Abstract
Background
Detection and management of female genital schistosomiasis (FGS) within primary healthcare is crucial for achieving schistosomiasis elimination, however, current technical strategies are not feasible in many settings. In Nigeria, there are currently no established standard operating procedures to support front-line health workers. This article presents an evaluation of piloting an FGS care package in two LGAs of Ogun State, Nigeria.
Methods
We used quantitative and qualitative analysis, including 46 interviews with patients, health workers and the quality improvement team; observations of training, learning sessions and supervision across 23 heath facilities; and records of patients detected and managed.
Results
Of 79 women and girls who were screened, 66 were treated and followed up. Health workers assimilated knowledge of FGS and effectively diagnosed and managed patients, demonstrating the feasibility of using symptomatic screening and treatment tools to diagnose and care for women or girls with suspected FGS. Challenges included establishing a referral pathway to tertiary care for patients with complications, insecurity, gender norms that limited uptake and sensitization, the limited capacity of the workforce, conflicting priorities and praziquantel acquisition.
Conclusions
Simple tools can be used in primary healthcare settings to detect and manage women and girls with FGS. Contextual challenges must be addressed. Sustainability will require political and financial commitments.
Keywords: co-production, female genital schistosomiasis, intervention, neglected tropical diseases, Nigeria, quality improvement
Introduction
Female genital schistosomiasis (FGS) is a neglected tropical disease (NTD) affecting girls and women living in urogenital schistosomiasis–endemic regions. FGS is caused by repeated exposure to Schistosoma haematobium parasites through infected water sources.1 FGS causes symptoms including genital itching, vaginal lesions, pain and bleeding during sex and abnormal vaginal discharge, which worsen when affected girls and women cannot access praziquantel treatment.2 This can result in severe conditions such as anaemia, cervical cancer, miscarriages, ectopic pregnancies and decreased fertility.3–5 Over several decades, prevention and control of schistosomiasis have primarily been through the vertical programme of mass drug administration (MDA) of praziquantel within schools and communities.6 However, there is growing evidence that MDA alone will not be enough to address FGS.2,6–8 In addition, due to its similarity to other differential gynaecological conditions, FGS is being misdiagnosed and not treated correctly, assumed to be cancer or a sexually transmitted infection (STI), thereby leading to potential stigma, wasted resources and costly health-seeking behaviour.9
Efforts are needed to reach and educate women and girls in endemic communities and to integrate FGS case detection and control within the primary healthcare (PHC) system where it can be accessed by those who need it most.10,11 However, current diagnosis is through inspection for internal lesions, which is often not practical in low resource contexts such as remote areas of Nigeria, as it requires extensive training, an uninterrupted power supply and equipment that is expensive to purchase, maintain and use.12,13 Therefore PHC-based interventions must consider the contextual realities of health systems and endemic communities. For example, there will be limitations and gaps around existing NTD policy and practice, technical skills and knowledge of health workers, availability of equipment and medicines, referral mechanisms to gynaecological care and availability of supportive supervision. Furthermore, interventions must consider a woman or girl's ability to access and accept diagnosis and treatment from health facilities, including their decision-making power, expenditures, security issues, cultural beliefs and potential stigma.11 Therefore a systems thinking, implementation research approach is necessary to understand what works and what doesn't in different contexts for point-of-care diagnosis and management of FGS.14,15
As a result of dialogue and awareness-raising through the COUNTDOWN consortium, drawing on information from a Ghanaian situational analysis,16 the Nigerian Federal Ministry of Health and Sightsavers (Nigeria) requested that we work together to better understand how to address challenges related to the diagnosis, treatment and management of FGS.5 A co-production implementation research project on FGS was designed and implemented by the COUNTDOWN research consortium in collaboration with the federal and Ogun State Ministries of Health. It is the first context-specific PHC intervention to diagnose and treat women and girls with FGS in Nigeria. An FGS care package was co-designed through a quality improvement (QI) approach of plan, do, study and act.5 The package supports health workers to diagnose, treat, counsel, follow up and refer when necessary women and girls with symptoms of FGS. During the initial workshops (presented in our associated publication5), we found that FGS knowledge and awareness among PHC, secondary and tertiary health workers in our study area was limited, therefore women and girls with symptoms of FGS were not being diagnosed or treated prior to our intervention.5
In this article we share the content and structure of the care pathway, which was developed and implemented with reflections on its challenges, opportunities and considerations in Nigeria. We also report on lessons learned, limitations and future research from piloting the FGS care package across two endemic local government areas (LGAs) in Nigeria.
Methods
Overview of the research process
Two QI cycles were implemented (4 weeks and 12 weeks). The first learning session was used to co-design and develop the FGS intervention. The results of this first learning session and the development of this FGS intervention have been reported in depth elsewhere.5 A further two learning sessions also took place towards the end of each of the two cycles to evaluate lessons learned and make changes to the intervention (Figure 1). Health workers across the two LGAs were trained on how to apply the tools. These training sessions and the roll-out of the intervention across the health facilities were staggered, in accordance with coronavirus 2019 (COVID-19) guidance and to support the QI process.
Figure 1.
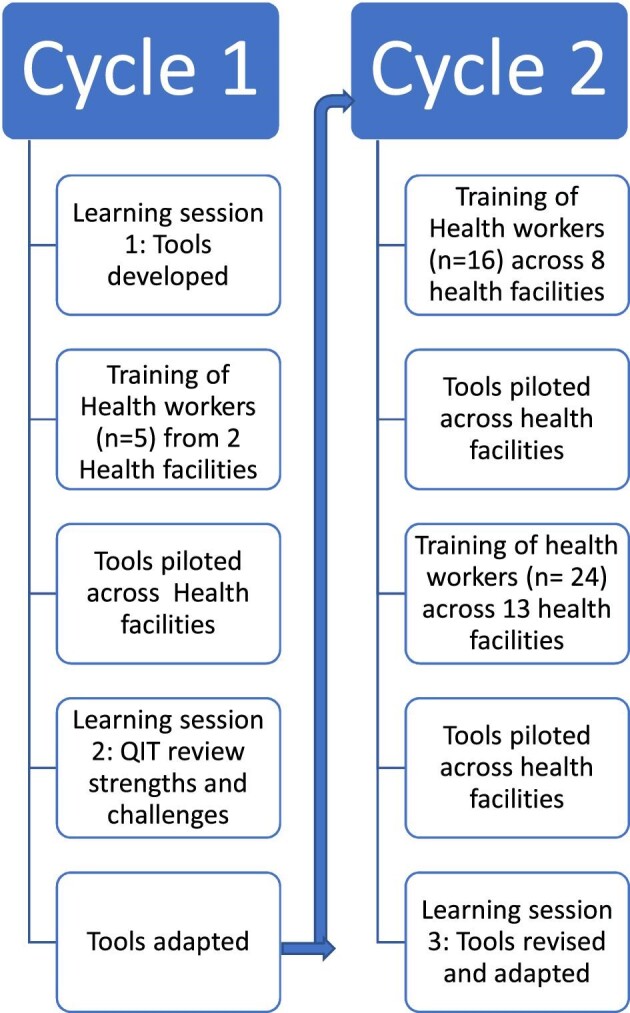
Quality improvement process to develop the FGS care package.
