Abstract
Objectives:
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) reduce heart failure (HF) in at-risk patients and may possess antitumor effects. We examined the effect of SGLT2i on HF and mortality among patients with cancer and diabetes.
Methods:
This was a retrospective propensity score-matched cohort study involving adult patients with type 2 diabetes mellitus diagnosed with cancer between January 2010 and December 2021. The primary outcomes were hospitalization for incident HF and all-cause mortality. The secondary outcomes were serious adverse events associated with SGLT2i.
Results:
From a total of 8640 patients, 878 SGLT2i-recipients were matched to non-recipients. During a median follow-up of 18.8 months, SGLT2i recipients had a 3-fold lower rate of hospitalization for incident HF compared with non-SGLT2i recipients (2.92 vs. 8.95 per 1000 patient-years, p=0.018). In Cox regression and competing regression models, SGLT2i were associated with a 72% reduction in the risk for hospitalization for HF (HR, 0.28 [95% CI: 0.11–0.77], p=0.013; SHR, 0.32 [95% CI: 0.12–0.84], p=0.021). The use of SGLT2i was also associated with a higher overall survival (85.3% vs. 63.0% at 2 years, p<0.001). The risk of serious adverse events such as hypoglycemia and sepsis was similar between the two groups.
Conclusions:
The use of SGLT2i was associated with a lower rate of incident HF and prolonged overall survival in cancer patients with diabetes mellitus.
Keywords: Sodium-glucose Cotransporter-2 Inhibitors, Cancer, Heart failure
Introduction
Survival with cancer has dramatically improved over time (1). This has led to a renewed focus on minimizing non-cancer-related sources of morbidity and mortality (2, 3). Consistent data have shown increased rates of cardiovascular disease among cancer survivors (4, 5). The presence of cancer itself and several standard cancer treatment approaches has been associated with an increased risk of heart failure (6–10). The development of heart failure has a detrimental effect on the prognosis of cancer (11, 12). Therefore, there is an important need to reduce heart failure and improve outcomes among patients with cancer.
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are novel cardiovascular therapies that lead to a 30–35% reduction in the risk of heart failure hospitalization among those with and without diabetes mellitus (13–18). Importantly, these cardiovascular benefits were observed in patients regardless of existing atherosclerotic cardiovascular disease or a history of heart failure (19). In fact, SGLT2i are recommended for the prevention of heart failure in Stage A patients with increased risk of heart failure (20, 21). There are no large clinical studies testing the effect of SGLT2i on incident heart failure among broad groups of patients with cancer. Nevertheless, in animal studies, the use of SGLT2i prior to anthracyclines was associated with the preservation of cardiac structure and function in mice (22, 23). Recently, a retrospective study reported that the use of SGLT2i is associated with lower rates of cardiac events among diabetes patients treated with anthracyclines (24). Whether SGLT2i confers cardiovascular outcomes benefits among patients with different types of cancer and a broad range of cancer treatments remains unknown. It is also unclear whether SGLT2i may affect survival outcomes in patients with cancer. Glycolysis plays a central role in tumor metabolism and growth; SGLT2 is upregulated in several cancer types and inhibition of SGLT2 has been shown to attenuate the proliferation of multiple different cancer cell lines (25, 26). However, the effect of SGLT2i on survival is incompletely understood. To address these knowledge gaps, we performed a retrospective cohort study to evaluate the cardiovascular and survival impact of SGLT2i among cancer patients.
Methods
Study Design
This was a retrospective propensity score-matched cohort study conducted at two tertiary referral centers in Taiwan. The Institutional Review Board of both hospitals approved this study (Chung Shan Medical University Hospital, CS1–22068; Taipei Tzu Chi Hospital, 11-X-035). Informed consent was waived. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of this research. Adult type 2 diabetes patients treated with cancer between January 2010 and December 2021 were identified using the International Classification of Diseases (ICD) codes. We excluded patients who had only one hospital visit and missing data. Patients were classified as having received SGLT2i if they received canagliflozin, dapagliflozin, or empagliflozin after a cancer diagnosis. This included patients who were started on SGLT2i before the diagnosis of cancer and continued the medication after the diagnosis. The index date was determined as the date of the first SGLT2i or non-SGLT2i diabetes medication after the diagnosis of cancer. In the non-SGLT2i group, the index date was chosen as the date of the first non-SGLT2i medication prescription after the diagnosis of cancer. We utilized the electronic medical records to collect data such as age, sex, cancer information, underlying comorbidities, previous cancer therapies, and use of diabetes medications and other cardiovascular medications. For patients who developed heart failure, we also collected data on the left ventricular ejection fraction (LVEF).
