Abstract
Rationale
Shared symptoms and genetic architecture between coronavirus disease (COVID-19) and lung fibrosis suggest severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may lead to progressive lung damage.
Objectives
The UK Interstitial Lung Disease Consortium (UKILD) post–COVID-19 study interim analysis was planned to estimate the prevalence of residual lung abnormalities in people hospitalized with COVID-19 on the basis of risk strata.
Methods
The PHOSP–COVID-19 (Post-Hospitalization COVID-19) study was used to capture routine and research follow-up within 240 days from discharge. Thoracic computed tomography linked by PHOSP–COVID-19 identifiers was scored for the percentage of residual lung abnormalities (ground-glass opacities and reticulations). Risk factors in linked computed tomography were estimated with Bayesian binomial regression, and risk strata were generated. Numbers within strata were used to estimate posthospitalization prevalence using Bayesian binomial distributions. Sensitivity analysis was restricted to participants with protocol-driven research follow-up.
Measurements and Main Results
The interim cohort comprised 3,700 people. Of 209 subjects with linked computed tomography (median, 119 d; interquartile range, 83–155), 166 people (79.4%) had more than 10% involvement of residual lung abnormalities. Risk factors included abnormal chest X-ray (risk ratio [RR], 1.21; 95% credible interval [CrI], 1.05–1.40), percent predicted DlCO less than 80% (RR, 1.25; 95% CrI, 1.00–1.56), and severe admission requiring ventilation support (RR, 1.27; 95% CrI, 1.07–1.55). In the remaining 3,491 people, moderate to very high risk of residual lung abnormalities was classified at 7.8%, and posthospitalization prevalence was estimated at 8.5% (95% CrI, 7.6–9.5), rising to 11.7% (95% CrI, 10.3–13.1) in the sensitivity analysis.
Conclusions
Residual lung abnormalities were estimated in up to 11% of people discharged after COVID-19–related hospitalization. Health services should monitor at-risk individuals to elucidate long-term functional implications.
Keywords: COVID-19, hospitalization, HRCT, lung damage, lung abnormalities
At a Glance Commentary
Scientific Knowledge on the Subject
Current studies highlight persistent breathlessness and radiological patterns suggestive of lung fibrosis, as well as shared genetic architecture with idiopathic pulmonary fibrosis, in people discharged after severe coronavirus disease (COVID-19) hospitalization. Survivors of COVID-19 may develop parenchymal abnormalities consistent with lung fibrosis.
What This Study Adds to the Field
This study assesses the risk factors for residual lung abnormalities, provides evidence of persistent abnormalities within 1 year of discharge from over 200 computed tomography scans, and estimates the prevalence of lung abnormalities after discharge to be up to 11% in a broad range of COVID-19 severity. The findings emphasize the importance for health services to undertake active radiological and physiological monitoring to assess progression or resolution over time.
Long-term symptoms of coronavirus disease (COVID-19) have been widely reported and can have a severe impact on quality of life, frequently characterized by chronic breathlessness (1–3). Postmortem studies on patients with COVID-19 have highlighted diffuse parenchymal alterations, including alveolar damage, exudation, and the development of pulmonary fibrosis, which may explain chronic respiratory symptoms in survivors (4–6).
A number of studies have identified similarities between severe COVID-19 and idiopathic pulmonary fibrosis, an archetypal interstitial lung disease (ILD). These include shared genetic etiology (7, 8), circulating biomarkers (9, 10), similarities in pulmonary function, and radiological features (11). Viral injury may promote lung fibrosis, and chronic viral infection has been shown to be associated with developing idiopathic pulmonary fibrosis (12). Consequently, survivors of COVID-19 may develop parenchymal abnormalities consistent with ILD, including radiological patterns of ground-glass opacities and reticulations.
To understand the potential risk of COVID-19 leading to the development of longer-term ILD and fibrosis, the UK Interstitial Lung Disease Consortium (UKILD) post–COVID-19 study aims to investigate the risk factors and nature of long-term lung damage from COVID-19 in a longitudinal observational study. To support clinical and research management, this planned interim analysis of the UKILD post–COVID-19 study addresses the extent of residual lung abnormalities after hospitalization after completion of an early follow-up visit of the prospective PHOSP–Covid-19 (Post-Hospitalization COVID-19) study (13).
Some of the results of these studies have been previously reported in the form of a preprint (medRxiv, 16 March 2022; https://www.medrxiv.org/content/10.1101/2022.03.10.22272081v2).
Methods
Participants
This interim analysis was restricted to participants of the PHOSP–COVID-19 study, a prospective longitudinal cohort study of adults discharged from National Health Service hospitals across the United Kingdom after admission for confirmed or clinically diagnosed COVID-19, previously described in detail (14).
Individuals withdrawing consent from PHOSP–COVID-19 were excluded. Individuals being managed for an a priori diagnosed ILD or pulmonary fibrosis, as recorded by site teams using hospital notes, were identified by hand searches of comorbidities and subsequently excluded.
Interim Study Design
Interim participants were discharged by the end of March 2021, representing wave one of the pandemic; interim data were collected up to October 2021 and were restricted to within 240 days of discharge. Analyses were performed with data recorded through routine follow-up (PHOSP–COVID-19 Tier 1) and those with completed early research follow-up visits (PHOSP–COVID-19 Tier 2). Clinically indicated thoracic computed tomography (CT) scans were identified through the PHOSP–COVID-19 study via linkage to a radiological database; linked CT scans were requested at clinical discretion. The presence of residual lung abnormalities on volumetric CTs was scored on a lobar basis; the percentage involvement of ground-glass opacities, reticulations, or the sum of involvement was averaged across lobes to quantify residual abnormality (15). The primary outcome was visually scored residual abnormalities greater than 10% lung involvement on CT (15).
Risk factors implicated in worse outcomes after COVID-19 hospitalization of individuals with ILD were described (16). These included sex, age, ethnicity, body mass index, and IMD (Index of Multiple Deprivation). A modified WHO (World Health Organization) clinical progression scale was used to define the severity of admission: 1) no supplemental oxygen; 2) supplemental oxygen only; 3) continuous positive airway pressure (CPAP); and 4) invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO). Abnormal chest X-ray (CXR) reports were classified at follow-up, defined as “suggestive of lung fibrosis,” “extensive, persistent changes greater than one-third of lung involvement,” and “indeterminate,” compared with “other” or “normal.” Breathless and cough symptoms were recorded at follow-up with the patient symptom questionnaire developed for the PHOSP–COVID-19 study (14). Percent predicted values for FVC (ppFVC) and DlCO (ppDlCO) were obtained at follow-up visits and calculated using global lung initiative reference equations.
Statistical Analysis
Risk factor data were presented descriptively overall, according to PHOSP–COVID-19 tier, and within the sample of linked and scored CTs. Chi-square tests were performed on nonmissing categories. Residual abnormalities on paired CT scans were tested with paired t test; changes in scored residual lung abnormalities over time were estimated using linear mixed effect models, with random effects of timing at the level of the individual, adjusted for sex and IMD. A random sample of 70 CT scans was tested for interrater agreement by Cohen’s kappa (κ), with a second radiologist blinded to scores.
