Abstract
Diet plays critical roles in defining our immune responses, microbiome, and progression of human diseases. With recent progress in sequencing and bioinformatic techniques, increasing evidence indicates the importance of diet-microbial interactions in cancer development and therapeutic outcome. Here, we focus on the epidemiological studies on diet-bacterial interactions in the colon cancer. We also review the progress of mechanistic studies using the experimental models. Finally, we discuss the limits and future directions in the research of microbiome and diet in cancer development and therapeutic outcome. Now, it is clear that microbes can influence the efficacy of cancer therapies. These research results open new possibilities for the diagnosis, prevention, and treatment of cancer. However, there are still big gaps to apply these new findings to the clinical practice.
Keywords: Barrier function, Colon cancer, Chemotherapy, Diet, Immunotherapy, Queuine, Microbiome, Metabolites, SCFA, TMAO, Tight Junctions
1. Introduction
Recent studies suggest that a combination of host factors, microbial factors, environmental factors, and lifestyle determine the risk of cancer and the eventual clinical outcome. The role of gut microbiota in carcinogenesis has been heavily investigated since the recent advance in sequencing technology, bioinformatic techniques, and “omics” methods. Food/diet plays critical roles in defining our immune responses, microbiome, and progression of diseases. More and more studies indicate the importance of diet-microbial interactions in cancer development 1 and therapeutic outcome. Over 20,000 research papers (with key words: microbiome/microbiota + diet/dietary search) have been published in the past decade according to PubMed. It is impossible to cover all new papers in this article. Here, we intend to focus on emerging dietary risk factors, which may have interactions with gut microbial biological activities in modulating the risk of developing cancer in human. As direct interactions between microbiota and mucosal cells are a central issue, colorectal cancer (CRC) is the main focus of this article as well. On one hand, risk factors for CRC, e.g., inflammatory bowel disease (IBD), diabetes, obesity, and smoking, modify the profile and function of microbiome. On the other hand, the microbiota can affect the efficacy and toxicity of chemotherapy, radiotherapy, and immunotherapy. Here, we review the epidemiological evidence (primarily within recent 5 years) of diet-bacterial interactions in the CRC. We discuss the progress of mechanistic studies using the experimental models. Finally, we will discuss the limits and future directions in the research of microbiome and diet in cancer development and treatment, including CRC.
2. Diet-gut microbiome and CRC studies in humans
2.1. Diet-gut microbiome interaction in cancer development
Epidemiologically speaking, there are two types of diet-bacterial interactions to be considered. One is synergistic or antagonistic interaction, where combined effect of diet and bacteria is significantly higher (synergistic) or lower (antagonistic) than that expected from the effects of diet or bacteria alone. Thus far, only a few studies have directly addressed this type of interactions. The second is mediation, where a part of effect of diet on cancer risk is mediated through gut microbial activities. While investigators have extensively studied the associations between gut microbiota-derived dietary metabolites and cancer risk, a few papers to date estimated separately indirect (mediated though microbiome) and direct effects of specific diet on cancer and other related endpoints 2,3. The opposite direction of mediations is possible, in which the effect of gut microbiome on cancer risk is partially medicated through changes in dietary intake. Growing evidence suggests that microbial activities affect satiety and eating behaviors of humans 4–6. Thus, such microbial mediation may be involved in the pathogenesis of obesity, a major risk factor for not only CRC but also postmenopausal breast, endometrial, pancreatic and liver cancer 7. Furthermore, obesity is associated with increased intestinal permeability and thus higher incidence of endotoxemia 8,9. A recent systematic review and meta-analysis including 26 randomized controlled trials (RCTs) and 1720 individuals showed a significant effect of probiotics in reducing body weight, body mass index (BMI), waist circumference, fat mass and fasting insulin levels 10. The long-term effects of probiotics on other appetite related hormones, such as leptin and adiponectin, however, are yet to be established 11.
2.2. Interactions between specific Dietary patterns and gut microbiome in CRC risk
To addresses the complexity of diet, i.e., multicollinearity between dietary components, dietary patterns methods, a key diet index-based approach, have been increasingly used in assessing the associations of diet with various chronic diseases including cancer. Studies relevant to CRC are summarized below.
2.1.1. Dietary Inflammatory Index (DII) and empirical dietary inflammatory pattern (EDIP)
DII / EDIP were developed to estimate overall effect of diet to modulate a set of inflammatory biomarkers, including IL-1β, IL-4, IL-6, IL-10, TNF-α, TNFRSF1B, and C-reactive protein 12,13. A recent meta-analysis based on 12 studies have confirmed increased risk of CRC (RR:1.16; 95% CI, 1.05–1.27) associated with high DII score with a dose-response trend, which was more pronounced in cohort studies 14. In addition, a higher DII score was significantly associated with higher risk of Crohn’s disease 15 (OR: 1.42; 95% CI: 1.05, 1.92) and was positively correlated with disease activity. Morganella morganii and Veillonella parvula were increased while Coprococcus eutactus was decreased in the pro-inflammatory diets group, as well as in CD 16. On the other hand, two recent RCTs have demonstrated that anti-inflammatory diet, which was designed to increase the intake of dietary fiber, probiotics, antioxidants, and omega-3 fatty acids and to decrease the intake of red meat, processed meat, and added sugar, increased fecal Bifidobacteriaceae, Lachnospiraceae, and Ruminococcaceae and reduced subclinical inflammation in ulcerative colitis (UC) patients in clinical remission and induced deep remission in mild-moderate UC 17,18. Interestingly, a prospective cohort studies in US nurses (NHS) and US health care professionals (HPFS) documented that higher EDIP scores were associated with increased risk of F nucleatum–positive CRC with the hazard ratio (HR) for the highest vs lowest EDIP tertile of 1.63 (95% CI, 1.03–2.58), while EDIP scores did not associate with F nucleatum–negative CRC 19. This suggests potential synergistic interaction between F. nucleatum and pro-inflammatory diet on CRC development, rather than diet fostering colonization of F. nucleatum, as previous studies have not identified F. nucleatum is more abundant in gut microbiota of individuals with high DII diet 16,20 and as potential etiological involvement of F. nucleatum has been supported in a meta-analysis of 57 studies comparing tissue level f of F. nucleatum in CRC cases and controls 21.
2.1.2. Sulfur microbial diet
Sulfur metabolizing bacteria are a specialized group of phylogenetically diverse microbes, including those from the Parabacteroides genera, Ruminococcus spp, and Desulfovibrio desulfuricans. These bacteria have the capacity to metabolize organic compounds for energy and to reduce dietary sulfur to H2S, which may lead to epithelial DNA damage and promote alterations in immune cell compositions 22,23 and thus may increase the risk of CRC. Nguyen et al developed the sulfur microbial diet score that reflects abundance of sulfur-metabolizing bacteria in stool and consists primarily of high processed meats and low vegetables and legumes consumption 23. Applying this score to the HPFS, they demonstrated increased sulfur microbial diet scores were associated with risk of distal colon and rectal cancers (highest vs lowest quartile RR 1.43; 95% CI 1.14–1.81) 23. In addition, the sulfur microbial diet score was associated with increased incidence of early onset CRC precursors, i.e., colorectal adenoma, in women who were enrolled in the Nurses’ Health Study II 24. The RR for highest vs lowest quartile was 1.31 (95% CI, 1.10-1.56) and the risk was further pronounced for early-onset adenomas with greater malignant potential villous/tubulovillous histology (OR(Q4 vs Q1), 1.65; 95% CI, 1.12-2.43). Finally, in the HPFS the authors observed differential associations of the sulfur microbial diet with distal CRC by the presence of intra-tumoral Bifidobacterium spp 25. Specifically, the association was only present for Bifidobacterium-negative distal CRC 25, suggesting potential antagonistic interaction between sulfur metabolizing bacterial and diet on CRC risk or that between and sulfur metabolizing bacteria and Bifidobacterium within gut community.
