To the Editor:
The risk of deterioration during acute hypoxemic respiratory failure (AHRF) treated by noninvasive respiratory support demands monitoring of the patient’s inspiratory effort (1, 2) to avoid delay to mechanical ventilation (MV) (3–5). Despite the fact that respiratory rate (RR) and composite indices such as respiratory rate-oxygenation (ROX) (6) and heart rate, acidosis, consciousness, oxygenation, and respiratory rate (HACOR) (7) might recognize patients at major failure risk, these only indirectly account for the inspiratory effort. Esophageal manometry provides an accurate quantification of effort; however, it is unpractical in real life (8, 9). The nasal pressure swing (ΔPnose) during tidal breathing is highly correlated with the esophageal pressure swing (ΔPes) in patients with AHRF (10). The aim of this post hoc analysis of a prospective study (www.clinicaltrials.gov, NCT 03826797) was to assess the accuracy of ΔPnose in predicting early (24-h) failure of high-flow nasal oxygen (HFNO) to treat AHRF.
Consecutive patients with AHRF who were admitted into the respiratory intensive care unit (RICU) of the University Hospital of Modena in Modena, Italy, between January 1, 2021 and June 30, 2022 and started on HFNO were eligible for enrollment (Optiflow and AIRVO, Fisher and Paykel Healthcare Ltd.). Verbal or written informed consent was obtained as appropriate. An age >18 years, peripheral SpO2 <90% under conventional oxygen supply by Venturi mask with an inspiratory fraction of 0.5 and consent to receive nasal manometry were criteria for inclusion. The need for immediate intubation, use of noninvasive ventilation (NIV) or MV within the same admission, concomitant hypercapnia, cardiogenic pulmonary edema, chronic obstructive pulmonary disease, chest wall neuromuscular diseases, parenchymal interstitial abnormalities, nasal tract anatomical alterations, and long-term oxygen regimen were criteria for exclusion.
Patients’ characteristics were collected on admission into the RICU when all patients started HFNO (Time 1 [T1]). ΔPnose was measured by attending staff who were blinded to the purpose of the study. In 69 patients (68%) out of the total, ΔPes recording was simultaneously taken. At T1 and 2 hours after HFNO initiation (Time 2 [T2]), ΔPnose, ΔPes, arterial blood gases, PaO2/FiO2 ratio, RR, HACOR, and ROX were assessed.
The decision to escalate from HFNO either to helmet/facemask NIV or MV (i.e., failure) was taken by the attending physician (8), who was blinded to the results for ΔPnose.
The primary outcome was the accuracy of ΔPnose in predicting failure of HFNO at T2. The comparison between ΔPnose and the ROX index in predicting failure and the correlation between ΔPnose and ΔPes at different time points were also considered.
Receiver operating characteristic curves and the area under the curve (AUC) were calculated to test accuracy. The optimal cutoff of ΔPnose was chosen according to Youden’s J statistic to maximize the sum of sensitivity and specificity.
The comparison of accuracy between ΔPnose and the ROX index tailed was assessed using Delong’s test. Correlation analysis using Pearson’s r or Spearman’s ρ coefficient, as appropriate, was conducted at different time points.
Post hoc, we tested the accuracy and the optimal cutoff of ΔPnose in predicting escalation to MV and the correlations between ΔPnose, and ΔPes, and the ROX index. A two-sided test P < 0.05 was considered statistically significant (SPSS package, version 25.0; IBM Corp.).
Of 210 eligible patients, 102 were enrolled in accordance with the exclusion criteria. Of these, 91 (89.2%) were diagnosed with coronavirus disease (COVID-19)–related pneumonia, and 43 had been included in a previous publication (10). Of the enrolled patients, 35 (34.3%) failed HFNO within 24 hours (between 6 and 12 h).
Table 1 shows the patients’ characteristics. At any time, those who failed showed higher ΔPnose and ΔPes compared with those who succeeded; at T2, group differences were observed in HACOR, ROX, PaO2/FiO2 ratio, PaCO2, RR, and breathing effort.
