Abstract
The monkeypox virus is a virus that has 90% genomic homology with the human (smallpox), but it is naturally transmitted between different wild animal reservoirs and is considered a zoonosis. Throughout the 20th century, different vaccines based on the vaccinia poxvirus were developed and used for vaccination against smallpox. After the eradication of smallpox, these vaccines were no longer used. Current vaccines against monkeypox virus are classified by the WHO as replicative (ACAM2000), minimally replicative (LC16m8) and non-replicative (MVA-BN), the latter being the one currently used. The 2022 extra-African monkeypox virus epidemic has highlighted the lack of vaccines with proven efficacy and low reactogenicity. It is considered that the use of this vaccine in the current outbreak may play a role in the prevention or attenuation of the disease as pre-exposure prophylaxis in close contacts of confirmed cases.
Keywords: Monkeypox virus, Vaccines, Vaccination
Abstract
El virus de la viruela de los monos es un virus que presenta un 90% de homología genómica con el humano (smallpox), pero se trasmite de forma natural entre diferentes reservorios animales salvajes y es considerado una zoonosis. A lo largo del siglo XX se desarrollaron diferentes vacunas basadas en el poxvirus vaccinia que fueron utilizadas para la vacunación frente a la viruela humana. Tras la erradicación de la viruela humana estas vacunas dejaron de utilizarse. Las vacunas actuales frente a la viruela de los monos se clasifican por la OMS como replicativas (ACAM2000), mínimamente replicativas (LC16m8) y no replicativas (MVA-BN), siendo esta última la utilizada en la actualidad. La epidemia extraafricana de viruela de los monos de 2022 ha puesto en evidencia la falta de vacunas de eficacia demostrada y de baja reactogenicidad. Se considera que la utilización de esta vacuna en el brote actual puede desempeñar un papel en la prevención o atenuación de la enfermedad como profilaxis preexposición en contactos estrechos de casos confirmados.
Palabras clave: Viruela del mono, Vacunas, Vacunación
Introduction
Monkeypox virus (MV) was identified in 1958 in Denmark as a new member of the Orthopoxvirus genus as a consequence of the use of tissues and organs from these animals in safety studies and isolation of the poliomyelitis virus for use in human vaccines.1, 2 It presents a double-stranded DNA-type genome that contains about 110–260,000 base pairs, a very complex structure, and measures a considerable size (200–250 nm).1
The human MV infection was first described in 1970 in the Democratic Republic of the Congo. From then on, outbreaks began to occur in neighbouring African countries.2, 3, 4 In 2003 there was an outbreak of 47 cases in the USA as a result of the African importation of rodents that subsequently transmitted the infection to prairie dogs, and their use as pets lead to infections in humans.4, 5 The geographical distribution of this infection has not been established, since few studies of carriers, either people or wild animals, have been conducted, but it is most likely located in all those areas or countries where the animal species that act as a natural reservoir are found.2, 6
The smallpox virus is the only member of the Orthopoxvirus genus that exclusively infects humans.7, 8 On the contrary, the MV virus (monkeypox) is a virus that presents 90% genomic homology with humans, but it is transmitted naturally between different wild animal reservoirs (squirrels and rodents) and is considered a zoonosis, being able to infect humans through direct contact with these animals.9 Interhuman transmission has also been described, especially in rural areas and closed family environments.7, 8 Two distinct phylogenetic lineages of the MV virus have been characterised and are distributed throughout western and central Africa. The epidemiological and virological data seem to indicate differences in virulence or severity of the infection in each of them; thus the central lineage seems to show greater severity (11%–17% lethality) and more clinical complications in both humans and primates.2, 4, 7