2.1.3. Western style (WS) diet/ prudent diet
Western-style diet is characterized by a high intake of red and processed meat, sugar, and refined grains and low intake of vegetables and legumes and a meta-analysis of 33 studies have reported the increased risk of CRC (OR 1.40, 95% CI 1.26 − 1.56) associated with the highest WS diet score compared with the lowest score groups 26. WS diet patterns have also been to alter gut microbiome and functions. In a cross-sectional study in Poland involving 200 participants, gut microbiome of individuals on WS diet were more enriched with of Bacteroidota and Escherichia-Shigella and repressed with Faecalibacterium, compared with those on healthy diet 27. In the American Gut Project that profiled fecal microbiota of 744 participants, standard WS diet was associated with increased relative abundance of Fusobacterium and reduced relative abundance of Lactococcus in comparison prudent dietary patterns, which were plant-based or ovo-vegetarian diets 15. Intriguingly, Arima et al found in the same NHS and HPFS cohorts that the association between WS dietary patterns and CRC risk was dependent on the presence and abundance of pks Island-carrying Escherichia coli in CRC tissue. The HR for top vs bottom tertile WS diet score was 3.45 (95% CI 1.53-7.78) in pks high CRC, while it was 1.10 (95% CI 0.85-1.42) in pks-negative CRC 28. Because E coli has been described to be increased with WS diet 27, these differential associations are more likely to reflect mediation effects of WS diet in modulating gut microbiome composition, while carcinogenic effect of E coli PKS has been demonstrated in a meta-analysis of 12 studies (OR 2.27, 95% CI 1.13–4.57) 29.
Prudent dietary patterns—rich in fruits, vegetables, and whole grains—, contrasting healthier dietary patterns to WS diet, have been associated with a lower risk of CRC 26. The same group of the investigators earlier analyzed NHS and HPFS data for its association with CRC risk according to tissue F. nucleatum status. The prudent diet score was associated with a lower risk of F nucleatum–positive CRC (HR 0.43; 95% CI, 0.25-0.72, for the highest vs the lowest prudent score quartile) but not with F nucleatum–negative cancers. On the other hand, the association with WS diet score did not vary with CRC F nucleatum status 30. These data suggestive of antagonistic interaction may be ascribed to mediation effect of prudent diet in reducing F nucleatum abundance in the gut, as described above 15.
2.1.4. Mediterranean diet (MedDiet)
The MedDiet is a plant-based pattern characterized by high amounts of fruits, vegetables, nuts, legumes, fish, and cereals moderate intake of alcohol, while reducing intake of red, processed meat, eggs and dairy 31. Many of these dietary components, such as fruits, nuts and seeds, and beans, show inversely associations with fecal bile acids concentrations 32 and serum bile acids concentrations (particularly secondary BA (SBA)) have been associated with increased risk of CRC in two of the protective cohorts 33. The updated systemic review and meta-analysis including 3,202,496 participants from 117 studies has shown protective effect of high adherence to MedDiet on risk of several types of cancer including CRC, based on 17 studies (pooled relative risk (RR): 0.83, 95% CI 0.76, 0.90). Among the 11 components of the MedDiet, high whole gran, vegetable and fruit and moderate alcohol intakes had significant effects on all endpoints combined 31. A meta-analysis of gut microbiota from individuals with various diets and colon diseases has found that gut microbiota associated with MedDiet it was enriched in bacteria that promote an anti-inflammatory environment such as Akkermansia, but low in taxa with pro-inflammatory properties, e.g., Fusobacterium 34. More recent systematic review including RCTs and focusing on MedDiet has reported multiple studies pointing to a decrease in Escherichia coli counts and an increase in Faecalibacterium (a butyrate-producing bacterium), while limited studies of fecal metabolite analysis have indicated increases in short chain fatty acids (SCFA), e.g., acetate, propionate and butyrate 35. To date, there have been no studies reporting the synergistic effects between SCFA producing bacteria and MedDiet on CRC risk.
Table 1 summarizes potential interaction between dietary patterns associated with CRC risk and gut bacteria, and their presumed modes of interactions as discussed in the sections above. These observations suggest that global dietary patterns may modulate CRC risk through modifying gut microbial composition as well as augmenting or suppressing virulence of pathogenic bacteria.
Table 1.
Potential interaction between dietary patterns associated with CRC risk and gut bacteria, and their presumed modes of interaction
| Dietary patterns | Effect on the risk of CRC or its precursors | Gut bacteria involved | Presumed mode of interaction | References |
|---|---|---|---|---|
| Pro-inflammatory/ Anti-inflammatory diets | Up/down | Fusobacterium nucleatum | Synergistic interaction to increase pro-inflammatory effect of diet | 19 |
| Sulfur microbial diet | Up | Bifidobacterium spp | Antagonistic interaction to mitigate the toxic effect of H2S | 25 |
| Western style diet | Up | pks-positive Escherichia coli | Mediation through increasing pathogenetic bacteria | 28 |
| Prudent diet | Down | Fusobacterium nucleatum | Mediation through reducing pathogenic bacteria | 30 |
| Mediterranean diet | Down | Escherichia coli, Fusobacterium, Faecalibacterium, Akkermansia | Mediation through fostering symbiotic community structure | 34,35 |
3. Emerging microbial metabolites of dietary component and CRC risk
There are many microbial metabolites of human dietary components 17,36, yet not many such metabolite have been convincingly associated with the risk of CRC. The following sections briefly summarize the findings from studies in humans.
3.1. Trimethylamine N-oxide (TMAO)
TMAO is a choline derived metabolite produced by gut microbiota. Gut microbial metabolic activities on dietary choline, l-carnitine and betaine results in the formation of trimethylamine 37, which is concomitantly converted into TMAO by host liver flavin monooxygenase 3 (FMO3) 38–40. A wide range of bacterial species have been described to catalyze this reaction 38,40. The plasma level of TMAO is determined by host genetic variation, diet, and composition of gut microbiota 40,41. Dietary substrates for TMA are rich in high protein foods such as red meat, fish, offal, eggs, and beans 40,42–44. A randomized crossover trial comparing plant versus animal-based meat diets has confirmed increased serum TMAO concentrations on animal-meat diet 45. Thus, there is a credible link to CRC because high red meat intake is an established risk factor for CRC 46. Owing to its proinflammatory and pro-insulin resistance properties, TMAO has been postulated to be a key mediator for the relation among diet, gut microbiota, and various health conditions, including cardiovascular disease, type 2 diabetes and CRC 41,47,48. A recent systemic review and meta-analysis on TMAO and cancer incidence reported the summary odds ratio (OR) of 9 studies to be 1.64 (95% confidence intervals (CI) 1.35–1.99) per an increase in serum/plasma TMAO concentrations for all cancer combined, although it was not clear how effects sizes were standardized across the studies 49. Among those included, 4 were CRC studies, and 2 of them were from prospective cohort studies 49, supporting the potential link.
