Abstract
BACKGROUND
Peutz-Jeghers syndrome (PJS) is a clinically rare disease with pigmented spots on the lips and mucous membranes and extremities, scattered gastrointestinal polyps, and susceptibility to tumors as clinical manifestations. Effective preventive and curative methods are still lacking. Here we summarize our experience with 566 Chinese patients with PJS from a Chinese medical center with regard to the clinical features, diagnosis, and treatment.
AIM
To explore the clinical features, diagnosis, and treatment of PJS in a Chinese medical center.
METHODS
The diagnosis and treatment information of 566 cases of PJS admitted to the Air Force Medical Center from January 1994 to October 2022 was summarized. A clinical database was established covering age, gender, ethnicity, family history, age at first treatment, time and sequence of appearance of mucocutaneous pigmentation, polyp distribution, quantity, and diameter, frequency of hospitalization, frequency of surgical operations, etc. The clinical data was retrospectively analyzed using SPSS 26.0 software, with P < 0.05 considered statistically significant.
RESULTS
Of all the patients included, 55.3% were male and 44.7% were female. Median time to the appearance of mucocutaneous pigmentation was 2 years, and median time from the appearance of mucocutaneous pigmentation to the occurrence of abdominal symptoms was 10 years. The vast majority (92.2%) of patients underwent small bowel endoscopy and treatment, with 2.3% having serious complications. There was a statistically significant difference in the number of enteroscopies between patients with and without canceration (P = 0.004, Z = -2.882); 71.2% of patients underwent surgical operation, 75.6% of patients underwent surgical operation before the age of 35 years, and there was a statistically significant difference in the frequency of surgical operations between patients with and without cancer (P = 0.000, Z = -5.127). At 40 years of age, the cumulative risk of intussusception in PJS was approximately 72.0%, and at 50 years, the cumulative risk of intussusception in PJS was approximately 89.6%. At 50 years of age, the cumulative risk of cancer in PJS was approximately 49.3%, and at 60 years of age, the cumulative risk of cancer in PJS was approximately 71.7%.
CONCLUSION
The risk of intussusception and cancer of PJS polyps increases with age. PJS patients ≥ 10 years old should undergo annual enteroscopy. Endoscopic treatment has a good safety profile and can reduce the occurrence of polyps intussusception and cancer. Surgery should be conducted to protect the gastrointestinal system by removing polyps.
Keywords: Peutz-Jeghers syndrome, Management, Intussusception, Canceration, STK11
Core Tip: Peutz-Jeghers syndrome (PJS) is a clinically rare autosomal dominant inherited disease with pigmented spots on the lips and mucous membranes and extremities, scattered gastrointestinal polyps, and susceptibility to tumors as clinical manifestations. Effective preventive and curative methods are still lacking. Here we summarize our experience with 566 Chinese patients with PJS from a Chinese medical center with regard to the clinical features, diagnosis, and treatment, in order to improve the understanding of PJS in Chinese patients and improve its clinical diagnosis and treatment.
INTRODUCTION
Peutz-Jeghers syndrome (PJS) is clinically characterized by labial mucosa and extremity terminal pigmentation and gastrointestinal multiple hamartoma polyposis[1-3]. Mucocutaneous pigmentation generally does not require specific treatment, but PJS polyposis is clinically serious. Gastrointestinal polyps cause secondary rupture, bleeding, intussusception, intestinal obstruction, abdominal pain, abdominal distension, hematochezia, and other symptoms, and their further progression causes enteric necrosis, intestinal perforation, and even cancer[4-7]. Therefore, it is essential to develop safe and effective treatments for PJS polyps. From January 1994 to October 2022, we diagnosed and treated 566 patients with PJS at the Air Force Medical Center, China, thereby accumulating some clinical experience for the management of this disease. This paper report our experience with this disease in order to explore the clinicopathological features, diagnosis, and treatment of PJS in Chinese patients.
MATERIALS AND METHODS
Materials
Patients diagnosed with PJS (ICD-9, ICD-10 disease code Q85.801) from January 1994 to October 2022 in the Air Force Medical Center's electronic medical record system were screened according to the standard reported previously[8,9]. Suspicious diagnoses and misdiagnoses were excluded, leaving a total of 566 patients. Statistical parameters explored in this study included: (1) General information: Gender, age, ethnicity, family history, etc.; (2) Clinical information: Age at onset, location of mucocutaneous pigmentation, time from mucocutaneous pigmentation to the appearance of abdominal symptoms (such as abdominal pain, intestinal obstruction, and gastrointestinal bleeding), polyp distribution, polyp burden, maximum- polyp diameter, polyp pathology and canceration etc.; and (3) Diagnosis and treatment information: Age at initial treatment, age at follow-up, frequency of hospitalization, frequency of surgical operations, frequency of endoscopy and comorbidities, etc. A database of clinical parameters was created and retrospective statistical analysis was performed.
Statistical analysis
SPSS 26.0 software was used for descriptive statistical analyses. A normal testing method was applied to evaluate the quantitative data, which are expressed as the mean ± standard deviation (SD); t-test was used for comparisons between groups. Skewed distribution data are described as medians (interquartile ranges); the Mann-Whitney U test was used for comparisons between groups. Qualitative data are expressed as percentages; the comparison of proportion and correlation analysis was evaluated by the χ2 test. P < 0.05 was considered statistically significant.