Study site
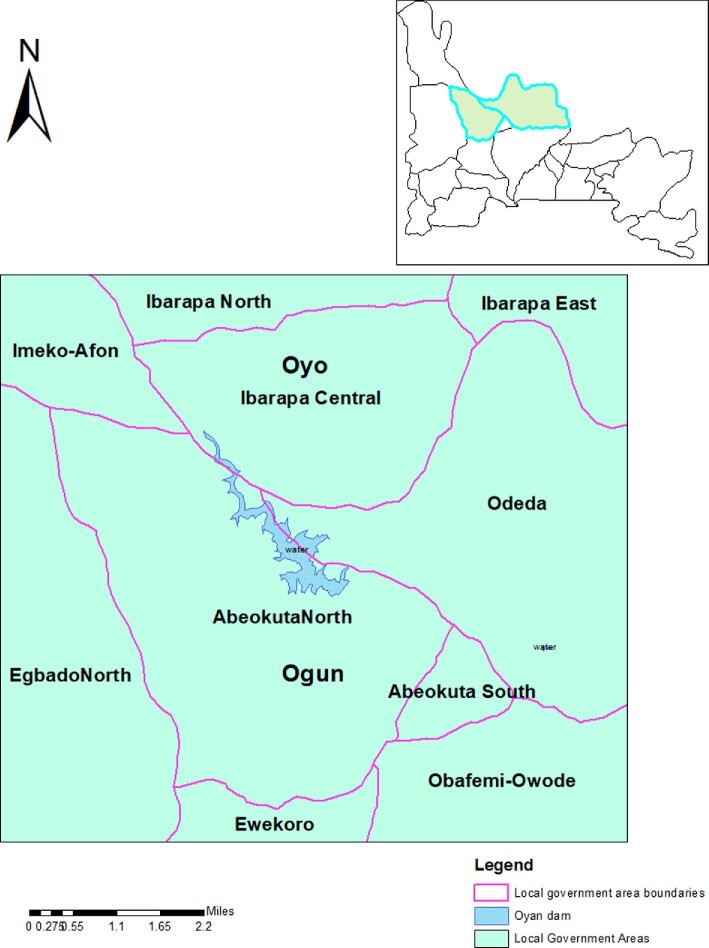
Ogun State is one of the most schistosomiasis-endemic states in Nigeria.17 Abeokuta North and Odeda LGAs, Ogun State (Figure 2), were purposively selected for piloting, as they are hotspots for schistosomiasis disease in the state with prevalences of 84% and 67%, respectively.18 The high endemicity is due to the Oyan River Dam, which is a source of schistosomiasis infection for communities living around it.1 Twelve health facilities were purposively selected for evaluation based on their closeness and easy accessibility to hotspot areas for schistosomiasis.
Figure 2.
Map showing the study area in the red circle around the Oyan River Dam (developed using GIS software).
Qualitative data collection and participant recruitment
Semi-structured interviews were undertaken with 22 women and girls who received treatment, 14 health workers and 10 quality improvement team (QIT) members to evaluate the success and challenges of implementing the FGS care package as well as identify recommendations from different perspectives (Table 1). While the research team endeavoured to interview women and girls across age ranges, there were limitations in recruiting individuals 15–17 y of age. Health workers initially asked women and girls receiving praziquantel if researchers could contact them for interviews, therefore patient interviews were opportunistic. There was no previous relationship between researchers and interviewees and all interviews followed a semi-structured guide to add quality and consistency. Across both cycles, a 2-d train-the-trainer workshop and three 4-d health worker training workshops were observed. Observations of learning sessions were also documented. Weekly updates from health facilities were also captured in researcher notes.
Table 1.
Qualitative data collection
| Number of events or participants | ||
|---|---|---|
| Dataset | Cycle 1 | Cycle 2 |
| In-depth interviews with health workers | 3 | 11 |
| In-depth interviews with patients (<18 y of age) | 0 | 2 |
| In-depth interviews with patients (>18 y of age) | 3 | 17 |
| Key informant interviews with QI team members | N/A | 10 |
| Observation reports of QI meetings | 3 | 1 |
| Health facility reports | 1 | 5 |
| Supervision reports | 1 | 1 |
Qualitative analysis
Semi-structured interviews were audio recorded and used to develop verbatim transcripts. The researchers observed meetings and workshops (in Yoruba and English) using observation grids (audio recordings were also used to inform reports). The researchers translated the interviews into English when transcribing, however, some key sentences in Yoruba remain to explain key concepts. Rapid analysis was used to pragmatically identify key issues in cycle 1, which fed into learning session 2. Following the completion of both cycles, a framework approach was used to analyse all the qualitative data across both cycles. The researchers first familiarised themselves with the transcripts and agreed on a coding framework to code all transcripts and reports. Charts were produced that analysed the data by dataset and time point to generate summaries. Overarching themes were agreed upon by all authors and are presented in the results section.
Quantitative data collection
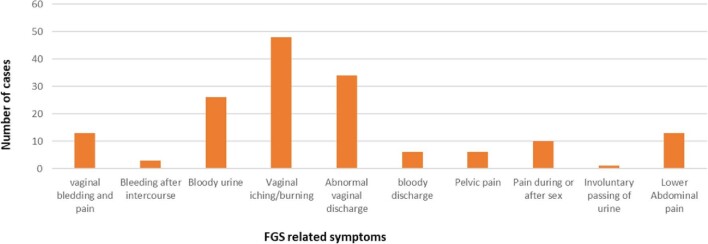
To assess the uptake of the intervention and effectiveness of treatment and to estimate the burden of FGS within study communities, quantitative data on the numbers of persons screened for FGS, diagnosed with FGS, treated for FGS, treated for other conditions, excluded and referred to other services were captured in an FGS register. Women and girls who presented to PHC with any gynaecological issues were screened using the core symptoms checklist and environmental risk assessment. The various symptoms presented by the patients at their initial visit to the health facility were also captured, enabling us to understand the symptoms and severity of FGS within the community, which may inform further training needs and treatment pathways (Figure 3).