Table 1.
Characteristics of the Study Population and Measurements (Baseline and 2 h after High-flow Nasal Oxygen Initiation) Grouped by Outcome (Failure or Success)
| Variable | Overall (N = 102; 100%) |
Outcome |
P Value | |
|---|---|---|---|---|
| Failure (n = 35; 34.3%) |
Success (n = 67; 65.7%) |
|||
| Age, yr, median (IQR) | 69 (56–75) | 67 (56–78) | 70 (56–75) | 0.6 |
| Male, n (%) | 71 (67) | 26 (74.3) | 45 (67.2) | 0.5 |
| BMI, kg/m2, median (IQR) | 23 (19–27) | 24 (21–27) | 22.5 (18–26) | 0.3 |
| Diagnosis and test | ||||
| COVID-19, n (%) | 91 (89.2) | 33 (94.3) | 58 (86.6) | 0.3 |
| Non–COVID-19, n (%) | 11 (10.7) | 2 (5.7) | 9 (13.4) | 0.3 |
| GCS, score, median (IQR) | 15 (15–15) | 15 (15–15) | 15 (15–15) | 0.9 |
| APACHE II score, median (IQR) | 11 (7–15) | 11 (7–14) | 11 (9–15) | 0.8 |
| SAPS II score, median (IQR) | 28 (23–33) | 29 (24–33) | 28 (23–34) | 0.9 |
| SOFA score, median (IQR) | 3 (3–3) | 3 (3–3) | 3 (3–3) | 0.6 |
| Baseline (Time 1) | ||||
| HACOR score, median (IQR) | 4 (3–5) | 5 (4–6) | 4 (3–5) | 0.1 |
| ROX index score, median (IQR) | 6.9 (5.8–8.6) | 6.6 (5.5–7.7) | 7.4 (6.1–9.1) | 0.1 |
| PaO2/FiO2, mm Hg, median (IQR) | 133 (115–152) | 125 (102–141) | 140 (123–160) | 0.1 |
| FiO2, %, median (IQR) | 50 (45–60) | 55 (50–60) | 50 (40–60) | 0.1 |
| PaO2, mm Hg, median (IQR) | 66 (60.4–72) | 64 (62.5–71.4) | 66 (60–72) | 0.7 |
| PaCO2, mm Hg, median (IQR) | 32.7 (31.2–34) | 32 (29.9–34.1) | 33 (31.2–34.5) | 0.1 |
| HR, bpm, median (IQR) | 93 (78–102) | 95 (76–102) | 93 (72–98) | 0.6 |
| RR, bpm, median (IQR) | 26 (24–28) | 26 (25–30) | 26 (24–28) | 0.1 |
| ΔPes, cm H2O, median (IQR) | 13.5 (11–16.3) | 15.2 (12.6–18) | 12.2 (10–15.8) | 0.04 |
| ΔPnose, cm H2O, median (IQR) | 6 (4.6–8) | 6.8 (5.6–8.2) | 5.6 (4.2–7) | 0.03 |
| 2 h after HFNO (Time 2) | ||||
| HACOR score, median (IQR) | 4 (3–5) | 5 (4–5) | 4 (3–4) | <0.0001 |
| ROX index score, median (IQR) | 7.9 (5.9–10.9) | 5.6 (5.2–6) | 9.2 (8–11.6) | <0.0001 |
| PaO2/FiO2, mm Hg, median (IQR) | 131 (112–152) | 111 (101–127) | 144 (130–175) | <0.0001 |
| FiO2, %, median (IQR) | 50 (45–60) | 65 (60–70) | 45 (35–55) | <0.0001 |
| PaO2, mm Hg, median (IQR) | 67.4 (62.2–72.6) | 72 (64–77) | 67 (62–70) | 0.01 |
| PaCO2, mm Hg, median (IQR) | 34.5 (32.4–36.7) | 32 (30–34) | 36.1 (33.7–37) | <0.0001 |
| HR, bpm, median (IQR) | 90 (78–100) | 96 (78–102) | 88 (80–100) | 0.7 |
| RR, bpm, median (IQR) | 24 (21–26) | 26 (25–27) | 21 (20–24) | <0.0001 |
| ΔPes, cm H2O, median (IQR) | 8 (6–14) | 16 (14–17) | 6.5 (5–8) | 0.01 |
| ΔPnose, cm H2O, median (IQR) | 3.2 (2.7–6) | 7 (6–8) | 3 (2.1–3.2) | <0.0001 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; BMI = body mass index; bpm = beats per minute; COVID-19 = coronavirus disease; GCS = Glasgow Coma Scale; HACOR = heart rate, acidosis, consciousness, oxygenation, and respiratory rate; HFNO = high-flow nasal oxygen; HR = heart rate; IQR = interquartile range; ROX = respiratory rate-oxygenation; RR = respiratory rate; SAPS II = Simplified Acute Physiology Score II; SOFA = Sequential Organ Failure Assessment; ΔPes = esophageal pressure swing; ΔPnose = nasal pressure swing.