The efficacy outcome was hospitalization for incident heart failure (HF). The cardiovascular events were adjudicated by two investigators, using a combination of ICD codes, medication use, and clinical/imaging findings (Supplemental Table 1), and blinded to exposure status. The primary safety outcome was all-cause mortality, and the secondary safety outcomes were serious adverse events reported as associated with the use of SGLT2i, including diabetic ketoacidosis, urosepsis, sepsis, hypoglycemia, acute kidney injury, and Fournier’s gangrene. In both SGLT2i and non-SGLT2i cohorts, only cardiac or adverse events that occurred for the first time after the initiation of the SGLT2i or non-SGLT2i diabetes medications were considered. To ensure that patients in the non-SGLT2i cohort were also receiving therapy for diabetes mellitus, patients were included if they received non-SGLT2i diabetes medications.
Statistical analysis
We conducted a 1:1 propensity score matching to minimize the baseline differences between SGLT2i and the non-SGLT2i cohorts. The propensity score was built based on the following predetermined variables: age, sex, cancer type, year of cancer diagnosis, institution treated, the presence of metastatic disease, underlying cardiac and non-cardiac comorbidities, use of cardiovascular medications, and the type of cancer therapy. These variables were selected because they predict the likelihood of the exposure, are potential confounders, or are related to the primary outcomes, which were shown to yield optimal propensity score matching models (27). We used a nearest neighbor matching approach, with a caliper set at 0.2 of the standard deviation of the logit of the propensity score. After matching, we compared the differences in baseline characteristics between patients who were treated with SGLT2i and non-SGLT2i diabetes medications using standardized mean differences. The time to cardiovascular events and overall survival between patients treated with SGLT2i and non-SGLT2i medications were estimated using the Kaplan-Meier method. We evaluated the time to hospitalization for HF using the cumulative incidence competing risk method. The association between SGLT2i use and the risk of each of the endpoints was evaluated using the Cox proportional hazard model. The proportional hazard ratio assumption was assessed using the Schoenfeld Residuals. We considered death as a competing risk for the occurrence of cardiovascular and adverse events and calculated the sub-distribution hazard ratio (SHR) using the Fine and Gray competing risk regression model. We performed a time-varying Cox analysis to account for patients who discontinued SGLT2i treatment during the study. We conducted additional analyses to evaluate the effects of SGLT2i on hospitalization for incident HF and all-cause mortality in pre-specified subgroups that include age, sex, cancer type, metastatic disease, underlying comorbidities, and use of cardiovascular medications and chemotherapeutic agents at baseline. Incidence rates were calculated as the number of incident HF or all-cause mortality divided by the total follow-up periods. Incidence rate ratios were calculated by dividing the incidence rates of exposed to unexposed. Because SGLT2i were only used after 2015 in our study sites, we performed additional sensitivity analyses to evaluate if controls selected before or after 2015 would influence the results. We further tested the effects of the length of SGLT2i prescription and different classes of SGLT2i and non-SGLT2i controls on the outcomes. A p-value less than 0.05 for a two-sided test was used to indicate statistical significance. For subgroup analyses, p-values indicating statistical significance were corrected using Bonferroni correction. All analyses were conducted using Stata version 16.0 (StataCorp LLC, College Station, TX).
Results
Patient Demographics
We identified 8640 cancer patients with type 2 diabetes mellitus between 2010 and 2021. After excluding patients who had only one hospital visit and missing data, there remained 878 and 7556 patients in the SGLT2i and non-SGLT2i cohorts (Figure 1). As expected, patients who were treated with SGLT2i had higher proportions of cardiovascular risk factors and were more commonly prescribed cardiac medications than patients who were not treated with SGLT2i (Supplemental Table 2). After propensity score matching, all covariates including underlying comorbidities, previous cardiovascular diseases, and the use of cardiac medications were well-balanced between the two groups (Table 1 and Supplemental Figure 1). The most common malignancy for both groups were gastrointestinal and genitourinary cancers. For common cardiotoxic agents, 136 (8%) received anthracyclines, 71 (4%) received tyrosine kinase inhibitors, and 47 (3%) received radiotherapy. Among patients treated with an SGLT2i; empagliflozin (49%) was the most commonly prescribed, followed by dapagliflozin (38%) (Supplemental Table 3). Among patients treated with non-SGLT2i medications, metformin was the most commonly prescribed (45%), followed by insulin (36%). The distribution of non-SGLT2i diabetes medications was similar between the SGLT2i and non-SGLT2i cohorts before cancer diagnosis (Supplemental Table 4). The median time from cancer diagnosis to the index date was 6.8 (IQR, 1.0–24.0) months and 1.0 (IQR, 0.2–7.8) months for the SGLT2i and non-SGLT2i groups, respectively. The median follow-up time was 20.4 (IQR, 8.3–35.8) months and 18.0 (IQR, 5.1–47.6) months for the SGLT2i and non-SGLT2i groups, respectively.
