Abstract
Hepatocellular carcinoma (HCC) is most commonly found in the context of liver cirrhosis and, in rare cases, in a healthy liver. Its prevalence has risen in recent years, particularly in Western nations, due to the increasing frequency of non-alcoholic fatty liver disease. Advanced HCC has a poor prognosis. For many years, the only proven therapy for unresectable HCC (uHCC) was sorafenib, a tyrosine kinase inhibitor. Recently, the synergistic effect of an immune checkpoint inhibitor, atezolizumab, and bevacizumab outperformed sorafenib alone in terms of survival, making it the recommended first-line therapy. Other multikinase inhibitors, lenvatinib and regorafenib, were also recommended as first and second-line drugs, respectively. Intermediate-stage HCC patients with retained liver function, particularly uHCC without extrahepatic metastasis, may benefit from trans-arterial chemoembolization. The current problem in uHCC is selecting a patient for the best treatment while considering the preexisting liver condition and liver function. Indeed, all study patients had a Child-Pugh class A, and the best therapy for other individuals is unknown. Additionally, in the absence of a medical contraindication, atezolizumab could be combined with bevacizumab for uHCC systemic therapy. Several studies are now underway to evaluate immune checkpoint inhibitors in combination with anti-angiogenic drugs, and the first findings are encouraging. The paradigm of uHCC therapy is changing dramatically, and many obstacles remain for optimum patient management in the near future. The purpose of this commentary review was to give an insight into current systemic treatment options for patients with uHCC who are not candidates for surgery to cure the disease.
Keywords: Hepatocellular carcinoma, Unresectable hepatocellular carcinoma, Non-alcoholic fatty liver disease, Tyrosine kinase inhibitor, Sorafenib, Lenvatinib, Immune checkpoint inhibitor, Atezolizumab, Bevacizumab
Core Tip: Hepatocellular carcinoma (HCC) is a major health problem that is the fourth leading cause of cancer-related mortality worldwide. The 5-year survival rate was nearly 19%, but only 2% in metastatic HCC. The first oral multikinase inhibitor for the systemic treatment of advanced or unresectable HCC (uHCC) was sorafenib. However, when compared to sorafenib, the combination of atezolizumab and bevacizumab increased survival rates and was authorized as first-line treatment for uHCC. Regorafenib and cabozantinib are suggested for use as second-line drugs in the event that the disease progresses. Transarterial chemoembolization for palliative care or downstaging is also suggested. This review focused on systemic therapy for uHCC patients who are not appropriate for liver-directed therapy.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common kind of primary liver cancer and a serious public health concern globally. With 905677 new cases and 830180 fatalities in 2020, liver cancer is the sixth most common malignancy and the third major cause of cancer mortality. The age-adjusted global incidence is 9.5 deaths per 100000 and the mortality rate is 8.7 per 100000 people[1]. HCC typically develops in the presence of cirrhosis, less frequently in chronic liver disease that is not cirrhotic, and very rarely in a healthy liver. The risk factors for HCC are well-known and vary by geographic location. In Asia and Africa, viral hepatitis is the main cause of HCC, but in North America and Western Europe, fatty liver disease and obesity are the main causes. The length of time that these risk factors have been detected in human populations is strongly correlated with the global increase in HCC incidence[2-4]. Depending on the tumor stage and liver function, the prognosis and treatment plan for HCC in cirrhotic livers are determined (Child–Pugh score). With a 5-year relative survival rate of 18.4%, overall survival (OS) is poor. Patients with localized, regional, and metastasis have 5-year survival rates of 33%, 10%, and 2%, respectively[5]. HCC may be treatable in its early stages by resection, liver transplantation, or ablation. However, patients are typically identified at intermediate or advanced stages due to a lack of symptoms.
For patients with HCC, the Barcelona Clinic Liver Cancer (BCLC) staging system employs many factors to direct the treatment course of action. The Child-Pugh score is comprised of the Eastern Cooperative Oncology Group (ECOG) performance status (PS), tumor burden (including portal invasion status and hepatic spread), and an estimation of the underlying liver function that should be estimated in addition to the Child-Pugh score using the Model for End-Stage Liver Disease score in cases of decompensated cirrhosis or the alpha-fetoprotein (AFP) concentration and albumin-bilirubin score in cases of compensated liver disease. HCC patients are classified into four stages: Very early (BCLC 0), early (BCLC A), intermediate (BCLC B), unresectable (BCLC C), and end-stages (BCLC D). Nearly 40% of HCC patients have an early diagnosis, making them eligible for curative procedures such as local radiofrequency ablation or surgery (liver transplantation or hepatic resection). A systemic, chemoembolization, or radioembolization treatment is necessary for more than half of them, which is in the intermediate stage and is unresectable (BCLC B and C)[6].
Unfortunately, systemic therapy is the only treatment available for individuals with HCC because more than 50% of cases are discovered at an advanced stage. In addition, recurrences occur in around 70% of individuals who have had their main tumor surgically removed[7]. When liver-directed medicines are not a possibility or the patient has experienced a significant recurrence, systemic therapy is chosen. Systemic treatments, including tyrosine kinase inhibitors (TKIs), monoclonal antibodies, and immune checkpoint inhibitors, have been made possible by recent technological advancements. The FDA has officially authorized sorafenib and lenvatinib as first-line therapies for advanced HCC. Second-line treatments for individuals who advanced or did not tolerate sorafenib include cabozantinib, regorafenib, ramucirumab, nivolumab, and pembrolizumab. The United States FDA recently approved the use of neurotrophic tyrosine receptor kinase (NTRK) inhibitors, larotrectinib or entrectinib, in HCC patients with NTRK fusion-positive solid tumors. Although cytokine treatment [interferon alpha-2b, interleukin (IL)-12] produced disappointing results, nivolumab and pembrolizumab have shown promising outcomes in phase II studies in terms of progression-free survival (PFS)[8-11]. Nivolumab in first-line treatment and pembrolizumab in second-line therapy phase III trials' primary endpoints, however, were not achieved[12,13]. The preliminary findings of a phase III study showed that atezolizumab with bevacizumab improved OS and PFS when compared to sorafenib as first-line therapy for HCC[14].
The development of effective HCC therapeutics is complicated by tumor heterogeneity caused by multiple risk factors. Greater understanding of the heterogenic tumorigenic pathways should provide information on tumor biomarkers, genomes, and other tumor characteristics that predict response to targeted treatment in HCC. In this review, we aimed to address the carcinogenesis of HCC, clinical trials that assessed medications targeting these tumorigenic pathways, and future directions of targeted treatment in advanced HCC.
PATHOGENESIS
Several pathways have been associated with the development of HCC. These pathways have served as the primary targets for systemic therapy development.
Mitogen-activated protein kinase pathway
In order to activate or deactivate their target, the mitogen-activated protein kinases (MAPK) phosphorylate either their own dual serine and threonine residues or those present on their substrates. As a common downstream pathway for numerous tyrosine kinase receptors, the MAPK pathway is a biological signaling system that controls crucial physiological processes such as cell proliferation, cell differentiation, stress responses, apoptosis, and immune defense. When external growth stimuli bind to these tyrosine kinase receptors, the MAPK cascade is initiated. An MAP3K stimulates an MAP2K, which in turn activates an MAPK, in an MAPK module. MAPK protein phosphatases, which dephosphorylate both phosphothreonine and phosphotyrosine residues on MAPKs, can inhibit MAPK phosphorylation processes. The most prevalent tyrosine kinase receptors are the insulin-like growth factor receptor, platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR), and epidermal growth factor receptor (EGFR). Growth factors that bind to these receptors cause a phosphorylation cascade, which in turn activates the adaptor molecular complex (growth factor receptor-bound protein 2-Src homology and collagen-son of sevenless). The proto-oncogenes rat sarcoma virus (Ras), rapidly accelerated fibrosarcoma (Raf), and guanosine triphosphatase are subsequently activated by this complex, leading to the activation of mitogen-activated protein kinase (MEK) 1/2 and extracellular signal-regulated kinase (ERK) 1/2 downstream. Eventually, this pathway results in the upregulation of gene transcription, which encourages cell proliferation, through the transcriptional activators c-Jun and c-Fos[15,16]. It has also been demonstrated that ERK phosphorylates proteins are involved in cell proliferation, apoptosis resistance, and angiogenesis in HCC[17,18]. Ras mutations were discovered to occur often in HCC, according to several studies, and overexpression of this pathway, particularly in high-grade tumors, has been reported in HCC[19,20]. Sorafenib, which inhibits the Raf serine/threonine kinase as well as several other receptors, was developed on the basis of this pathway[21,22].
Phosphoinositide 3-kinase–Ak strain transforming–mammalian target of rapamycin pathway
Cell development and regulation also depend on the phosphoinositide 3-kinase (PI3K)-Ak strain transforming (AKT)-mammalian target of rapamycin (mTOR) signaling pathway. Growth factors including epidermal growth factor and IL-2 bind to their associated tyrosine kinase receptors to activate PI3K. Phosphoinositol triphosphate, a lipid second messenger produced by PI3K, activates the serine/threonine kinase AKT (protein kinase B). After that, AKT phosphorylates a number of proteins, including the proapoptotic protein Bcl-2 opponent of cell death, to activate the mTOR family of proteins (BAD). To promote cell cycle progression and unrestricted growth and proliferation, mTOR regulates the phosphorylation of the translational repressor proteins ribosomal protein S6 kinase beta-1 (P70S6 kinase) and eukaryotic translation initiation factor 4E-binding protein 1 (EIF4EBP1 or 4E-BP-1)[23-25]. Studies have shown that cancers with abnormal expression of the pathway including phosphatase and tensin homolog (PTEN), AKT, and PS6 are more severe and have a poorer prognosis overall than tissues with normal expression[26,27]. In approximately half of all HCC patients, expression of the tumor suppressor gene product PTEN, which generally suppresses PI3K activity, is significantly decreased. Due to the gene deletion process, PTEN is inactivated, which results in uncontrolled PI3K activity and downstream phosphorylation of proteins that inhibit apoptosis and encourage tumor growth[28,29].