Univariate relative risk ratios of threshold greater than 10% residual abnormalities and difference in involvement on CT were modeled with dichotomized exposure variables. Bayesian binomial and linear associations were estimated using 12,500 Markov Chain Monte Carlo (MCMC) iterations, including a burn-in of 2,500 and 10,000 subsequent simulations using random-walk Metropolis-Hastings sampling. Noninformative, flat priors were selected, and estimates were reported with a 95% CrI. Linear associations were additionally adjusted for demographics of sex and IMD.
Clinical risk factors with consistent significant effects were selected to develop risk strata of suspected residual lung abnormalities after COVID-19 hospitalization. For the indexing of risk strata, missing data on clinical indicators were imputed to the reference (lowest risk) category. The percentage of participants within moderate- to very-high–risk strata and no CT scored were defined as at-risk. Hospital admissions were compared between at-risk groups using chi-square, and 15 index admission variables were selected from 61 by least absolute shrinkage and selection operator.
Bayesian inference with a binomial distribution of at-risk cases and noncases (17) was used to estimate the prevalence of suspected residual lung abnormalities after COVID-19 hospitalization within 240 days of discharge, reported with a 95% CrI. MCMC simulations were run as described above. Noninformative, uniform β priors were used and compared in sensitivity analyses with uniform Jeffrey’s priors, as well as skeptical and power priors informed by published population studies of ILD (18, 19). Sensitivity analyses were performed in PHOSP–COVID-19 Tier 2 research follow-up participants in which data completeness was greater. Analyses were performed in Stata SE16.0 within the Scottish National Safe Haven Trusted Research Environment.
Results
Cohort Demographics and Patterns of Lung Damage
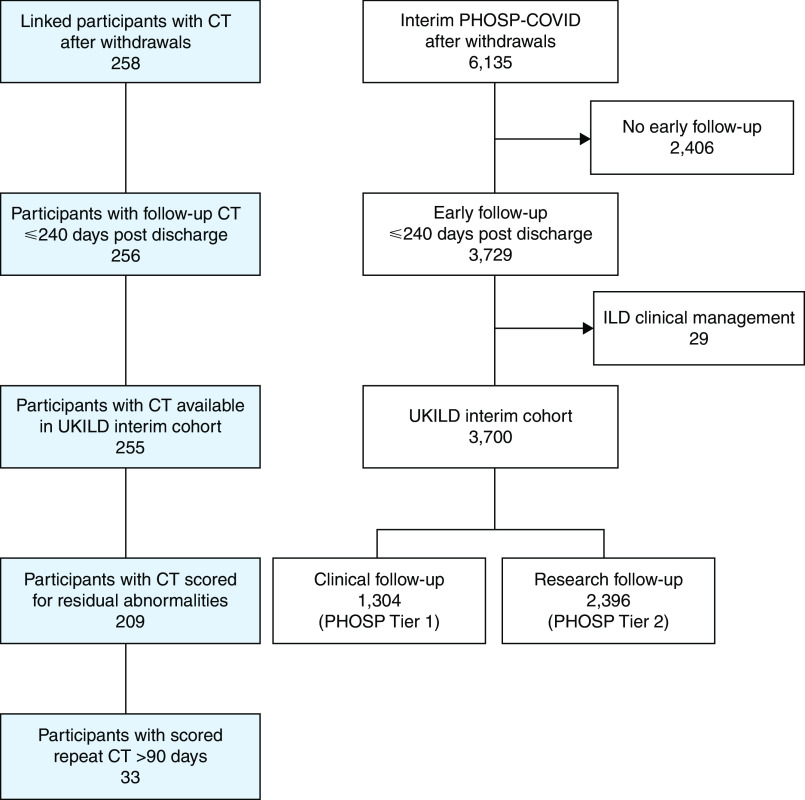
A total of 3,700 PHOSP–COVID-19 participants reached the criteria for inclusion in the interim UKILD post–COVID-19 study cohort. This included 1,304 patients with data available through routine clinical care (Tier 1) and 2,396 who had completed an early follow-up research visit within 240 days of discharge (Tier 2) (Figure 1). We observed that 255 of 3,700 (6.9%) participants in the interim cohort had a linkable thoracic CT scan performed, 220 of 2,396 (9.2%) Tier 2 participants had CT scans performed, and 35 of 1,304 (2.7%) of Tier 1 participants had CT scans performed (P < 0.001). Of 255 participants with linked CT scans within 240 days of discharge (median, 113 days; interquartile range [IQR], 69–166) (Figure E1 in the online supplement), a total of 209 (82.0%) were visually scored with interrater agreement on 70% of scans (Cohen’s κ, 0.33). Participants with a CT scored were majority male (68.4%), White (68.9%), had a median age of 58 (52–67), and had a median time to early follow-up visit of 140 days (IQR, 106–170) (Table 1).
Figure 1.
CONSORT flow diagram of UKILD post–COVID-19 study interim cohort definition. White boxes derived from the PHOSP-COVID database. Blue boxes represent computed tomography samples linked with Post-Hospitalization COVID (PHOSP-COVID) identifiers in a radiological database. CONSORT = Consolidated Standards of Reporting Trials; CT = computed tomography; ILD = interstitial lung disease; UKILD = UK interstitial lung disease consortium post–COVID-19 study.
Table 1.