The disease caused by MV is transmitted by direct contact and respiratory secretions and is very similar to human smallpox. After an incubation period of between 4–24 days, most patients present a fever and headaches. A few days later they develop a very characteristic rash that is initially papular, then it becomes vesicular and finally pustular. Although it is a benign disease in the healthy population, it can present a lethality of 1%–5% and be associated with serious complications or sequelae in 74% of unvaccinated people.2, 4, 7, 10 The main differential diagnosis must be made with varicella, (designated as chickenpox), although it is not produced by a poxvirus but by a herpesvirus.8, 10
Since the appearance of the different African outbreaks very few cases of MV had been described outside these countries. However, as of April 2022, there has been an extra-African outbreak that has led to the confirmation of more than 60,000 cases. This current outbreak is characterised by preferentially affecting (95%) men who have sex with other men, so that this has become the main risk group and the epidemic has spread in this group.11 The control of the MV epidemic outbreak must be carried out by applying public health measures aimed at the risk groups, establishing epidemiological surveillance systems such as the isolation and control of infected people.12 Antiviral drugs against MV are scarce and of variable efficacy13 and for this reason, only vaccines specifically for the human smallpox virus, which protect 95% against MV, could be used in certain risk situations.12
First generation vaccines
Throughout the 20th century, various vaccines have been developed for use against smallpox, based on the vaccinia poxvirus with differing biological characteristics. These vaccines contained active and competent replicative viruses with variable human reactogenicity. During the different campaigns to eradicate human smallpox, different strains of the vaccinia virus were used as vaccines, such as Lister/Elstree, New York City Board of Health (NYCBH, Dryvax® vaccine), EM-63 and Tian-Tan, chosen for their high level of safety compared to other strains such as Copenhagen or Bern.14, 15 The difference with these first-generation vaccines was their ability to infect different tissues; thus, the Lister vaccine was grown in the chick embryo chorioallantoic membrane (CAM) and the NYCBH on calf or water buffalo skin cells. They also differed in their form of presentation, either frozen or lyophilized.14, 16 The production of these vaccines decreased as the eradication of smallpox progressed, until those remaining were stored in high-security laboratories. In the USA vaccination for the general public ended in 1972, and in 1989 for the military population. The production of the Dryvax® vaccine was discontinued in 1978, although 15 million doses were stored as a preventative measure.14, 15, 16
Second generation vaccines
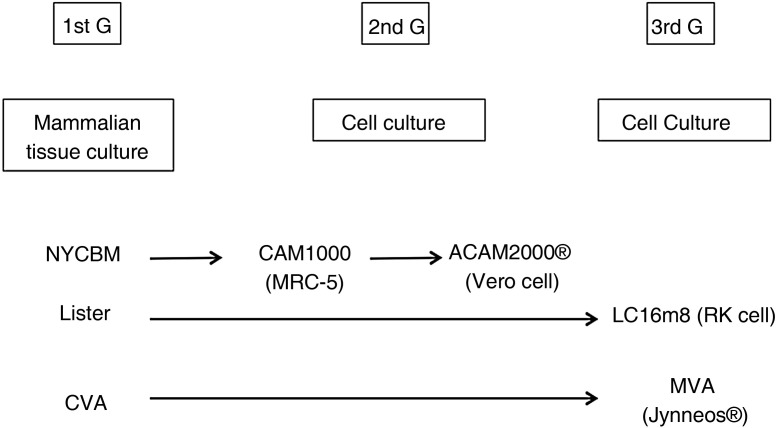
In the 1990s when faced with the possible use of the vaccinia virus as a bioterrorism weapon, the American government decided to restart the studies aimed at improving the safety and immunogenicity of the Dryvax® vaccine; this vaccine was obtained from the pustules caused by the virus on the skin of calves, which was the reason why it presented many impurities and unwanted effects. The company Acambis, Inc. (Cambridge, MA) lead the way to conducting the molecular studies of adaptation and improvement of the new vaccine against smallpox. The first analyses showed that this vaccine was heterogeneous and made up of 6 virological clones that differed in terms of virulence and immunogenicity. Out of all of them, it was clone 2 (CL2) that seemed to present the average behavior of the vaccine itself and the one with the lowest neurovirulence in animals. This CL2 clone was passaged 7 times in the MRC-5 human fibroblast line and named ACAM1000. To increase its attenuation, this new strain was passaged 10 times in the Vero cell line. (African Green monkey kidney epithelial cells) giving rise to the definitive strain and vaccine designated as ACAM2000 (Emergent BioSolutions Inc. Gaithersburg, Maryland, USA) (Fig. 1 ).17, 18
Fig. 1.