Figure 3.
FGS-related symptoms presented by patients at the health facilities.
Quantitative analysis
Descriptive statistics using frequency were used to describe the quantitative data collected through the FGS health facility register. Graphical representations of the various symptoms presented were developed.
Results
Quantitative outcomes
A total of 79 girls and women self-presenting with gynaecological issues were screened for FGS using symptomatic questionnaires. Of these, 66 suspected cases of FGS were treated (Table 2). Major FGS symptoms reported by those who accessed care include vaginal itching, burning sensation, vaginal discharge, pain during sex and contact bleeding (Figure 3). A total of 65 health providers were trained in FGS case management (Tables 2 and 3).
Table 2.
Quantitative results of participants included
| Participants included | Values, n |
|---|---|
| Health facility trained | 23 |
| Health workers trained | 65 |
| Women and girls presenting with symptoms of FGS | 79 |
| Diagnosed with FGS | 66 |
| Followed up | 66 |
| Referred or treated for an additional condition | 1 |
Table 3.
Quantitative results of participants excluded from treatment
| Reasons patients excluded from treatment | Cases, n |
|---|---|
| Pregnancy | 2 |
| Breastfeeding | 6 |
| Comorbidities or concomitant medications | 3 |
| Declined | 1 |
| Previous MDA within 6 months | 1 |
Qualitative outcomes
The qualitative results are presented in three sections. The first section explores the key strengths and limitations of the care pathway. Issues reported here include the use and understanding of tools for diagnosis, eligibility for praziquantel, referral pathways for further investigations and specialist advice and follow-up. We also report on resources needed to support the care pathway. In the second section we explore the success and challenges of capacity strengthening for the care pathway, and in the third, we explore the contextual realities of implementing the care pathway.
Reflections on the care pathway
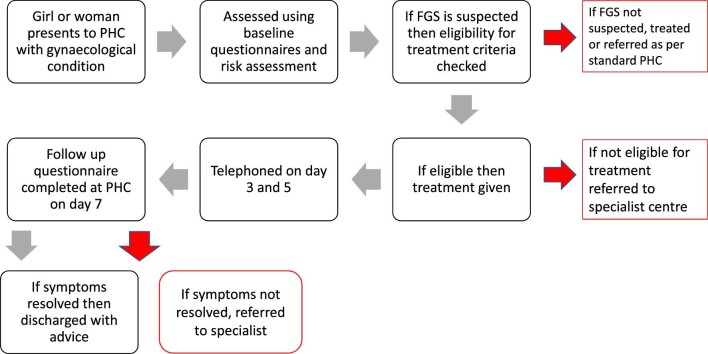
This section presents challenges and successes identified along the care pathway (Figure 4). In summary, the diagnostic tools appeared appropriate to use and were well understood by the health workers. However, the checklist for severe symptoms was not always used correctly in the first roll-out and therefore enhanced training of this was embedded into the next cycle. All women and girls who were assessed using the diagnostic tools as having presumed FGS were assessed to see if they could receive praziquantel at the PHC facility. Eligibility criteria were developed based on the protocols used for MDA by the Nigerian Federal Ministry of Health (FMoH). Health workers expressed that further clarification by the FMoH on these criteria would be beneficial. For this pilot study, women and girls with presumed FGS but ineligible to receive praziquantel at a PHC facility were referred to a tertiary care centre. A referral pathway was set up during the pilot but was not adequately evaluated during this research due to limited women being referred. The majority of women who attended follow-up in PHC reported that symptoms had resolved.
Figure 4.
Flow diagram of care pathway.
Diagnosing FGS severity and differential diagnosis
Health workers used symptomatic questionnaires to diagnose FGS at the PHC level. The questionnaires were developed based on current literature and highlight the main symptoms and complications of FGS that women and girls may present with.3,19–21 If women or girls presented with a gynaecological complaint, the symptomatic questionnaires were used to support the diagnosis of presumed FGS, with or without additional conditions. If the women or girls were assessed to have presumed FGS, they were treated, where appropriate, with praziquantel on the day of assessment. If the women or girls showed signs of severe symptoms, had complications arising from FGS, had additional conditions or where ineligible for praziquantel within PHC, they were then referred to tertiary facilities for specialist care as appropriate. Other medications such as analgesia could also be recommended in accordance with healthcare protocols.
The challenge of differentiating FGS from other conditions such as STIs and cancer was recognised early on. Health workers were trained to assess if a woman or girl had contact with schistosomiasis-infected water. The five tools used to support diagnosis are outlined in Table 4.
Table 4.
Five tools to support diagnosis of FGS, severity and other conditions (see ref. 23)
| Tool | Description |
|---|---|
| Initial symptom questionnaire | The questionnaire included core symptoms of FGS as outlined by the World Health Organization: vaginal discharge, bloody discharge, bleeding during or after sexual intercourse, genital itching or burning sensation, pelvic pain or pain during sexual intercourse.22 These symptoms were presented in a grid, with mild, moderate or severe categories. If women and girls had any symptoms (of any severity) at the baseline assessment, then it was followed up by the environmental risk assessment questions. |
| Environmental risk assessment | The rationale behind the environmental risk assessment was to establish contact with infected water sources. Other questions are has the woman/girl had or ever had blood in urine and have people in the community reported similar symptoms? A scoring system was used with scores <4 indicating low/no risk of FGS and scores of 4–10 indicating high risk of exposure to schistosomiasis and therefore a higher risk of having FGS. Women and girls with moderate or severe symptoms, a risk assessment score of ≥4 and eligible for treatment should be treated at the health facility level and then referred to appropriate facilities for further investigation and management. |
| Complications of FGS/other conditions checklist | This questionnaire was used to determine if the women and girls required further investigations or specialist management. Health workers were trained that it is not always possible to make a clinical diagnosis of FGS based on symptoms alone and therefore some may require internal vaginal examinations or further tests, such as vaginal swabs, for confirmation of diagnosis. Health workers were trained to work in accordance with local health facility protocols, if they had established capacity and resources to conduct these tests/examinations. However, all women and girls with either moderate/severe core symptoms or complications should be treated and/or referred to the designated tertiary hospital. A referral pathway was set up to facilitate this. |
| Discharge colour chart | Health workers were guided that the discharge colour chart was a supportive instrument that, in conjunction with clinical knowledge and the tools listed above, could indicate a vaginal condition. The health workers were trained that the colour of discharge should be used to support diagnosis. There were grades of colours with interpretations that help the health worker to know if the colour discharge is normal or abnormal. This information was to be recorded on referral forms. |
| Follow-up questionnaire | The follow-up questionnaire was used 7 d after praziquantel treatment to determine if core symptoms had resolved. If not resolved, the patient should be referred to the tertiary hospital for further investigation, as this could either indicate that the woman/girl has an additional condition or severe FGS that requires specialist management. |
Understanding and application of diagnostic tools
Symptoms and complications of FGS were well understood during training. Role play was used for the health workers to practice the diagnosis steps and feedback was given. The environmental risk assessment and core symptoms were understood by many of the health workers. This was assessed through role play during the training. Questions and responses observed and documented during the training indicated a good understanding of the use of the environmental risk assessment and core symptom checklist. During health worker interviews, participants were asked about their understanding of the tools, and the majority of participants could explain the rational for using the tools and how they were used. However, it was not clear whether the ‘complications of FGS/other conditions’ checklist was being used on all women and girls who presented with initial symptoms. Women and health workers reported severe symptoms such as urinary incontinence and challenges with fertility, however, at this time point, no patients were referred to tertiary services and severity was not being documented. During the intervention, the care pathway was revised so that the ‘complications of FGS/other conditions checklist’ was used by health workers earlier in the diagnostic pathway and emphasis was placed on using this questionnaire on all women and girls.