UKILD Post–COVID-19 Study Interim Cohort Demographics
| Interim |
CT score |
Tier 1 |
Tier 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 3700 | % | n = 209 | % | n = 1304 | % | n = 2396 | % | χ2 P value | |
| Sex | 0.091 | ||||||||
| Male | 2,247 | 60.7 | 143 | 68.4 | 768 | 58.9 | 1,479 | 61.7 | — |
| Female | 1,450 | 39.2 | 66 | 31.6 | 535 | 41.0 | 915 | 38.2 | — |
| Age | 0.027 | ||||||||
| ⩾60 | 1,801 | 48.7 | 99 | 47.4 | 667 | 51.2 | 1,134 | 47.3 | — |
| <60 | 1,895 | 51.2 | 110 | 52.6 | 636 | 48.8 | 1,259 | 52.5 | — |
| Ethnicity | <0.001 | ||||||||
| White | 2,804 | 75.8 | 144 | 68.9 | 1,015 | 77.8 | 1,789 | 74.7 | — |
| Asian | 467 | 12.6 | 40 | 19.1 | 144 | 11.0 | 323 | 13.5 | — |
| Black | 223 | 6.0 | 15 | 7.2 | 56 | 4.3 | 167 | 7.0 | — |
| Other | 131 | 3.5 | 6 | 2.9 | 31 | 2.4 | 100 | 4.2 | — |
| Missing | 75 | 2.0 | — | — | 58 | 4.4 | 17 | 0.7 | — |
| IMD | 0.031 | ||||||||
| 1 (most) | 867 | 23.4 | 38 | 18.2 | 326 | 25.0 | 541 | 22.6 | — |
| 2 | 817 | 22.1 | 40 | 19.1 | 268 | 20.6 | 549 | 22.9 | — |
| 3 | 666 | 18.0 | 41 | 19.6 | 251 | 19.2 | 415 | 17.3 | — |
| 4 | 659 | 17.8 | 38 | 18.2 | 241 | 18.5 | 418 | 17.4 | — |
| 5 (least) | 667 | 18.0 | 50 | 23.9 | 210 | 16.1 | 457 | 19.1 | — |
| Missing | 24 | 0.6 | — | — | 8 | 0.6 | 16 | 0.7 | — |
| BMI | 0.491 | ||||||||
| <25 | 262 | 7.1 | 22 | 10.5 | 45 | 3.5 | 217 | 9.1 | — |
| 25–<30 | 612 | 16.5 | 59 | 28.2 | 84 | 6.4 | 528 | 22.0 | — |
| 30–<40 | 880 | 23.8 | 67 | 32.1 | 121 | 9.3 | 759 | 31.7 | — |
| ⩾40 | 230 | 6.2 | 12 | 5.7 | 30 | 2.3 | 200 | 8.3 | — |
| Missing | 1,716 | 46.4 | 49 | 23.4 | 1,024 | 78.5 | 692 | 28.9 | — |
| WHO severity | 0.826 | ||||||||
| No O2 (i) | 624 | 16.9 | 35 | 16.7 | 223 | 17.1 | 401 | 16.7 | — |
| Noninvasive O2 (ii) | 1,567 | 42.4 | 77 | 36.8 | 557 | 42.7 | 1,010 | 42.2 | — |
| CPAP (iii) | 860 | 23.2 | 34 | 16.3 | 306 | 23.5 | 554 | 23.1 | — |
| IMV (iv) | 645 | 17.4 | 63 | 30.1 | 217 | 16.6 | 428 | 17.9 | — |
| CXR at follow-up | <0.004 | ||||||||
| Normal | 1,289 | 34.8 | 70 | 33.5 | 511 | 39.2 | 778 | 32.5 | — |
| Other | 325 | 8.8 | 19 | 9.1 | 140 | 10.7 | 185 | 7.7 | — |
| Abnormal | 162 | 4.4 | 21 | 10.0 | 45 | 3.5 | 117 | 4.9 | — |
| Missing | 2,139 | 57.8 | 36 | 41.4 | 677 | 52.2 | 1,462 | 60.8 | — |
| CT at follow-up | — | ||||||||
| Linked records | 255 | 6.9 | 209 | 100.0 | 35 | 2.7 | 220 | 9.2 | <0.001 |
| Scored | 209 | 5.6 | 209 | 100.0 | 29 | 2.2 | 180 | 7.5 | <0.001 |
| Symptoms at follow-up | 0.636 | ||||||||
| Present (worsen) | 850 | 23.0 | 74 | 35.4 | 21 | 1.6 | 829 | 34.6 | — |
| Present (no change) | 319 | 8.6 | 21 | 10.0 | 11 | 0.8 | 308 | 12.9 | — |
| Not present/improved | 359 | 9.7 | 24 | 11.5 | 9 | 0.7 | 350 | 14.6 | — |
| Missing | 2,172 | 58.7 | 90 | 43.1 | 1,263 | 96.9 | 909 | 37.9 | — |
| ppFVC at follow-up, % | — | ||||||||
| ⩾80 | 786 | 21.2 | 53 | 25.4 | — | — | 773 | 32.3 | — |
| <80 | 297 | 8.0 | 29 | 13.9 | — | — | 294 | 12.3 | — |
| Missing | 2,617 | 70.7 | 127 | 60.8 | 1,288 | 98.8 | 1,329 | 55.5 | — |
| ppDlCO at follow-up, % | — | ||||||||
| ⩾80 | 333 | 9.0 | 37 | 17.7 | — | — | 333 | 13.9 | — |
| <80 | 177 | 4.8 | 25 | 12.0 | — | — | 175 | 7.3 | — |
| Missing | 3,190 | 86.2 | 147 | 70.3 | 1,302 | 99.8 | 1,888 | 78.8 | — |
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| Age, yr | 59 | (50–68) | 58 | (52–67) | 60 | (51–70) | 59 | (50–67) | — |
| ppFVC | 90.3 | (78.6–101.7) | 87.0 | (75.0–98.8) | — | — | 90.2 | (78.6–101.6) | — |
| ppDlCO | 87.6 | (74.2–101.3) | 84.7 | (69.9–96.2) | — | — | 87.5 | (74.0–101.3) | — |
| Time to follow-up, d | 127 | (91–173) | 140 | (106–170) | 101 | (82–138) | 141 | (100–180) | — |
Definition of abbreviations: BMI = body mass index; CT = computed tomography–chest; CXR = chest X-ray; IMD = index of multiple deprivation in quintiles; IQR = interquartile range; ppDlCO = percent predicted DlCO; ppFVC = percent predicted FVC; Symptoms = Patient Symptom Questionnaire breathless or cough; WHO = modified World Health Organization severity score.
Small numbers of less than 5 have been suppressed. χ2 performed between Tier 1 and Tier 2 on nonmissing categories.
Residual lung abnormalities greater than 10% were observed in 166 of 209 (79.4%) participants. Visual scoring of involvement indicated ground-glass opacities affected a mean of 25.5 ± 5.9% of the lung, reticulation a mean of 15.1 ± 11.0%, with residual abnormalities involved a mean of 40.6 ± 20.8% of the lung (Figure 2A). A total of 33 people had a repeat CT visually scored after a minimum of 90 days (median, 161 d; IQR, 109–187), 28 of 33 (84.8%) of whom were classified with residual abnormalities greater than 10% on the initial scan, with 26 of 28 (92.9%) observed to have greater than 10% involvement in subsequent scans. In paired analysis, the overall change in residual lung abnormalities was −3.62% (95% confidence interval [CI], −6.10 to −1.13; P = 0.006) (Figure 2B). The involvement of lung reticulations and ground-glass opacities did not significantly change with a mean difference of −2.08% (95% CI, −4.66 to 0.51; P = 0.112) and −1.54% (95% CI, −4.74 to 1.39; P = 0.293), respectively (Figures 2C and 2D). Using all scored CT scans, the mean weekly change in lung involvement was estimated at −0.13% per week (95% CI, −0.20 to −0.05) for reticulations and −0.13% per week (95% CI, −0.22 to −0.04) for ground-glass opacities (Figure 2E). The weekly change in residual lung abnormalities was −0.20% per week (95% CI, −0.28 to −0.11) (Figure 2F). Representative CT images of residual lung abnormality demonstrated persistent involvement more than 100 days after discharge (Figure 3).
Figure 2.