3.2. Short Chains fatty acids (SCFA)
Despite the fact that a number of cancer preventive properties of SCFAs, in particular, acetate, propionate, and butyrate, which are the major products from the microbial fermentative activity in the gut, have been demonstrated in laboratory studies 50,51, direct evidence from epidemiological/clinical studies to link SCFA concentrations in body fluids to CRC risk is rather limited. Loftfield et al recently analyzed the serum samples from two prospective cohorts and reported that only women from one of the cohort (not men in either cohort) had reduced risk of CRC associated with higher concentrations of acetate, but not butyrate or propionate 33. A meta-analysis of fecal SCFAs summarizing 4 recent cross-sectional studies, which involved 14-26 CRC cases and 14-38 controls, found significantly lower fecal acetate and butyrate concentrations in CRC cases compared with controls 52. The retrospective nature of the latter studies certainly precludes inferring a causa association, but their results may indicate that intraluminal rather than systemic exposure to SCFAs plays more vital role in modulating CRC risk. Finally,10-year follow-up of a RCT with resistant starch supplementation for Lynch Syndrome patients reported the null effect on CRC incidence (HR 0.92; 95% CI 0.62–1.34), while significant reduction in non-CRC-Lynch-related cancers (HR 0.54, 95% CI 0.33–0.86) 53. Thus, overall epidemiological data to support cancer preventive potential of SCFAs or dietary fiber remain inconsistent, and more emphasis should be placed on mechanistic pathways derived from laboratory experiments.
3.3. Endogenous ethanol
Alcohol drinking is another established risk factor for CRC 46. Recent studies in gut microbiome have discovered microbial metabolic capacity to synthesize ethanol from dietary carbohydrates, especially excessive sugar (e.g., glucose, sucrose, and fructose) is consumed 54,55. Bacteria from Lactobacillaceae and Enterobacteriaceae families, such as Lactobacillus and Klebsiella and some yeasts, such as Candida and Saccharomyces, have been shown to be ethanol producers 55–57. Microbially derived endogenous ethanol has been implicated in the development of non-alcoholic fatty liver disease (NAFLD) 54,57–59. But, importantly increased intraluminal ethanol production by gut microbiota may more directly damage the intestinal epithelial cell and mucosal barrier than systemically ingested alcohol. Excessive sugar intake especially added sugars from sugary foods and beverages, which are prepared with sucrose and high-fructose corn syrup, has attracted growing health concern, because it has been associated with traits of the metabolic syndrome, i.e., obesity and insulin resistance 60. These traits are also acknowledged to increase the risk of CRC 61. In particular, intestinal uptake of fructose is limited compared to glucose, resulting in more transit to the colon, where it is metabolized by the microbiota 62. Overall, the association between excessive sugar intake and cancer risk has been still controversial, but Makarem et al recently conducted a systematic review of cohort studies, including 21 for gastrointestinal tract cancer, 6 of which were CRC studies 60. While most studies reporting risk estimates for total sugar and sucrose intake found a null association, evidence was mixed for fructose, as four out of nine studies reported higher risks of pancreatic and colorectal cancer with increased intake. Especially, two cohort studies in the United States, the Health Professionals Follow-Up Study and the Women’s Health Study 63,64, reported, respectively, a 37% increase and a >twofold higher risk of CRC with higher fructose intake. Thus, further studies on fructose and CRC risk that incorporate microbial markers are warranted.
4. Pre and pro-biotic and dietary supplements in cancer directed therapies
4.1. Dietary supplements
Use of dietary supplements after a cancer diagnosis is common 65,66. However, there has been concern if use of dietary supplements during treatment could reduce treatment efficacy 67. One of the common components in dietary supplements that has been linked to gut microbiome is iron, because iron is required for growth by the majority of colonic bacteria 68. A small clinical comparing oral versus intravenous iron supplement on CRC patients reported that oral iron-treated patients had a greater abundance of colorectal cancer-enriched genera such as Coprococcus and Prevotella 69 and a similar study comparing the two iron regimens in patients with IBD showed that oral iron treatment was associated with decreased abundances of some beneficial taxonomic units such as Faecalibacterium prausnitzii 70. Indeed, a study among breast cancer patients enrolled in a cooperative group clinical trial reported that use of iron supplement during chemotherapy was significantly associated with disease recurrence (HR, 1.79; 95% CI 1.20 -2.67) 67. Certainly, more studies among CRC patients are warranted.
4.2. Ketogenetic diet (KD)
The ketogenic diet is a dietary regime focused on substantially reducing carbohydrate intake and increasing fat intake with adequate amounts of protein, leading to a state of ketosis. The ketogenic diet has gained much popularity over the years due to its effects on promoting weight loss, but recently has received increasing interest as a promising adjuvant agent to standard cancer therapies by exploiting reprogramed metabolism of cancer cells 71. Most of the data to date are from case reports or pilot/feasibility studies, but despite the lack of RCTs, several individual observations have supported the antitumor effects of KDs, including those for brain cancer and rectal cancer in conjunction with radiation therapy 71,72. Its potential effects on cancer immune therapy have just begun to emerge in an animal model 73. The exact mechanism by which a ketogenic diet exert potential anticancer activities remains unknown, but recent evidence points towards a crucial role for the gut microbiota 74,75. An interesting study in both mice and humans found that a KD led to decreased fecal Bifidobacterium abundance, which led to lower levels of intestinal and visceral fat pro-inflammatory Th17 cells 76. While KD reduced overall alpha diversity, it increased the relative abundance of Akkermansia muciniphila, a known SCFA producer, in some studies 75,77. A RCT to investigate the effects of a 2 month KD, followed by a 2 month low-caloric diet concluded that the KD resulted in a beneficially altered gut microbiota composition, i.e., reduced relative abundances of Serratia, Erwinia, and Citrobacter and increased relative abundances of Oscillospira and Butyricimonas 78. However, overall changes in gut microbiome composition induced by KD in the literature are very inconsistent, suggesting need for more well-designed studies 74,75.
4.3. Anti-, pre-, probiotics and fecal microbiota transplantation (FMT)
Outcomes of systemic cancer therapies are modulated by gut microbiota as well as tumor-associated bacteria. Antibiotic use during chemotherapy was associated with improved overall survival in patients with metastatic CRC, metastatic pancreatic cancer and locally advanced head and neck cancer 79–81, pointing to a possibility enzymic inactivation of chemotherapeutic agents by intra-tumor bacteria 37. On the other hand, systematic review and meta-analysis of 33 studies including 5565 solid cancer patients who were treated with immune checkpoint inhibitors 44 revealed a significantly reduced overall survival (HR 1.76, 95% CI 1.41–2.19) associated with antibiotic exposure82. This detrimental association was primarily based on studies in patients with non-small cell cancer and renal cancer carcinoma 82. Several specific bacteria, such as Bifidobacterium spp. and Faecalibacterium. spp 83,84, have been have associated with improved response in retrospective clinical studies, suggesting that they may optimize the efficacy of immunotherapy 85. Accordingly, a number of RCTs using various formulation of single or multiple strain probiotics, prebiotics/diet or FMT have been initiated, although most of the FMT trials are still in Phase 1 and many others are at stages of phase 2, not reporting trial results concerning efficacy and adverse reactions to specific immunotherapies 86,87. Thus, it will take some time to get such microbial modification approaches translated into clinical practice as viable adjuvant therapies.