Wingless and Int-1/β-catenin pathway
The Wingless and Int-1 (WNT)/β-catenin signaling system regulates embryonic development, cellular proliferation, and differentiation. It is a highly conserved and carefully regulated molecular process. Notably, growing data suggests that abnormal WNT/β-catenin signaling increases the formation and progression of HCC. The two distinct WNT signaling pathways, known as non-canonical and canonical, with the latter including β-catenin activation. Comprehensive genomic analyses have revealed that β-catenin-encoding CTNNB1 and AXIN1 gain-of-function mutations are present in about 35% of human HCC samples. Human HCCs with activated WNT/β-catenin pathways display unique gene expression patterns and malignant properties. Through its downstream effectors, activated WNT/β-catenin interacts with a variety of signaling pathways to encourage the development of HCC. As a result, medications that target WNT/β-catenin have being looked into as possible HCC treatments[30,31].
SYSTEMIC TREATMENT FOR UNRESECTABLE HCC
First-line systemic therapy
Single drug multikinase inhibitor: Sorafenib-A multikinase inhibitor (MTKI), sorafenib blocks tyrosine kinases and pathways essential for angiogenesis and cell proliferation. VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-β, KIT, RET, RAS/RAF/MAPK, FLT-3, and Janus kinase/signal transducer and activator of transcription protein are among the receptors that it inhibits (STAT)[32]. The multicenter phase III European Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) research and the Asia-Pacific trial on OS benefit compared with placebo both supported the efficacy of first-line sorafenib for uHCC patients with Child-Pugh class A cirrhosis. The inclusion criteria for both studies were the same: Advanced HCC with detectable illness, mostly Child-Pugh class A cirrhosis, no prior systemic therapy, adequate hematological, renal, and hepatic function, and a life expectancy of at least 12 wk. Six hundred and two untreated uHCC patients from Europe, North America, South America, and Australia were included in the SHARP study. Two groups of patients were randomly treated with either sorafenib 400 mg (n = 299) twice daily or a placebo (n = 303). Treatment with sorafenib was continued until the illness became worse, the toxicity was too much, or someone passed away. Patients receiving sorafenib experienced response rates of just 2%, compared with only 1% in the placebo group. Clinical outcomes were achieved in 43% and 32% of patients in the sorafenib and placebo groups, respectively (P = 0.002). The project was halted after a second planned interim analysis showed that the median OS with sorafenib was significantly longer at 10.7 mo compared to 7.9 mo with placebo (P < 0.01). With sorafenib, 1-year survival rates were 44%, while with placebo, they were 33% (P < 0.01). The most common treatment-related side effects in people taking sorafenib were diarrhea, weight loss, hand-foot syndrome (HFS), and hypophosphatemia[33] (Table 1).
Table 1.
Summary of phase 3 trials of sorafenib and lenvatinib for treatment of unresectable hepatocellular carcinoma
|
Ref.
|
No. of patients (Child-Pugh A/B)
|
Treatment or placebo
|
Median OS in mo
|
Response rate, %
|
Control rate, %
|
Median TP in mo
|
Dose reduction, % patients
|
Discontinuation due to AE, % patients
|
| Llovet et al[33] (SHARP) | 298 (284/14) | Sorafenib (400 mg × 2/d) | 10.7 | 2 | 43 | 5.5 | 26 | 11 |
| 303 (297/6) | Placebo | 7.9 | 1 | 32 | 2.8 | 7 | 5 | |
| Cheng et al[34] (Asia-Pacific) | 150 (146/4) | Sorafenib (400 mg × 2/d) | 6.5 | 3.3 | 35.3 | 5.5 | 30.9 | 19.5 |
| 76 (74/2) | Placebo | 4.2 | 1.3 | 15.8 | 2.8 | 2.7 | 13.3 | |
| Kudo et al[47] (REFLECT) | 478 (478/0) | Lenvatinib (12 mg/d) | 13.6 | 40.6 | 73.8 | 7.4 | 37 | 9 |
| 476 (476/0) | Sorafenib (400 mg × 2/d) | 12.3 | 12.4 | 56.4 | 3.7 | 38 | 7 |
AE: Adverse event; OS: Overall survival; SHARP: Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol; TP: Time to progression.
The Asia-Pacific study, which ran alongside the SHARP trial, was designed to look at the safety and activity of sorafenib in uHCC patients. They randomly assigned 150 of the 226 participants who were drawn from 23 locations in China, South Korea, and Taiwan to treat with sorafenib 400 mg twice daily or a placebo (n = 76). Similar to the SHARP study, sorafenib had a low response rate (3.3% vs 1.3% for placebo). Sorafenib had a disease control rate of 35.3% whereas a placebo had a rate of 15.8% (P < 0.001). The median OS periods for sorafenib and the placebo were 6.5 mo and 4.2 mo, respectively (P = 0.014). Despite the fact that both studies utilized the identical eligibility standards and treatment strategy, the Asia-Pacific trial's patients had more extrahepatic disease, more hepatic lesions, a worse PS, more advanced disease, and a higher rate of AFP elevation. Because of this, their sorafenib-related survival in the Asia-Pacific study (6.5 mo) was lower than in the SHARP trial (10.7 mo). In the subgroup analysis, sorafenib showed higher efficacy in patients without extrahepatic spread [hazard ratio (HR): 0.55 vs 0.84], with hepatitis C-associated illness (HR: 0.47 vs 0.81), and with a low neutrophil-to-lymphocyte ratio (HR: 0.59 vs 0.84)[34] (Table 2).
Table 2.
Summary of the 2 large prospective observational studies of sorafenib for treatment of unresectable hepatocellular carcinoma
|
Ref.
|
No. of patients (Child-Pugh A or Child-Pugh B)
|
Treatment
|
Median OS in mo
|
Response rate, %
|
Control rate, %
|
Median TP in mo
|
Dose reduction, % patients
|
Discontinuation due to AE, % patients
|
| Abou-Alfa et al[68] | 98 | Sorafenib (400 mg × 2/d) | 10.7 | 2 | 43 | 5.5 | 26 | 11 |
| 38 | Sorafenib (400 mg × 2/d) | 7.9 | 1 | 32 | 2.8 | 7 | 5 | |
| Cheng et al[34] (Asia-Pacific) | 150 (146/4) | Sorafenib (400 mg × 2/d) | 6.5 | 3.3 | 35.3 | 5.5 | 30.9 | 19.5 |
| 76 (74/2) | Placebo | 4.2 | 1.3 | 15.8 | 2.8 | 2.7 | 13.3 |
AE: Adverse event; OS: Overall survival; TP: Time to progression.
In a subset analysis of the SHARP trial, Bruix et al[35] looked at how the etiology of the illness, the tumor load, PS, the tumor stage, and past treatments affected survival, disease control, time to progression (TTP), and safety. Compared to placebo, sorafenib treatment significantly enhanced survival and disease control regardless of the etiology, tumor load, PS, tumor stage, or previous therapy. Except for individuals who were hepatitis B virus (HBV)-positive, sorafenib also reliably reduced the median time to progression (MTP). The relationship between the pre-study liver state and the effects of sorafenib on liver function were investigated in a second subgroup analysis of the SHARP trial data. Individuals with baseline transaminase, AFP, or bilirubin levels elevation showed shorter survival periods than those with normal baseline values, regardless of therapy. Patients with normal or high liver markers experienced the same level of safety. The investigators came up with the conclusion that sorafenib was safe and efficient independent of the liver biomarkers present at baseline and that bilirubin levels, which are used to evaluate hepatic function, were consistent during sorafenib treatment. It should be highlighted that the majority of the trial participants had Child-Pugh class A cirrhosis. Therefore, individuals with more severe cases (Child-Pugh class B and C) should not extrapolate from these findings[36].
Sorafenib was also further studied in two large prospective observational trials, GIDEON and INSIGHT. The GIDEON research, or Global Investigation of Therapeutic DEcisions in HCC and of its Treatment with Sorafenib, examined the safety and tolerability of sorafenib in patients with advanced HCC in actual clinical settings. All treatment choices in this study were made by the patient's attending physician. When therapy first started, out of 2708 people, 72.7% were categorized as Child-Pugh class A, 24.5% as class B, and 2.7% as class C. Sorafenib's starting dosage was 400 mg twice daily. Child-Pugh class A, B, and C patients experienced adverse events (AEs) at rates of 69%, 64%, and 39%, respectively, whereas 9%, 14%, and 3% of patients experienced serious medication responses. The most frequent AEs were HFS (32%, 17%, and 5%), diarrhea (28%, 26%, and 11%), and exhaustion (16%, 14%, and 14%) in patients with Child-Pugh classes A, B, and C scores. Patients in Child-Pugh class A had a longer median OS (13.6 mo) than those in Child-Pugh class B or C (5.2 mo) (2.6 mo). The GIDEON study showed that the most prevalent drug-related side effects, as well as sorafenib tolerance, were comparable between Child-Pugh class A and Child-Pugh class B patients. They discovered that some individuals with Child-Pugh class B cirrhosis might receive sorafenib safely. Because of the variety of individuals with Child-Pugh class B conditions, patients should be carefully selected for sorafenib treatment based on a thorough evaluation of their hepatic condition[37].
The INSIGHT study was a prospective multicenter trial that recruited 788 uHCC patients who were treated with sorafenib. The main objective was to assess sorafenib's safety and efficacy, especially TTP and OS. Sorafenib was generally given at a dosage of 800 mg daily. The prevalence of cirrhosis among the patients was 56.7% Child-Pugh class A, 23.3% Child-Pugh class B, and 3.3% Child-Pugh class C. Patients in Child-Pugh classes A, B, and C experienced an OS of 17.6, 8.1, and 5.6 mo, respectively (P < 0.01). Sorafenib-related AEs occurred in 64.9% of patients and were regarded as severe in 9.8%. The most frequent serious adverse medication event (5.2%) was diarrhea. HFS (16.5%), nausea (8.0%), and exhaustion (7.9%) were other notable medication side effects. Both MTP and survival decreased dramatically when Child-Pugh scores increased. Patients having a Child-Pugh score of 7 and those with a score of 8 had comparable MTP and survival rates. Patients with a 9 on the Child-Pugh scale had substantially reduced MTP and survival (P < 0.01 and P = 0.003, respectively)[38].