Figure 1.
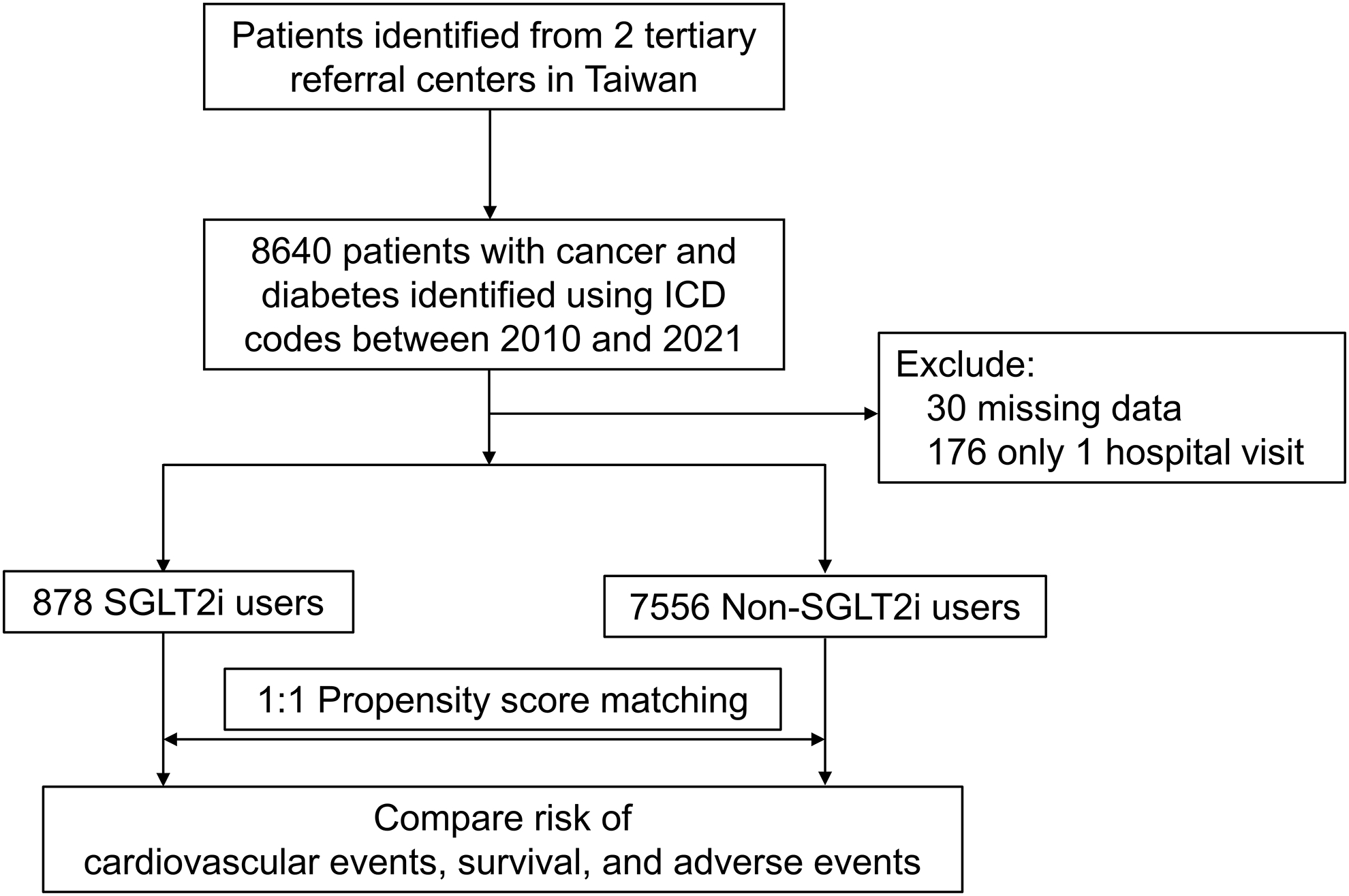
Flowchart showing patient inclusion.