5. Mechanistic/molecular bases of diet-microbial interactions in cancer development
Mechanisms by which dietary, including fiber deprivation, impacts the microbiota and alters cancer risk are studied using experimental cancer models, e.g., AOM-treated mice and Apcmin/+ mice. The gnotobiotic mouse model, in which animals were colonized with a synthetic human gut microbiota composed of commensal bacteria, was used in mechanistic studies.
During chronic or intermittent dietary fiber deficiency, the gut microbiota resorts to host-secreted mucus glycoproteins as a nutrient source, leading to erosion of the colonic mucus barrier 88. Dietary fiber deprivation, together with a fiber-deprived, mucus-eroding microbiota, promotes greater epithelial access and lethal colitis by the mucosal pathogen, Citrobacter rodentium 88. This study indicates that Intricate pathways linking diet, the gut microbiome, and intestinal barrier dysfunction could be exploited to improve health using dietary therapeutics.
On one hand, in human CRC, the bile acid metabolism is among the top biomarkers of patients 89. Quantitively profiled metabolites derived from host-microbial co-metabolism using the unbiased method will help to identify key players in the colonic carcinogenesis. We found the changes in primary bile acid metabolism and secondary bile acid metabolism in VDRΔIEC mice. The fold change ratios of the identified bile acid species were significantly higher in the VDRΔIEC mice, which were susceptible to carcinogenesis 90. Microbiota from patients with IBD produce DNA damage metabolites, e.g., indolimines. Indolimines were produced by the bacteria Morganella morganii 91. In the AOM/DSS colon cancer mice, tumor burden was increased by M. morganii , but not by a mutant of M. morga unable to produce indolimine. Indolimine-producing M. morganii exacerbated colon tumorigenesis in gnotobiotic mice, as well. On the other hand, microbial metabolites, e.g., Lactobacillus reuterin, from healthy mice or humans are growth-repressive, and this response is attenuated in mice and patients with CRC 92. A recent study has shown that Lactobacillus reuteri and its metabolite, reuterin, are downregulated in mouse and human CRC. Reuterin changes redox balance and suppresses proliferation in colon cancer cells. Reuterin induces selective protein oxidation and inhibits ribosomal biogenesis and protein translation. Exogenous Lactobacillus reuteri was able to restrict tumor growth, increases tumor reactive oxygen species, and decreases protein translation 92. A 2022 study by Dmitrieva-Posocco et al. identify the mechanism of ketogenic diets in suppressing CRC through the beneficial metabolite beta-hydroxybutyrate (BHB) in vivo 93. BHB is able to reduces the proliferation of colonic crypt cells and potently suppresses intestinal tumor growth. BHB acts through the surface receptor Hcar2 and induces the transcriptional regulator Hopx, thereby inhibiting cell proliferation. In humans, increased BHB and active HOPX are associated with reduced intestinal epithelial proliferation 93. Thus, actionable insights by oral or systemic interventions with a single metabolite or metabolites might complementing current prevention and treatment methods for CRC.
Bacteria and their metabolites are known not only contribute to CRC, but also the other cancers. Intestinal microbiome showed enriched queuosine producing bacteria in obese mice and increased S-adenosyl methionine (SAM) producing bacteria in control diet-fed mice. Supplementation of obese animals with SAM sensitized pancreatic tumors to chemotherapy. Queuine (Q) is a micronutrient from diet and microbiome. In eukaryotes, Q modification is dependent on diet or gut microbiome in multicellular organisms 94. Although tRNA Q-modification has been studied for a long time regarding its properties in decoding and tRNA fragment generation, how QTRT1 affects tumorigenesis and the microbiome is still poorly understood. We have shown that 95 QTRT1 gene and tRNA Q-modification altered cell proliferation, junctions, and microbiome in tumors and the intestine, thus playing a critical role in breast cancer development. Thus, understanding the protective role of diet, bacteria and metabolites will help to develop better strategies for cancer prevention and therapy.
Bacterial pathogens, such as H. pylori, E. coli psk, and Salmonella, are known to increase the risk of GI cancers, as reviewed in recent papers 96,97. Systemic S. Typhi infections are linked to gallbladder cancer, whereas non-typhoidal Salmonella infections are associated with colon cancer 98. Fungal component of the intestinal microbiome is also altered in CRC. In fact, an abundance of a specific fungal genus actually promotes the disease. Two 2022 studies reported the association between fungal species and various human cancers, including colon cancer 99 100. The fugal DNA was identified in tumors. However, there are no studies on whether fungi directly responsible for cancer progression. Fungi metabolites, Mycotoxins, may impact the physiological functions, e.g. barrier role, of the intestine, however still lack in vivo data 101.
Intestinal archaea may be critical for maintaining immune homeostasis by activating antigen-specific adaptive immune responses 102. Archaea act as electron acceptors for substrates originating from anaerobic digestive processes of gut bacteria 103. Methanomassiliicoccales are less-known members of the human gut archaeome 104. Archaea contribute to removal of trimethylamine from the gut. Methanogenic archaea, Methanobrevibacter smithii, Methanosarcina mazei, and Methanomicrococcus blatticola, use TMA as growth substratese and reduced plasma TMAO in mice 105. Thus, further research is needed to understand the novel role of archaea and metabolites in the development of CRC.
There are dynamic bidirectional interactions among the three key factors: diet, immune system, and intestinal microbiome, in the host 106. Diet has dominant influence on the profile and function of microbiota, in return, influences nutrient absorption. Diet also has a profound influence on the immune system (e.g., vitamin A, vitamin D, AHR). The immune system can affect nutrient uptake as well. The immune system can exert control over microbial composition and localization, whereas microbial signals and products are critical for development and function of the immune system in health and disease 1,97.