RESULTS
General data
The general information of 566 patients with PJS is shown in Table 1. As of the final follow-up age, 236 cases were married and 330 were unmarried. There were 183 cases married with children and 106 patients with healthy children after marriage; 77 children born after marriage had PJS; considering that some healthy children born to 106 patients were still too young for disease characteristics to appear, the diagnosis may not be clear, and the actual proportion of children with the disease may be higher.
Table 1.
General data of 566 patients with Peutz-Jeghers syndrome
|
General data
|
Classification
|
Cases
|
Percentage (%)
|
| Gender | Male | 313 | 55.3 |
| Female | 253 | 44.7 | |
| Ethnic distribution | Han nationality | 539 | 95.4 |
| Manchu | 11 | 1.9 | |
| Hui nationality | 7 | 1.2 | |
| Mongolian | 4 | 0.7 | |
| Tibetan | 2 | 0.4 | |
| Tujia nationality | 1 | 0.2 | |
| Zhuang nationality | 1 | 0.2 | |
| She minority | 1 | 0.2 | |
| Family history of PJS | Yes | 330 | 58.3 |
| No | 236 | 41.7 | |
| ABO blood group | A | 149 | 27.1 |
| B | 175 | 31.8 | |
| O | 167 | 30.4 | |
| AB | 59 | 10.7 | |
| Deletion | 16 | 2.8 | |
| Rh blood group | Rh+ | 530 | 97.2 |
| Rh— | 0 | 0 | |
| Deletion | 16 | 2.8 | |
| Marital history | Unmarried | 330 | 58.3 |
| Married | 236 | 41.7 | |
| Offspring inheritance | Yes | 77 | 42.1 |
| No | 106 | 57.9 |
PJS: Peutz-Jeghers syndrome.
Clinical data
The clinical data of 566 patients with PJS is shown in Table 2. Age distribution ranged from 0.5 to 60 years old, with a median age of 15 years old.
Table 2.
Clinical data of 566 patients with Peutz-Jeghers syndrome
|
Clinical data
|
Median
|
(P25, P75)
|
Extreme value
|
| First treatment age | 15 | (9, 22) | 0.5, 60 |
| Follow-up age | 26 | (18, 34) | 4, 65 |
| Age of appearance of mucocutaneous pigmentation | 2 | (1, 4) | 0, 33 |
| Interval time between age of mucocutaneous pigmentation and occurrence of gastrointestinal symptoms | 10 | (5.5, 18) | 0, 58 |
| Gastric polyp burden | 2 | (1, 5) | 1, 100 |
| Maximum diameter of gastric polyps (mm) | 8 | (5, 15) | 2, 80 |
| Small intestinal polyp burden | 3 | (2, 8) | 1, 100 |
| Maximum diameter of small intestinal polyps (mm) | 40 | (25, 50) | 1, 160 |
| Colorectal polyps burden | 2 | (1, 5) | 1, 100 |
| Maximum diameter of colorectal polyps (mm) | 30 | (15, 45) | 1, 120 |
| Cumulative hospitalizations | 2 | (1, 3) | 1, 11 |
| Number of operations | 1 | (0, 2) | 0, 7 |
| Frequency of small intestinal enteroscopic examinations | 2 | (1, 3) | 0, 12 |
| Frequency of gastroenterographic examinations | 1 | (0, 2) | 0, 16 |
| Frequency of colonoscopic examinations | 1 | (0, 2) | 0, 16 |
First treatment age: A total of 407 patients (71.9%) received initial treatment before 20 years old, and 513 (90.6%) received initial treatment before 30 years old. There was a statistically significant difference in the age of initial treatment between patients with and without a family history of PJS (P = 0.035, Z = -2.114); the age of initial treatment of patients with a family history of PJS was later than that of patients without. There was no significant difference in first treatment age according to gender (Z = -0.105, P = 0.310). There was no significant difference in first treatment age according to blood group (H = 1.652, P = 0.648). There was a significant difference in first treatment age between patients with and without malignant tumors (P = 0.009, Z = -2.631). The median age of patients with malignant tumors was significantly higher than that of patients without, which suggests that the later the age of initial treatment, the more likely the occurrence of malignancy.
Mucocutaneous pigmentation: A total of 563 cases (99.5%) had mucocutaneous pigmentation, which appeared before 7 years old in 507 cases (90.1%). As to the order of the appearance of mucocutaneous pigmentation, 402 cases (71.0%) had mucocutaneous pigmentation on both the lips and limbs, 45 (8.0%) had mucocutaneous pigmentation on the lips and then on the limbs, 4 (0.7%) had mucocutaneous pigmentation on the limbs and then on the lips, and 16 cases (2.8%) developed mucocutaneous pigmentation in an unknown order. There was a statistically significant difference in the age of the appearance of mucocutaneous pigmentation age between patients with and without a family history of PJS (P = 0.016, Z = -2.415), and the age of the appearance of mucocutaneous pigmentation in cases with a family history of PJS was significantly lower than that of patients without. There was no significant difference in the age of the appearance of mucocutaneous pigmentation and gender (P = 0.686, Z = -0.404). There was no significant difference in the age of the appearance of mucocutaneous pigmentation according to blood group (H = 2.3, P = 0.512). The age of the appearance of mucocutaneous pigmentation was positively correlated with the age of initial treatment (r = 0.197, P = 0.000). The age of initial treatment was positively correlated with small intestinal polyp burden (r = 0.097, P = 0.034) and colorectal polyp burden (r = 0.208, P = 0.000), and negatively correlated with the maximum diameter of colorectal polyps (r = -0.120, P = 0.024).