McNamara et al[39] performed a meta-analysis of sorafenib therapy in patients with Child-Pugh class B cirrhosis, including 30 trials and 8678 participants. Most of the investigations were retrospective or prospective single-institution studies. Child-Pugh class A participants had an objective response rate of 4.6%, whereas Child-Pugh class B participants had an objective response rate of 4.2%. The distribution of Child-Pugh status was 79% Child-Pugh class A and 19% Child-Pugh class B. The assessed median OS for Child-Pugh class A patients was 8.8 mo and 4.6 mo for Child-Pugh class B patients. There was an inverse relationship between OS and Child-Pugh class B hepatic dysfunction. Child-Pugh class B patients had considerably lower survival, according to four studies that compared patients with Child-Pugh classes A and B using several variables (P < 0.001). In 35% of individuals with Child-Pugh class A or B, there was grade 3 or 4 toxicities. The rates of treatment termination without progress and treatment-related mortality were comparable. The study's findings revealed that while sorafenib response, safety, and tolerability were unaffected by the Child-Pugh score, survival was strongly impacted by these factors. No research involving patients with Child-Pugh class B cirrhosis were controlled trials, despite the large number of such studies that have been done, and the information that is now available is insufficient to draw firm conclusions on the use of sorafenib in such individuals. This meta-analysis proved that certain individuals with Child-Pugh class B cirrhosis can get sorafenib safely. Patients with Child-Pugh scores of 7 or less seem to be the safest group to treat with sorafenib, and patients with Child-Pugh scores ≥ 8 should be cautioned. Selected Child-Pugh class B patients may benefit from sorafenib even if Child-Pugh class A patients have much higher survival rates than Child-Pugh class B patients.
Other MTKIs such as brivanib, sunitinib, linifanib, erlotinib, and everolimus were also investigated, but none of them demonstrated superiority over sorafenib[40-44]. Prior to regorafenib's approval for second-line therapy in April 2017 and lenvatinib’s approval for first-line therapy in August 2018, sorafenib was the sole FDA-approved therapeutic option for uHCC[45,46].
Lenvatinib-Lenvatinib is a MTKI that targets VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-β, FGFR-1, FGFR-2, FGFR-3, FGFR-4, KIT, and RET. Lenvatinib is approved as the first-line treatment for uHCC patients. Patients who weigh 60 kg or more should take 12 mg daily, while those who weigh less should take 8 mg. Patients with uHCC who have Child-Pugh class A cirrhosis should not have their doses reduced, and there is no suggested dose for those with Child-Pugh class B or C cirrhosis. Based on the REFLECT trial, a noninferiority phase 3 research, lenvatinib was authorized. In this trial, there were 954 untreated patients with uHCC with Child-Pugh class A cirrhosis from 154 locations in 20 nations throughout the Asia-Pacific, European, and North American continents. Lenvatinib at a daily dose of 12 mg (n = 478) (for patients’ weight > 60 kg) or 8 mg (for patients weighing 60 kg) was given to patients at random, while sorafenib (n = 476) was given at a dose of 400 mg twice daily. OS was the primary aim, and patients were treated up until their radiological state worsened or they developed significant toxicity. Response rate, TTP, and PFS were secondary endpoints. Lenvatinib was comparable to sorafenib in terms of survival, with a median OS of 13.6 mo and 12.3 mo, respectively [HR: 0.92; 95% confidence interval (95%CI): 0.79-1.06]. According to baseline features, these effects were discovered to be constant across all patient groupings. In terms of all secondary objectives, lenvatinib was considerably more successful than sorafenib, according to an independent imaging analysis. Response rates were 40.6% vs 12.4% (P < 0.001) for lenvatinib vs sorafenib; disease control rates were 73.8% vs 58.4%; MTP rates were 7.4 mo vs 3.7 mo (P < 0.001); and median PFS rates were 7.3 mo vs 3.6 mo (P < 0.001). When compared to sorafenib, lenvatinib was related with more incidences of hypertension associated with treatment (42% vs 30%), proteinuria (25% vs 11%), and hypothyroidism (16% vs 2%), and lower rates of alopecia (3% vs 25%), HFS (27% vs 52%), and diarrhea (39% vs 46%). The reductions of dosage, treatment suspensions, and drugs discontinuations were reported by 40%, 37%, and 9% of lenvatinib-treated patients, respectively, and 32%, 38%, and 7% of patients who were treated with sorafenib, respectively. During the REFLECT trial's follow-up period, 33% of patients who were treated with lenvatinib and 39% of patients who were treated with sorafenib received antineoplastic drugs. The median survival time among patients who did not get further treatment with lenvatinib was 11.5 mo and 9.1 mo with sorafenib. Patients who underwent further therapy following lenvatinib therapy and sorafenib therapy had median survival lengths of 20.8 mo and 17.0 mo, respectively. This was one of the earliest signs that sequential treatment might enhance survival[47].
Finn et al[48] investigated the relationships between serum or tissue biomarkers and effectiveness outcomes from the REFLECT study. ELISA was used to assess serum biomarkers [VEGF, angiopoietin-2 (ANG2), FGF19, FGF21, and FGF23]. The nCounter PanCancer Pathways Panel assessed gene expression in tumor tissues. Pharmacodynamic variations in serum biomarker levels from baseline were investigated, as were clinical outcome relationships with baseline biomarker levels. They included 407 people in the serum analysis group (lenvatinib n = 279, sorafenib n = 128) and 58 people in the gene-expression analysis group (lenvatinib n = 34, sorafenib n = 24). They observed that, whereas both treatments were associated with increases in VEGF, only lenvatinib was associated with increases in FGF19 and FGF23 across the whole study period. Responders had greater levels of FGF19 and FGF23 on cycle 4, day 1 than non-responders (FGF19: 55.2% vs 18.3%, P = 0.014; FGF23: 48.4% vs 16.4%, P = 0.002, respectively). In both therapy groups, higher baseline VEGF, ANG2, and FGF21 Levels were associated with a shorter OS. With greater baseline FGF21, lenvatinib had a longer OS than sorafenib [10.9 mo vs 6.8 mo, respectively; HR: 0.53; 95%CI: 0.33-0.85; P = 0.0397]. In a biomarker examination of tumor tissue, VEGF/FGF-enriched groups outlived the intermediate VEGF/FGF group (HR: 0.39; 95%CI: 0.16-0.91; P = 0.0253). They concluded that shorter OS may be predicted by increased baseline levels of VEGF, FGF21, and ANG2. Lenvatinib's superior OS vs sorafenib may be predicted by higher baseline FGF21, but further research is required to prove this.
Now, uHCC has an additional first-line MTKI option in lenvatinib. In March 2018, it was approved in Japan. It was also approved in the United States in August 2018. The European Association for the Study of the Liver, European Society for Medical Oncology (ESMO), National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and all agreed that lenvatinib should be used in the first-line setting but only for patients with Child-Pugh class A cirrhosis[49-52].
Combination therapy of immune check-point inhibitor and VEGF inhibitor
Atezolizumab plus bevacizumab: The importance of VEGF and VEGFR signaling pathways in angiogenesis and tumor formation has been highlighted through research into the etiology of HCC. Both healthy and pathological angiogenesis are regulated by the VEGF protein family[53]. When VEGF overexpression was observed in these tumors, their function as therapeutic targets in uHCC was found. It is not unexpected that the idea of targeting tumor angiogenesis as a potential therapeutic method was offered as early as 1971 given the knowledge that a significant phase in tumor development requires oxygen and nourishment supply for sustained growth. As previously stated, VEGF expression is increased in HCC. Sorafenib targets the VEGF signaling system via MTKI, resulting in the therapeutic advantage already documented. However, the tangible benefit of sorafenib in terms of VEGF inhibition prompted researchers to look for other pathways targeting tumor angiogenesis and VEGF inhibition.
Because it develops in persistently inflamed livers from both viral and non-viral origins, HCC is usually referred to as an immunogenic malignancy. Furthermore, tumor-associated antigen expression and particular gene alterations that result in unique neoantigens lead to HCC immunogenicity. Innovative immunomodulation treatment options in uHCC have emerged as a result of investigations into the tumor microenvironment (TME) in HCC. Hepatitis viruses' persistent inflammation and the parenchyma's production of cytokines and growth factors coexist in a complex microenvironment. Due to restricted T cell activation that results in the generation of tumor-related antigens, the liver is also known to have an intrinsic immunosuppressive environment. The majority of the immune system's anti-tumor defenses are T cells, and tumor cells that have overexpressed the programmed cell death ligand 1 (PD-L1) have created an immunosuppressive milieu. Immunological checkpoints are coinhibitory membrane glycoproteins that largely block T cell immune overactivation during inflammatory and infectious conditions. Normally, this avoids collateral tissue damage, but in the TME, their expression plays a critical role in encouraging T cell exhaustion and immunological tolerance. The presence of Cytotoxic T Lymphocyte-Associated protein 4 (CTLA-4) and PD-1, immunological checkpoints involved in T cell activation and other inflammatory responses in malignancies, is well understood. In tumor expression on T cell activation and other inflammatory responses, CTLA-4 and PD-1 are immunological checkpoints that have been thoroughly studied. Additionally, expressed on regulatory T cells, CTLA-4 prevents T cells from co-stimulating when antigen is given. This is accomplished by the competitive binding of CD80 and CD86 receptors on antigen-presenting cells, which results in decreased CD28 stimulation and immune escape. As PD-1 binds to its ligands, PD-L1 and PD-L2, the CD28 pathway is also affected, which prevents CD8+ T cell activation and results in immunological inactivation. By expressing PD-L1 and PD-L2, cancer cells use this strategy to escape immune monitoring. Enduring antigen T cell tiredness is caused by exposure to the TME and is demonstrated by an increase in PD-L1 in tumor cells and antigen-presenting cells, which is induced by reactive T cells that express PD-1. As a result, the prognosis is poorer and the tumor grows larger with less effective tumor suppression[54-56].