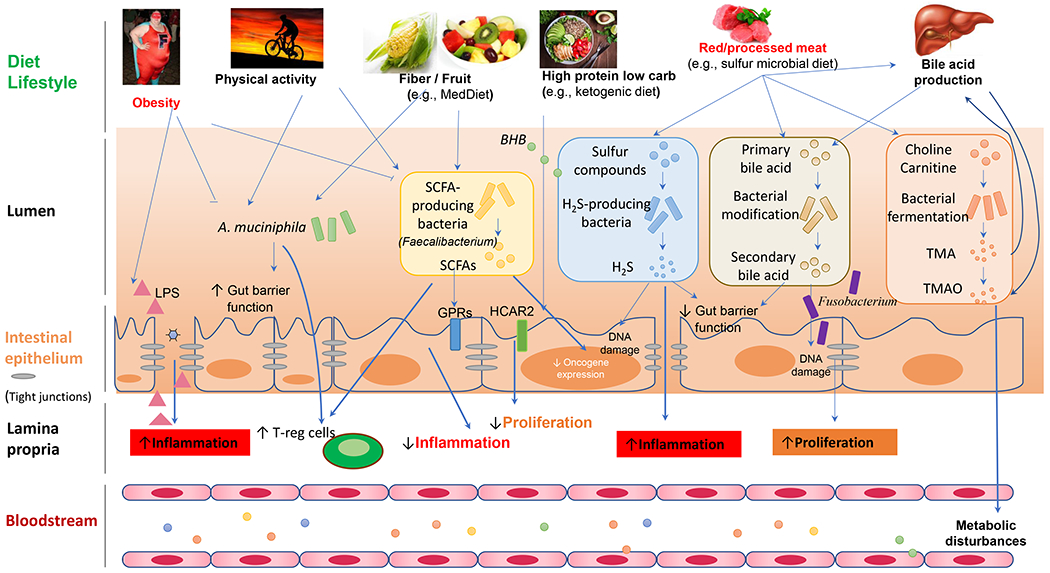
Taken together, gut microbiota mainly affects the occurrence and development of CRC by damaging host DNA, promoting inflammatory microenvironment, transforming cells for hyperproliferation, breaking intestinal barriers, changing microbial metabolites, and affecting host immune responses. Figure 1 illustrates a working model of interactions among the host, microbes, and diets in the development of CRC 1. Host-microbial interactions can be mediated by a plethora of microbe-derived effector molecules that include metabolites (TMAO, BHB, and SCFAs), immune and inflammatory modulators (PAMPs, MAMPs), and small natural products. Perturbations of physiological host-microbial interactions have significant consequences to immune and metabolic homeostasis at the local and systemic levels.
6. Microbial metabolites in cancer treatment
Lida et al 107 showed that disruption of the microbiota impairs the response of subcutaneous tumors to CpG-oligonucleotide immunotherapy and platinum chemotherapy. Optimal responses to cancer therapy require an intact microbiota that mediates its effects by modulating myeloid-derived cell functions in the tumor microenvironment. It is reported 37 that bacteria can metabolize the chemotherapeutic drug gemcitabine (2’,2’-difluorodeoxycytidine) into its inactive form, 2’,2’-difluorodeoxyuridine. Metabolism was dependent on the expression of a long isoform of the bacterial enzyme cytidine deaminase (CDDL), seen primarily in Gammaproteobacteria. In a colon cancer mouse model, gemcitabine resistance was induced by intratumor Gammaproteobacteria, dependent on bacterial CDDL expression, and abrogated by cotreatment with the antibiotic ciprofloxacin. Gemcitabine is commonly used to treat pancreatic ductal adenocarcinoma (PDAC). In human PDACs, 76% (86/113) was positive for bacteria, mainly Gammaproteobacteria 37. Thus, precise analysis of the gut microbiota of individual in the development of CRC is critically essential for modified treatment.
In a recent study, Teng et al. 108 report that Bacteroides vulgatus-mediated nucleotide biosynthesis attenuates the response of rectal cancer patients to chemoradiotherapy; uric acid is a potential prognosis marker for rectal cancer patients receiving chemoradiotherapy. The findings provide insights into the cancer cells-microbiota crosstalk during cancer therapies.
7. Conclusion /Future Directions
Microbial dysbiosis contributes to inflammation, tumor growth, and therapy response. The microbiota is a potential biomarker of diagnosis or clinical outcome of CRC. By elucidating the mechanisms and microbial contributions to the development and progression of colon cancers, researchers hope to target the microbiota (bacteria, viruses, fungi, archaea, and other microbes) as a promising strategy and to restore the healthy host-microbial interactions to achieve better clinical outcomes.
Probiotics can introduce missing microbial components with known beneficial functions for the host. Prebiotics can maximize sustainable changes in the human microbiome by enhancing the proliferation of beneficial microbes or probiotics. Prebiotics or probiotics might target the microbiome for cancer prevention, especially in high-risk populations. Dietary intervention, probiotics supplementation, and FMT may improve treatment efficacy 109. However, more clinical trials evaluating gut microbiota-mediated therapies are necessary to improve outcomes of CRC treatment.
Accumulating evidence has revealed the critical roles of commensal microbes and pathogens in cancer progression. It is now clear that the microbial community is closely related to the efficacy of chemotherapy and immunotherapies. The future direction will consider not only bacteria but also virome and fungi in determining biomarkers for diagnosis and strategies for effective treatment in CRC. Compelling links between the gut microbiota, the host diet, and host physiology has emerged 109. Precisely modulation diet-microbiome interactions will provide new opportunities to use bugs as drugs. We need personalized prebiotics/probiotics, FMT, and dietary for the clinical practice. Modeling and machine learning for microbiome data and metabolites will help us to better predict the stage of CRC and effectiveness of treatment.
REFERENCES
- 1.Song M, Chan AT, Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 2020;158(2):322–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serrano D, Pozzi C, Guglietta S, et al. Microbiome as Mediator of Diet on Colorectal Cancer Risk: The Role of Vitamin D, Markers of Inflammation and Adipokines. Nutrients 2021;13(2) (In eng). DOI: 10.3390/nu13020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano CP, Wilkens LR, Shvetsov YB, et al. Associations of the Dietary Inflammatory Index with total adiposity and ectopic fat through the gut microbiota, LPS, and C-reactive protein in the Multiethnic Cohort-Adiposity Phenotype Study. Am J Clin Nutr 2022;115(5):1344–1356. DOI: 10.1093/ajcn/nqab398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox TO, Lundgren P, Nath K, Thaiss CA. Metabolic control by the microbiome. Genome Med 2022;14(1):80. (In eng). DOI: 10.1186/s13073-022-01092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J Nutr 2017;147(5):727–745. (In eng). DOI: 10.3945/jn.116.240481. [DOI] [PubMed] [Google Scholar]
- 6.Han H, Yi B, Zhong R, et al. From gut microbiota to host appetite: gut microbiota-derived metabolites as key regulators. Microbiome 2021;9(1):162. (In eng). DOI: 10.1186/s40168-021-01093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AICR. Body Fatness & Weight Gain. January/27/2020. (https://www.aicr.org/research/the-continuous-update-project/body-fatness-weight-gain/).