Interval time between age of mucocutaneous pigmentation and occurrence of gastrointestinal symptoms: A total of 529 cases (93.5%) had clear records; 3 cases (0.5%) had no data on gastrointestinal symptoms and 34 (6.0%) had missing data. The interval time was negatively correlated with the age of pigmentation (r = -0.175, P = 0.000). There was a statistical difference in the interval time of gastrointestinal symptoms according to the burden of colorectal polyps (χ2 = 70.476, P = 0.015). The greater the burden of colorectal polyps, the shorter the interval until the occurrence of gastrointestinal symptoms.
Gastrointestinal polyps: PJS polyps were distributed in the stomach in 421 cases (74.4%), in the small intestine in 546 (96.5%), and in the colorectum in 445 (78.6%). There were 26 cases (4.6%) with gallbladder polyps, 4 (0.7%) with nasal polyps, and 2 (0.35%) with uterine polyps. Regarding pathological type, there were 433 cases of hamartoma, 75 cases of adenoma, 32 cases of canceration, and 23 cases of hyperplastic and inflammatory polyps. There was no statistically significant difference between the maximum diameter of gastric polyps and that of small intestinal polyps (χ2 = 656.319, P = 0.991), but there was a statistically significant difference between the maximum diameter of gastric polyps and that of colorectal polyps (χ2 = 639.396, P = 0.026). There was a statistically significant difference between the diameters of small intestinal polyps and colorectal polyps (χ2 = 1443.082, P = 0.000). There was a statistically significant difference between the gastric polyp burden and small intestinal polyp burden (χ2 = 1000.592, P = 0.000), between the gastric polyp burden and colorectal polyp burden (χ2 = 468.22, P = 0.000), and between the small intestinal polyp burden and colorectal polyp burden (χ2 = 1739.598, P = 0.000).
Endoscopic examination and treatment: Among the 566 patients in this study, 522 (92.2%) underwent enteroscopy and treatment, and a total of 1381 small intestinal enteroscopies were completed (841 transoral small intestinal enteroscopies and 533 transanal small intestinal enteroscopies). About 7236 polyps were found through endoscopy (1959 gastric polyps, 3555 small intestinal polyps and 1722 colorectal polyps). 346 polyps < 5 mm were not treated, 1489 polyps between 5-10 mm were electrocoagulated and removed by argon plasma coagulation (APC), 917 polyps between 10-20 mm were removed by endoscopic mucosal resection (EMR) and snare polypectomy (SP), and 4484 polyps > 20 mm were removed by endoscopic mucosal dissection (ESD). After endoscopic treatment, 60 cases developed clinical symptoms (10 cases of abdominal discomfort, 35 cases of incomplete intestinal obstruction and 15 cases of gastrointestinal perforation and bleeding), and 47 cases (91.7%) were cured. However, 13 cases (2.3%) developed gastrointestinal perforation and bleeding, and accepted surgical operations for the perforation repair or partial resection of the small intestine. 329 patients (58.1%) underwent gastroscopy 673 times, 393 patients (69.4%) underwent colonoscopy 868 times, 38 patients (6.7%) underwent capsule endoscopy 41 times and 9 patients (1.6%) underwent gastroenterography examinations. There was a statistically significant difference in the number of small intestinal enteroscopies between patients with and without canceration (P = 0.004, Z = -2.882), which suggests that the greater number of follow-up enteroscopies, the easier the detection of polyp canceration.