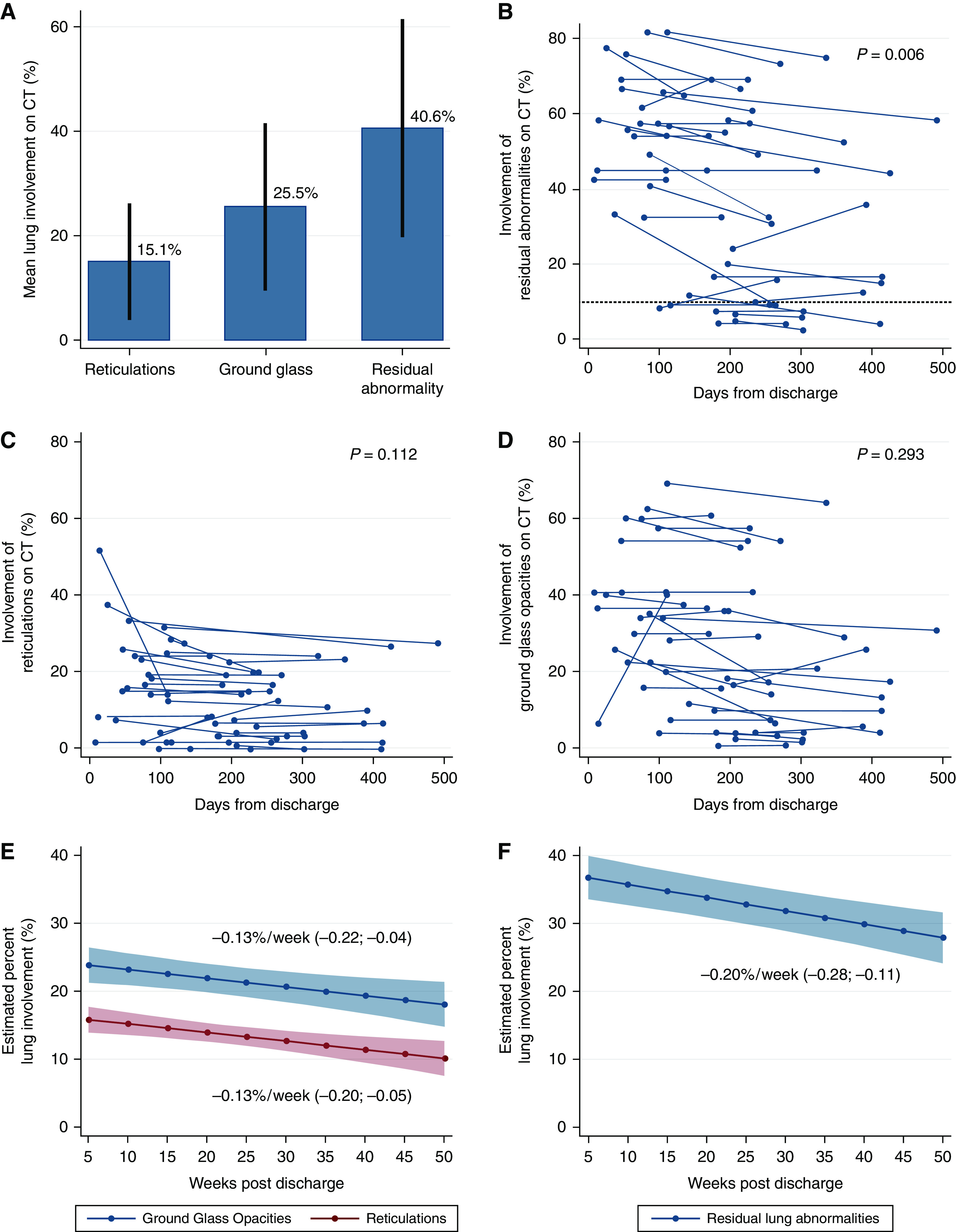
Extent of residual lung abnormalities on linked computed tomography. (A) Mean percentage lung involvement of reticulations, ground-glass opacities, and residual abnormalities within 240 days of discharge with visually scored involvement greater than 10%, presented with standard deviation (n = 166). Percentage lung involvement of (B) residual abnormalities, (C) reticulations, and (D) ground-glass opacities at initial and repeat computed tomography scans with greater than 90 days between (n = 33), with P values from paired t test. (E) Estimated percent lung involvement of ground-glass opacities (top, blue) and reticulations (bottom, red) from linear mixed effects by weeks after discharge (n = 209, scans = 242). (F) Estimated percent lung involvement of residual abnormalities from linear mixed effects by weeks after discharge, presented with mean weekly effect and 95% confidence intervals (n = 209, scans = 242). CT = computed tomography.
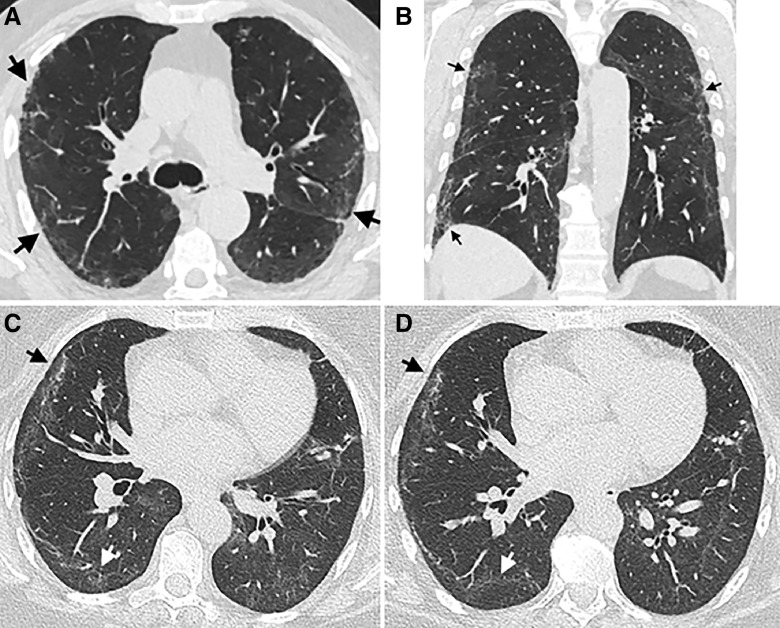
Figure 3.
Representative computed tomography (CT) images of residual lung abnormalities. Representative (A) coronal and (B) axial noncontrast CT imaging from the same individual performed 137 days after discharge after a coronavirus disease (COVID-19) admission scored with 52.5% total lung involvement of residual lung abnormality, of which 18.3% was reticulation and 34.2% ground-glass opacity. Peripheral reticulation (arrows) is evident, surrounded by faint areas of ground-glass opacity. Representative coronal CT images from the same individual at (C) 114 days after discharge scored 56.8% lung involvement (28.5% reticulation and 28.3% ground-glass opacity), and (D) 239 days after discharge scored 49.2% total lung involvement (20.0% reticulation and 29.2% ground-glass opacity). Peripheral areas of reticulation (black arrow) and ground-glass opacity (white arrow) in the right lung.