Bevacizumab, a VEGF monoclonal antibody and atezolizumab, an anti-PD-L1 antibody, have been used in combination therapy for uHCC patients, which has resulted in the most recent and notable advancement in the treatment of HCC[14]. In the TME, VEGF overexpression has been seen to support immunological tolerance and evasion in malignancies. The main function of VEGF is angiogenesis, which paradoxically results in a hypoxic and acidotic TME and attracts immune-suppressive cells like regulatory T cells. VEGF also increases the expression of PD-1 on tumor-infiltrating T cells[57-59]. Targeting VEGF lowers immune suppression, and combining immune checkpoint inhibitors leads in enhanced immunological reactivation via increased T cell activity and tumor cell penetration. The phase III IMbrave150 research randomly allocated 501 patients who had not previously undergone systemic treatment to atezolizumab-bevacizumab or sorafenib in a 2:1 ratio. The study found that atezolizumab-bevacizumab improved OS by 67.2% (95%CI: 61.3%-73.1%) at 12 mo while sorafenib improved OS by 54.6% (95%CI: 45.2%-64.0%)[59]. Atezolizumab-bevacizumab had an objective response rate (ORR) of 27.3% (95%CI: 22.5%-32.5%) and sorafenib had an ORR of 11.9% (95%CI: 7.4%-18.0%) according to Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1). This combination's adverse event profile was consistent with the established safety profiles of each medication and the underlying condition. Proteinuria, tiredness, and an increase in aspartate aminotransferase (AST) levels were also observed in 15% of patients with grade 3-4 hypertension. In the atezolizumab-bevacizumab arm, approximately 15% of patients withdrew therapy due to side effects, compared to 10% in the sorafenib arm. The most common reported reason for withdrawal was gastrointestinal side effects. Bleeding is a recognized consequence of bevacizumab due to its anti-angiogenic properties. A typical consequence of cirrhosis is upper gastrointestinal bleeding, which has the potential to be a life-threatening hemorrhage. This was observed in 7% of patients in the atezolizumab-bevacizumab group versus 4.5% in the sorafenib group. Due to the increased risk of catastrophic bleeding with bevacizumab, patients must not have esophagogastric varices prior to beginning treatment. This encouraging advancement has rendered this combination, the FDA-approved preferred strategy for treating uHCC and is currently advised as the first-line treatment in uHCC following the 2021 recommendations[60].
Atezolizumab plus cabozantinib: Kelley et al[61] conducted an open-label, randomized, phase 3 study (COSMIC-312) in 837 uHCC patients from 178 centers in 32 countries to compare cabozantinib + atezolizumab against sorafenib as first-line systemic therapy. Patients required to have Child-Pugh class A, ECOG PS 0 or 1, detectable illness as defined by RECIST 1.1, BCLC stage B or C, and adequate organ and marrow function. Through a web-based interactive response system, they were randomly assigned (2:1:1 = 432:217:188) to receive cabozantinib 40 mg once daily plus atezolizumab 1200 mg intravenously (IV) every 3 wk, sorafenib 400 mg twice daily, or cabozantinib 60 mg once daily. Intention to treat (ITT) population had a median follow-up of 13.3 mo, whereas the PFS ITT group had a median follow-up of 15.8 mo (IQR: 14.5-17.2), (IQR: 10.5-16.0). The authors showed that median PFS in the combination therapy arm was 6.8 mo (99%CI: 5.6-8.3) vs 4.2 mo (99%CI: 2.8-7.0) in the sorafenib arm (HR: 0.63, 99%CI: 0.44-0.91, P = 0.0012). In the combination treatment group, the median OS was 15.4 mo, compared to 15.5 mo in the sorafenib group (HR: 0.90, 96%CI: 0.69-1.18; P = 0.44). The most frequent grade 3 or 4 AEs included a 9% (38/429) increase in alanine aminotransferase in the combination treatment arm, compared to 3% (6/207) in the sorafenib arm, and 6% (12/188) in the cabozantinib arm. Hypertension was found in 9%, 8%, and 12%, HFS was found in 8%, 8%, and 9%, serious treatment-related AEs occurred in 18%, 16%, and 13% of the combination treatment arm, sorafenib arm, and cabozantinib arm, respectively. Treatment-related grade 5 events occurred in 1% of the combination treatment arm, < 1% of the sorafenib arm, and < 1%) of the cabozantinib arm. They suggested that cabozantinib in combination with atezolizumab might be a therapy option for some uHCC patients[62].
Combination therapy of anti-PD-L1 and CTLA-4 antibody
Durvalumab and tremelimumab: Several studies have shown that extended exposure to CTLA-4 inhibitors may not be required for long-term anti-tumor effects. In metastatic melanoma, Eroglu et al[63] found that a single dosage of the CTLA-4 inhibitor tremelimumab might result in exceptionally extended durations of objective anti-tumor responses lasting more than 12 years. In advanced non-small cell lung cancer, a phase Ib research discovered that tremelimumab 1 mg/kg plus durvalumab 20 mg/kg every 4 wk provided an anti-tumor action while being tolerated. In a recent phase I/II study, durvalumab 20 mg/kg with tremelimumab 1 mg/kg every 4 wk for 4 doses was evaluated. The results showed acceptable tolerability and encouraging early efficacy in the second-line setting. The T-300/D-1500 high-dose had the highest risk and benefit outcome with a median OS and ORR of 18.7 mo and 22.7 mo, respectively, in the expanded phase 2 study that examined combinations of 75 mg tremelimumab with 1500 mg durvalumab (T-75/D-1500) and T-300/D-1500. In the phase 3 HIMALAYA study, the T-300/D-1500 group was examined as first-line treatment. Patients with uHCC were divided into four groups: (1) T-300/D-1500 followed by D-1500 every 4 wk (STRIDE); (2) D-1500 every 4 wk; (3) sorafenib 400 mg twice daily; and (4) T-75 every 4 wk followed by D-1500 every 4 wk (T-75/D). T-75/D enrollment was halted due to a planned analysis found no significant difference between D-1500 and T-75/D. D was noninferior to sorafenib alone in terms of OS (16.6 mo vs 13.8 mo; HR: 0.86; 96%CI: 0.73-1.03). The T-300/D-1500 ORR was 20.1%, 17% with durvalumab alone, and 5.1% with sorafenib alone. Nevertheless, no statistically significant change in PFS was seen. Durvalumab showed a good safety profile and wasn't worse than sorafenib. Durvalumab generated grade 3/4 AEs in 12.9% of patients treated with the combo group, and sorafenib in 25.8% of patients[64-66]. As a result, the combination of durvalumab plus tremelimumab is a realistic first-line option for patients who are not candidates for atezolizumab or bevacizumab, such as those with a high risk of bleeding[67].
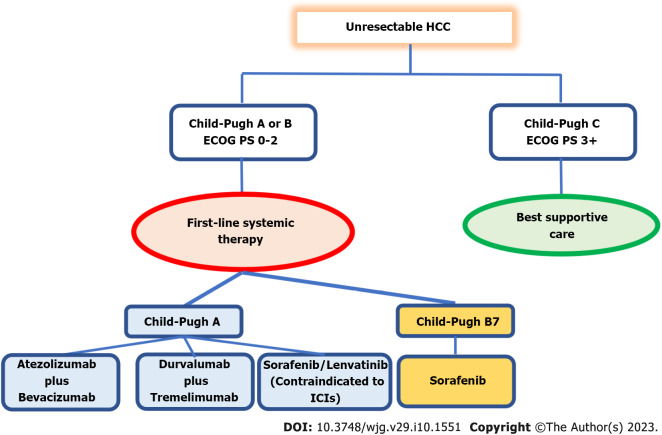
For uHCC patients with a Child-Pugh class A cirrhosis without anticoagulant therapy, an ECOG score of 0 or 1, and treatment for esophageal varices, current ASCO and ESMO guidelines recommend atezolizumab-bevacizumab combination therapy rather than lenvatinib or sorafenib monotherapy. Tremelimumab plus durvalumab is an alternative to bevacizumab for patients who are unsuitable to receive it. Monotherapy with sorafenib or lenvatinib is an alternative treatment for Child-Pugh class B cirrhosis patients with no worse than score 7, or when double immunotherapy is considered unsafe, or when the clinical condition of the patients is less fit, or with multiple comorbidities with predicted poor acceptance to combined immunotherapy. Only individuals with cirrhosis that is no worse than Child-Pugh class A are advised to take lenvatinib. Lenvatinib often causes fewer HFS and alopecia than sorafenib and has a lower overall toxicity profile. According to the REFLECT study, it also had a higher ORR, better PFS, and a longer TTP. As a result, if monotherapy is indicated, the majority of physicians now choose to begin with lenvatinib. However, considering the extended amount of experience and the noninferior median OS observed in the REFLECT study, sorafenib may still be preferable (Figure 1).
Figure 1.
First-line systemic therapy in unresectable hepatocellular carcinoma. ECOG PS: Eastern Cooperative Oncology Group Performance Status; ICIs: Immune checkpoint inhibitors; HCC: Hepatocellular carcinoma.
Second-line systemic therapy
Single drug-multikinase inhibitor: Regorafenib-Regorafenib was proposed as a possible second-line therapy for uHCC in the RESORCE study in patients who had progressed on sorafenib. The EGFR and VEGF receptors are the targets of the MTKI regorafenib. The phase III, randomized, double-blind trial compared OS to placebo in 567 Child-Pugh class A patients who had been receiving sorafenib and tolerated treatment for at least 20 of the 28 d prior to discontinuation. After enrollment, patients were randomized to receive regorafenib 160 mg daily or a placebo. When compared to the placebo, the median OS with regorafenib was 10.6 mo as opposed to 7.8 mo (HR: 0.63, 95%CI: 0.50-0.79; P < 0.01). Hypertension, hand-foot skin reactions, tiredness, and diarrhea were all grade 3 or 4 AEs. They stated that regorafenib is the only systemic therapy that has been demonstrated to improve survival in HCC patients who are advancing on sorafenib treatment. Regorafenib should be studied in combination with more systemic medications in the future, as well as third-line therapy for patients who do not respond to or tolerate the sorafenib-regorafenib regimen[45].