- 8.Ott B, Skurk T, Hastreiter L, et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep 2017;7(1):11955. (In eng). DOI: 10.1038/s41598-017-12109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokkala K, Pellonperä O, Röytiö H, Pussinen P, Rönnemaa T, Laitinen K. Increased intestinal permeability, measured by serum zonulin, is associated with metabolic risk markers in overweight pregnant women. Metabolism 2017;69:43–50. (In eng). DOI: 10.1016/j.metabol.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Pontes K, Guedes MR, Cunha MRD, et al. Effects of probiotics on body adiposity and cardiovascular risk markers in individuals with overweight and obesity: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr 2021;40(8):4915–4931. (In eng). DOI: 10.1016/j.clnu.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Cabral LQT, Ximenez JA, Moreno KGT, Fernandes R. Probiotics have minimal effects on appetite-related hormones in overweight or obese individuals: A systematic review of randomized controlled trials. Clin Nutr 2021;40(4):1776–1787. (In eng). DOI: 10.1016/j.clnu.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17(8):1689–96. (In eng). DOI: 10.1017/s1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and Validation of an Empirical Dietary Inflammatory Index. The Journal of Nutrition 2016;146(8):1560–1570. DOI: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syed Soffian SS, Mohammed Nawi A, Hod R, et al. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients 2022;14(8) (In eng). DOI: 10.3390/nu14081555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotillard A, Cartier-Meheust A, Litwin NS, et al. A posteriori dietary patterns better explain variations of the gut microbiome than individual markers in the American Gut Project. Am J Clin Nutr 2022;115(2):432–443. (In eng). DOI: 10.1093/ajcn/nqab332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Z, Zhuang X, Zhuo S, et al. Dietary inflammatory potential mediated gut microbiota and metabolite alterations in Crohn’s disease: A fire-new perspective. Clin Nutr 2022;41(6):1260–1271. (In eng). DOI: 10.1016/j.clnu.2022.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Keshteli AH, Valcheva R, Nickurak C, et al. Anti-Inflammatory Diet Prevents Subclinical Colonic Inflammation and Alters Metabolomic Profile of Ulcerative Colitis Patients in Clinical Remission. Nutrients 2022;14(16):3294. (https://www.mdpi.com/2072-6643/14/16/3294). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kedia S, Virmani S, S KV, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut 2022;71(12):2401–2413. DOI: 10.1136/gutjnl-2022-327811. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Tabung FK, Zhang X, et al. Diets That Promote Colon Inflammation Associate With Risk of Colorectal Carcinomas That Contain Fusobacterium nucleatum. Clin Gastroenterol Hepatol 2018;16(10):1622–1631 e3. DOI: 10.1016/j.cgh.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng J, Hoffman KL, Chen JS, et al. Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br J Nutr 2020;124(9):931–942. (In eng). DOI: 10.1017/s0007114520001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villar-Ortega P, Expósito-Ruiz M, Gutiérrez-Soto M, Ruiz-Cabello Jiménez M, Navarro-Marí JM, Gutiérrez-Fernández J. The association between Fusobacterium nucleatum and cancer colorectal: A systematic review and meta-analysis. Enferm Infecc Microbiol Clin (Engl Ed) 2022;40(5):224–234. (In eng). DOI: 10.1016/j.eimce.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res 2007;5(5):455–9. (In eng). DOI: 10.1158/1541-7786.Mcr-06-0439. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen LH, Ma W, Wang DD, et al. Association Between Sulfur-Metabolizing Bacterial Communities in Stool and Risk of Distal Colorectal Cancer in Men. Gastroenterology 2020;158(5):1313–1325. (In eng). DOI: 10.1053/j.gastro.2019.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen LH, Cao Y, Hur J, et al. The Sulfur Microbial Diet Is Associated With Increased Risk of Early-Onset Colorectal Cancer Precursors. Gastroenterology 2021;161(5):1423–1432.e4. (In eng). DOI: 10.1053/j.gastro.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikavi DR, Nguyen LH, Haruki K, et al. The Sulfur Microbial Diet and Risk of Colorectal Cancer by Molecular Subtypes and Intratumoral Microbial Species in Adult Men. Clin Transl Gastroenterol 2021;12(8):e00338. (In eng). DOI: 10.14309/ctg.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng YL, Shu L, Zheng PF, et al. Dietary patterns and colorectal cancer risk: a meta-analysis. Eur J Cancer Prev 2017;26(3):201–211. (In eng). DOI: 10.1097/cej.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 27.Malinowska AM, Kok DE, Steegenga WT, Hooiveld G, Chmurzynska A. Human gut microbiota composition and its predicted functional properties in people with western and healthy dietary patterns. Eur J Nutr 2022;61(8):3887–3903. (In eng). DOI: 10.1007/s00394-022-02928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arima K, Zhong R, Ugai T, et al. Western-Style Diet, pks Island-Carrying Escherichia coli, and Colorectal Cancer: Analyses From Two Large Prospective Cohort Studies. Gastroenterology 2022;163(4):862–874. (In eng). DOI: 10.1053/j.gastro.2022.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaab ME, Lozano PO, Ibañez D, et al. A Meta-Analysis on the Association of Colibactin-Producing pks+ Escherichia coli with the Development of Colorectal Cancer. Lab Med 2022. (In eng). DOI: 10.1093/labmed/lmac072. [DOI] [PubMed] [Google Scholar]
- 30.Mehta RS, Nishihara R, Cao Y, et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol 2017;3(7):921–927. (In eng). DOI: 10.1001/jamaoncol.2016.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morze J, Danielewicz A, Przybyłowicz K, Zeng H, Hoffmann G, Schwingshackl L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur J Nutr 2021;60(3):1561–1586. (In eng). DOI: 10.1007/s00394-020-02346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitry P, Wawro N, Sharma S, et al. Associations between usual food intake and faecal sterols and bile acids: results from the Cooperative Health Research in the Augsburg Region (KORA FF4) study. Br J Nutr 2019;122(3):309–321. (In eng). DOI: 10.1017/s000711451900103x. [DOI] [PubMed] [Google Scholar]
- 33.Loftfield E, Falk RT, Sampson JN, et al. Prospective Associations of Circulating Bile Acids and Short-Chain Fatty Acids With Incident Colorectal Cancer. JNCI Cancer Spectr 2022;6(3) (In eng). DOI: 10.1093/jncics/pkac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illescas O, Rodríguez-Sosa M, Gariboldi M. Mediterranean Diet to Prevent the Development of Colon Diseases: A Meta-Analysis of Gut Microbiota Studies. Nutrients 2021;13(7) (In eng). DOI: 10.3390/nu13072234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimble R, Gouinguenet P, Ashor A, et al. Effects of a mediterranean diet on the gut microbiota and microbial metabolites: A systematic review of randomized controlled trials and observational studies. Crit Rev Food Sci Nutr 2022:1–22. (In eng). DOI: 10.1080/10408398.2022.2057416. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Zhernakova DV, Kurilshikov A, et al. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nature Medicine 2022;28(11):2333–2343. DOI: 10.1038/s41591-022-02014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357(6356):1156–1160. (In eng). DOI: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19(5):576–85. (In eng). DOI: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koeth RA, Levison BS, Culley MK, et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab 2014;20(5):799–812. (In eng). DOI: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fennema D, Phillips IR, Shephard EA. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab Dispos 2016;44(11):1839–1850. (In eng). DOI: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canyelles M, Tondo M, Cedó L, Farràs M, Escolà-Gil JC, Blanco-Vaca F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int J Mol Sci 2018;19(10) (In eng). DOI: 10.3390/ijms19103228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krüger R, Merz B, Rist MJ, et al. Associations of current diet with plasma and urine TMAO in the KarMeN study: direct and indirect contributions. Mol Nutr Food Res 2017;61(11) (In eng). DOI: 10.1002/mnfr.201700363. [DOI] [PubMed] [Google Scholar]
- 43.Mei Z, Chen G-C, Wang Z, et al. Dietary factors, gut microbiota, and serum trimethylamine-N-oxide associated with cardiovascular disease in the Hispanic Community Health Study/Study of Latinos. The American Journal of Clinical Nutrition 2021;113(6):1503–1514. DOI: 10.1093/ajcn/nqab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lombardo M, Aulisa G, Marcon D, et al. Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods. Nutrients 2021;13(5) (In eng). DOI: 10.3390/nu13051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crimarco A, Springfield S, Petlura C, et al. A randomized crossover trial on the effect of plant-based compared with animal-based meat on trimethylamine-N-oxide and cardiovascular disease risk factors in generally healthy adults: Study With Appetizing Plantfood—Meat Eating Alternative Trial (SWAP-MEAT). The American Journal of Clinical Nutrition 2020;112(5):1188–1199. DOI: 10.1093/ajcn/nqaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AICR. Colorectal Cancer. January/09/2020. (https://www.aicr.org/research/the-continuous-update-project/colorectal-cancer/).