Overall, the median time to follow-up in the UKILD interim cohort (N = 3,700) was 127 days (IQR, 91–173), the median age was 59 (IQR, 50–68), and the cohort was majority male (60.7%). Tier 1 participants (n = 1,304) had a median time to follow-up of 101 days (IQR, 82–138), a median age of 60 (IQR, 51–70), and the majority were male (58.9%); demographics were similar in Tier 2 participants (n = 2,396) with a median time to research visit of 141 days (IQR, 100–180), a median age of 59 (IQR, 50–67), and the majority male (61.7%) (Table 1). There was minimal evidence of systematic bias in the characteristics between Tier 2 and Tier 1 participants in nonmissing data (Table 1), although the representation of people aged below 60 was greater in Tier 2 participants (52.5% vs. 48.8%; P = 0.027); similarly, there were small differences in the representation of ethnicity (P < 0.001), greater representation of the lowest deprivation quintile (19.1% vs. 16.1%; P = 0.031), as well as lower representation of normal CXR (32.5% vs. 39.2%; P = 0.004).
Tier 2 participants had a median ppFVC at research follow-up of 90.2% (IQR, 78.6–101.6) with missing records at 55.5%, whereas median ppDlCO was 87.5% (IQR, 74.0–101.3) with missing records at 78.8%; lung function was largely missing in the routine follow-up of Tier 1 participants. We observed 34.6% of people reported worsening cough or dyspnea since discharge in Tier 2. ILD diagnostic criteria of lung function (ppFVC and ppDlCO), CXR, and symptoms were frequently missing, particularly in Tier 1 of clinical follow-up (Figure E2). In Tier 1, 578 of 1,304 (44.3%) participants were missing data on all four characteristics at interim analysis, whereas in Tier 2, 362 of 2,396 (15.1%) participants were missing data on all four characteristics. In contrast, a total of 202 (8.4%) Tier 2 participants had complete data on all, and no Tier 1 participants had complete lung function, CXR, or symptom data. In the subsample of participants with scored CTs, data was missing at a rate similar to Tier 2 for lung function (ppDlCO, 70.3% and ppFVC, 60.8%), CXR (47.4%), and Patient Symptom Questionnaire (43.1%) (Table 1).
Risk of Residual Lung Abnormalities and Persistence Over Time
Univariate risk ratios were calculated to assess the risk of residual lung abnormalities greater than 10% on CT. A greater risk was observed in males (risk ratio [RR], 1.42; 95% CrI, 1.17–1.77) and in those over 60 years of age (RR, 1.22; 95% CrI, 1.06–1.40). Clinical indicators, including severe illness on admission requiring CPAP, IMV, or ECMO (RR, 1.40; 95% CrI, 1.23–1.63), abnormal CXR findings (RR, 1.40; 95% CrI, 1.22–1.61), and ppDlCO less than 80% (RR, 1.26; 95% CrI, 1.02–1.58) were also associated with greater risk, with consistent effects for the relative mean difference of percent involvement after adjustment for sex and deprivation quintile (Table 2).
Table 2.
Risk Factors of Residual Lung Abnormalities on Computed Tomography
| Characteristic | Risk Factor Present, % | Risk Factor Absent, % | Univariate Risk Ratio | 95% Credible Interval | Estimated Mean Difference, % | 95% Credible Interval | Adjusted Mean Difference, % | 95% Credible Interval |
|---|---|---|---|---|---|---|---|---|
| Male | 87.4 | 62.1 | 1.42 | (1.17 to 1.77) | 12.46 | (5.76 to 19.59) | 11.26 | (4.24 to 18.04) |
| Age ⩾60, yr | 87.9 | 71.8 | 1.22 | (1.06 to 1.40) | 8.29 | (2.11 to 14.44) | 8.57 | (3.61 to 6.16) |
| Non-White | 78.5 | 79.9 | 0.97 | (0.84 to 1.12) | 3.48 | (−3.78 to 10.88) | 3.84 | (−4.95 to 9.37) |
| IMD (Q1 and 2) | 87.2 | 74.4 | 1.17 | (1.02 to 1.34) | 6.91 | (0.38 to 13.33) | 6.28 | (−0.31 to 12.91) |
| BMI >30 | 87.3 | 71.6 | 1.22 | (1.04 to 1.45) | 3.93 | (−3.70 to 11.52) | 4.54 | (−2.40 to 11.65) |
| CPAP/IMV | 93.8 | 67.0 | 1.40 | (1.23 to 1.63) | 20.56 | (14.80 to 26.36) | 20.14 | (14.34 to 25.69) |
| aCXR | 100.0 | 73.0 | 1.40 | (1.22 to 1.61) | 14.96 | (3.89 to 25.78) | 11.54 | (0.53 to 21.59) |
| ppFVC <80 | 86.2 | 79.3 | 1.07 | (0.85 to 1.31) | 10.40 | (−0.90 to 22.00) | 11.99 | (−0.14 to 23.52) |
| ppDlCO <80 | 96.0 | 75.7 | 1.26 | (1.02 to 1.58) | 19.04 | (7.65 to 30.71) | 15.31 | (2.84 to 28.06) |
| PSQ worse | 78.4 | 80.0 | 0.99 | (0.81 to 1.21) | 4.49 | (−4.58 to 13.54) | 4.71 | (−4.31 to 13.87) |
Definition of abbreviations: aCXR = abnormal chest X-ray; BMI = body mass index; CPAP/IMV = continuous positive airway pressure or invasive mechanical ventilation; IMD = Index of Multiple Deprivation; Q = quintile; ppDlCO = percent predicted DlCO; ppFVC = percent predicted FVC; PSQ = Patient Symptom Questionnaire.
Percentage of nonmissing case observations reaching greater than 10% threshold of residual lung abnormalities according to risk factor being present or absent. Univariate risk ratio of greater than 10% threshold of residual lung abnormalities and 95% credible interval derived from binomial regression, mean effect difference in the percentage lung involvement in which risk factor present relative to risk factor absent estimated from univariate linear regression and adjusted for sex and index of multiple deprivation.
Three significant clinical indicators were selected to index the risk of residual lung abnormalities after COVID-19 in the remaining cohort (n = 3,491) on the basis of combined thresholds: ppDlCO less than 80%; abnormal CXR; and severe illness on admission. Individuals were considered to be at very high risk when reaching the defined thresholds in all three indicators (risk index four), high risk when two thresholds were reached (risk index three), or moderate risk if reaching ppDlCO or CXR thresholds alone (risk index two). Individuals reaching the threshold of the severity of illness on admission alone were considered low risk in the absence of other indicators (risk index one). Those who did not reach any threshold were considered very low risk (risk index zero). A total of 14 of 3,419 (0.4%) participants were considered very high risk, 143 of 3,419 (4.1%) high risk, 116 of 3,419 (3.3%) moderate risk, 1,256 of 3,419 (36.0%) low risk, and 1,962 of 3,419 (56.2%) very low risk (Table 3). Combined, 273 of 3,419 (7.8%) participants in strata of moderate to very high risk were defined as at-risk, and 8 of 46 (17.4%) participants with an unscored clinically indicated CT were at-risk. In sensitivity analyses applying risk stratification to Tier 2 alone, 231 of 2,219 (10.4%) participants were at moderate to very high risk, including 20% of those with an unscored clinically indicated CT (Table 3).
Table 3.
