Nutrition Information. City of Industry, CA, USA: Prolacta Bioscience; 2014. [Google Scholar]
- 8.Moran-Lev H MF, Mimouni FB, OVental A, Mangel L, Mandel D, Lubetsky R. Circadian macronutrients variations over the first 7 weeks of human milk feeding of preterm infants. Breast feeding Med. 2015;10:(7):366–370. [DOI] [PubMed] [Google Scholar]
- 9.Gross SJ, David RJ, Bauman L, Tomarelli RM. Nutritional composition of milk produced by mothers delivering preterm. J Pediatr 1980;96(4):641–644. [DOI] [PubMed] [Google Scholar]
- 10.Anderson GH, Atkinson SA, Bryan MH. Energy and macronutrient content of human milk during early lactation from mothers giving birth prematurely and at term. Am J Clin Nutr. 1981;34(2):258–265. [DOI] [PubMed] [Google Scholar]
- 11.Lemons JA, Moye L, Hall D, Simmons M. Differences in the composition of preterm and term human milk during early lactation. Pediatr Res. 1982;16(2):113–117. [DOI] [PubMed] [Google Scholar]
- 12.Bulut O, Coban A, Ince Z. Macronutrient analysis of preterm human milk using mid-infrared spectrophotometry. J Perinat Med. 2019;47(7):785–791. [DOI] [PubMed] [Google Scholar]
- 13.Zachariassen G, Fenger-Gron J, Hviid MV, Halken S. The content of macronutrients in milk from mothers of very preterm infants is highly variable. Dan Med J. 2013;60(6):1–5. [PubMed] [Google Scholar]
- 14.Global Health Observatory (GHO) Data. Accessed September 1,2019. https://apps.who.int/gho/data/view.main.BMI25CREGv?lang=en.
- 15.Koletzko B PB, Uauy R. Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. Nutritional Care of Preterm Infants. Scientific Basis and Practical Guidelines. Basel, Switzerland: Karger; 2010:297–299. [DOI] [PubMed] [Google Scholar]
- 16.Burianova I, Bronsky J, Pavilkova M, Janota J, Maly J. Maternal body mass index, parity and smoking are associated with human milk macronutrient content after preterm delivery. Early Hum Dev. 2019;137:104832. [DOI] [PubMed] [Google Scholar]
- 17.Yang T, Zhang Y, Ning Y, et al. Breast milk macronutrients composition and the associated factors in urban Chinese mothers. Chin Med J (Engl). 2014;127(9):1721–1725. [PubMed] [Google Scholar]
- 18.Bzikowska A, Czerwonogrodzka-Senczyna A, Weker H, Wesolowska A. Correlation between human milk composition and maternal nutrition status. Rocz Panstw Zakl Hig. 2018;69(4):363–367. [DOI] [PubMed] [Google Scholar]
- 19.Mahajan S, Chawla D, Kaur J, Jain S. Macronutrients in breastmilk of mothers of preterm infants. Indian Pediatr. 2017;54(8):635–637. [DOI] [PubMed] [Google Scholar]
- 20.Zeigler E, O’Donnell A, Nelson S, et al. Body composition of the reference fetus. Growth. 1976;40:329–341. [PubMed] [Google Scholar]
- 21.Narang AP, Brains HS, Kansal S, Singh D. Serial composition of human milk in preterm and term mothers. Indian J Clin Biochem. 2006;21(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moya F, Sisk P Walsh K, Berseth CL. A new liquid human milk fortifier and linear growth in preterm infants. Pediatrics. 2012;130(4):928–935. [DOI] [PubMed] [Google Scholar]
- 23.Butte NF, Garza C, Johnson CA Smith EO, Nichols BL. Longitudinal changes in milk composition of mothers delivering preterm and term infants. Early Hum Dev. 1984;9(2):153–162. [DOI] [PubMed] [Google Scholar]
- 24.Schanler RJ, Oh W. Composition of breast milk obtained from mothers of premature infants as compared to breast milk obtained from donors. J Pediatr. 1980;96(4):679–681. [DOI] [PubMed] [Google Scholar]
- 25.Widdowson EM, Spray CM. The chemical composition of the human body. Clin Sci. 1951;10:113–115. [PubMed] [Google Scholar]
- 26.Ichikawa G, Watabe Y, Suzumura T, Muto T, Arisaka O. Hypophosphatemia in small for gestational age extremely low birth weight infants receiving parenteral nutrition in the first week after birth. J Pediatr Endocrinol Metab. 2012;25(3-4):317–321. [DOI] [PubMed] [Google Scholar]