Cabozantinib-in uHCC patients who had previously been treated with sorafenib and had suffered disease progression on at least one systemic therapy, the MTKI cabozantinib has demonstrated clinical effectiveness with good outcomes. Cabozantinib acts by targeting the VEGF, MET, and AXL receptors. Antiangiogenic resistance, epithelial mesenchymal transition, invasion, and metastasis have all been related to MET and AXL receptors. High levels of MET and AXL expression have been associated with a poor prognosis in HCC, and higher levels of MET activity have been observed in previously treated patients who develop sorafenib resistance. In this double-blind, randomized phase III study (CELESTIAL), 707 patients who had previously taken sorafenib, who had progressed on at least one systemic therapy for HCC, or who had received up to two prior systemic therapies, were randomly assigned to receive cabozantinib 60 mg daily as opposed to a placebo. OS was the main objective, with PFS and ORR as additional endpoints. Patients who received cabozantinib showed a longer OS (10.2 mo vs 8.0 mo; HR: 0.76; 95%CI: 0.63-0.92; P = 0.005). When cabozantinib and placebo have been used, PFS was 5.2 mo vs 1.9 mo, and ORR was 4% and 1%, respectively. Sixty-eight percent of cabozantinib patients developed grade 3 or 4 adverse effects, with hand-foot skin reactions, hypertension, fatigue, diarrhea, and an increase in liver AST levels being the most frequent. They concluded that in patients with advanced HCC who had already had treatment, cabozantinib therapy produced longer OS and PFS than placebo. Nearly twice as many major side events occurred in the cabozantinib group than they did in the placebo group[68].
Single drug-VEGF inhibitor
Ramucirumab: For the treatment of uHCC patients who have previously had sorafenib therapy and have an AFP of 400 ng/mL or more, ramucirumab has a license. Inhibiting ligand-stimulated VEGFR2, cell proliferation, and angiogenesis, ramucirumab binds to VEGFR2 and blocks the binding of VEGFR ligands VEGF-A, VEGF-C, and VEGF-D. The dose for uHCC patients is 8 mg/kg IV every 2 wk until the condition progresses or there is significant toxicity. For those with mild to moderate hepatic impairment, the manufacturer advises against dose adjustment, but it also notes that clinical deterioration has been observed in patients with Child-Pugh class B and class C cirrhosis who received ramucirumab. Child-Pugh class B or class C cirrhosis patients should only consider ramucirumab if the potential benefits are deemed to exceed the risks of clinical worsening. In a phase 2 trial including 42 uHCC patients, ramucirumab was used as a first-line treatment. Ramucirumab was administered to patients at a dose of 8 mg/kg every 2 wk until the condition progressed or unacceptable toxicity developed. PFS was the primary objective, with response and survival serving as additional goals. The median PFS, MTP, and survival were each 4.0, 4.2, and 12.0 mo, respectively. The median response time was 14.1 mo, and the disease control rate was 69.0%. There were 4 partial responses (9.5% of patients)[69]. The main ramucirumab trial was the phase 3 REACH trial, which included uHCC patients who were unresponsive to locoregional treatment. All of the patients had previously undergone sorafenib treatment. In addition to best supportive care (BSC), patients were randomly treated with ramucirumab 8 mg/kg IV (n = 283) or a placebo (n = 282) every 2 wk, or until disease progresses, intolerable toxicity, or death. PFS, response, and disease control were secondary goals, with OS serving as the primary objective. The median survival time with ramucirumab was 9.2 mo compared to 7.6 mo with placebo (P = 0.14). In the ramucirumab and placebo groups, the median PFS times were 2.8 mo and 2.1 mo, respectively (P < 0.01). MTP for ramucirumab was 3.5 mo and 2.6 mo for the placebo (P < 0.01). In comparison to the placebo group, which only saw two partial responses (1% of patients), the ramucirumab group showed a full resolution and 19 partial responses (7% of patients) (P < 0.01). Fifty six percent of ramucirumab-treated individuals and 46% of placebo receivers achieved disease control (P = 0.011). The reductions of dosage were required in 7% of patients who were treated with ramucirumab and less than 1% of placebo patients, with dose omission rates of 22% and 10%, respectively; 10% and 3% of patients in the ramucirumab and placebo groups were discontinued due to unfavorable drug side effects. The most frequent grade 3 or 4 AEs were ascites (5% of patients who were treated with ramucirumab vs 4% of placebo patients), AST elevation (5% vs 8%), thrombocytopenia (5% vs 1%), hypertension (12% vs 4%), asthenia (5% vs 2%), and hyperbilirubinemia (1% vs 5%). Investigations were conducted on a predetermined sample of individuals having a baseline AFP level of 400 ng/mL. With ramucirumab, the median survival time was 7.8 mo as opposed to 4.2 mo with a placebo (P = 0.006). Survival was 11.8 mo with placebo and 10.1 mo with ramucirumab in patients with an AFP value less than 400 ng/mL (P = 0.51). These results led the researchers to propose that a high baseline AFP level may be a predictor of who may respond favorably to ramucirumab treatment[70].
The objective of the phase 3 study (REACH-2) was to address the efficacy of ramucirumab treatment in baseline AFP levels of 400 ng/mL or above patients. They were recruited because they had an ECOG PS of 0 or 1, intolerance to sorafenib, Child-Pugh class A cirrhosis, a history of disease progression, and they weren't candidates for locoregional therapy or resistant to it. Every 2 wk, patients were treated with ramucirumab 8 mg/kg (n = 197) or a placebo (n = 95), along with BSC, until the illness progressed or the side effects became unbearable. When ramucirumab was used instead of a placebo, the response rates were 5% and 1%, respectively (P = 0.1697), while the rates of disease control were 59.9% and 38.9%, respectively (P < 0.001). When compared to placebo, treatment with ramucirumab was related with significantly prolonged median PFS (2.8 mo vs 1.6 mo, P < 0.001) and median survival (8.5 mo vs 7.3 mo, P = 0.019). The REACH-2 study revealed that ramucirumab gave significantly better survival than placebo when taken as follow-up treatment following sorafenib in patients with an AFP value of 400 ng/mL or more. Ramucirumab did not cause HFS, a well-known side effect of other targeted medications[71]. Post hoc analysis of the REACH and REACH-2 studies confirmed the relevance of AFP as a predictive indicator, with AFP response considerably greater in individuals treated with ramucirumab than placebo (P < 0.0001). Survival was considerably enhanced with an AFP response (13.6 mo vs 5.6 mo; HR: 0.45; P < 0.0001)[72]. Ramucirumab is advised by NCCN as a category 1 medication for further therapy following sorafenib usage in individuals with an AFP level of 400 ng/mL or more[52].
Single drug-immune check-point inhibitor
Pembrolizumab: In the phase II KEYNOTE-224 research, pembrolizumab, an anti-PD1 monoclonal antibody, demonstrated comparable clinical antitumor effectiveness and safety when given to patients who had previously taken sorafenib for uHCC patients[11]. Finn et al[13] performed a phase 3 randomized, double-blind trial at 119 healthcare centers in 27 countries. With a follow-up length of 13.8 mo for pembrolizumab and 10.6 mo for placebo, they randomly allocated 413 patients to receive pembrolizumab plus BSC or placebo plus BSC. The median OS for pembrolizumab was 13.9 mo compared to 10.6 mo for placebo. At the end of the study, the median PFS for pembrolizumab was 3.0 mo vs 2.8 mo for placebo. Pembrolizumab was used in 147 (52.7%) and 62 (46.3%) patients, respectively; treatment-related events occurred in 52 (18.6%) and 10 patients (7.5%), respectively. No new cases of hepatitis C or B were discovered. They concluded that OS and PFS did not satisfy the criteria for statistical significance. Pembrolizumab offers a positive risk-to-benefit ratio in this population, according to the results, which are similar to those of KEYNOTE-224.
Drug-combination of PD-1 and CTLA-4 antibody
Nivolumab and ipilimumab: The CTLA-4 molecule, a crucial signaling checkpoint required for T-cell activation, is the target of the immune check-point inhibitor (ICI) ipilimumab. They successfully target two different immunological checkpoints when combined with nivolumab, inducing the modify immune response. The United States FDA approved nivolumab with ipilimumab as a second-line therapy in March 2020. The efficacy of combination treatment was proven in the phase 1/2 CHECKMATE-040 trial, which included 148 patients who were treated with sorafenib and clinically were better than Child-Pugh class A. The study evaluated 3 different regimens: Arm A: Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 wk for 4 cycles, followed by biweekly nivolumab 240 mg; Arm B: Nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 wk for 4 cycles, followed by biweekly nivolumab 240 mg; Arm C: Nivolumab 3 mg/kg every 2 wk plus ipilimumab 1 mg/kg every 6 wk. The suggested regimen is Arm A. According to the findings, the proposed regimen had the best ORR of 32%. CR was obtained by 8% of patients, and PR by 24%. The duration of response was 17 mo on average. The disease control rate was comparable among the three groups. However, larger sample size experiments are required to corroborate this conclusion[73]. In this CHECKMATE-040 cohort, individuals with or without hepatitis B or C had similar patterns of AEs, however Arm A was associated with higher TRAEs. Due to TRAEs, treatment was stopped in 18% of Arm A patients, 6% of Arm B patients, and 2% of Arm C patients. Rashes, adrenal insufficiency, hypothyroidism or thyroiditis, colitis, pneumonitis, and infusion-related complications were all observed in 35%, 18%, 22%, 10%, 10%, and 8% of patients, respectively.