Evolution of the three generations of vaccines against smallpox.
CVA: Chorioallantoic Vaccinia; MVA: Modified Vaccinia Virus Ankara.
ACAM2000 is a live attenuated vaccine that was approved by the FDA in August 2007 to replace the older Dryvax® vaccine based on a vaccinia virus.18, 19 This vaccine is indicated for immunization against human smallpox in people at risk over 18 years of age who are immunocompetent. It is not indicated for anyone immunocompromised, or pregnant or breast-feeding women. Only one dose should be administered and each dose contains between 2.5–12.5 × 105 infectious units and is administered by the percutaneous route (scarification) using a bifurcated needle. Great care must be taken during its administration as the virus can be transmitted to people who are in contact with the vaccinated person, including the personnel who perform the vaccination. The main adverse effects occur at the inoculation site, although general effects (headache, malaise, fever) have also been described.18, 19, 20
In a person vaccinated for the first time, a pustular-like skin reaction occurs at the injection site; this lesion evolves giving rise to a papule after 2–5 days. The papule becomes vesicular after 8–10 days and dries up and forms a scab after 14–21 days, following the classic evolution of vaccination prior to 1980.19 Inadvertent transmission only occurs with this vaccine and can lead to vertical transmission, resulting in severe fetal smallpox. In the ACAM2000 vaccine, myocarditis and/or pericarditis has been described with a frequency of 5.7/100,000 vaccinated and post-vaccination encephalitis.20, 21
Third generation vaccines
In 1959, the German microbiologist Anton Mayr began to infect cells derived from the chick embryo chorioallantoic membrane (CAM) with the vaccine strain (vaccinia) against human smallpox with the aim of obtaining a new attenuated strain.22 After several years of cell passages (>500), he discovered that this virus had changed and was no longer capable of infecting human cells. However, when experimentally inoculated to laboratory animals, mice and chimpanzees, it induced an intense immune response that protected them from human smallpox. In 1968, and after 516 passages in CAM, the virus was named Modified Vaccinia Virus Ankara (MVA) because the original strain came from a patient from this Turkish city. The comparison of the genomic maps of the original and the modified Ankara virus (Fig. 2 ) showed important deletions and mutations that shortened the length of the viral genome from 208 kb to 178 kb.21 The main changes corresponded to: a) the inability of the strain to infect and replicate in mammalian cells (host restriction); b) loss of the genes that determined the formation of type A intracytoplasmic inclusions and c) shortening of the viral hemagglutinin coding sequence which made it difficult for it to bind to the cellular receptor. All these changes converted the MVA strain into a useful new tool for genomics and vaccine production with high safety and a protective immune response against human smallpox and monkeypox. The MVA strain was used by the Bavarian State Institute for Vaccines (Bavarian Nordic, Hellerup, Denmark) for the industrial production of vaccines against smallpox.23, 24
Fig. 2.
Genetic modifications of the original Ankara virus after 572 passages in chick embryonic cells.