- 27.Ross JR, Finch C, Ebeling M, Taylor SN. Refeeding syndrome in very-low birth weight intrauterine growth-restricted neonates. J Perinatol. 2013;33:(9):717–720. [DOI] [PubMed] [Google Scholar]
- 28.Moltu SJ, Strommen K, Blackstad EW, et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia-a randomized, controlled trial. Clin Nutr. 2013;32(2):207–212. [DOI] [PubMed] [Google Scholar]
- 29.Hsu YC, Chen CH, Lin MC, Tsai Cr, Liang JT, Wang TM. Changes in preterm breast milk nutrient content in the first month. Pediatr Neonatol. 2013;55(6):449–454. [DOI] [PubMed] [Google Scholar]
- 30.Fusch C JF. Water, sodium, potassium and chloride. Nutritional Care of Preterm Infants. Scientific Basis and Practical Guidelines. Basel: Karger; 2010:99–120. [Google Scholar]
- 31.Al-Dahhan J, Haycock GB, Chantler C, Stimmler L. Sodium homeostasis in term and preterm neonates. II. Gastrointestinal aspects. Arch Dis Child. 1983;58:(5):343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumbhat MM KS, Bijur AM, Jadhav CS. Breastmilk composition in relation to gestation. Indian Pediatr. 1985;22:229–233. [PubMed] [Google Scholar]
- 33.Magnesium. In National Institutes of Health Office of Dietary Supplements. 2020. ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- 34.Widdowson EM, Spray CM. The chemical composition of the human body. Clin Sci. 1951;10:113–115. [PubMed] [Google Scholar]
- 35.Butts CA, Hedderley DJ, Herath TD, et al. Human milk composition and dietary intakes of breastfeeding women of different ethnicity from Manawatu-Wanganui region of New Zealand. Nutrients. 2018;10(9):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isemann B, Mueller EW, Narandran V, Akinbi H.. Impact of early sodium supplementation on hyponatremia and growth in premature infants: a randomized controlled trial. J Parenter Enteral Nutr. 2016;40(3):342–349. [DOI] [PubMed] [Google Scholar]
- 37.Obladen M, Loui A, Kampmann W, Renz H. Zinc deficiency in rapidly growing preterm infants. Acta Paediatr. 1998;87(6):685–691. [DOI] [PubMed] [Google Scholar]
- 38.Atkinson SA, Whelam D, Whyte RK, Lonerdal B. Abnormal zinc content in human milk. Am J Dis Child. 1989;143(5):608–611. [DOI] [PubMed] [Google Scholar]
- 39.Krebs NF, Miller LV, Hambridge M. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatr Int Child Health. 2014;34(4):279–289. [DOI] [PubMed] [Google Scholar]
- 40.Kiechl-Kohlendorfer U, Fink FM, Steichen-Gersdorf E. Transient symptomatic zinc deficiency in a breast fed preterm infant. Pediatric Dermatol. 2007;24(5):536–540. [DOI] [PubMed] [Google Scholar]
- 41.Griffin I, Domelof M, Bhatia J, Anderson DM, Kjer N. Zinc and copper requirments in preterm infants: An examination of the current literature. Early Hum Dev. 2013;89:s29–s34. [DOI] [PubMed] [Google Scholar]
- 42.Aumeistere L, Ciprovica I, Zavadska D, Bavrins K, Borisova A. Zinc content in breastmilk and its association with maternal diet. Nutrients. 2018;10(10):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trugo NM, Donagelo CM, Koury JC, Silva MI, Freitas LA. Concentration and distribution pattern of selected micronutrients in preterm and term milk from urban Brazilian mothers during early lactation. Eur J Clin Nutr. 1987;42:497–507. [PubMed] [Google Scholar]
- 44.Atinmo T, Omololu A. Trace element content of breastmilk from mothers of preterm infants in Nigeria. Early Hum Dev. 1982;6(3):309–313. [DOI] [PubMed] [Google Scholar]
- 45.Mendelson RA, Anderson GH, Bryan MH. Zinc, copper and iron content of milk from mothers of preterm and full-term infants. Early Hum Dev. 1982;6(2):145–151. [DOI] [PubMed] [Google Scholar]