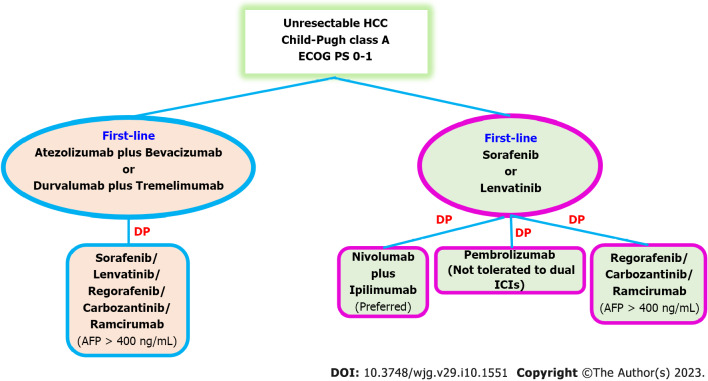
In conclusion, the appropriate second-line therapy regimen and sequencing are not well established and rely on the patient's PS, liver function, and choice of first-line therapy. TKIs like sorafenib, lenvatinib, or cabozantinib are suggested as second-line treatments for patients who have previously received atezolizumab plus bevacizumab or durvalumab plus tremelimumab. Given the possibility for a greater ORR than single medications, combination immunotherapy with nivolumab-ipilimumab is preferred for patients who have become worse on TKIs such sorafenib or lenvatinib. Pembrolizumab is an alternative if the patients are unable to take double ICIs. Regorafenib or cabozantinib may be considered as second-line options if sorafenib or lenvatinib have been chosen as the first-line therapy and the patients have a contraindication to ICIs. Patients with an AFP level > 400 ng/mL are advised to use ramucirumab[74] (Figure 2).
Figure 2.
Second-line systemic therapy in unresectable hepatocellular carcinoma. DP: Disease progress; ECOG PS: Eastern Cooperative Oncology Group Performance Status; ICIs: Immune checkpoint inhibitors; HCC: Hepatocellular carcinoma; AFP: Alpha-fetoprotein.
FUTURE TRENDS IN SYSTEMIC COMBINATION THERAPY
Since the rapid FDA approval of atezolizumab and bevacizumab, combining checkpoint inhibitors with multikinase inhibitors-especially anti-angiogenesis therapy-has gained widespread acceptance. Numerous studies have shown that the synergistic interaction of ICIs and TKIs promotes vascular remodeling and tumor immune activation[75-77]. In a phase Ib study, lenvatinib and pembrolizumab were investigated. The median PFS, OS, and ORR for this combination were 9.7 mo, 20.4 mo, and 46%, respectively, showing substantial anti-tumor effectiveness. Most of the AEs might be controlled by changing the dosage[78]. The combination of camrelizumab, an anti-PD-1 antibody, and apatinib, an orally active VEGFR-2 inhibitor, was also investigated in a dose-expansion and escalation phase I research. The suggested dosage of camrelizumab 200 mg every 2 wk with apatinib 250 mg daily showed therapeutic advantages with a 50% ORR. This regimen was thus investigated in the phase 2 RESCUE study. For the treatment of naïve uHCC patients or those who had previously failed or were intolerant to TKIs, apatinib 250 mg was administered orally every day combined with camrelizumab 200 mg IV (body weight > 50 kg) or 3 mg/kg (body weight 50 kg) every 2 wk. A total of 70 patients and 120 patients, who were mainly HBV-infected (88.3%) in the first-line and second-line settings, respectively, were included. The median time since the cutoff for the data was 29.1 mo. The 2-year OS was 43.3% and the median OS was 20.1 mo in the first-line setting. The median OS in the second-line condition was 21.8 mo, with a 2-year OS of 44.6%. A phase 3 study is underway to evaluate its effectiveness in the first-line situation compared to that of sorafenib[79].
To assess the effectiveness and tolerability of lenvatinib plus camrelizumab vs lenvatinib monotherapy as first-line therapy, Li et al[80] performed a multicenter, retrospective cohort investigation of 92 uHCC patients. In contrast, 44 patients received oral lenvatinib 12 mg or 8 mg daily and 48 patients received intravenous camrelizumab 200 mg every 3 wk. The ORR in the combination group was shown to be significantly higher than in the monotherapy group (RECIST 1.1: 37.5% vs 13.6%, P = 0.009). The median OS in the monotherapy group was 13.9 mo (95%CI: 13.3-18.3), but not in the combination group (P = 0.015). Lenvatinib with camrelizumab had a 1-year survival rate of 79.2%, compared to lenvatinib monotherapy's 56.8%. Lenvatinib with camrelizumab had a significantly longer median PFS than lenvatinib monotherapy (10.3 mo vs 7.5 mo, P = 0.009). Subgroup analysis revealed that combination therapy was associated with a longer OS in males, patients with a Child-Pugh score < 7, patients with three or more tumors, patients with AFP levels greater than 200 ng/mL, HBV-positive patients, patients with vascular invasion, and patients without hypertension. In the lenvatinib plus camrelizumab and lenvatinib monotherapy groups, the AEs that affected more than 20% of patients were HFS (22.9% vs 25.0%, P = 0.81), hypertension (33.3% vs 38.6%, P = 0.59), diarrhea (31.2% vs 31.8%, P = 0.95), and loss of appetite (41.7% vs 40.9%, P = 0.90). There were no statistically significant variations in the occurrences of any AEs between the two groups. They concluded that first-line lenvatinib with camrelizumab treatment may benefit patients with uHCC better than lenvatinib alone. There were no new safety signals, and the toxicity and tolerance profiles of the two treatment protocols appeared to be comparable. Further research is necessary before deciding if lenvatinib and camrelizumab treatment together can provide uHCC patients a unique therapeutic alternative.
CONCLUSION
Systemic therapy for HCC has significantly advanced with a major breakthrough since the FDA approved sorafenib in 2007. Although several MTKIs have shown promise in the therapy of uHCC, the discovery of ICIs has completely changed the field, bringing about remarkable ORR and OS benefit. This is demonstrated by the innovative outcomes from the HIMALAYA trial, the IMbrave150 study, and the CHECKMATE-040 trial for the second-line combination of nivolumab-ipilimumab, atezolizumab-bevacizumab, durvalumab-tremelimumab, and atezolizumab-bevacizumab, respectively. Each of the aforementioned regimens has a somewhat distinct toxicity profile, and combination therapy is correlated with greater toxicities. In clinical practice, single-agent therapies are explored for patients who are less fit or have severe medical comorbidities, whereas combination treatments are studied for very fit patients, such as those with a status of performance 0 to 1 and no importance medical comorbidities. Additionally, an esophago-gastro-duodenoscopy must be performed before starting atezolizumab-bevacizumab therapy in order to address any esophageal varices due to the relatively high dose of bevacizumab used-15 mg/kg-which is associated with a higher bleeding risk.
Because of this, it is still unclear which patient groups may benefit from or tolerate a certain combination of therapies better than others. Additionally, there are very few clinical trials investigating the function of future therapy in individuals who respond to ICIs. This topic is being actively investigated by a number of current studies, including a phase II study looking at cabozantinib in the post-ICI situation and other studies comparing the combination of atezolizumab-lenvatinib to sorafenib in the post-atezolizumab-bevacizumab scenario. Recently, anti-PD-1 medication has been controversially used in patients with non-alcoholic steatohepatitis-related HCC (NASH-HCC)[81]. In addition, Pfister et al[82] examined anti-PD-1 therapy animal models with NASH in both therapeutic and preventative contexts. They demonstrated that anti-PD-1 therapy accelerated hepatocarcinogenesis and produced hepatic fibrosis in NASH-mice without tumors. Treatment with anti-PD-1 did not result in tumor regression in NASH-mice carrying HCC. Surprisingly, it has, on the contrary, sped up the tumor's growth. They conducted a meta-analysis of 1656 patients from 3 significant studies to determine whether similar outcomes were also observed in human HCC (CHECKMATE-459, IMBrave150, and KEYNOTE-240). In accordance with the underlying etiology of HCC, they also evaluated the survival results of HCC treated with immunotherapy. Individuals with viral HCC had a longer survival time with immunotherapy (HR: 0.64; 95%CI: 0.48-0.94), but those with non-viral HCC did not (HR: 0.92; 95%CI: 0.77-1.11). According to a research, non-viral HCC patients who received anti-PD-1 medication had the same ORR and PFS as patients with viral HCC. Alternative explanations have been developed in response to these seemingly incongruous findings, including the varied population with non-viral HCC and the dearth of knowledge surrounding follow-up therapies[83]. It's also essential to remember that these worse outcomes for immunotherapy-treated non-viral HCC patients were retrospectively determined. As a result, based on the etiology of HCC, this cannot result in a shift in clinical management for uHCC patients. To clarify these concerns, more clinical studies with predetermined stratification should be planned.
Unanswered questions about therapeutic drug resistance and predictive biomarkers still exist. Drug resistance is prevalent and is generally believed to be the leading cause of therapeutic failure. EGFR activation, the existence of cancer stem cells, tumor-initiating cells, and the epithelial-mesenchymal transition (EMT) are examples of potential pathways. Cancer stem cells are created when EMT signals are activated, and these cancer stem cells have the ability to self-renew and differentiate. In human liver cell lines, prolonged sorafenib exposure results in EMT, which is characterized by changes in cell shape, loss of E-cadherin, and elevated vimentin expression[84,85]. In a genetically engineered mouse model of HCC, it was discovered that β-catenin activation encourages immune evasion and resistance to PD-1 inhibitors[86]. Although additional study is required to translate this bench-to-bedside data, these biomarkers have the potential to affect treatment choices. Our knowledge of tumor biology is still lacking, and there is an unmet need for cutting-edge drugs to deal with these resistance mechanisms. To accurately predict prognosis and the therapeutic response to target treatment or immunotherapy, there are yet no meaningful molecular indications. In actuality, imaging methods are used to identify the majority of HCCs, and the available treatments are much the same. It makes sense to look at biomarkers that might inform treatment choices in the era of personalized medicine. The most extensively researched immunotherapy biomarkers, including as tumor mutational burden, microsatellite instability, and PD-L1 expression, are presently employed very seldom in uHCC[87]. Although it has been shown that circulating markers including AFP, tumor necrosis factor-alpha, and IL-6 associated with HCC treatment outcomes, further studies are required to confirm these findings. A gene expression test has also recently been investigated as a possible biomarker for predicting immunotherapy response[88].