QIT members and health workers identified that previously they may have misdiagnosed FGS and highlighted that the tools were simple to use and could act as an aide-mémoire.
…you know before when people presents with vaginal discharge or abdominal pain, all our thinking and all our thoughts is that it is just an infection, probably it could be PID – pelvic inflammatory disease – or STI but now for us to now come up with, that it cannot be PID nor STD but the first thing is that have you had contact with water and the person said yes, with all the signs and symptoms, it makes us to understand that this person is having FGS. (QIT interview)
QIT members recognised the need for the diagnostic tools to be simple and context specific for primary care. Some QIT members expected gynaecologists to make the diagnosis of FGS through colposcopy examination, using an outreach service or referring all women and girls to tertiary care. Others highlighted that this was not appropriate at the PHC level, suggesting that the use of colposcopy or speculum vaginal examinations may have strengthened the clinical diagnosis.
Initially at the beginning we were thinking it will be impossible to make a diagnosis without having a gynaecologist on the field, having to use a colposcopy, all of that to make to definitive diagnosis but with the tool we found out that yes it was appropriate for that level of health care delivery. (QIT interview)
Eligibility criteria for treatment
The inclusion and exclusion criteria developed for the intervention were based on national guidelines produced by the Nigerian FMoH for MDA.20,23 During the implementation, the main exclusions for the PHC level were breastfeeding and pregnant women following the discussion around FMoH policy. Other exclusion criteria included not treating people with specific chronic conditions, such as epilepsy and sickle cell anaemia, or on specific medicines that are contraindicated with praziquantel.24 The QIT reflected on some of the exclusion criterion within the FMoH guidance that they found surprising and highlighted the need for more guidance around treating people with comorbidities so that people are not continuously missed from treatment and treated safely.
For the people with sickle cell, that they said [FMOH guidance] they should not be given praziquantel, I really want to know whether if they are in crisis we cannot give or if they have, it shouldn't be given at all. (QIT interview)
Referral processes and pathways
It was agreed that there were specific points along the FGS care pathway where patients should be referred to other facilities, either for further investigations that cannot be conducted at the specific PHC facility or they require specialist care because of contraindicated medications or conditions.
To establish a referral pathway from primary care to tertiary care, the QIT paid an advocacy visit to a regional specialist hospital in Ogun State. It was agreed that patients would be seen on the same day they present. However, as this protocol may take time to establish with routine practice, in the interim the patients would be seen by two members of the QIT (at the new referral centre). One QIT member who was interviewed said the referral pathway had been challenging to establish, but they had prioritised it because of the recognised challenges patients face with travelling to the referral centre and the associated costs.
So, knowing that these women will not have that liberty of time and even the luxury of finance to be coming again and again we had that challenge of eventually coming to a compromise of how they will land in the community medicine department, all their biodata everything captured, and then immediately referred for the gynaecologist's review. I think we have as much as tidied up the process now. (QIT interview)
Barriers to referral
Although it was recognised that patients with severe symptoms or a complicated diagnosis should be referred to other facilities, this rarely happened during the intervention, and thus the referral pathway was not adequately tested during the study period. During the intervention only one patient was referred to the referral centre. The woman who was referred potentially had a dual diagnosis of FGS and STI and had subsequently become pregnant after praziquantel treatment.
Referral to other health facilities, such as laboratory services, was not documented in the FGS registers. One health worker highlighted that she had referred a woman with presumed FGS who was excluded from treatment because she was currently breastfeeding, however, the health worker reported that this patient would not attend the referral because of transportation costs. Another health worker reported that she had referred one woman to see a doctor, as she believed she had an underlying condition, but this was not documented in the FGS register. Other health workers explained that while they had referred women to laboratory services for scans, some patients did not attend them because of the associated costs or preference for attending other facilities.
To ensure that all patients who require further investigation or management are referred appropriately and that documentation is captured, clearer guidance was added to the final intervention guides and subsequent training workshops.
Follow-up and symptom resolution
Health workers attempted to telephone patients on the third and fifth day post-treatment and many patients reported that their symptoms had decreased. The follow-up questionnaire was administered at the health facility 7 d after the baseline visit. Most patients interviewed reported improvement in their symptoms, which included resolution of discharge and itching and not experiencing pain during sexual intercourse. Those who had improved or resolved symptoms were keen to express that they would encourage others to attend if they had similar symptoms. However, two women reported that symptoms decreased, but following sexual intercourse the pain and other symptoms returned. Another patient also reported that symptoms had not resolved and believed she had malaria, for which she has since started treatment. Some cases were discussed by the QIT at learning session 3 to ensure patients had been followed up and referred appropriately.
Resources and structures to support the care pathway
Availability of medications and the need for policy
Long-term availability of praziquantel at health facilities was a concern expressed by the QIT, the health workers and some patients. For the study, Ogun State requested additional praziquantel. However, during normal MDA campaigns, any leftover praziquantel is recalled and is not permitted by donors to remain in facilities. Therefore, ensuring that health facilities had a continuous supply was highlighted as a potential barrier throughout this study and beyond. A QIT member also suggested that there was a need for medications to be available to treat other conditions with symptoms similar to FGS. It was recognised by the QIT that policy was needed to ensure the long-term availability of praziquantel (and other medications) for treating symptoms.