Risk Stratification of Residual Lung Abnormalities in Unscored UKILD Post–COVID-19 Study Interim Cohort
| Interim Cohort | ||||
|---|---|---|---|---|
| Strata | Unscored (n = 3,491), n | % | Sensitivity (n = 2,219), n | % |
| Very high | 14 | 0.4 | 14 | 0.6 |
| High | 143 | 4.1 | 123 | 5.5 |
| Moderate | 116 | 3.3 | 94 | 4.2 |
| Low | 1,256 | 36.0 | 767 | 34.6 |
| Very low | 1,962 | 56.2 | 1,221 | 55.0 |
| Linked CT: Unscored | ||||
|---|---|---|---|---|
| Interim (n = 46), n | % | Sensitivity (n = 40), n | % | |
| At-risk | 8 | 17.4 | 8 | 20.0 |
| Low risk | 38 | 82.6 | 32 | 80.0 |
Definition of abbreviation: CT = computed tomography.
Risk strata: Very high (all three risk factors present [abnormal chest X-ray, percent predicted DlCO less than 80%, and severe admission requiring continuous positive airway pressure or invasive mechanical ventilation]); High (at least two risk factors present); Moderate (either abnormal chest X-ray or percent predicted DlCO less than 80% present); Low (severe admission present only); Very low (risk factors not present). Missing data were imputed at the reference category. Percent denominator is the interim cohort without linked, scored computed tomography (n = 3,491) and sensitivity analysis within Tier 2 research visit participants (n = 2,219). Moderate to very high risk combined with at-risk and low to very low risk combined with low risk quantified in people with unscored linked computed tomography.
No differences were observed between at-risk participants (n = 273) and participants with greater than 10% residual abnormalities on CT (n = 166) according to a representation of males, older age, ethnicity, deprivation, body mass index, severity of admission, ppFVC less than 80%, or Patient Symptom Questionnaire (Table E1). There was a lower representation of normal CXR in the at-risk group (14.7% vs. 30.1%; P < 0.001) and more representation of ppDlCO less than 80% (55.3% vs. 14.5%; P < 0.001). The percentage of people who did not have a severe admission requiring CPAP, ECMO, or IMV was similar in both groups (44.3% vs. 45.2%), whereas CXR was missing in 26.0% of the at-risk group and 48.2% of people with residual abnormalities scored.
Comparing at-risk participants to low-risk participants, there were more records of immunosuppressant (18.3% vs. 9.9%; P = 0.001) and corticosteroid treatment (35.3% vs. 26.5%; P = 0.019) preadmission, intensive care unit stays (50.0% vs. 33.4%; P < 0.001), and complications of acute respiratory distress syndrome (ARDS; 25.0% vs. 13.7%; P < 0.001) (Table E2). In addition, there were more recorded unscheduled emergency visits after discharge (34.8% vs. 25.2%; P = 0.001), with a greater representation of visits in which patients presented with symptoms of shortness of breath (33.7% vs. 24.3%; P = 0.046). Findings were similar in comparisons of CT-scored residual lung abnormalities greater than 10% compared with those not reaching this threshold, although statistical significance was not always met (Table E2).
On the basis of the distribution of at-risk cases, the prevalence of residual lung abnormalities after COVID-19 hospitalization was estimated at 8.51% (95% CrI, 7.56–9.51) using noninformative priors, or 6.49% (95% CrI, 5.75–7.27) with skeptical priors on the basis of ILD population prevalence estimated at 1 in 1,000 (Table 4 and Figure E3) (18, 19). In sensitivity analyses on the basis of Tier 2 distribution, the prevalence of residual lung abnormalities after COVID-19 hospitalization was estimated at 11.67% (95% CrI, 10.28–13.14) using noninformative priors, or 7.74% (95% CrI, 6.79–8.72) using skeptical priors.
Table 4.
Prevalence Estimate of Residual Lung Abnormalities Greater than 10% After COVID-19 Hospitalization
| Model | Prevalence, % | 95% CrI | Prior | a | b | DIC |
|---|---|---|---|---|---|---|
| 1 | 8.51 | (7.56–9.51) | Uniform | 1 | 1 | 9.38 |
| 1-i | 8.48 | (7.52–9.49) | Jeffreys | 0.5 | 0.5 | 9.45 |
| 1-ii | 6.49 | (5.75–7.27) | Skeptical | 1 | 1,000 | 28.67 |
| 1-iii | 7.37 | (6.53–8.24) | Power | 1 | 1,000 | 14.99 |
| 2 | 11.67 | (10.28–13.14) | Uniform | 1 | 1 | 9.20 |
| 2-i | 11.61 | (10.19–13.04) | Jeffreys | 0.5 | 0.5 | 9.27 |
| 2-ii | 7.74 | (6.79–8.72) | Skeptical | 1 | 1,000 | 45.97 |
| 2-iii | 9.32 | (8.17–10.54) | Power | 1 | 1,000 | 20.91 |
Definition of abbreviations: Crl = credible interval; DIC = deviance information criterion.
The estimated prevalence of greater than 10% residual lung abnormalities on computed tomography after hospitalization for coronavirus disease (COVID-19), derived from posterior mean and 95% credibility interval using binomial distributions of at-risk versus low-risk numbers in interim UK Interstitial Lung Disease Consortium (UKILD) post–COVID-19 study cohort. Model 1, overall; Model 2, Tier 2 research visit participants. Uniform priors and sensitivity analysis with Jeffreys noninformative (i), skeptical informative priors (ii), and skeptical informative priors with power at 50% weighting (iii). β prior distributions defined using cases (a) and noncases (b). DIC presented to interpret the model.
Discussion
These data demonstrate that residual lung abnormalities were visually identifiable on clinically indicated thoracic follow-up CT imaging in a substantial proportion of patients within 8 months of discharge after COVID-19 hospitalization. The involvement of scored residual lung abnormalities minimally declined per week after discharge, and minimal resolution was observed in paired subsequent scans at least 90 days apart. Key clinical risk factors associated with residual abnormalities in the early follow-up period included abnormal CXR, ppDlCO less than 80%, and severe admissions requiring invasive support (IMV, CPAP, or ECMO). In those without a scored CT, 0.4% were in very-high–risk strata (all three indicators present), 4.1% in high-risk strata (any two indicators present), and 3.3% in moderate-risk strata (presence of either ppDlCO less than 80% or abnormal CXR, alone). Combining these risk strata, 7.8% of the interim cohort had suspected residual lung abnormalities after COVID-19 hospitalization, which increased to 10.4% in sensitivity analysis on those with planned research follow-up. On the basis of Bayesian modeling, we estimate the prevalence of suspected residual lung abnormalities with greater than 10% lung involvement to be up to 11.7% in people hospitalized with acute COVID-19 infections before March 2021.
This UKILD Post–COVID-19 Study interim analysis of residual abnormalities in patients hospitalized for COVID-19 offers the largest assessment of prevalence in hospitalized individuals to date and is consistent with findings from a number of smaller studies that demonstrate persistent radiological patterns and impaired gas transfer during extended follow-up of patients with COVID-19 (20–23). At the time of this interim analysis, it is not possible to determine whether the observed residual lung abnormalities represent early ILD with potential for progression or whether they reflect residual pneumonitis that may be stable or resolve over time (24). The 10% threshold used was determined to support the distinction of interstitial lung damage from interstitial lung abnormalities (15). Longer-term follow-up and mechanistic studies will be required to determine the clinical trajectory of these observations.