Surgical treatment
Laparotomy is the most common type of surgery for the treatment of PJS, in which multiple polyps in the gastrointestinal are treated by intraoperative endoscopic resection or small incision removal, and it is necessary to removal part of the bowel in some severe cases. Due to the progress of endoscopic techniques in the treatment of PJS, we advise removing all the polyps that can be reached at the same time. Current indications for surgery include: (1) The endoscope could not reach the lesion; (2) The occurrence of acute intussusception and intestinal obstruction symptoms prevents endoscopic treatment; (3) Patients with sessile flat polyps, giant polyps, or densely distributed polyps which are difficult to remove by endoscopy; (4) Polyps pathologically confirmed to have become malignant or highly suspected of canceration under endoscopic observation; (5) The occurrence of perforation during endoscopic polyp resection or postoperative perforation which could not be treated conservatively; (6) The occurrence of major bleeding during or after endoscopic treatment which does not respond to conservative medical treatment; and (7) Patients or their family members request the disposable surgical removal of polyps. A total of 405 patients (71.6%) underwent 655 surgical procedures, 305 (75.6%) underwent abdominal surgery before the age of 35, 168 (29.7%) underwent surgical treatment twice or more, and 1 underwent surgical treatment seven times. With regard to the number of times of operation, these patients underwent nasopharyngeal polypectomy (n = 1), subtotal gastrectomy (n = 2), partial small bowel resection (n = 570), pancreaticoduodenectomy (n = 7), partialcolectomy (n = 22), left-semicolon resection (n = 2), right hemicolectomy resection (n = 4), pancolectomy (n = 11), radical resection of small bowel cancer (n = 18), radical operations for colon cancer (n = 6), radical resection of rectal carcinoma (n = 2), radical surgery for ovarian cancer (n = 3), radical hysterectomy (n = 3), radical resection of pulmonary carcinoma (n = 3), and modified radical mastectomy (n = 1). There was a statistically significant difference in the number of operations between patients with and without cancer (P = 0.000, Z = -5.127), and patients with cancer required more surgery.
Drug therapy
Of nine patients who were treated with oral celecoxib capsules 400 mg once daily after small intestinal enteroscopies, three developed allergic reactions and three developed hematochezia, for which the treatment was ceased. Two patients completed a 6-mo course of treatment and one completed a 9-mo course of treatment. The reexamination of small intestinal enteroscopies showed that the drug therapy was effective in only one case. Traditional Chinese medicine (TCM) was used in 30 cases and was shown to inhibit the growth of some small polyps < 1 cm, but its inhibitory effects on the growth of polyps ≥ 1 cm were unsatisfactory, with an overall effective rate of only 40.0%.
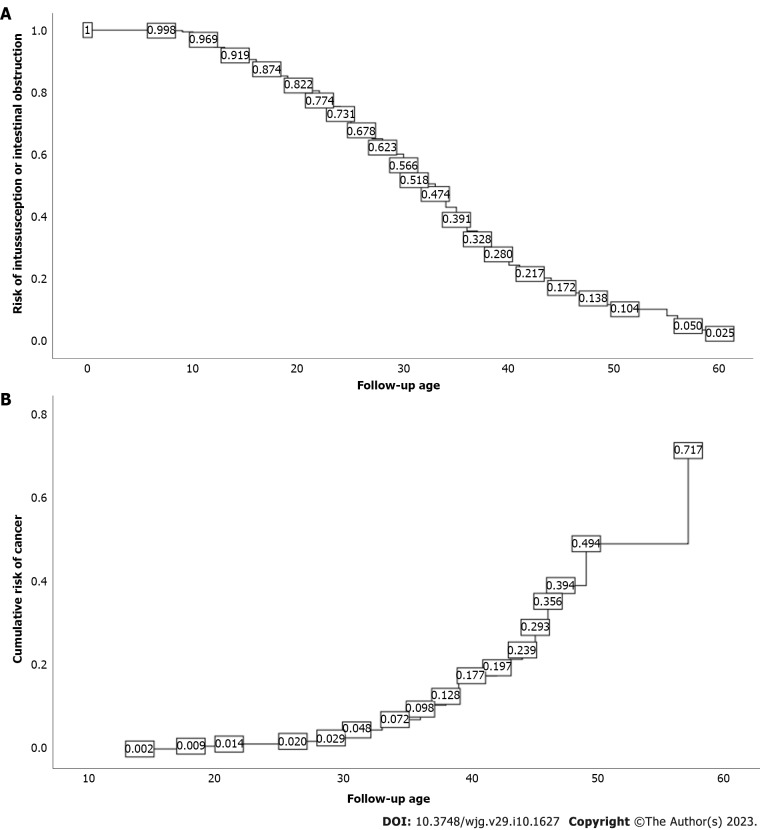
Follow-up
As of the final follow-up date of this study, 361 patients were followed more than twice at our hospital, and no death occurred. A total of 338 cases (189 males and 149 females with a median age of 27.5 years) had previously or concurrently developed intussusception, intestinal obstruction, or intestinal perforation. Kaplan-Meier survival analysis showed that, at the age of 40 years, the cumulative risk of intussusception in PJS patients could reach 72.0%, and at 50 years, the cumulative risk of intussusception could reach 89.6% (Figure 1A). Forty-six patients had malignant tumors (27 males and 19 females with a median age of 36.5 years); there were 18 cases of small bowel cancer, 8 cases of colorectal cancer, 6 cases of duodenal cancer, 3 cases of ovarian cancer, 3 cases of cervical cancer, 3 cases of lung cancer, 2 cases of gastric cancer, and 1 case each of cholangiocarcinoma, breast cancer, and nasopharyngeal cancer. Kaplan-Meier survival analysis showed that, at the age of 50 years, the cumulative risk of cancer in PJS patients could reach 49.3%, and at 60 years, the cumulative risk of cancer could reach 71.7% (Figure 1B).
Figure 1.
Cumulative risk. A: Intussusception; B: Cancer.