Abbreviations: ICD, International Classification of Diseases; SGLT2i, Sodium-glucose Cotransporter 2 Inhibitors
Table 1.
Patient demographics after propensity score matching
| Total N=1,756 | non-SGLT2i N=878 | SGLT2i N=878 | SMD (%) | |
|---|---|---|---|---|
| Age | 65 (58–73) | 65 (59–75) | 65 (58–71) | −0.2 |
| Male | 931 (53%) | 456 (52%) | 475 (54%) | 4.3 |
| Year of cancer diagnosis | 2016 (2013–2019) | 2016 (2013–2019) | 2016 (2013–2019) | −2.7 |
| Institution | ||||
| Taipei Tzu Chi Hospital | 539 (31%) | 277 (32%) | 262 (30%) | 3.6 |
| Chung Shan Medical University Hospital | 1,217 (69%) | 601 (68%) | 616 (70%) | |
| Cancer type | ||||
| Gastrointestinal | 623 (35%) | 311 (35%) | 312 (36%) | |
| Genitourinary | 311 (18%) | 145 (17%) | 166 (19%) | |
| Thoracic | 222 (13%) | 116 (13%) | 106 (12%) | |
| Head and neck | 180 (10%) | 95 (11%) | 85 (10%) | 1.4 |
| Breast | 199 (11%) | 97 (11%) | 102 (12%) | |
| Hematologic | 83 (5%) | 52 (6%) | 31 (4%) | |
| Skin | 27 (2%) | 17 (2%) | 10 (1%) | |
| Others | 111 (6%) | 45 (5%) | 66 (8%) | |
| Metastatic disease | 322 (18%) | 158 (18%) | 164 (19%) | 1.7 |
| Comorbidities | ||||
| Cardiovascular disease* | 325 (19%) | 162 (18%) | 163 (19%) | 0.3 |
| Heart failure | 84 (5%) | 43 (5%) | 41 (5%) | |
| Myocardial infarction | 59 (3%) | 23 (3%) | 36 (4%) | |
| Ischemic stroke | 62 (4%) | 37 (4%) | 25 (3%) | |
| Arrhythmia | 188 (11%) | 98 (11%) | 90 (10%) | |
| Hypertension | 1,319 (75%) | 674 (77%) | 645 (73%) | −7.1 |
| Dyslipidemia | 1,090 (62%) | 548 (62%) | 542 (62%) | −1.4 |
| Chronic kidney disease | 245 (14%) | 122 (14%) | 123 (14%) | 0.3 |
| COPD | 177 (10%) | 89 (10%) | 88 (10%) | −0.4 |
| Cardiovascular medications | ||||
| ACEI/ARB | 992 (56%) | 508 (58%) | 484 (55%) | −5.6 |
| Beta-blockers | 1,034 (59%) | 527 (60%) | 507 (58%) | −4.7 |
| Diuretics | 748 (43%) | 383 (44%) | 365 (42%) | −4.2 |
| Calcium channel blockers | 955 (54%) | 481 (55%) | 474 (54%) | −1.6 |
| Statin | 990 (56%) | 492 (56%) | 498 (57%) | 1.5 |
| Aspirin | 390 (22%) | 203 (23%) | 187 (21%) | −4.5 |
| Cancer therapy | ||||
| Alkylating agents | 82 (5%) | 37 (4%) | 45 (5%) | 4.7 |
| Antimetabolites | 310 (18%) | 153 (17%) | 157 (18%) | 1.3 |
| Platinum | 216 (12%) | 112 (13%) | 104 (12%) | −3 |
| Plant alkaloids | 190 (11%) | 100 (11%) | 90 (10%) | −4.1 |