When linked longitudinal scans were available, most patients did not show evidence of substantial improvement, although such clinically requested CTs may be overrepresented by those with slower recovery. However, approximately half the people with visually scored residual abnormalities above the 10% threshold did not require CPAP, IMV, or ECMO during their admission and less than one quarter had ARDS recorded as a complication, suggesting medium- and longer-term disability consequent to severe COVID-19 infection, consistent with prior studies (18).
The risk factors for a residual abnormality scored in the CT subsample (abnormal CXR, ppDlCO less than 80%, and severe admissions requiring invasive support) were applied to the remaining hospitalized cohort to generate clinically applicable risk strata. For participants in receipt of a clinically indicated but unscored CT, 17.4% of people were in moderate- to very-high–risk strata for residual lung abnormalities (sensitivity, 20.0%). These rates were similar to meta-analysis estimates of the percentage of clinically indicated CT scans with radiological patterns suggestive of fibrosis (29%; 95% CI, 22–37%) and people with impaired gas transfer (17%; 95% CI, 13–23), neither of which were associated with the timing of follow-up within the first year after COVID-19 (25). In paired CT scans greater than 90 days apart, we demonstrate no significant difference in the mean change for percent involvement of reticulations and ground-glass opacities, whereas the scored involvement of reticulations and ground-glass opacities on the basis of all CT scans declined by 0.13% per week of study from discharge, suggesting persistence over time in at-risk groups.
Differences between individuals at moderate to very high risk and those at lower risk suggested more immunosuppressant and corticosteroid treatment preadmission, ICU stays, and ARDS complications, as well as further unscheduled emergency visits after discharge both overall and including a presentation with breathlessness. Classification of at-risk participants using clinically applicable strata identified those who may have had a more severe viral injury and inflammatory response during acute infection, as well as subsequent respiratory exacerbations after COVID-19. Recent analysis identified a hyperinflammatory phenotype of COVID-19–related ARDS was associated with worse outcomes, with better survival linked to corticosteroid treatment (26). Surviving a hyperinflammatory response to COVID-19 may be consistent with residual lung abnormalities, including fibrosing nonspecific interstitial pneumonia and alveolar damage (27).
Residual lung abnormalities after COVID-19 were not uncommon in this hospitalized population and may persist long-term, but indicators that could support diagnosis and clinical management of lung disease were frequently unavailable. Considering approximately 280,000 people were discharged after confirmed COVID-19 admission in the United Kingdom National Health Service by the end of March 2021 (28), these results emphasize the importance for health services to undertake active radiological and physiological monitoring, especially in people at moderate or above risk (15).
Strengths and Limitations
The UKILD long–COVID-19 Study interim cohort excluded participants with any evidence of ILD before hospitalization, and we used informative skeptical priors and power priors for more conservative estimates of prevalence, which continued to suggest a substantial burden of residual lung abnormalities after COVID-19 hospitalization. The approach we report can be reasonably applied to other cohorts and time points, with current findings used as informative priors for updating Bayesian inference.
Although included CTs were assumed to be representative of clinically indicated radiology, this is limited by local management protocols, the timing of services, and changes to healthcare service prioritization during the COVID-19 pandemic, which increases the chances of selection and ascertainment bias. Furthermore, individuals with linked CT may have unrecorded preexisting disease or present with radiological patterns other than reticulation and ground-glass opacities. Fair interrater agreement (κ, 0.33) of CT scoring was observed, representing agreement in 70% of scans.
We recognize these interim findings may also be limited by misclassification. Descriptive analyses identified substantial missing data in clinical risk factors, limiting multiple imputation techniques. We used dichotomized thresholds with single data imputation at the reference category to support risk strata classification, maintain denominators, and provide conservative estimates. In contrast, lung involvement of reticulation and ground-glass opacities was frequently scored on CTs that were clinically indicated, contributing to selection bias. It is similarly likely that repeat CT scans reflect a sample of individuals that did not experience clinical improvement over time. We report estimates from multilevel models to support the interpretation of residual lung abnormalities over time.
Although our findings are on the basis of people hospitalized with mixed severity of COVID-19 infection, we recognize that they may not be generalizable to all populations, especially those not admitted to the hospital. Severe admissions requiring CPAP or IMV were overrepresented in the PHOSP–COVID-19 dataset relative to hospitalized survivors of COVID-19 (14). Linked clinical admission data suggested 50% of at-risk individuals and those scored with residual abnormalities attended ICUs during admission, and up to 25% had complications of anemia and ARDS. Furthermore, these data reflect people who were discharged before the end of March 2021 and do not represent later severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants in fully vaccinated populations that more frequently led to milder infections.
Conclusions
Thresholds of ppDlCO, CXR, and severity of admission can stratify the risk of residual abnormalities on CT involving more than 10% of the lung, informing clinical management, particularly of individuals meeting moderate- to very-high–risk strata. Longitudinal analysis of CT scans suggested the persistence of abnormalities over study time, although the longer-term functional consequence is unknown and may be limited by clinical indication. These findings highlight the importance of radiological and physiological monitoring of patients at both early and later follow-ups and suggest up to 11% of people discharged from an acute COVID-19 admission are at risk of residual lung abnormalities. Further study is required to elucidate the progressive development of radiological patterning or resolution over time.
Acknowledgments
Acknowledgment
The authors would like to acknowledge the support of the electronic Data Research and Innovation Service team (Public Health Scotland) for their involvement in obtaining approvals, provisioning, and linking data, and the use of the secure analytical platform within the National Safe Haven. This study would not be possible without all the participants who have given their time and support. The authors thank all the participants and their families. The authors thank the many research administrators, healthcare and socialcare professionals who contributed to setting up and delivering the study at all of the 65 NHS trust health boards and 25 research institutions across the United Kingdom, as well as all the supporting staff at the NIHR Clinical Research Network, Health Research Authority, Research Ethics Committee, Department of Health and Social Care, Public Health Scotland, and the United Kingdom Health Security Agency, and support from the ISARIC Coronavirus Clinical Characterisation Consortium (ISARIC4C). The authors thank Kate Holmes at the NIHR Office for Clinical Research Infrastructure (NOCRI) for her support in coordinating the charities group. The PHOSP–COVID-19 industry framework was formed to provide advice and support in commercial discussions, and we thank the Association of the British Pharmaceutical Industry, as well as Ivana Poparic and Peter Sargent at NOCRI, for coordinating this. The authors are very grateful to all the charities that have provided insight into the study: Action Pulmonary Fibrosis, Alzheimer’s Research UK, Asthma + Lung UK, British Heart Foundation, Diabetes UK, Cystic Fibrosis Trust, Kidney Research UK, MQ Mental Health, Muscular Dystrophy UK, Stroke Association Blood Cancer UK, McPin Foundations, and Versus Arthritis. The authors thank the NIHR Leicester Biomedical Research Centre patient and public involvement group and the Long Covid Support Group.