Data are presented as n (%) for dichotomous values or median (IQR) for continuous values. Continuous variables were compared using the Student’s t test or Mann-Whitney U test, as appropriate. Differences in categorical variables were assessed with the chi-square test or Fisher exact test, as appropriate.
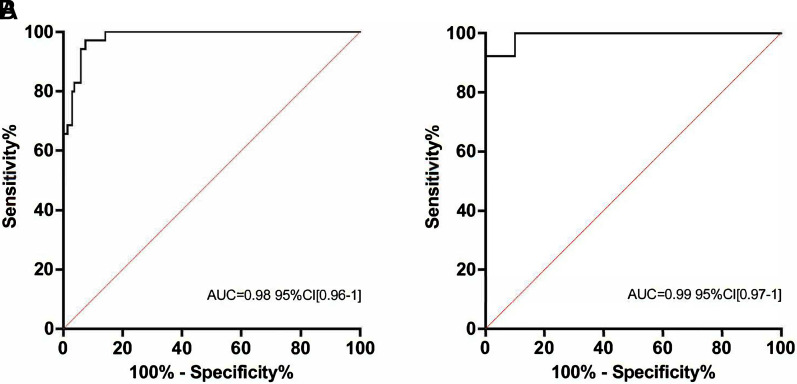
ΔPnose at T2 accuracy of prediction was high (Figure 1A), being 5.1 cm H2O, the cutoff value of risk. At T2, no difference was found when comparing the AUC of ΔPnose with ROX (AUC = 0.98; 95% confidence interval [CI], 0.96–1, P < 0.0001), whose threshold value of risk for failure was 6.52. Among patients without esophageal manometry (n = 69), ΔPnose still showed a high accuracy of prediction (Figure 1B).
Figure 1.
Receiver operating characteristic analyses for high-flow nasal oxygen (HFNO) early failure (24 h). ΔPnose 2 hours after HFNO initiation in the whole population (A) and in those patients without esophageal pressure assessment (B). AUC = area under the curve; CI = confidence interval; ΔPnose = nasal pressure swings.
Only three of those patients who failed reported ROX >6.52, whereas all three showed ΔPnose >5.1 cm H2O (6.7 cm H2O, 7.5 cm H2O, and 6.5 cm H2O). Two patients with ΔPnose <5.1 cm H2O failed, whereas the reported ROX was <6.52 (4.21 and 6.03, respectively). ΔPnose and ΔPes showed significantly high correlation (R2 = 0.91, P < 0.0001) that persisted at any time point (average ΔPes/ΔPnos ratio = 2.21, SD = 0.32). Moreover, an inverse correlation was found between ROX and both ΔPnose (R2 = 0.34, P < 0.0001) and ΔPes (R2 = 0.35, P < 0.0001). ΔPnose accuracy prediction to MV (n = 12) was high (0.917; 95% CI, 0.86–0.98, P < 0.0001), being 6 cm H2O, the risk threshold value.