| Anthracyclines | 136 (8%) | 70 (8%) | 66 (8%) | −1.9 |
| Tyrosine kinase inhibitors | 71 (4%) | 35 (4%) | 36 (4%) | 0.6 |
| HER2 inhibitors | 32 (2%) | 14 (2%) | 18 (2%) | 4 |
| VEGF inhibitors | 29 (2%) | 15 (2%) | 14 (2%) | −1.1 |
| Immune checkpoint inhibitors | 22 (1%) | 11 (1%) | 11 (1%) | 0 |
| Radiotherapy | 47 (3%) | 23 (3%) | 24 (3%) | 0.8 |
Abbreviations: ACEI-ARB, Angiotensin-converting enzyme inhibitors-angiotensin receptor blockers; COPD, chronic obstructive pulmonary disease; HER2, Human epidermal growth factor receptor 2; SGLT2i, Sodium-glucose cotransporter 2 inhibitors; SMD, standardized mean differences; VEGF, Vascular endothelial growth factor
History of heart failure, myocardial infarction, ischemic stroke, and arrhythmia
Hospitalization for incident HF
During a median follow-up period of 18.8 months, the incidence rate for hospitalization for incident HF for SGLT2i and non-SGLT2i cohorts was 2.92 and 8.95 per 1000 patient-years, with an incidence rate ratio of 0.33 [95% CI: 0.10–0.91] (p = 0.018) (Table 2). Kaplan-Meier analysis showed that those prescribed SGLT2i had a lower cumulative incidence of HF compared with those prescribed non-SGLT2i medications (Log-rank, p = 0.009) (Figure 2). In a Cox proportional hazard model, SGLT2i were associated with a 72% reduction in the risk for hospitalization for HF (HR, 0.28 [95% CI: 0.11–0.77], p = 0.013) (Table 2). Using a competing risk analysis, the cumulative incidence and risk of HF remained lower in the SGLT2i group (SHR, 0.32 [95% CI: 0.12–0.84], p = 0.021) (Table 2 and Supplemental Figure 2). In a time-varying Cox analysis, the use of SGLT2i was associated with a lower risk of hospitalization for heart failure (HR 0.22 [95% CI: 0.05–0.93], p = 0.040) (Supplemental Table 5). Among the 24 patients who were hospitalized with incident HF, 5 received SGLT2i, while 19 were treated with non-SGLT2i medications. Among patients with available echocardiography data, 11 patients had reduced LVEF, and 10 had preserved LVEF (Supplemental Table 6).
Table 2.
Incidence rate ratio and cox proportional analysis of cardiovascular outcomes and all-cause mortality between SGLT2i and non-SGLT2i cohorts