- 46.Moran JR, Vaughan R, Stroop S, Coy S, Johnston H, Green HL. Concentrations and total daily output of micronutrients in breast milk of mothers delivering preterm: a longitudinal study. J Pediatr Gastroenterol Nutr. 1983;2(4):629–634. [DOI] [PubMed] [Google Scholar]
- 47.Lonnerdal B. Copper nutrition during infancy and childhood. Am J Clin Nutr. 1998;67(5):1046S–1053S. [DOI] [PubMed] [Google Scholar]
- 48.Kim SY, Park JH, Kim EA, Lee-Kim YC. Longitudinal study on trace mineral compositions (selenium, zinc, copper, manganese) in Korean human preterm milk. J Korean Med Sci. 2012;27(5):532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi YK, Kim JM, Lee JE, et al. Association of maternal diet with zinc, copper, and iron concentrations in transitional human milk produced by Korean mothers. Clin Nutr Res 2016;5(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bulut O CA, Uzunhan O, Ince Z. Effects of targeted versus adjustable protein fortification of breast milk on early growth in very low birth weight preterm infants: a randomized clinical trial. Nutr Clin Prac. 2020;35(2):335–342. [DOI] [PubMed] [Google Scholar]
- 51.Morlacchi L MD, Gianni ML, Roggero P, Amato O, Piemotese D, Mosca F. Is targeted fortification of human breast milk an opitmal nutrition strategy for preterm infants? An Intervential Study. J Transl Med. 2016;14(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rochow NFG, Choi A, Chessell L,et al. Target fortification of breast milk with fat, protein, and carbohydrates for preterm infants. J Pediatr. 2013;163(4):1001–1007. [DOI] [PubMed] [Google Scholar]
- 53.Guerrini P, Bosi G, Chierici R, Fabbri A. Human milk: relationship of fat content with gestational age. Early Hum Dev. 1981;5(2):187–194 [DOI] [PubMed] [Google Scholar]
- 54.Anderson DM, Williams FH, Merkatz RB, Schulman PK, Kerr DS, Pittard WB 3rd. Length of gestation and nutritional composition of human milk. Am J Clin Nutr. 1983;37(5):810–814. [DOI] [PubMed] [Google Scholar]
- 55.Ehrenkranz RA, Ackerman BA, Nelli CM. Total lipid content and fatty acid composition of preterm human milk. J Pediatr Gastroenterol Nutr. 1984:3(5):755–758. [DOI] [PubMed] [Google Scholar]
- 56.Jitta JN, Musoke RN, Bwibo NO, Kioni J. Composition of early human milk of Kenyan mothers of preterm and term infants. East Afr Med J. 1986:693–698. [PubMed] [Google Scholar]
- 57.Darwish Ael M, Dakroury AM, el-Feel MS, Nour NM. Comparative study on breast milk of mothers delivering preterm and term infants—protein, fat, lactose. Nahrung. 1989;3(3):249–251. [DOI] [PubMed] [Google Scholar]
- 58.Aquilo E, Spagnoli R, Seri S, Bottone G, Spennati G. Trace element content of human milk during lactation of preterm newborns. Bio Trace Elem Res. 1996;51(1):63–70. [DOI] [PubMed] [Google Scholar]
- 59.Maas C, Mathes M, Bleeker C, et al. Effect of increased enteral protein intake on growth in human milk-fed preterm infants: a randomized clinical trial. JAMA Pediatr. 2017;171(1):16–22. [DOI] [PubMed] [Google Scholar]
- 60.Faerk J, Skafte L, Peterson S, Peitsersen B, Michaelsen KF. Macronutrients in milk from mothers delivering preterm. Adv Exp Med Biol. 2001:409–413. 10.1007/978-1-4615-1371-1_51. [DOI] [PubMed] [Google Scholar]
- 61.Dutta S, Saini S, Prassad R. Changes in preterm human milk composition with particular reference to introduction of mixed feeding. Indian Pediatr. 2014;51(12):997–999. [DOI] [PubMed] [Google Scholar]
- 62.Kreissl A, Zwiauer V, Repa A, et al. Human milk analyser shows that the lactation period affects protein levels in preterm breastmilk. Acta Paediatrica. 2016;105(6):635–640. [DOI] [PubMed] [Google Scholar]
- 63.Hascoet JM, Chauvin M, Pierret C, et al. Impact of maternal nutrition and perinatal factors on breast milk composition after premature delivery. Nutrients. 2019;366:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