Ok some of the facilitators for sustaining the intervention is that; there must be a recording process that is enough praziquantel is available for FGS treatment at the health care facility. For now, I think there is no policy on it. So, we prefer Ministry of Health to come out with a policy on FGS specifically to address FGS. (QIT interview)
Supervision and monitoring
Supervision took place through face-to-face visits to the health facilities, weekly telephone calls with the research team and a WhatsApp platform where health workers and QIT members could interact. The on-site supervision was an opportunity to correct errors made or incompleteness in documentation and reporting while ensuring implementation as stated in the guideline. In addition, health workers had the opportunity to ask questions regarding areas where they were unsure about the intervention guide or documentation.
The importance of monitoring and documentation was recognised by the QIT, who saw that this could contribute to the development of FGS policy and the sustainability of the intervention. The QIT discussed that there was a need for a monthly monitoring form to be developed and completed by the health workers and sent to the state to ensure that errors were not made.
Our supervisors, they did not leave us, they keep calling us to know if we have seen clients…patients so that at least is giving us the feeling that we are not left alone. (Health worker interview, HF03)
Embedding learning to strengthen capacity and patient–provider relationships to manage FGS
This section explores the participatory-style training programme that was developed to support the care package. We found through the use of role play and interviews that health workers increased their knowledge of FGS and had a good understanding of the care package. Importantly, the training also enabled a space for dialogue about stigma and appeared to improve the relationship between health workers and patients. Barriers to embedding capacity of the new care package included limitations in workforce capacity due to the number of staff available and the rotation system used for PHC in Ogun State.
Training format and facilitation tools for embedded learning
Capacity strengthening took place across multiple levels within the health system with intervention tools developed to guide training. The trainer's manual included participatory activities such as role play, template presentation slides and agendas.22 The participatory style of teaching, which included role play, was appreciated by the health workers and facilitated their understanding and practice.
Some health workers trained on the intervention also cascaded training to their colleagues, such as pharmacists, pharmacist technicians and health attendants, and they expressed that they were ready and willing to provide support to their colleagues where needed.
…it is the role play that stays longer with us. When the person acted like an angry patient…I still remember the roleplay on how we are to behave to and receive clients. I sometimes want to get angry with patients but once I remember the training on client relationship, it makes me calm down. So if they can allow the role play to be a huge part of the training it would be good. We attend trainings all the time, but the fact is that not everyone will have time to go over the training materials but whatever we do, we remember. (Health worker interview, HF10)
Workforce capacity
The health workers had a range of experience from 4 to 30 y. While some had been in their current health facility for years, others had been there for only 1 month. Health workers mostly appeared to move around the LGA, rotating between health facilities. It was recognised that sustainability of the intervention requires retraining of the workforce and supervision to ensure that all health workers new to the facilities have the knowledge required and that the momentum of implementing the FGS package is maintained. As well as health workers at the PHC level rotating to different localities, it was suggested that the LGA-level NTD teams are also subject to change. Furthermore, a position within the team is not always filled when someone rotates. Therefore it was suggested that training should include more permanent stakeholders, such as community members themselves, medical officers of health/directors of public health and apex nurses (the most senior nurse in the LGA).
Health workers reported that they were often understaffed and have numerous activities that they are involved in, including, at that time, training for COVID-19 vaccine administration. A lack of workforce was recognised by a QIT member as a reason for inadequate documentation during the pilot.
…it is a new thing to us. If there is no continuity in the training, because this set of people that we have trained now I can tell you that for the next one year they would not be found in that duty post again. So, if you don't continue with the training there might be issues in the training…. (QIT interview)
Knowledge uptake of FGS
There was evidence of increased awareness among most patients and health workers related to FGS transmission and symptoms. Most patients reported that contact with river water was the main cause of FGS and that this should be avoided either with protective clothing or using alternative water sources. Previous misconceptions included infection from toilets, dogs urinating and sexual intercourse. A woman reported that she heard from another community member that her symptoms ‘are not always associated with infection due to sexual contact but that it can also come as a result of infection from the river’ (Patient interview, HF14). This motivated her to attend the health facility. However, a few other patients misunderstood the mode of transmission, relating it to a dog's urination.
Addressing patient–health worker relations for FGS management
Enabling space for dialogue
Health education and counselling is an important aspect of the treatment guidelines and the health workers were trained on this aspect. The training delivered for FGS also addressed the patient–health worker relationship and creating positive spaces for treatment. This included how to meet and greet patients, how to address aggression or patients with language barriers, how to build trust for open discussions and how to ensure privacy and confidentiality. There was also an emphasis in the training on how to ask some sensitive referral questions related to fertility and to think carefully about the words used. Within the training for this pilot project, patient confidentiality was also covered, including confidentiality of data, privacy of consultations and finding safe spaces to discuss and counsel women and girls.
Developing awareness of stigma
Training around different types of stigma was delivered through interactive discussions and group works. Health workers recorded on sticky notes their understanding of what can create stigma; however, initially many of the notes were related to confidentiality rather than stigma specifically (Box 1).
Box 1. What is stigma? Captured in the health worker training report
Unable to welcome her or unable to make her comfortable
Not making her comfortable
No privacy when attending to her
Not paying adequate attention to the client
Tagging or calling her with her disease condition
Use of abusive words by health workers
Shouting when talking to the patient
By not keeping her secret
Ignoring client complaints
By inviting a fellow health worker to come and listen to her (not in the context of a language barrier)
By abusing her with her medical condition
The facilitators addressed these using slides and examples of stigmatisation that they had witnessed within the health system in relation to other conditions such as tuberculosis and Buruli ulcer, giving examples of experienced, internalised and anticipated stigmatisation.25 Discussions around stigmatisation were held and openly addressed in the training. At the end of the training, health workers expressed their changed views on what stigma is; how it can be caused, such as misinformation, beliefs and fear; and what the outcomes of stigma are for patients, such as depression. Impacts of relationships were also mentioned as well as suicidal thoughts.
Contextual realities of implementation
During this pilot we identified a number of challenges to implementing the care package that reflect the contextual realities on the ground, including limitations in reaching community members through our advocacy campaign. There were also issues of insecurity, safety and competing priorities that limited the uptake of the intervention.