Ultimately, more research is necessary to determine any potential indirect drug interactions between antibiotics, proton pump inhibitors, ICIs, or multiple therapies. It is known that administering immunotherapy and antibiotics at the same time has a detrimental impact on the effectiveness of anti-cancer treatment. Numerous studies have shown the close relationship between ICIs and gut flora. Intestinal homeostasis and the reduction of systemic inflammation are crucially dependent on the microbiota. Antibiotics have a deleterious effect by causing dysbiosis, which changes the immune system's systemic anti-tumor response during, or in the 1st few weeks before commencing ICIs[89].
Since sorafenib was the only first-line therapy option for uHCC for 15 years, three alternative first-line therapeutic options have emerged. This represents a significant improvement in the management of uHCC (atezolizumab-bevacizumab, sorafenib, and lenvatinib). Cabozantinib, regorafenib, and ramucirumab are now included in the second-line therapy alternatives (AFP > 400 ng/mL). Nivolumab and pembrolizumab, ICIs, have been suggested as second-line treatments for individuals who are intolerant to MTKIs.
Footnotes
Conflict-of-interest statement: All the authors report no having relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 27, 2022
First decision: January 3, 2023
Article in press: February 22, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dang SS, China; Pandit R, United States; Huang CF, Taiwan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
Contributor Information
Wattana Leowattana, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand. wattana.leo@mahidol.ac.th.
Tawithep Leowattana, Department of Medicine, Faculty of Medicine, Srinakharinwirot University, Bangkok 10110, Thailand.
PathompThep Leowattana, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand.
References
- 1.IARC Fact sheets by Population-Globocan-IARC. [cited 31 July 2022]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf .
- 2.Samant H, Amiri HS, Zibari GB. Addressing the worldwide hepatocellular carcinoma: epidemiology, prevention and management. J Gastrointest Oncol. 2021;12:S361–S373. doi: 10.21037/jgo.2020.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razavi H. Global Epidemiology of Viral Hepatitis. Gastroenterol Clin North Am. 2020;49:179–189. doi: 10.1016/j.gtc.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, Bianchi L, Ayuso C, Vargas V, Rodés J, Bruix J. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000;31:54–58. doi: 10.1002/hep.510310111. [DOI] [PubMed] [Google Scholar]
- 9.Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, Benito A, Larrache J, Pueyo J, Subtil JC, Olagüe C, Sola J, Sádaba B, Lacasa C, Melero I, Qian C, Prieto J. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22:1389–1397. doi: 10.1200/JCO.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 10.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 12.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 13.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 15.Dimri M, Satyanarayana A. Molecular Signaling Pathways and Therapeutic Targets in Hepatocellular Carcinoma. Cancers (Basel) 2020;12 doi: 10.3390/cancers12020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25:212–225. doi: 10.1055/s-2005-871200. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt CM, McKillop IH, Cahill PA, Sitzmann JV. Increased MAPK expression and activity in primary human hepatocellular carcinoma. Biochem Biophys Res Commun. 1997;236:54–58. doi: 10.1006/bbrc.1997.6840. [DOI] [PubMed] [Google Scholar]
- 18.Xing S, Chen S, Yang X, Huang W. Role of MAPK activity in PD-L1 expression in hepatocellular carcinoma cells. J BUON. 2020;25:1875–1882. [PubMed] [Google Scholar]
- 19.Hu J, Cai D, Zhao Z, Zhong GC, Gong J. Suppression of Heterogeneous Nuclear Ribonucleoprotein C Inhibit Hepatocellular Carcinoma Proliferation, Migration, and Invasion via Ras/MAPK Signaling Pathway. Front Oncol. 2021;11:659676. doi: 10.3389/fonc.2021.659676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo S, Fang D, Zhao S, Thai Hoa PT, Zhou C, Liang T, He Y, Yu T, Chen Y, Qin W, Han Q, Su H, Zhu G, Luo X, Peng T, Han C. Down regulated oncogene KIF2C inhibits growth, invasion, and metastasis of hepatocellular carcinoma through the Ras/MAPK signaling pathway and epithelial-to-mesenchymal transition. Ann Transl Med. 2022;10:151. doi: 10.21037/atm-21-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 23.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 25.Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis A, Tsoukalas D, Spandidos DA, Margina D. The Akt pathway in oncology therapy and beyond (Review) Int J Oncol. 2018;53:2319–2331. doi: 10.3892/ijo.2018.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Zhang Y, Qin X, Geng H, Zuo D, Zhao Q. PI3K/AKT/mTOR pathway-related long non-coding RNAs: roles and mechanisms in hepatocellular carcinoma. Pharmacol Res. 2020;160:105195. doi: 10.1016/j.phrs.2020.105195. [DOI] [PubMed] [Google Scholar]
- 28.Hu TH, Huang CC, Lin PR, Chang HW, Ger LP, Lin YW, Changchien CS, Lee CM, Tai MH. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer. 2003;97:1929–1940. doi: 10.1002/cncr.11266. [DOI] [PubMed] [Google Scholar]
- 29.Hu TH, Wang CC, Huang CC, Chen CL, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM, Changchien CS, Tai MH. Down-regulation of tumor suppressor gene PTEN, overexpression of p53, plus high proliferating cell nuclear antigen index predict poor patient outcome of hepatocellular carcinoma after resection. Oncol Rep. 2007;18:1417–1426. [PubMed] [Google Scholar]
- 30.He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. doi: 10.1016/j.biopha.2020.110851. [DOI] [PubMed] [Google Scholar]
- 31.Xu C, Xu Z, Zhang Y, Evert M, Calvisi DF, Chen X. β-Catenin signaling in hepatocellular carcinoma. J Clin Invest. 2022;132 doi: 10.1172/JCI154515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranieri G, Gadaleta-Caldarola G, Goffredo V, Patruno R, Mangia A, Rizzo A, Sciorsci RL, Gadaleta CD. Sorafenib (BAY 43-9006) in hepatocellular carcinoma patients: from discovery to clinical development. Curr Med Chem. 2012;19:938–944. doi: 10.2174/092986712799320736. [DOI] [PubMed] [Google Scholar]
- 33.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 34.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 35.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Raoul JL, Bruix J, Greten TF, Sherman M, Mazzaferro V, Hilgard P, Scherubl H, Scheulen ME, Germanidis G, Dominguez S, Ricci S, Nadel A, Moscovici M, Voliotis D, Llovet JM. Relationship between baseline hepatic status and outcome, and effect of sorafenib on liver function: SHARP trial subanalyses. J Hepatol. 2012;56:1080–1088. doi: 10.1016/j.jhep.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JH, de Guevara LL, Papandreou C, Takayama T, Sanyal AJ, Yoon SK, Nakajima K, Lehr R, Heldner S, Lencioni R. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol. 2016;65:1140–1147. doi: 10.1016/j.jhep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Ganten TM, Stauber RE, Schott E, Malfertheiner P, Buder R, Galle PR, Göhler T, Walther M, Koschny R, Gerken G. Sorafenib in Patients with Hepatocellular Carcinoma-Results of the Observational INSIGHT Study. Clin Cancer Res. 2017;23:5720–5728. doi: 10.1158/1078-0432.CCR-16-0919. [DOI] [PubMed] [Google Scholar]
- 39.McNamara MG, Slagter AE, Nuttall C, Frizziero M, Pihlak R, Lamarca A, Tariq N, Valle JW, Hubner RA, Knox JJ, Amir E. Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma-a meta-analysis. Eur J Cancer. 2018;105:1–9. doi: 10.1016/j.ejca.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, Lu L, Chao Y, Boucher E, Han KH, Paik SW, Robles-Aviña J, Kudo M, Yan L, Sobhonslidsuk A, Komov D, Decaens T, Tak WY, Jeng LB, Liu D, Ezzeddine R, Walters I, Cheng AL. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 41.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, Omata M, Pitman Lowenthal S, Lanzalone S, Yang L, Lechuga MJ, Raymond E. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 42.Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC, Gorbunova V, Eskens FA, Qian J, McKee MD, Ricker JL, Carlson DM, El-Nowiem S. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, Leberre MA, Jensen M, Meinhardt G, Kang YK. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 44.Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL, Dorval E, Peck-Radosavljevic M, Santoro A, Daniele B, Furuse J, Jappe A, Perraud K, Anak O, Sellami DB, Chen LT. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 45.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 46.Nair A, Reece K, Donoghue MB, Yuan WV, Rodriguez L, Keegan P, Pazdur R. FDA Supplemental Approval Summary: Lenvatinib for the Treatment of Unresectable Hepatocellular Carcinoma. Oncologist. 2021;26:e484–e491. doi: 10.1002/onco.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 48.Finn RS, Kudo M, Cheng AL, Wyrwicz L, Ngan RKC, Blanc JF, Baron AD, Vogel A, Ikeda M, Piscaglia F, Han KH, Qin S, Minoshima Y, Kanekiyo M, Ren M, Dairiki R, Tamai T, Dutcus CE, Ikezawa H, Funahashi Y, Evans TRJ. Pharmacodynamic Biomarkers Predictive of Survival Benefit with Lenvatinib in Unresectable Hepatocellular Carcinoma: From the Phase III REFLECT Study. Clin Cancer Res. 2021;27:4848–4858. doi: 10.1158/1078-0432.CCR-20-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 50.Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E. Correction to: "Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Ann Oncol. 2019;30:871–873. doi: 10.1093/annonc/mdy510. [DOI] [PubMed] [Google Scholar]