- 47.Ilyas A, Wijayasinghe YS, Khan I, et al. Implications of trimethylamine N-oxide (TMAO) and Betaine in Human Health: Beyond Being Osmoprotective Compounds. Front Mol Biosci 2022;9:964624. (In eng). DOI: 10.3389/fmolb.2022.964624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiNicolantonio JJ, McCarty M, J OK. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019;6(1):e000890. (In eng). DOI: 10.1136/openhrt-2018-000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khodabakhshi A, Monfared V, Arabpour Z, Vahid F, Hasani M. Association between Levels of Trimethylamine N-Oxide and Cancer: A Systematic Review and Meta-Analysis. Nutr Cancer 2022:1–13. (In eng). DOI: 10.1080/01635581.2022.2129080. [DOI] [PubMed] [Google Scholar]
- 50.Dalal N, Jalandra R, Bayal N, et al. Gut microbiota-derived metabolites in CRC progression and causation. J Cancer Res Clin Oncol 2021;147(11):3141–3155. (In eng). DOI: 10.1007/s00432-021-03729-w. [DOI] [PubMed] [Google Scholar]
- 51.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016;165(6):1332–1345. (In eng). DOI: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 52.Alvandi E, Wong WKM, Joglekar MV, Spring KJ, Hardikar AA. Short-chain fatty acid concentrations in the incidence and risk-stratification of colorectal cancer: a systematic review and meta-analysis. BMC Med 2022;20(1):323. (In eng). DOI: 10.1186/s12916-022-02529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathers JC, Elliott F, Macrae F, et al. Cancer Prevention with Resistant Starch in Lynch Syndrome Patients in the CAPP2-Randomized Placebo Controlled Trial: Planned 10-Year Follow-up. Cancer Prev Res (Phila) 2022;15(9):623–634. (In eng). DOI: 10.1158/1940-6207.Capr-22-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Faria Ghetti F, Oliveira DG, de Oliveira JM, de Castro Ferreira L, Cesar DE, Moreira APB. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. Eur J Nutr 2018;57(3):861–876. (In eng). DOI: 10.1007/s00394-017-1524-x. [DOI] [PubMed] [Google Scholar]
- 55.Elshaghabee FM, Bockelmann W, Meske D, et al. Ethanol Production by Selected Intestinal Microorganisms and Lactic Acid Bacteria Growing under Different Nutritional Conditions. Front Microbiol 2016;7:47. (In eng). DOI: 10.3389/fmicb.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bayoumy AB, Mulder CJJ, Mol JJ, Tushuizen ME. Gut fermentation syndrome: A systematic review of case reports. United European Gastroenterol J 2021;9(3):332–342. (In eng). DOI: 10.1002/ueg2.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meijnikman AS, Davids M, Herrema H, et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat Med 2022;28(10):2100–2106. (In eng). DOI: 10.1038/s41591-022-02016-6. [DOI] [PubMed] [Google Scholar]
- 58.Zhou D, Fan JG. Microbial metabolites in non-alcoholic fatty liver disease. World J Gastroenterol 2019;25(17):2019–2028. (In eng). DOI: 10.3748/wjg.v25.i17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57(2):601–9. (In eng). DOI: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 60.Makarem N, Bandera EV, Nicholson JM, Parekh N. Consumption of Sugars, Sugary Foods, and Sugary Beverages in Relation to Cancer Risk: A Systematic Review of Longitudinal Studies. Annu Rev Nutr 2018;38:17–39. (In eng). DOI: 10.1146/annurev-nutr-082117-051805. [DOI] [PubMed] [Google Scholar]
- 61.Shen X, Wang Y, Zhao R, et al. Metabolic syndrome and the risk of colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2021;36(10):2215–2225. (In eng). DOI: 10.1007/s00384-021-03974-y. [DOI] [PubMed] [Google Scholar]
- 62.Jones HF, Butler RN, Brooks DA. Intestinal fructose transport and malabsorption in humans. Am J Physiol Gastrointest Liver Physiol 2011;300(2):G202–6. (In eng). DOI: 10.1152/ajpgi.00457.2010. [DOI] [PubMed] [Google Scholar]
- 63.Higginbotham S, Zhang ZF, Lee IM, et al. Dietary glycemic load and risk of colorectal cancer in the Women’s Health Study. J Natl Cancer Inst 2004;96(3):229–33. (In eng). DOI: 10.1093/jnci/djh020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michaud DS, Fuchs CS, Liu S, Willett WC, Colditz GA, Giovannucci E. Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 2005;14(1):138–47. (In eng). [PubMed] [Google Scholar]
- 65.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol 2008;26(4):665–73. (In eng). DOI: 10.1200/jco.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 66.Kato I, Neale AV. Does use of alternative medicine delay treatment of head and neck cancer? A surveillance, epidemiology, and end results (SEER) cancer registry study. Head Neck 2008;30(4):446–54. (In eng). DOI: 10.1002/hed.20721. [DOI] [PubMed] [Google Scholar]
- 67.Ambrosone CB, Zirpoli GR, Hutson AD, et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J Clin Oncol 2020;38(8):804–814. (In eng). DOI: 10.1200/jco.19.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puga AM, Samaniego-Vaesken ML, Montero-Bravo A, Ruperto M, Partearroyo T, Varela-Moreiras G. Iron Supplementation at the Crossroads of Nutrition and Gut Microbiota: The State of the Art. Nutrients 2022;14(9) (In eng). DOI: 10.3390/nu14091926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phipps O, Al-Hassi HO, Quraishi MN, et al. Oral and Intravenous Iron Therapy Differentially Alter the On- and Off-Tumor Microbiota in Anemic Colorectal Cancer Patients. Cancers (Basel) 2021;13(6) (In eng). DOI: 10.3390/cancers13061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee T, Clavel T, Smirnov K, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017;66(5):863–871. (In eng). DOI: 10.1136/gutjnl-2015-309940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer - Where do we stand? Mol Metab 2020;33:102–121. (In eng). DOI: 10.1016/j.molmet.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kato I, Dyson G, Snyder M, Kim HR, Severson RK. Differential effects of patient-related factors on the outcome of radiation therapy for rectal cancer. J Radiat Oncol 2016;5(3):279–286. (In eng). DOI: 10.1007/s13566-016-0245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 2021;6(2) (In eng). DOI: 10.1172/jci.insight.145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Attaye I, van Oppenraaij S, Warmbrunn MV, Nieuwdorp M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2021;14(1) (In eng). DOI: 10.3390/nu14010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, Piccini F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes (Basel) 2019;10(7) (In eng). DOI: 10.3390/genes10070534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ang QY, Alexander M, Newman JC, et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020;181(6):1263–1275.e16. (In eng). DOI: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng D, Xie M. A review of a potential and promising probiotic candidate—Akkermansia muciniphila. Journal of Applied Microbiology 2021;130(6):1813–1822. [DOI] [PubMed] [Google Scholar]