DISCUSSION
This study found that 90.1% of PJS patients developed mucocutaneous pigmentation before the age of 7 years. The median time between the appearance of mucocutaneous pigmentation and the occurrence of abdominal symptoms was 10 years. Therefore, as PJS patients are often outpatients undergoing dermatological and stomatological treatment for mucocutaneous pigmentation, which is easy for doctors unfamiliar with the disease to misdiagnose, and valuable early treatment opportunities may thus be missed, clinicians should be more vigilant. PJS that does not cause the malignant transformation of mucocutaneous pigmentation on the lips and limbs generally does not need to be treated[10]. Some scholars used a 755 nm picosecond laser to treat mucocutaneous pigmentation on the lips, which showed a reduction in pigmentation of 50%-75% after 3 mo, and good recovery after surgery[11].
At present, there is no effective treatment for PJS polyps. Some scholars used everolimus (mechanistic target of rapamycin inhibitor, a derivative of rapamycin) to treat two PJS patients, and the results showed that pancreatic cancer progressed after 2 mo in one patient, while the other refused to continue treatment due to severe toxicity, so the trial was discontinued[12]. One study[13] found that patients with PJS (2/6) responded well to celecoxib and had fewer gastric polyps, suggesting that COX-2 inhibitors may be beneficial in PJS therapy. However, in our study, celecoxib capsules were used to prevent polyps, and it was effective in only one case. This suggests that COX-2 inhibitors may not be ideal drugs for PJS therapy, as they had a high proportion of side effects (6/9) such as allergic reaction and gastrointestinal bleeding. We also treated 30 PJS patients with TCM, which had an effective rate for small polyps of only 40%, while being basically ineffective for large polyps. This study showed that PJS polyps could be distributed in the stomach (74.4%), small intestine (96.5%), and colorectum (78.6%). The burden and diameter of small intestine polyps were much higher than those of stomach polyps, which was the root cause of intussusception and intestinal obstruction. We found that some PJS patients had concurrent gallbladder polyps, nasal polyps, cervical polyps, etc. Whether these are the extragastrointestinal manifestations of PJS polyps still needs to be supported by pathological evidence. Endoscopic treatment methods for PJS polyps include EMR and ESD[14,15]. Our study showed that the incidence of gastrointestinal bleeding, perforation, and other serious complications during colonoscopy and endoscopic treatment was 2.3%. Overall, the endoscopic treatment of PJS polyps is safe and feasible.
Due to gastrointestinal polyps occurring in multiple places, patients often undergo multiple laparotomy operations, which can cause severe intraperitoneal adhesion. Laparoscopic surgery is difficult and not recommended as a routine treatment method for PJS. Laparoscopic surgery and small intestinal endoscopic surgery could be used for abdominal exploration or patients with moderate abdominal adhesions. Laparoscopic perforation repair or hemostatic suture can be used as adjuvant treatments for endoscopic complications when removing large localized polyps and dense intestinal segments of polyps with perforation or bleeding during endoscopic treatment.
Open surgery for PJS patients should be explored to determine the distribution of polyps in the whole gastrointestinal tract. Following the principle of organ protection, it is recommended to perform intestinal adhesion release and try to avoid large sections of intestinal resection causing short bowel syndrome. Multiple small incisions can be made in the intestinal wall to remove all palpable polyps, but when encountering necrotic, perforated, or dense bowel polyps, bowel segment resection is recommended. For patients diagnosed with malignant transformation, radical resection and regional lymph node dissection should be performed on cancerous intestinal segments.
PJS is a tumor-susceptible syndrome[16-18], and it has been previously reported that PJS polyps have a evolvement sequence of hamartoma-adenoma-cancer[19]. In our study, 46 cases were pathologically confirmed to have a malignant tumor, of which 35 (76.1%) were malignant transformation of PJS polyps and the rest were breast cancer, cervical cancer, lung cancer, ovarian cancer, nasopharyngeal cancer, and cholangiocarcinoma. The cumulative cancer risk of PJS patients reached 49.3% at the age of 50 years and 71.7% at the age of 60 years. Considering the short follow-up time for some cases, the actual cumulative risk of carcinogenesis may be higher. For PJS patients, we should also pay attention to their mental health, especially adolescent PJS patients. With the deepening of their understanding of PJS, e.g., it is a genetic disease which requires long-term hospitalization, they may gradually develop the psychology of hating their parents and retaliating against society. We also found that PJS patients have different degrees of concern about the risk of cancer, which reminds us that we should strengthen psychological counseling and provide professional interpretation for PJS patients to reduce their anxiety and depression, and where necessary, psychologists should be involved in the treatment process of PJS patients.
Our study showed that there was a statistically significant difference in the age of the occurrence of mucocutaneous pigmentation between patients with and without a family history of PJS (P = 0.016, Z = -2.415). As such, we suggest that suspected or already confirmed PJS patients should undergo next generation sequencing, which can reliably quantify the incidence, penetrance, mutation type, and expression of PJS. When adult patients are pregnant, they can receive preimplantation genetic testing or preimplantation genetic screening to predict whether there is a correlation with STK11 or other gene mutation[20,21]. If screening finds that the fetus is a carrier of the STK11 gene mutation, the parents can terminate the pregnancy; thus, this approach makes it possible to have healthy children born.