- 51.European Association for the Study of the Liver. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma" [J Hepatol 69 (2018) 182-236] J Hepatol. 2019;70:817. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan LA, Brekken RA. The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs. 2010;2:165–175. doi: 10.4161/mabs.2.2.11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cariani E, Missale G. Immune landscape of hepatocellular carcinoma microenvironment: Implications for prognosis and therapeutic applications. Liver Int. 2019;39:1608–1621. doi: 10.1111/liv.14192. [DOI] [PubMed] [Google Scholar]
- 55.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 56.Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong Y, Wong JSL, Sugimura R, Lam KO, Li B, Kwok GGW, Leung R, Chiu JWY, Cheung TT, Yau T. Recent Advances and Future Prospects in Immune Checkpoint (ICI)-Based Combination Therapy for Advanced HCC. Cancers (Basel) 2021;13 doi: 10.3390/cancers13081949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y, Hu H, Yuan X, Fan X, Zhang C. Advances in Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma. Front Immunol. 2022;13:896752. doi: 10.3389/fimmu.2022.896752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 60.Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen YL, Xu Y, Liu J, Zhao H, Pierce WF, Mehta S, Goldberg KB, Theoret MR, Kluetz PG, Pazdur R, Lemery SJ. FDA Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable or Metastatic Hepatocellular Carcinoma. Clin Cancer Res. 2021;27:1836–1841. doi: 10.1158/1078-0432.CCR-20-3407. [DOI] [PubMed] [Google Scholar]
- 61.Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Banerjee K, Hazra S, Fawcett J, Yau T. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 62.Cammarota A, Zanuso V, D'Alessio A, Pressiani T, Personeni N, Rimassa L. Cabozantinib plus atezolizumab for the treatment of advanced hepatocellular carcinoma: shedding light on the preclinical rationale and clinical trials. Expert Opin Investig Drugs. 2022;31:401–413. doi: 10.1080/13543784.2022.2032641. [DOI] [PubMed] [Google Scholar]
- 63.Eroglu Z, Kim DW, Wang X, Camacho LH, Chmielowski B, Seja E, Villanueva A, Ruchalski K, Glaspy JA, Kim KB, Hwu WJ, Ribas A. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur J Cancer. 2015;51:2689–2697. doi: 10.1016/j.ejca.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, Rizvi NA. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, Yong WP, Cheng AL, Gasbarrini A, Damian S, Bruix J, Borad M, Bendell J, Kim TY, Standifer N, He P, Makowsky M, Negro A, Kudo M, Abou-Alfa GK. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kudo M. Durvalumab Plus Tremelimumab: A Novel Combination Immunotherapy for Unresectable Hepatocellular Carcinoma. Liver Cancer. 2022;11:87–93. doi: 10.1159/000523702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maestri M, Pallozzi M, Santopaolo F, Cerrito L, Pompili M, Gasbarrini A, Ponziani FR. Durvalumab: an investigational agent for unresectable hepatocellular carcinoma. Expert Opin Investig Drugs. 2022;31:347–360. doi: 10.1080/13543784.2022.2033208. [DOI] [PubMed] [Google Scholar]
- 68.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu AX, Finn RS, Mulcahy M, Gurtler J, Sun W, Schwartz JD, Dalal RP, Joshi A, Hozak RR, Xu Y, Ancukiewicz M, Jain RK, Nugent FW, Duda DG, Stuart K. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res. 2013;19:6614–6623. doi: 10.1158/1078-0432.CCR-13-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chau I, Peck-Radosavljevic M, Borg C, Malfertheiner P, Seitz JF, Park JO, Ryoo BY, Yen CJ, Kudo M, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Okusaka T, Bowman L, Cui ZL, Girvan AC, Abada PB, Yang L, Zhu AX. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: Patient-focused outcome results from the randomised phase III REACH study. Eur J Cancer. 2017;81:17–25. doi: 10.1016/j.ejca.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 72.Zhu AX, Finn RS, Kang YK, Yen CJ, Galle PR, Llovet JM, Assenat E, Brandi G, Motomura K, Ohno I, Daniele B, Vogel A, Yamashita T, Hsu CH, Gerken G, Bilbruck J, Hsu Y, Liang K, Widau RC, Wang C, Abada P, Kudo M. Serum alpha-fetoprotein and clinical outcomes in patients with advanced hepatocellular carcinoma treated with ramucirumab. Br J Cancer. 2021;124:1388–1397. doi: 10.1038/s41416-021-01260-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yau T, Tai D, Chan SL, Huang YH, Choo SP, Hsu C, Cheung TT, Lin SM, Yong WP, Lee J, Leung T, Shum T, Yeung CSY, Tai AY, Law ALY, Cheng AL, Chen LT. Systemic Treatment of Advanced Unresectable Hepatocellular Carcinoma after First-Line Therapy: Expert Recommendations from Hong Kong, Singapore, and Taiwan. Liver Cancer. 2022;11:426–439. doi: 10.1159/000525582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D, Michael IP, Bergers G. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60. doi: 10.1186/s12943-019-0974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kudo M. Scientific Rationale for Combined Immunotherapy with PD-1/PD-L1 Antibodies and VEGF Inhibitors in Advanced Hepatocellular Carcinoma. Cancers (Basel) 2020;12 doi: 10.3390/cancers12051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, Shao G, Xu L, Yin T, Liu J, Ren Z, Xiong J, Mao X, Zhang L, Yang J, Li L, Chen X, Wang Z, Gu K, Pan Z, Ma K, Zhou X, Yu Z, Li E, Yin G, Zhang X, Wang S, Wang Q. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res. 2021;27:1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 80.Li Q, Cao M, Yuan G, Cheng X, Zang M, Chen M, Hu X, Huang J, Li R, Guo Y, Ruan J, Chen J. Lenvatinib Plus Camrelizumab vs. Lenvatinib Monotherapy as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Cohort Study. Front Oncol. 2022;12:809709. doi: 10.3389/fonc.2022.809709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelley RK, Greten TF. Hepatocellular Carcinoma - Origins and Outcomes. N Engl J Med. 2021;385:280–282. doi: 10.1056/NEJMcibr2106594. [DOI] [PubMed] [Google Scholar]
- 82.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, Müller F, Sinha A, Friebel E, Engleitner T, Lenggenhager D, Moncsek A, Heide D, Stirm K, Kosla J, Kotsiliti E, Leone V, Dudek M, Yousuf S, Inverso D, Singh I, Teijeiro A, Castet F, Montironi C, Haber PK, Tiniakos D, Bedossa P, Cockell S, Younes R, Vacca M, Marra F, Schattenberg JM, Allison M, Bugianesi E, Ratziu V, Pressiani T, D'Alessio A, Personeni N, Rimassa L, Daly AK, Scheiner B, Pomej K, Kirstein MM, Vogel A, Peck-Radosavljevic M, Hucke F, Finkelmeier F, Waidmann O, Trojan J, Schulze K, Wege H, Koch S, Weinmann A, Bueter M, Rössler F, Siebenhüner A, De Dosso S, Mallm JP, Umansky V, Jugold M, Luedde T, Schietinger A, Schirmacher P, Emu B, Augustin HG, Billeter A, Müller-Stich B, Kikuchi H, Duda DG, Kütting F, Waldschmidt DT, Ebert MP, Rahbari N, Mei HE, Schulz AR, Ringelhan M, Malek N, Spahn S, Bitzer M, Ruiz de Galarreta M, Lujambio A, Dufour JF, Marron TU, Kaseb A, Kudo M, Huang YH, Djouder N, Wolter K, Zender L, Marche PN, Decaens T, Pinato DJ, Rad R, Mertens JC, Weber A, Unger K, Meissner F, Roth S, Jilkova ZM, Claassen M, Anstee QM, Amit I, Knolle P, Becher B, Llovet JM, Heikenwalder M. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan LL, Chan SL. Novel Perspectives in Immune Checkpoint Inhibitors and the Management of Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma. Cancers (Basel) 2022;14 doi: 10.3390/cancers14061526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun H, Zhu MS, Wu WR, Shi XD, Xu LB. Role of anti-angiogenesis therapy in the management of hepatocellular carcinoma: The jury is still out. World J Hepatol. 2014;6:830–835. doi: 10.4254/wjh.v6.i12.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, Wang Q, Wang S, Rong D, Reiter FP, De Toni EN, Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. doi: 10.1038/s41392-020-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M, Villacorta-Martin C, Singhi AD, Moghe A, von Felden J, Tal Grinspan L, Wang S, Kamphorst AO, Monga SP, Brown BD, Villanueva A, Llovet JM, Merad M, Lujambio A. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singal AG, Hoshida Y, Pinato DJ, Marrero J, Nault JC, Paradis V, Tayob N, Sherman M, Lim YS, Feng Z, Lok AS, Rinaudo JA, Srivastava S, Llovet JM, Villanueva A. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology. 2021;160:2572–2584. doi: 10.1053/j.gastro.2021.01.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Debes JD, Romagnoli PA, Prieto J, Arrese M, Mattos AZ, Boonstra A On Behalf Of The Escalon Consortium. Serum Biomarkers for the Prediction of Hepatocellular Carcinoma. Cancers (Basel) 2021;13 doi: 10.3390/cancers13071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buti S, Bersanelli M, Perrone F, Tiseo M, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, Spagnolo F, Rastelli F, Pergolesi F, Santini D, Russano M, Anesi C, Giusti R, Filetti M, Marchetti P, Botticelli A, Gelibter A, Occhipinti MA, Ferrari M, Vitale MG, Nicolardi L, Chiari R, Rijavec E, Nigro O, Tuzi A, De Tursi M, Di Marino P, Conforti F, Queirolo P, Bracarda S, Macrini S, Gori S, Zoratto F, Veltri E, Di Cocco B, Mallardo D, Santoni M, Patruno L, Porzio G, Ficorella C, Pinato DJ, Ascierto PA, Cortellini A. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer. 2021;142:18–28. doi: 10.1016/j.ejca.2020.09.033. [DOI] [PubMed] [Google Scholar]