Challenges to community advocacy and sensitisation
There was evidence that community engagement and sensitisation efforts were not reaching the target populations and that they were not on a large-enough scale to create the level of awareness needed to direct women and girls to the facilities in endemic areas. An advocacy visit was held with the head of the local government service administration and permission was gained to deliver sensitisation to women in the area. Efforts to engage some community leaders were limited due to issues of insecurity within the community and health worker workload. While considered expensive and time consuming, they were perceived as important for reaching community members. Health workers also asked women seen within their health facility to pass on the message about FGS to their communities. FGS posters were posted in the health facility and leaflets were distributed in the community. These materials were reported to aid understanding and prompted patients to ask about FGS.
The challenge of reaching women in Purdah (a religious and social practice of female seclusion) was also discussed and it was suggested there is a need for peer education and training, such as a ‘community-based person’ to address some of the access challenges. The challenges of the language barrier were also discussed, and it was agreed that jingles and information, education and communication (IEC) material should be translated into different languages.
Other sensitisation techniques suggested by health workers included sharing FGS information with people who visit the clinic, in markets, through schools and other community structures and through stakeholders. Additional sensitisation channels suggested included radio jingles, live drama, television, megaphone announcements in communities along the river, large banners and social media. The level of sensitisation needed for a disease like FGS was compared to that of human immunodeficiency virus (HIV).
…you should do more publicity, publicise more on radio, television, if the publicity is much like you have in HIV and other diseases, people will know that it is not something you hide, it can be treated, go to the health facility. (QIT interview)
Insecurity, safety and competing priorities
In some areas insecurity, risks of kidnapping, COVID-19 prevention and vaccination activities, difficult terrain and distance from the health facility to endemic communities were issues for access to facilities and outreach for both patients and health workers, sensitisation efforts and supervision by the health system. Health workers highlighted that one of the challenges with the FGS intervention is that community members live far from the health facilities. Road networks may be unsafe and the cost of travelling to health facilities may be a barrier to seeking care. Health workers suggested that funding was needed to support health workers in accessing the community and they expressed concerns that their messages were not reaching enough people.
…because of the issue of the crisis that is going on the country, the issue of kidnapping, herdsmen, whatever, whatever and so on and so forth, and you know because of the terrain of the environment of our health centres, so, there are so many challenges. (QIT interview)
Even with these challenges, two health facilities managed to conduct outreach visits to communities. During this time the health workers took the opportunity to sensitise the communities and to treat where appropriate. A total of 19 women were identified during the outreach and 18 were given praziquantel treatment. One patient reported receiving praziquantel in the last 6 months and thus was excluded. Only four women subsequently visited the health facility for follow-up.
Discussion
This study is the first of its kind and has successfully kick-started the process of tackling FGS in Nigeria and beyond. The co-production approach allowed for contextualised systems thinking that considered and embedded the viewpoints of patients, health workers, policymakers and NTD implementers. By piloting the intervention in cycles, lessons were learned in real time and applied, meaning that the health system was strengthened and women could access treatment immediately. However, the study was time limited and therefore just the first step towards sustainable change.
Through qualitatively evaluating the intervention, the research has demonstrated the importance of applying systems thinking using the six building blocks of a health system identified by the World Health Organization.26,27 For example, participants have identified the need for ongoing awareness and sensitisation strategies in the community for service delivery, embedding learning about FGS for the health workforce, sustainable mechanisms to ensure that essential medicines and praziquantel are available in PHC, health information systems for monitoring and evaluation to ensure quality of service and tracking of results and financing and leadership to ensure that policy is developed or integrated considering equity and embedding of the latest knowledge, which is continuously growing for this neglected disease. Health system stakeholders, especially policymakers working within the schistosomiasis-endemic setting, must drive the process, as this will ensure local ownership of the intervention approach.28
We further add that a person-centred approach is required to ensure that the woman or girl is placed at the centre of the service design and treated as a person first, especially due to the potential consequences related to stigmatisation of a condition that affects sexual and reproductive health.29 This means developing care pathways and health worker training that consider and empathise with the individual experience of women and girls attending services. Therefore, management of FGS requires skilled health workers who have effective communication and counselling skills that can support women and girls to seek diagnosis and receive treatment. The health workers must have good knowledge of the disease and its mode of transmission, effective communication skills for health education and counselling to allay patients’ fears and the ability to reduce stigma.5,30,31
The results section clearly highlights the complexity of developing and piloting an intervention that can identify women and girls with FGS without stigmatising them in the process, train and support PHC workers to deliver effective diagnosis, treatment and follow-up without the use of complex equipment, challenge and interrogate existing policy related to praziquantel access and develop advocates that can drive the agenda forward.6,32,33 Therefore, gradual scaling up of the intervention will be needed with cycles of learning built in that reflect on different contexts and challenges in a timely manner. For example, as shown here, there was an implementation gap related to effective referral systems to secondary and tertiary healthcare for the management of complicated cases or cases where symptoms are unresolved. There is also a risk of missing a differential or additional diagnosis, such as cancer or STIs, therefore a robust referral system is needed to ensure that these women are not missed. Having praziquantel available in facilities is still a challenge due to international donor conditions, which will require dialogue, as highlighted by participants in this study. Therefore the intervention requires further testing and adaptation to national policy. Political and financial support are also required at a global and national level.
Strengths and limitations of the research
Our study was time limited, with smaller numbers of women and girls accessing the service than originally predicted. Due to the global pandemic of COVID-19, the intervention period of the study was reduced and a staggered/cautious approach to roll-out of the intervention was needed. While this enabled in-depth lessons to be learned and tools to be changed, longer QI cycles may have enabled more women and girls to be detected and treated. Due to security challenges in some communities, outreach and follow-up were also limited. This reflected the reality of managing FGS in endemic communities where issues of insecurity and risk need to be carefully considered. Due to funding constraints and cuts, the scale-up of the intervention is not currently being evaluated by COUNTDOWN, which has now ended. However, the FMoH and Ogun State MoH are continuing to develop and roll out the FGS programme from the information of this initial pilot. Results from this are likely to be seen in coming years.
We found that the participatory style training and tools produced during this research were useful in supporting awareness of FGS among health workers. Importantly, it also strengthened relationships and trust between health workers and patients. Some women reported that they would recommend to friends and family that they should access PHC if they have similar symptoms, and one woman even recommended to her husband that they change from a private facility to the PHC facility because of the trust she had in the health worker. This may further increase uptake of the intervention. This was also echoed by health workers during their interviews.
Suggestions for further research
Further research could consider how different awareness and advocacy campaigns may increase uptake of the intervention. It would be important to consider here socio-economic and demographic information such as how age, (dis)ability, religion, culture, distance from the PHC facility, economic status and education status, among others, may impact uptake of the intervention.