At present, there is no unified protocol for the follow-up of PJS polyps. Our study showed that the occurrence time of mucocutaneous pigmentation in most patients is earlier than the occurrence time of gastrointestinal symptoms, which is a good window period in which to intervene in the development of polyps. We suggest that PJS patients should start endoscopic examinations when they are 10 years old, and the endoscopic treatment of gastrointestinal polyps ≥ 10 mm in diameter should be performed to prevent intussusception and intestinal obstruction. The whole gastrointestinal tract should be explored by oral and transanal small intestinal enteroscopy as far as possible. Cold forceps polypectomy and cold SP or APC can be used on polyps < 10 mm which are sessile or flat; combined with high-frequency electroresection, effective eradication can be achieved without complications such as bleeding and perforation. If small intestinal enteroscopy exploration finds moderate intussusception, incomplete intestinal obstruction, and a perforation area < 1 cm, conservative treatment methods can be used, such as fasting, fluid infusion, gastrointestinal decompression, endoscopic balloon reduction, or perforation repair with titanium clips. If polyps ≥ 10 mm are detected, EMR or ESD will be required to remove them, but the possibility of perforation should be noted. Early surgical treatment should be performed for irretrievable intussusception, complete intestinal obstruction, intestinal perforation area ≥ 1 cm, malignant polyps, and failure of conservative treatment. PJS patients < 10 years old may undergo noninvasive examinations such as abdominal ultrasound, CT, capsule endoscopy, gastrointestinal contrast, or magnetic resonance imaging to assess the burden and diameter of their gastrointestinal polyps. If polyps < 10 mm are found, resection can be attempted for a smaller quantity of polyps, but if they cannot be removed, a biopsy will be required to determine the pathology of the polyps and estimate the next follow-up time, and the growth rate of the polyps needs to be closely monitored. For giant polyps, multiple endoscopic resections should be carried out, but for diffuse distributions of polyps, the complete resection of all polyps in the entire intestinal canal should not be excessively pursued in order to prevent short bowel syndrome, and the patients should be monitored every 1 to 2 years (Figure 2).
Figure 2.
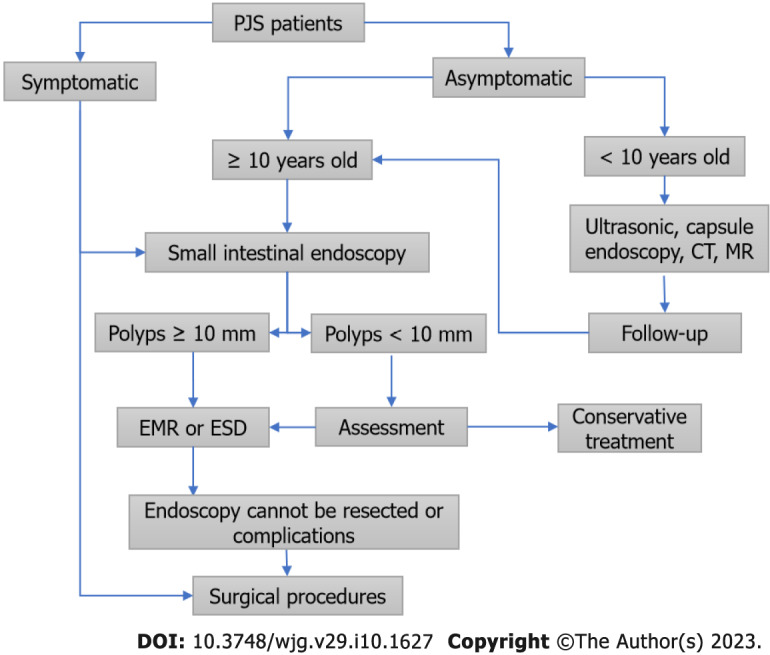
Diagnosis and treatment process of Peutz-Jeghers syndrome patients. PJS: Peutz-Jeghers syndrome; CT: Computed tomography; MR: Magnetic resonance imaging; EMR: Endoscopic mucosal resection; ESD: Endoscopic mucosal dissection.
CONCLUSION
The development of gastrointestinal symptoms in PJS patients is closely related to the age of the appearance of mucocutaneous pigmentation and polyp burden and diameter. The later the age of mucocutaneous pigmentation, the more severe the gastrointestinal symptoms, and patients receive more frequent operations and hospitalization. As PJS polyps have a high risk of intussusception and carcinogenesis, patients aged ≥ 10 years should undergo a small intestinal enteroscopy every 1–2 years. Endoscopic treatment has a good safety profile and can have significant beneficial effects for PJS patients, and timely endoscopic treatment can reduce the risk of the intussusception and carcinogenesis of polyps.
ARTICLE HIGHLIGHTS
Research background
Peutz-Jeghers syndrome (PJS) is a clinically rare autosomal dominant inherited disease with pigmented spots on the lips and mucous membranes and extremities, scattered gastrointestinal polyps, and susceptibility to tumors as clinical manifestations. Effective preventive and curative methods are still lacking.