Jynneos® (also called Imvamune, Imvanex or MVA-BN) is a live attenuated, non-replicating viral vector vaccine produced from the genetic modification of the vaccinia Ankara-Bavarian Nordic (MVA-BN strain) virus grown in chicken embryo fibroblasts. Each dose of 0.5 ml contains between 0.5 and 3.95 × 108 of infectious units.19 It was approved by the FDA in September 2019 and indicated for the prevention of human smallpox and the monkeypox in people over 18 years of age at risk of acquiring these infections.25 This vaccine was later approved in Europe under the name Imvanex®. It was administered in two doses of 0.5 ml administered at least 28 days apart (4 weeks) subcutaneously or intramuscularly. In 2022, based on the results obtained by Frey et al.26 in 2015 with the MVA vaccine, the FDA authorised the administration of only 0.1 ml of this vaccine but intradermally. The high viral load of the original vaccine and the demonstration of an immune response similar to the previously recommended dose were the main reasons for authorising this new administration of the vaccine.27, 28 Adverse reactions were analysed in a randomised, double-blind clinical study versus placebo in a group of volunteers aged 18–40 years who received two doses of Jynneos® versus placebo. Most of the effects were at the level of the injection site (pain, redness, edema) and local (muscular pain).25
Analysis of the immunogenicity of the Jynneos® vaccine against ACAM2000 was performed in a randomised study in adults (18–42 years) who received two doses of the prior (220 subjects) and one of the latter (213 subjects). The primary objective of this study was to determine the geometric mean titer (GMT) for the neutralizing antibody induced by each of these vaccines. It was verified that the Jynneos® vaccine induced post-vaccinal GMTs almost twice (152.8 vs. 84.4) those obtained with the ACAM2000 vaccine.23, 24 Greenberg et al.24 conducted a randomised, double-blind phase II study to evaluate the safety and immunogenicity of MVA versus placebo in people aged 56–80 years, verifying that one or two doses of MVA is safe and immunogenic in this age group.
LC16m8 is a Japanese attenuated vaccine derived from the Lister vaccine strain; it was developed in the 1970s to improve the safety of those in use at that time. The attenuation was carried out through multiple passages in rabbit primary kidney cells at suboptimal temperatures, therefore it should be considered as a vaccine sensitive to human body temperature.14 Molecular data showed passage-induced mutations in the B5R gene, leading to a truncated B8 antigen and inefficient extracellular virion production. This vaccine induced robust protection against smallpox and immunity similar to conventional vaccines but with fewer adverse effects. However, the replicative capacity of the attenuated virus included in the vaccine and the possible reversion of the gene B5R, suggested that its attenuation process was less than that obtained in the MVA vaccine.29, 30
Regarding the other third-generation vaccines, it is worth mentioning NYVAC, which is attenuated and derived from the original Copenhagen strain. It has been generated through a deletion of 18 non-essential genes necessary to encode the virulence genes, giving rise to a highly secure attenuated strain. Recent studies seem to show that this vaccine induces low levels of humoral immunity in humans compared to previous vaccines, which is why it has ceased to be used.10, 14, 31 Furthermore, the defective dVV-L vaccine was generated by modifying the Lister strain through the deletion of the gene encoding the uracil-DNA-glucosylase gene, essential for viral replication. Although it induces a good immune response, its ability to prevent exogenous infection by the human smallpox virus has not been tested.31
Vaccination strategies
WHO has published a recommendation guide for the use of MV vaccines.12 It establishes the following conclusions: a) based on the risks, benefits, and scarce vaccine supply, mass vaccination of the unexposed population is not recommended since currently its risk is low; b) human-to-human spread of MV must be controlled by public health measures (detection, isolation and case follow-up); c) post-exposure preventive vaccination, preferably with a third generation vaccine, is recommended in the first 3–4 days of exposure (and up to 14 days in the absence of symptoms) in close contacts of infected people, but if it is administered between days 4–14 postexposure, the vaccine only reduces symptoms but does not prevent disease; and d) primary preventive pre-exposure vaccination is only recommended for healthcare workers at high risk of exposure, laboratory personnel working directly with any poxviruses, and personnel designated by the health authorities who are directly involved in treatment and contact with possible cases.12, 19
The current vaccines against MV are classified by the WHO as replication-competent (ACAM2000), minimally replicating (LC16m8) and non-replicating (MVA-BN) and the main differences and recommendations for use are described in Table 1, Table 2 ; these recommendations are very similar to those made by the ACIP in 2022 voting in favor of the preferential use of the non-replicating vaccine (Jynneos®).12 MVA-BN could be used in <18 years at a high risk of exposure, but its use would be off-label.12
Table 1.