An outreach service was successful in reaching some women. It is important to conduct further research on this, exploring how an outreach intervention with PHC could reach women and girls. This could be embedded into MDA, however, this would require further revision of existing FMoH protocols.
As this was a pilot study, we were unable to adequately evaluate the success of the referral pathway. This is an important aspect to consider, as the intervention is considered for scale-up across Ogun State.
New diagnostics and urine microscopy could be embedded into the diagnostic pathway and evaluated. It is also important to consider wider randomised controlled trials that could compare the care package with more invasive diagnostics such as colposcopy.
Future research would also benefit from an evaluation of health systems strengthening outcomes. This may also include further research to strengthen workforce capacity for detection and management of FGS at the secondary and tertiary levels within Nigeria.
A strength of the research was that we engaged with different stakeholders at the initial stages of planning and throughout the pilot roll-out. This research focused on embedding the FGS care package in PHC, however, future research could engage with the National AIDS and STI Control Programme in Nigeria. A similar care package could be integrated into different services.
Conclusions
In summary, our study highlights the feasibility of integrating FGS management into PHC within schistosomiasis-endemic regions. Taking a co-productive approach and listening to the voices of women and girls, health workers and specialists across the health system has enabled care and management of symptoms to be integrated within PHC while considering the contextual challenges and solutions of such an intervention. The authors call on funders and policymakers to build upon this pilot study through generating awareness of FGS across the health system and communities, ensuring that praziquantel is available in PHC and ensuring that capacity strengthening of health workers in endemic areas is prioritised and financially supported. This requires political commitment and support. The lessons presented here must be considered so that the lives of women and girls, who have for too long been neglected, can be improved.
Acknowledgements
The authors would like to thank other FGS working group members, all the health workers at the facilities, the patients who volunteered for interviews.
Contributor Information
Helen Piotrowski, Department of International Health, Liverpool School of Tropical Medicine, Liverpool, UK.
Akinola Oluwole, Department of Neglected Tropical Diseases, Sightsavers Nigeria Country Office, Kaduna P.O. Box 503, Kaduna Nigeria.
Victoria O Fapohunda, Department of Neglected Tropical Diseases, Sightsavers Nigeria Country Office, Kaduna P.O. Box 503, Kaduna Nigeria.
Josephine B Adejobi, Department of Neglected Tropical Diseases, Sightsavers Nigeria Country Office, Kaduna P.O. Box 503, Kaduna Nigeria.
Obiageli J Nebe, NTDs Division, Department of Public Health, Federal Ministry of Health, Abuja, Nigeria.
Islamiat Soneye, Neglected Tropical Diseases Unit, Department of Public Health, Ogun State Ministry of Health, Ogun State Nigeria.
Maryam Kafil-Emiola, Neglected Tropical Diseases Unit, Department of Public Health, Ogun State Ministry of Health, Ogun State Nigeria.
Ntuen Uduak Gideon, NTDs Division, Department of Public Health, Federal Ministry of Health, Abuja, Nigeria.
Uwem F Ekpo, Department of Pure and Applied Zoology, Federal University of Agriculture, Abeokuta, PMB 2240 Abeokuta 110001, Nigeria.
Aminat O Ahmed, Department of Obstetrics and Gynaecology, Federal Medical Centre Abeokuta, Ogun State Nigeria.
Hameedat Opeyemi Abdussalam, Department of Obstetrics and Gynaecology, Federal Medical Centre Abeokuta, Ogun State Nigeria.
Gloria B Imhonopi, Department of Community Medicine and Primary Care, Federal Medical Centre Abeokuta, PMB 3031, Ogun State, Nigeria.
Omobola Yetunde Ojo, Department of Community Medicine and Primary Care, Federal Medical Centre Abeokuta, PMB 3031, Ogun State, Nigeria.
Oluwafayokemi Y Odubena, Department of Obstetrics and Gynaecology, Federal Medical Centre Abeokuta, Ogun State Nigeria.
Ise Oluwa-Adelokiki Adebola, State Hospital, Sokenu, Ijaye, Abeokuta, Ogun State.
Festus O Soyinka, Neglected Tropical Diseases Unit, Department of Public Health, Ogun State Ministry of Health, Ogun State Nigeria.
Olusola O Ogunmola, NTDs Division, Department of Public Health, Federal Ministry of Health, Abuja, Nigeria.
Abosede F Olalupo, Ogun State Secretariat, Ogun State Ministry of Health, Abeokuta 234, Nigeria.
Sunday Isiyaku, Department of Neglected Tropical Diseases, Sightsavers Nigeria Country Office, Kaduna P.O. Box 503, Kaduna Nigeria.
Rachael Thomson, Department of International Health, Liverpool School of Tropical Medicine, Liverpool, UK.
Kim Ozano, Department of International Health, Liverpool School of Tropical Medicine, Liverpool, UK.
Authors’ contributions
KO, HP, RT, AO and SI conceived the study. HP, KO and AO designed the study protocol and drafted the manuscript. VOF, JBA and AO conducted the interviews. HP, KO, AO, JBA and VOF conducted the analysis and interpretation of data. UFE, AOA, HA, GBI, OYO, OYO, AIO, FOS, OOO and AFO contributed to interpretation of the data and revision of the tools. All authors contributed to the study design, implemented the study and read and approved the final manuscript.
Funding
The research was funded by the COUNTDOWN programme (grant PO 6407), which is a multidisciplinary research consortium dedicated to investigating cost-effective, scaled-up and sustainable solutions necessary to control and eliminate the seven most common NTDs. COUNTDOWN (2014–2021) was funded by UK Aid, part of the Foreign, Commonwealth and Development Office.
Competing interests
None declared.
Ethical approval
This study was approved by the Liverpool School of Tropical Medicine Research Ethics Committee (19-090) and the National Health Research Ethics Committee of Nigeria (NHREC01/01/2007).
The participants were provided with a participant information leaflet outlining the study. Girls 15–17 y of age who received praziquantel treatment were only approached for interview if they attended the health facility with a parent or guardian and provided written assent and their parents/guardians provided written informed consent. All other participants provided written consent for observations at meetings and interviews.
Data availability
Appropriate data is available in the manuscript. Raw data is unsuitable for sharing publicly. Data requests may be sent to Julie Irving (Julie.irving@lstmed.ac.uk).