Research motivation
Here we summarize out experience with 566 Chinese patients with PJS from a Chinese medical center our experience with 566 Chinese patients with PJS from a Chinese medical center with regard to the clinical features, diagnosis, and treatment, in order to promote the clinical understanding of PJS and improve its clinical diagnosis and treatment.
Research objectives
To explore the clinical features, diagnosis, and treatment of PJS in Chinese patients.
Research methods
The clinical data of 566 PJS cases admitted to the Air Force Medical Center from January 1994 to October 2022 was retrospectively analyzed, including age, gender, ethnicity, family history, first treatment age, time and sequence of appearance of mucocutaneous pigmentation, polyp distribution, polyp quantity and diameter, frequency of hospitalization, frequency of surgical operations, etc.
Research results
Of all the patients included, 55.3% were male and 44.7% were female. Median time to the appearance of mucocutaneous pigmentation was 2 years, and median time from the appearance of mucocutaneous pigmentation to the occurrence of abdominal symptoms was 10 years. The vast majority (92.2%) of patients underwent small bowel endoscopy and treatment, with 2.3% having serious complications. There was a statistically significant difference in the number of enteroscopies between patients with and without canceration (P = 0.004, Z = -2.882); 71.2% of patients underwent surgical operation, 75.6% of patients underwent surgical operation before the age of 35 years, and there was a statistically significant difference in the frequency of surgical operations between patients with and without cancer (P = 0.000, Z = -5.127). At 40 years of age, the cumulative risk of intussusception in PJS was approximately 72.0%, and at 50 years, the cumulative risk of intussusception in PJS was approximately 89.6%. At 50 years of age, the cumulative risk of cancer in PJS was approximately 49.3%, and at 60 years of age, the cumulative risk of cancer in PJS was approximately 71.7%.
Research conclusions
The risk of intussusception and cancer of PJS polyps increases with age. PJS patients ≥ 10 years old should undergo annual enteroscopy. Endoscopic treatment has a good safety profile and can reduce the occurrence of polyp intussusception and cancer. Surgery should be conducted to protect the gastrointestinal system by removing polyps.
Research perspectives
The clinical data of 566 PJS cases diagnosed and treated in a Chinese medical center were retrospectively analyzed, and the clinical characteristics and diagnosis and treatment process of Chinese PJS are summarized.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Air Force Medical Center, PLA Institutional Review Board (No. 2020-105-PJ01).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 12, 2022
First decision: January 11, 2023
Article in press: February 16, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Awai K, Japan; Sjoblom T, Sweden S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
Contributor Information
Zu-Xin Xu, Fifth Clinical College of Anhui Medical University, Air Force Clinical College of Anhui Medical University, Beijing 100142, China.
Li-Xin Jiang, Air Force Clinical College of China Medical University, Beijing 100142, China.
Yu-Rui Chen, Air Force Clinical College of China Medical University, Beijing 100142, China.
Yu-Hui Zhang, Department of General Surgery, Air Force Medical Center, Chinese People's Liberation Army, Beijing 100142, China.
Zhi Zhang, Department of General Surgery, Air Force Medical Center, Chinese People's Liberation Army, Beijing 100142, China.
Peng-Fei Yu, Department of General Surgery, Air Force Medical Center, Chinese People's Liberation Army, Beijing 100142, China.
Zhi-Wei Dong, Department of General Surgery, Air Force Medical Center, Chinese People's Liberation Army, Beijing 100142, China.
Hai-Rui Yang, Department of General Surgery, Air Force Medical Center, Chinese People's Liberation Army, Beijing 100142, China.
Guo-Li Gu, Department of General Surgery, Air Force Medical Center, Chinese People's Liberation Army, Beijing 100142, China. kzggl@163.com.
Data sharing statement
No additional data are available.