Footnotes
Supported by the National Institute of Health Research (COV0319) and UK Research and Innovation (MR/V027859/1).
Author Contributions: Conceptualization: I.S., J.J., J.M.W., J.C.P., P.L.M., P.M.G., R.J.A., J.K.B., S.L.B., P.B., S.M.B., J.F.B., N.C., G.C., E.K.D., A.D., L.F., M.A.G., F.V.G., B.G., I.P.H., N.A.H., M.H., T.E.H., S.R.J., M.G.J., F.K., R.L., P.M., M.P., K.P., J.K.Q., P.R.-O., M. Sereno, A.J.S., D.J.F.S., M. Spears, L.G.S., S.S., D.R.T., A.A.R.T., S.L.F.W., N.D.W., M.E.W., D.G.W., C.E.B., R.C.C., L.-P.H., K.P.H., L.V.W., and R.G.J. Data curation: S.A., E.M.H., M. Sereno, O.L., H.M., I.S., J.J., N.L., J.K.Q., and S.L.F.W. Formal analysis: I.S., J.J., and J.K.Q. Funding acquisition: C.E.B., L.V.W., R.A.E., J.D.C., L.-P.H., A.H., M.M., K.P., B.R., O.L., M.R., O.E., H.M., A. Shikotra, M. Sereno, R.S., V.H., L.H.-W., N.J.G., and R.G.J. Project administration: A. Shikotra, M.E.W., and A. Singapuri. Writing of original draft: I.S., J.J., P.M.G., L.V.W., and R.G.J. Writing and review & editing: I.S., J.J., P.M.G., P.L.M., J.C.P., R.J.A., J.K.B., S.L.B., P.B., S.M.B., J.F.B., J.D.C., R.C.C., N.C., C.C., G.C., E.K.D., A.D., O.E., R.A.E., L.F., M.A.G., F.V.G., B.G., N.J.G., B.G.G., I.P.H., N.A.H., V.H., E.M.H., M.H., T.E.H., A.H., L.H.-W., I.J., S.R.J., M.G.J., F.K., R.L., O.L., N.L., M.M., H.M., P.M., D.P., K.P.H., M.P., J.P., K.P., J.K.Q., B.R., M.R., P.R.-O., L.S., R.S., M.G.S., M. Sereno, A. Shikotra, A.J.S., A. Singapuri, D.J.F.S., M. Spears, L.G.S., S.S., D.T., A.A.R.T., M.T., S.L.F.W., S.W., N.D.W., J.M.W., D.G.W., C.E.B., L.P.H., L.V.W., and R.G.J.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202203-0564OC on December 1, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, Attwood M, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax . 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carfì A, Bernabei R, Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA . 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. ARC Study Group ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax . 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis . 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ducloyer M, Gaborit B, Toquet C, Castain L, Bal A, Arrigoni PP, et al. Complete post-mortem data in a fatal case of COVID-19: clinical, radiological and pathological correlations. Int J Legal Med . 2020;134:2209–2214. doi: 10.1007/s00414-020-02390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao L, Wang X, Xiong Y, Fan Y, Zhou Y, Zhu W. Correlation of autopsy pathological findings and imaging features from 9 fatal cases of COVID-19 pneumonia. Medicine (Baltimore) . 2021;100:e25232. doi: 10.1097/MD.0000000000025232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fadista J, Kraven LM, Karjalainen J, Andrews SJ, Geller F, Baillie JK, et al. COVID-19 Host Genetics Initiative Shared genetic etiology between idiopathic pulmonary fibrosis and COVID-19 severity. EBioMedicine . 2021;65:103277. doi: 10.1016/j.ebiom.2021.103277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allen RJ, Guillen-Guio B, Croot E, Kraven LM, Moss S, Stewart I, et al. Genetic overlap between idiopathic pulmonary fibrosis and COVID-19. Eur Respir J . 2022;60:2103132. doi: 10.1183/13993003.03132-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nasr El-Din A, Ata KAE, Abdel-Gawad AR, Fahmy NF. Impact of high serum levels of MMP-7, MMP-9, TGF-β and PDGF macrophage activation markers on severity of COVID-19 in obese-diabetic patients. Infect Drug Resist . 2021;14:4015–4025. doi: 10.2147/IDR.S329004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moin ASM, Sathyapalan T, Diboun I, Atkin SL, Butler AE. Identification of macrophage activation-related biomarkers in obese type 2 diabetes that may be indicative of enhanced respiratory risk in COVID-19. Sci Rep . 2021;11:6428. doi: 10.1038/s41598-021-85760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J . 2021;57:2003690. doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheng G, Chen P, Wei Y, Yue H, Chu J, Zhao J, et al. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta-analysis. Chest . 2020;157:1175–1187. doi: 10.1016/j.chest.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wild JM, Porter JC, Molyneaux PL, George PM, Stewart I, Allen RJ, et al. Understanding the burden of interstitial lung disease post-COVID-19: the UK interstitial lung disease-long COVID study (UKILD-Long COVID) BMJ Open Respir Res . 2021;8:e001049. doi: 10.1136/bmjresp-2021-001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. PHOSP-COVID Collaborative Group Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med . 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med . 2020;8:726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drake TM, Docherty AB, Harrison EM, Quint JK, Adamali H, Agnew S, et al. ISARIC4C Investigators Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med . 2020;202:1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoff P. A first course in Bayesian statistical methods. New York: Springer; 2009. [Google Scholar]
- 18. Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, Sanyal S, Brillet PY, Brauner M, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of greater Paris. Eur Respir J . 2017;50:1602419. doi: 10.1183/13993003.02419-2016. [DOI] [PubMed] [Google Scholar]
- 19. Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res . 2021;22:197. doi: 10.1186/s12931-021-01791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med . 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology . 2021;299:E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han X, Fan Y, Alwalid O, Zhang X, Jia X, Zheng Y, et al. Fibrotic interstitial lung abnormalities at 1-year follow-up CT after severe COVID-19. Radiology . 2021;301:E438–E440. doi: 10.1148/radiol.2021210972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou JN, Sun L, Wang BR, Zou Y, Xu S, Ding YJ, et al. The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT. PLoS One . 2021;16:e0248957. doi: 10.1371/journal.pone.0248957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta VK, Alkandari BM, Mohammed W, Tobar AM, Abdelmohsen MA. Ventilator associated lung injury in severe COVID-19 pneumonia patients - case reports: ventilator associated lung injury in COVID-19. Eur J Radiol Open . 2020;8:100310. doi: 10.1016/j.ejro.2020.100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabbri L, Moss S, Khan F, Chi W, Xia J, Robinson KA, et al. 2021.
- 26. Sinha P, Furfaro D, Cummings MJ, Abrams D, Delucchi K, Maddali MV, et al. Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med . 2021;204:1274–1285. doi: 10.1164/rccm.202105-1302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ravaglia C, Doglioni C, Chilosi M, Piciucchi S, Dubini A, Rossi G, et al. Clinical, radiological and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection. Eur Respir J . 2022;60:2102411. doi: 10.1183/13993003.02411-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NHS England. 2021. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-hospital-activity/