References
- 1. Ekpo U, Odeyemi O, Sam-Wobo Set al. Female genital schistosomiasis (FGS) in Ogun State, Nigeria: a pilot survey on genital symptoms and clinical findings. Parasitol Open. 2017;3:e10. [Google Scholar]
- 2. Kjetland EF, Leutscher PDC, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol. 2012;28(2):58–65. [DOI] [PubMed] [Google Scholar]
- 3. Christinet V, Lazdins-Helds JK, Stothard JRet al. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int J Parasitol. 2016;46(7):395–404. [DOI] [PubMed] [Google Scholar]
- 4. Friedman JF, Mital P, Kanzaria HKet al. Schistosomiasis and pregnancy. Trends Parasitol. 2007;23(4):159–64. [DOI] [PubMed] [Google Scholar]
- 5. Oluwole A, Bettee AK, Nganda MNet al. A quality improvement approach in co-developing a primary health care package for raising awareness and managing Female Genital Schistosomiasis (FGS) in Nigeria and Liberia. In review. [DOI] [PMC free article] [PubMed]
- 6. Bizimana P, Polman K, Van Geertruyden J-Pet al. Capacity gaps in health facilities for case management of intestinal schistosomiasis and soil-transmitted helminthiasis in Burundi. Infect Dis Poverty. 2018;7(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rollinson D, Knopp S, Levitz Set al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128(2):423–40. [DOI] [PubMed] [Google Scholar]
- 8. Utzinger J, Raso G, Brooker Set al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136(13):1859–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Søfteland S, Sebitloane MH, Taylor Met al. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int J Gynecol Obstet. 2021;153(2):190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortu G, Williams O. Neglected tropical diseases: exploring long term practical approaches to achieve sustainable disease elimination and beyond. Infect Dis Poverty. 2017;6(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masong MC, Wepnje GB, Marlene NTet al. Female genital schistosomiasis (FGS) in Cameroon: a formative epidemiological and socioeconomic investigation in eleven rural fishing communities. PLoS Glob Public Health. 2021;1(10):e0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basu P, Banerjee D, Mittal Set al. Evaluation of a compact, rechargeable, magnifying device to triage VIA and HPV positive women in a cervical cancer screening program in rural India. Cancer Causes Control. 2016;27(10):1253–9. [DOI] [PubMed] [Google Scholar]
- 13. Kjetland EF, Norseth HM, Taylor Met al. Classification of the lesions observed in female genital schistosomiasis. Int J Gynaecol Obstet. 2014;127(3):227–8. [DOI] [PubMed] [Google Scholar]
- 14. Bardosh K. Global aspirations, local realities: the role of social science research in controlling neglected tropical diseases. Infect Dis Poverty. 2014;3(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization, Joint United Nations Programme on HIV/AIDS . No more neglect — Female genital schistosomiasis and HIV — Integrating sexual and reproductive health interventions to improve women's lives. New York: Joint United Nations Programme on HIV/AIDS; 2019.
- 16. COUNTDOWN . Female genital schistosomiasis: a guide to inform equitable schistosomiasis control efforts in Ghana. Liverpool, UK: COUNTDOWN; 2020. [Google Scholar]
- 17. Ekpo UF, Hürlimann E, Schur Net al. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat Health. 2013;7(2):355–66. [DOI] [PubMed] [Google Scholar]
- 18. Nigeria Ministry of Heath . Ogun State Ministry of Health Report. Ogun, Nigeria: Ogun State Ministry of Health; 2009.
- 19. Engels D, Hotez P, Ducker Cet al. Integration of preventative and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull World Health Org. 2020;98(9):615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nigeria Federal Ministry of Health . Implementation guidelines for mass administration of deworming medicines in Nigeria. In: National Schistosomiasis and Soil-Transmitted Helminths Elimination Programmes. Abuja, Nigeria: Nigeria Federal Ministry of Health; 2020. [Google Scholar]
- 21. World Health Organization . Female genital schistosomiasis: a pocket atlas for clinical health-care professionals. Geneva: World Health Organization; 2015. [Google Scholar]
- 22. COUNTDOWN . Trainer manual: managing FGS at primary health care. Available from: https://countdown.lstmed.ac.uk/sites/default/files/centre/Trainer%20manual-%20Managing%20FGS%20at%20Primary%20Health%20Care.pdf [accessed 5 November 2022].
- 23. Neglected Tropical Diseases Department of Public Health . A Training Guide for Trainers of Community Implementers: on neglected tropical diseases control and elimination. Abuja, Nigeria: Federal Ministry of Health; 2014. [Google Scholar]
- 24. Drugs.com . Medications known to interact with praziquantel. Available from: https://www.drugs.com/drug-interactions/praziquantel.html [accessed 5 November 2022].
- 25. Dean L, Tolhurst R, Nallo Get al. Neglected tropical disease as a ‘biographical disruption’: listening to the narratives of affected persons to develop integrated people centred care in Liberia. PLoS Negl Trop Dis. 2019;13(9):e0007710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization . Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. Geneva: World Health Organization; 2010. [Google Scholar]
- 27. Glenn J, Kamara K, Umar ZAet al. Applied systems thinking: a viable approach to identify leverage points for accelerating progress towards ending neglected tropical diseases. Health Res Policy Syst. 2020;18(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Majid U, Kim C, Cako Aet al. Engaging stakeholders in the co-development of programs or interventions using intervention mapping: a scoping review. PLoS One. 2018;13(12):e0209826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization . WHO global strategy on people-centred and integrated health services: Interim Report. Geneva: World Health Organization; 2015. [Google Scholar]
- 30. Kukula VA, MacPherson EE, Tsey IHet al. A major hurdle in the elimination of urogenital schistosomiasis revealed: identifying key gaps in knowledge and understanding of female genital schistosomiasis within communities and local health workers. PLoS Negl Trop Dis. 2019;13(3):e0007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mazigo HD, Uisso C, Kazyoba Pet al. Primary health care facilities capacity gaps regarding diagnosis, treatment and knowledge of schistosomiasis among healthcare workers in north-western Tanzania: a call to strengthen the horizontal system. BMC Health Serv Res. 2021;21(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inocencio da Luz R, Linsuke S, Lutumba Pet al. Assessment of schistosomiasis and soil-transmitted helminths prevalence in school-aged children and opportunities for integration of control in local health services in Kwilu Province, the Democratic Republic of the Congo. Trop Med Int Health. 2017;22(11):1442–50. [DOI] [PubMed] [Google Scholar]
- 33. van der Werf MJ, Bosompem KM, de Vlas SJ. Schistosomiasis control in Ghana: case management and means for diagnosis and treatment within the health system. Trans R Soc Trop Med Hyg. 2003;97(2):146–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Appropriate data is available in the manuscript. Raw data is unsuitable for sharing publicly. Data requests may be sent to Julie Irving (Julie.irving@lstmed.ac.uk).