In a real-life cohort of patients with AHRF undergoing HFNO, ΔPnose showed excellent accuracy in predicting early failure, similar to that displayed by ROX. Given that the decision to upgrade to NIV or MV was based on clinical variables, the high accuracy of ROX in predicting failure of HFNO is not surprising. The similar accuracy of ΔPnose (the only measurement to which the staff and attending physician remained blinded in our clinical decision) strengthened the association with outcome, avoiding incorporation bias.
The inverse correlation between the ROX index and both ΔPes and ΔPnose was weak, although the significant level of correlation between ΔPnos and ROX might suggest that they are only partially measuring the same phenomenon. Although ROX can be easily measured without additional equipment, we feel that the integration of ΔPnose as a physiological variable might provide more thorough information in patients with AHRF at risk of deterioration, thus assisting clinicians in their decision-making process. In this line, tidal ΔPnose shows strong correlation with ΔPes, thus making it a valid surrogate marker of the patient’s inspiratory effort during spontaneous breathing (10).
Given the limits of the study (monocentric and explorative design, unbalanced population with the majority of patients with COVID-19, post hoc analysis, lack of a validation cohort, and issues related to ΔPes assessment), the present findings should be interpreted with caution. Moreover, the role of airflow in influencing ΔPnose during spontaneous breathing needs further investigation. Notwithstanding, should data be confirmed in multicentric and empowered studies, these might pave the way for a novel, minimally invasive, and practical tool that allows real-time monitoring of the breathing effort of patients with AHRF.
Footnotes
Author Contributions: A.M. explored and elucidated the physiological assumptions of the study and defined the study design and procedures. R.T. was responsible for the analysis and interpretation of physiological variables, wrote the paper, and produced the figures. R.F., G.B., I.C., and L.T. developed the prototype for nasal pressure measurements, enrolled the patients, and wrote the paper. L.B. and A.C. designed the study, enrolled the patients, analyzed the data, and wrote the paper. S.B. reviewed the literature and wrote the manuscript. E.C. designed the study and reviewed and edited the manuscript. R.T. and A.C. have contributed equally to the conception and realization of the study. All authors have read and approved the final version of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202210-1848LE on December 7, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Pelosi P, Tonelli R, Torregiani C, Baratella E, Confalonieri M, Battaglini D, et al. Different methods to improve the monitoring of noninvasive respiratory support of patients with severe pneumonia/ARDS due to COVID-19: an update. J Clin Med . 2022;11:1704. doi: 10.3390/jcm11061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grieco DL, Menga LS, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol . 2019;85:1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
- 3. Kang BJ, Koh Y, Lim CM, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med . 2015;41:623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 4. Ball L, Robba C, Herrmann J, Gerard SE, Xin Y, Pigati M, et al. GECOVID Group Early versus late intubation in COVID-19 patients failing helmet CPAP: a quantitative computed tomography study. Respir Physiol Neurobiol . 2022;301:103889. doi: 10.1016/j.resp.2022.103889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, et al. LUNG SAFE Investigators; ESICM Trials Group Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med . 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 6. Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med . 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 7. Duan J, Han X, Bai L, Zhou L, Huang S. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med . 2017;43:192–199. doi: 10.1007/s00134-016-4601-3. [DOI] [PubMed] [Google Scholar]
- 8. Tonelli R, Fantini R, Tabbì L, Castaniere I, Pisani L, Pellegrino MR, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure. A pilot study. Am J Respir Crit Care Med . 2020;202:558–567. doi: 10.1164/rccm.201912-2512OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. PLeUral pressure working Group (PLUG—Acute Respiratory Failure section of the European Society of Intensive Care Medicine) Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med . 2016;42:1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 10. Tonelli R, Cortegiani A, Marchioni A, Fantini R, Tabbì L, Castaniere I, et al. Nasal pressure swings as the measure of inspiratory effort in spontaneously breathing patients with de novo acute respiratory failure. Crit Care . 2022;26:70. doi: 10.1186/s13054-022-03938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]