Main differences between the current vaccines against smallpox and monkeypox.
| ACAM2000® | Jynneos® | |
|---|---|---|
| Virus | Attenuated | Modified |
| Virus origin | NYCBM | MVA-BN |
| Replication | Competent | Deficient |
| Dose | 1 | 2 (at 28 days) |
| Composition | 2.5−12.5 × 105 iu | 0.5−3.95 × 108 iu |
| Injection | Percutaneous | Subcutaneous |
| Skin reaction | Yes | No |
| Accidental inoculation | Yes | No |
| Self-inoculation | Yes | No |
| Vaccinal eczema | Yes | No |
| Immunocompromised | No | Yes |
| Pregnant | No | Yes |
iu: Infective units.
Table 2.
Type of vaccines recommended according to the situation of the exposed person.
| ACAM2000 | MVA-BN | LC16m8 | |
|---|---|---|---|
| Healthy adults | Yes | Yes | Yes |
| Immunosuppressed | No | Yes | No |
| Pregnant | No | Yes | Yes |
| Breast-feeding | No | Yes | Yes |
| Children <18 years | No | Yesa | No |
Off-label use.
In Spain, the health authorities have published some recommendations for the early detection and treatment of suspected cases of MV.32 In the current outbreak, the Imvamex® vaccine can act as primary preventive pre-exposure in close contacts of confirmed cases, and thereby play a role in disease prevention or attenuation. However, due to vaccine supply constraints, post-exposure vaccination should be prioritized.33 The vaccine has now become a measure which, together with the epidemiological and clinical control of the patients, is determining a progressive decrease in the current epidemic of monkeypox.34
Financing
No funding has been received for this work.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Jezek Z., Fenner F. Human monkeypox. Monogr Virol. 1988;17:1–40. [Google Scholar]
- 2.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 3.Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd-Smith J.O., Kisalu N.K., Kinketa T.L., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. PNAS. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reina J., Reina N. ¿Deberíamos empezar a preocuparnos por la viruela del mono? Med Clin (Barc) 2018;151:320–322. doi: 10.1016/j.medcli.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Reed K.D., Melski J.W., Craham M.B., Regnery T.L., Sotir M.J., Wegner M.V., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 6.Parker S., Buller R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damon I.K. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29(S4):D54–D59. doi: 10.1016/j.vaccine.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Jezek Z., Szceniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 9.Khodakevich L., Jezek Z., Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–99. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lum F.M., Torres-Ruesta A., Tay M.Z., Lin R.T., Lye D., Renia L., et al. Monkeypox: disease epidemiology, host immunity and cliniocal interventions. Nat Rev Immunol. 2022;5:1–17. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organizarion Monkeypox. 2022. https://www.who.int/health-topics/monkeypox Available from:
- 12.World Health Organization. Vaccines and immunization for monkeypox: interim guidance. 24 August 2022. [Accessed 13 September 2022]. Available from: https://www.who.int/publications/i/item/WHO-MPX-Immunization-2022.2-eng.
- 13.Sherwat A., Brooks J.T., Birnkrant D., Kim P. Tecovirimat and the treatment of monkeypox: past, present and future considerations. N Engl J Med. 2022;387:579–581. doi: 10.1056/NEJMp2210125. [DOI] [PubMed] [Google Scholar]
- 14.Melamed S., Israely T., Paran N. Challenges and achievements in prevention and treatment of smallpox. Vaccines. 2018;6:8. doi: 10.3390/vaccines6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenner F. A successful eradication campaign. Global eradication of smallpox. Rev Infect Dis. 1982;4:916–930. doi: 10.1093/clinids/4.5.916. [DOI] [PubMed] [Google Scholar]
- 16.Collier L.H. The development of a stable smallpox vaccine. J Hyg. 1955;53:76–101. doi: 10.1017/s002217240000053x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalca A., Zumbrum E.E. ACAM2000™. The new smallpox vaccine for United States strategic national stockpile. Drug Des Devel Ther. 2010;4:71–79. doi: 10.2147/dddt.s3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monath T.P., Caldwell J.R., Mundt W., Fusco J., Johnson C.S., Buller M., et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City board of health strain), a second generation smallpox vaccine for viological defense. Int J Infect Dis. 2004;S2:S31–S44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizk J.G., Lippi G., Henry B.M., Forthal D.N., Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022 doi: 10.1007/s40265-022-01742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voigt E.A., Kennedy R.B., Poland G.A. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197. doi: 10.1080/14760584.2016.1175305. 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ACAM2000 Package insert. Gaitherburg, MD: emergent product development Gaithersburg Inc 2007. [Accessed September 2022]. Available from: https://www.fda.gov/media/75792/dowload.