- 78.Gutiérrez-Repiso C, Hernández-García C, García-Almeida JM, et al. Effect of Synbiotic Supplementation in a Very-Low-Calorie Ketogenic Diet on Weight Loss Achievement and Gut Microbiota: A Randomized Controlled Pilot Study. Mol Nutr Food Res 2019;63(19):e1900167. (In eng). DOI: 10.1002/mnfr.201900167. [DOI] [PubMed] [Google Scholar]
- 79.Lu L, Zhuang T, Shao E, et al. Association of antibiotic exposure with the mortality in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy: A hospital-based retrospective cohort study. PLoS One 2019;14(9):e0221964. (In eng). DOI: 10.1371/journal.pone.0221964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohindroo C, Hasanov M, Rogers JE, et al. Antibiotic use influences outcomes in advanced pancreatic adenocarcinoma patients. Cancer Med 2021;10(15):5041–5050. (In eng). DOI: 10.1002/cam4.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nenclares P, Bhide SA, Sandoval-Insausti H, et al. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. European Journal of Cancer 2020;131:9–15. DOI: 10.1016/j.ejca.2020.02.047. [DOI] [PubMed] [Google Scholar]
- 82.Yang M, Wang Y, Yuan M, et al. Antibiotic administration shortly before or after immunotherapy initiation is correlated with poor prognosis in solid cancer patients: An up-to-date systematic review and meta-analysis. Int Immunopharmacol 2020;88:106876. (In eng). DOI: 10.1016/j.intimp.2020.106876. [DOI] [PubMed] [Google Scholar]
- 83.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359(6371):104–108. (In eng). DOI: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28(6):1368–1379. (In eng). DOI: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 85.Szczyrek M, Bitkowska P, Chunowski P, Czuchryta P, Krawczyk P, Milanowski J. Diet, Microbiome, and Cancer Immunotherapy-A Comprehensive Review. Nutrients 2021;13(7) (In eng). DOI: 10.3390/nu13072217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davar D, Zarour HM. Facts and Hopes for Gut Microbiota Interventions in Cancer Immunotherapy. Clin Cancer Res 2022;28(20):4370–4384. DOI: 10.1158/1078-0432.CCR-21-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020;39(26):4925–4943. (In eng). DOI: 10.1038/s41388-020-1341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Desai MS, Seekatz AM, Koropatkin NM, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016;167(5):1339–1353 e21. DOI: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li T, Apte U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv Pharmacol 2015;74:263–302. DOI: 10.1016/bs.apha.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang YG, Lu R, Wu S, et al. Vitamin D Receptor Protects Against Dysbiosis and Tumorigenesis via the JAK/STAT Pathway in Intestine. Cell Mol Gastroenterol Hepatol 2020;10(4):729–746. DOI: 10.1016/j.jcmgh.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao Y, Oh J, Xue M, et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science 2022;378(6618):eabm3233. DOI: 10.1126/science.abm3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bell HN, Rebernick RJ, Goyert J, et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 2022;40(2):185–200 e6. DOI: 10.1016/j.ccell.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dmitrieva-Posocco O, Wong AC, Lundgren P, et al. beta-Hydroxybutyrate suppresses colorectal cancer. Nature 2022;605(7908):160–165. DOI: 10.1038/s41586-022-04649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muller M, Legrand C, Tuorto F, et al. Queuine links translational control in eukaryotes to a micronutrient from bacteria. Nucleic Acids Res 2019;47(7):3711–3727. DOI: 10.1093/nar/gkz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J, Lu R, Zhang Y, et al. tRNA Queuosine Modification Enzyme Modulates the Growth and Microbiome Recruitment to Breast Tumors. Cancers (Basel) 2020;12(3). DOI: 10.3390/cancers12030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cullin N, Azevedo Antunes C, Straussman R, Stein-Thoeringer CK, Elinav E. Microbiome and cancer. Cancer Cell 2021;39(10):1317–1341. DOI: 10.1016/j.ccell.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Sun J Impact of bacterial infection and intestinal microbiome on colorectal cancer development. Chin Med J (Engl) 2022;135(4):400–408. DOI: 10.1097/CM9.0000000000001979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daphne M van Elsland JWD, Jilei Zhang, Virginie Stévenin, Yongguo Zhang, Lang Zha, Yinglin Xia, Eelco Franz, Jun Sun, Lapo Mughini-Gras, Jacques Neefjes,. Repetitive non-typhoidal Salmonella exposure is an environmental risk factor for colon cancer and tumor growth. Cell Reports Medicine 2023;3(12). DOI: 10.1016/j.xcrm.2022.100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Narunsky-Haziza L, Sepich-Poore GD, Livyatan I, et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 2022;185(20):3789–3806. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dohlman AB, Klug J, Mesko M, et al. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022;185(20):3807–3822. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koziel MJ, Ziaja M, Piastowska-Ciesielska AW. Intestinal Barrier, Claudins and Mycotoxins. Toxins (Basel) 2021;13(11). DOI: 10.3390/toxins13110758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bang C, Schmitz RA. Archaea associated with human surfaces: not to be underestimated. FEMS Microbiol Rev 2015;39(5):631–48. DOI: 10.1093/femsre/fuv010. [DOI] [PubMed] [Google Scholar]
- 103.Gaci N, Borrel G, Tottey W, O’Toole PW, Brugere JF. Archaea and the human gut: new beginning of an old story. World J Gastroenterol 2014;20(43):16062–78. DOI: 10.3748/wjg.v20.i43.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de la Cuesta-Zuluaga J, Spector TD, Youngblut ND, Ley RE. Genomic Insights into Adaptations of Trimethylamine-Utilizing Methanogens to Diverse Habitats, Including the Human Gut. mSystems 2021;6(1). DOI: 10.1128/mSystems.00939-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramezani A, Nolin TD, Barrows IR, et al. Gut Colonization with Methanogenic Archaea Lowers Plasma Trimethylamine N-oxide Concentrations in Apolipoprotein e−/− Mice. Sci Rep 2018;8(1):14752. DOI: 10.1038/s41598-018-33018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014;157(1):121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342(6161):967–70. DOI: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teng H, Wang Y, Sui X, et al. Gut microbiota-mediated nucleotide synthesis attenuates the response to neoadjuvant chemoradiotherapy in rectal cancer. Cancer Cell 2022. [DOI] [PubMed] [Google Scholar]
- 109.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016;165(6):1332–1345. DOI: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]