References
- 1.Sengupta S, Bose S. Peutz-Jeghers Syndrome. N Engl J Med. 2019;380:472. doi: 10.1056/NEJMicm1806623. [DOI] [PubMed] [Google Scholar]
- 2.Klimkowski S, Ibrahim M, Ibarra Rovira JJ, Elshikh M, Javadi S, Klekers AR, Abusaif AA, Moawad AW, Ali K, Elsayes KM. Peutz-Jeghers Syndrome and the Role of Imaging: Pathophysiology, Diagnosis, and Associated Cancers. Cancers (Basel) 2021;13 doi: 10.3390/cancers13205121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Jong MA, van Leerdam ME, Offerhaus GJAJ, Keller JJ. [100 years Peutz-Jeghers syndrome] Ned Tijdschr Geneeskd. 2022;166 [PubMed] [Google Scholar]
- 4.Kalliakmanis V, Perysinakis I, Koutsouvas K, Karras P, Margaris E, Angelakis C. Massive intussusception caused by a solitary Peutz-Jeghers type hamartomatous polyp. Ann R Coll Surg Engl. 2018;100:e91–e93. doi: 10.1308/rcsann.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto M, Iwamoto K, Suzuki R, Mukai Y, Takeoka T, Asukai K, Shinno N, Hara H, Kanemura T, Nakai N, Hasegawa S, Sugimura K, Haraguchi N, Nishimura J, Wada H, Takahashi H, Matsuda C, Yasui M, Omori T, Miyata H, Ohue M, Murata M. Laparoscopic-assisted disinvagination and polypectomy for multiple intussusceptions induced by small intestinal polyps in patients with Peutz-Jeghers syndrome: a case report. World J Surg Oncol. 2021;19:22. doi: 10.1186/s12957-021-02133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Wang Z, Wang Y, Wu J, Yu Z, Chen C, Chen J, Wu B, Chen Y. High risk and early onset of cancer in Chinese patients with Peutz-Jeghers syndrome. Front Oncol. 2022;12:900516. doi: 10.3389/fonc.2022.900516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SH, Kim ER, Park JJ, Kim ES, Goong HJ, Kim KO, Nam JH, Park Y, Lee SP, Jang HJ KSGE; Research Group of Capsule Endoscopy and Artificial Intelligence in Imaging. Cancer risk in patients with Peutz-Jeghers syndrome in Korea: a retrospective multi-center study. Korean J Intern Med. 2022 doi: 10.3904/kjim.2022.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latchford A, Cohen S, Auth M, Scaillon M, Viala J, Daniels R, Talbotec C, Attard T, Durno C, Hyer W. Management of Peutz-Jeghers Syndrome in Children and Adolescents: A Position Paper From the ESPGHAN Polyposis Working Group. J Pediatr Gastroenterol Nutr. 2019;68:442–452. doi: 10.1097/MPG.0000000000002248. [DOI] [PubMed] [Google Scholar]
- 9.Wagner A, Aretz S, Auranen A, Bruno MJ, Cavestro GM, Crosbie EJ, Goverde A, Jelsig AM, Latchford A, Leerdam MEV, Lepisto A, Puzzono M, Winship I, Zuber V, Möslein G. The Management of Peutz-Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline. J Clin Med. 2021;10 doi: 10.3390/jcm10030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu M, Krishnamurthy K. Peutz-Jeghers Syndrome. 2022 Aug 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan- [PubMed] [Google Scholar]
- 11.Zeng R, Wu Q, Guo L, Lin T. Successful treatment of oral pigmented spots in Chinese subjects with Peutz-Jeghers syndrome using a 755-nm picosecond laser. Indian J Dermatol Venereol Leprol. 2021;87:288–289. doi: 10.25259/IJDVL_151_20. [DOI] [PubMed] [Google Scholar]
- 12.de Brabander J, Eskens FALM, Korsse SE, Dekker E, Dewint P, van Leerdam ME, van Eeden S, Klümpen HJ. Chemoprevention in Patients with Peutz-Jeghers Syndrome: Lessons Learned. Oncologist. 2018;23:399–e33. doi: 10.1634/theoncologist.2017-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udd L, Katajisto P, Rossi DJ, Lepistö A, Lahesmaa AM, Ylikorkala A, Järvinen HJ, Ristimäki AP, Mäkelä TP. Suppression of Peutz-Jeghers polyposis by inhibition of cyclooxygenase-2. Gastroenterology. 2004;127:1030–1037. doi: 10.1053/j.gastro.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 14.Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270–297. doi: 10.1055/s-0043-102569. [DOI] [PubMed] [Google Scholar]
- 15.Yang DH, Luvsandagva B, Tran QT, Fauzi A, Piyachaturawat P, Soe T, Wong Z, Byeon JS. Colonoscopic Polypectomy Preferences of Asian Endoscopists: Results of a Survey-Based Study. Gut Liver. 2021;15:391–400. doi: 10.5009/gnl20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Friedl W, Møller P, Hes FJ, Järvinen H, Mecklin JP, Nagengast FM, Parc Y, Phillips RK, Hyer W, Ponz de Leon M, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen JT, Clark SK, Hodgson SV. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975–986. doi: 10.1136/gut.2009.198499. [DOI] [PubMed] [Google Scholar]
- 17.Tavusbay C, Acar T, Kar H, Atahan K, Kamer E. The patients with Peutz-Jeghers syndrome have a high risk of developing cancer. Turk J Surg. 2018;34:162–164. doi: 10.5152/turkjsurg.2017.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boland CR, Idos GE, Durno C, Giardiello FM, Anderson JC, Burke CA, Dominitz JA, Gross S, Gupta S, Jacobson BC, Patel SG, Shaukat A, Syngal S, Robertson DJ. Diagnosis and Management of Cancer Risk in the Gastrointestinal Hamartomatous Polyposis Syndromes: Recommendations From the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022;162:2063–2085. doi: 10.1053/j.gastro.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Latchford AR, Clark SK. Gastrointestinal aspects of Peutz-Jeghers syndrome. Best Pract Res Clin Gastroenterol. 2022;58-59:101789. doi: 10.1016/j.bpg.2022.101789. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan-Pyke C, Dokras A. Preimplantation Genetic Screening and Preimplantation Genetic Diagnosis. Obstet Gynecol Clin North Am. 2018;45:113–125. doi: 10.1016/j.ogc.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Leaver M, Wells D. Non-invasive preimplantation genetic testing (niPGT): the next revolution in reproductive genetics? Hum Reprod Update. 2020;26:16–42. doi: 10.1093/humupd/dmz033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.