- 22.Kupferschmidt K. Monkeypox vaccination plans take shape amid questions. Science. 2022;376:1142–1143. doi: 10.1126/science.add3743. [DOI] [PubMed] [Google Scholar]
- 23.Pittman P.R., Hahn M., Lee H.S., Koca C., Samy N., Schmidt D., et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccineagainst smallpox. N Engl J Med. 2022;381:1897–1908. doi: 10.1056/NEJMoa1817307. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg R.N., Hay C.M., Stapleton J.T., Marbury T.C., Wagner E., Kreitmeir E., et al. A randomized, double-blind, placebo-controled phase II trial investigating the safety and immunogenicity of modified vaccinia Ankara smallpox vaccine (MVA-BN) in 56-80-year-old subjects. PLoS One. 2016 doi: 10.1371/journal.pone.0157335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao A.K., Petersen B.W., Whiehill F., Razeq J.H., Isaacs S.N., Merchlinsky M.J., et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices. United States, 2022. Morb Mortal Wkly Rep. 2022;71:734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey S.E., Stapleton J.T., Ballas Z.K., Rasmussen W.L., Kaufman T.M., Blevins T.P., et al. Human antibody responses following vaccinia immunization using protein microarrays and correlation with cell-mediated immunity and antibody-depeddnt celular cytotoxicity responses. J Infect Dis. 2021;224:1372–1382. doi: 10.1093/infdis/jiab111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larkin H.D. FDA authorizes intradermal vaccine, streamlines rules to increase monkeypox treatment access. JAMA. 2022;328:819. doi: 10.1001/jama.2022.14692. [DOI] [PubMed] [Google Scholar]
- 28.Brooks J.T., Marks P., Goldstein R.H., Walensky R.P. Intradermal vaccination for monkeypox — benefits for individual and public health. N Engl J Med. 2022;387:1151–1153. doi: 10.1056/NEJMp2211311. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy J.S., Gurwith M., Deckker C.L., Frey S.E., Edwards K.M., Kenner J., et al. Safety and immunigenicity of LC16m8, an attenuted smallpox vaccine in vaccinia-naive adults. J Infect Dis. 2011;204:1395–1402. doi: 10.1093/infdis/jir527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eto A., Saito T., Yokote H., Kurane I., Kanatani Y. Recent advances in the study of live attenuated cell-cultured smallpox vaccine LC16m8. Vaccine. 2015;33:6106–6111. doi: 10.1016/j.vaccine.2015.07.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Midgley C.M., Putz M.M., Weber J.N., Smith G.L. Vaccinia virus strain nyvac induces substantially lower and qualitatively different human antibody responses compared with strains lister and dryvax. J Gen Virol. 2008;89:2992–2997. doi: 10.1099/vir.0.2008/004440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponencia de alertas y planes de preparación y respuesta. Protocolo para la detección precoz y manejo de casos ante la alerta de viruela de los monos (monkeypox) en España. Ministerio de Sanidad. [Accessed 5 August 2022]. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/alertaMonkeypox/home.htm.
- 33.Comisión de Salud Pública. Recomendaciones de vacunación en el brote actual de viruela del mono. Consejo interterritorial. Ministerio de Sanidad. [Accessed 9 June 2022]. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/MonkeyPox/docs/Propuesta_vacunacion_Monkeypox.pdf.
- 34.Poland G.A., Kennedy R.B., Tosh P.K. Prevention of monkeypox with vaccines: a rapid review. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00574-6. S1473-3099(22)00574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]