| Outcomes | Exposure | Cases | Incidence per 1000 patient-years | Incidence rate ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | SHR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Heart failure hospitalization | Non-SGLT2i | 19 | 8.95 | Reference | 0.018 | Reference | 0.013 | Reference | 0.021 |
| SGLT2i | 5 | 2.92 | 0.33 (0.10–0.91) | 0.28 (0.11–0.77) | 0.32 (0.12–0.84) | ||||
| All-cause mortality | Non-SGLT2i | 362 | 162.7 | Reference | <0.001 | Reference | <0.001 | - | |
| SGLT2i | 124 | 70.0 | 0.43 (0.35–0.53) | 0.35 (0.28–0.43) |
Abbreviations: CI, confidence interval; SGLT2i, Sodium-glucose Cotransporter-2 Inhibitors; SHR, sub-distribution hazard ratio
Figure 2.
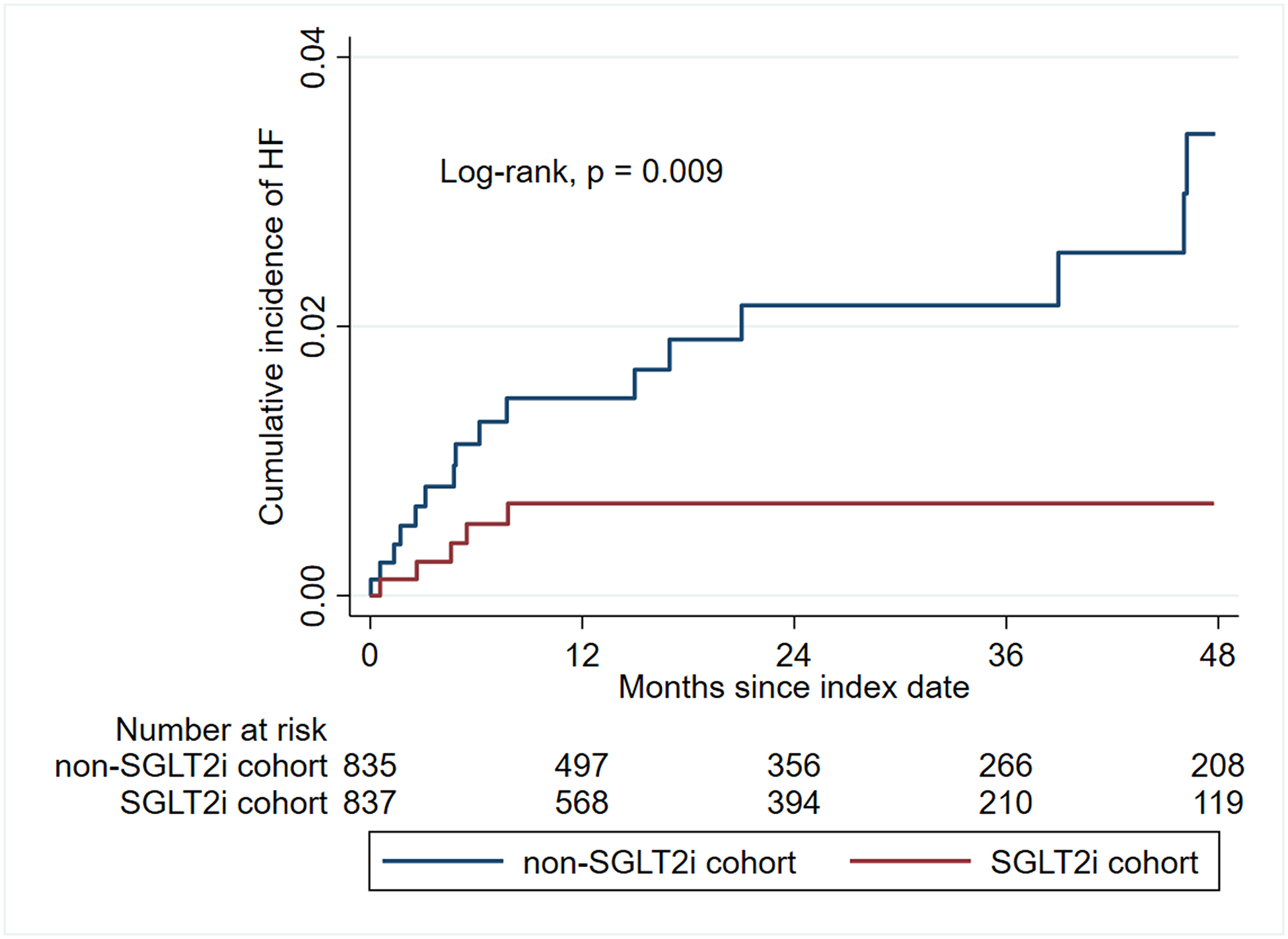
Kaplan-Meier curve showing cumulative incidence of hospitalization for heart failure.
Abbreviations: HF, heart failure; SGLT2i, Sodium-glucose Cotransporter 2 Inhibitors
In our subgroup analysis, the effects of SGLT2i were similar across different age and sex groups (Supplemental Figure 3). The use of SGLT2i was associated with a greater HF risk reduction in patients without chronic kidney disease than those with chronic kidney disease, but this association was not statistically significant (HR, 0.22 [95% CI: 0.06–0.75] vs. 0.53 [95% CI: 0.09–3.17], p for interaction = 0.337). Patients with dyslipidemia showed a trend toward greater benefit from the use of SGLT2i than patients without dyslipidemia; however, this difference was not statistically significant (HR, 0.09 [95% CI: 0.01–0.68] vs. 0.65 [95% CI: 0.18–2.32], p for interaction = 0.112) (Supplemental Figure 3).
Overall Survival
Compared with patients not treated with SGLT2i, those who received SGLT2i had a higher overall survival (e.g., at 2 years, 85.3% vs. 63.0%, log-rank, p < 0.001) (Figure 3). In a Cox proportional hazard model, the use of SGLT2i was associated with a 65% reduction in the risk for all-cause mortality (HR, 0.35 [95% CI: 0.28–0.43], p < 0.001) (Table 2). In a time-varying Cox analysis, the use of SGLT2i was associated with a lower risk of all-cause mortality (HR 0.08 [95% CI: 0.05–0.13], p <0.001) (Supplemental Table 5). In subgroup analysis, SGLT2i were associated with a lower risk of mortality regardless of underlying comorbidities, cancer type, and cancer therapy (Supplemental Figures 4 and 5). However, patients who received immune checkpoint inhibitors did not experience a reduction in mortality (Supplemental Figure 5).
Figure 3.
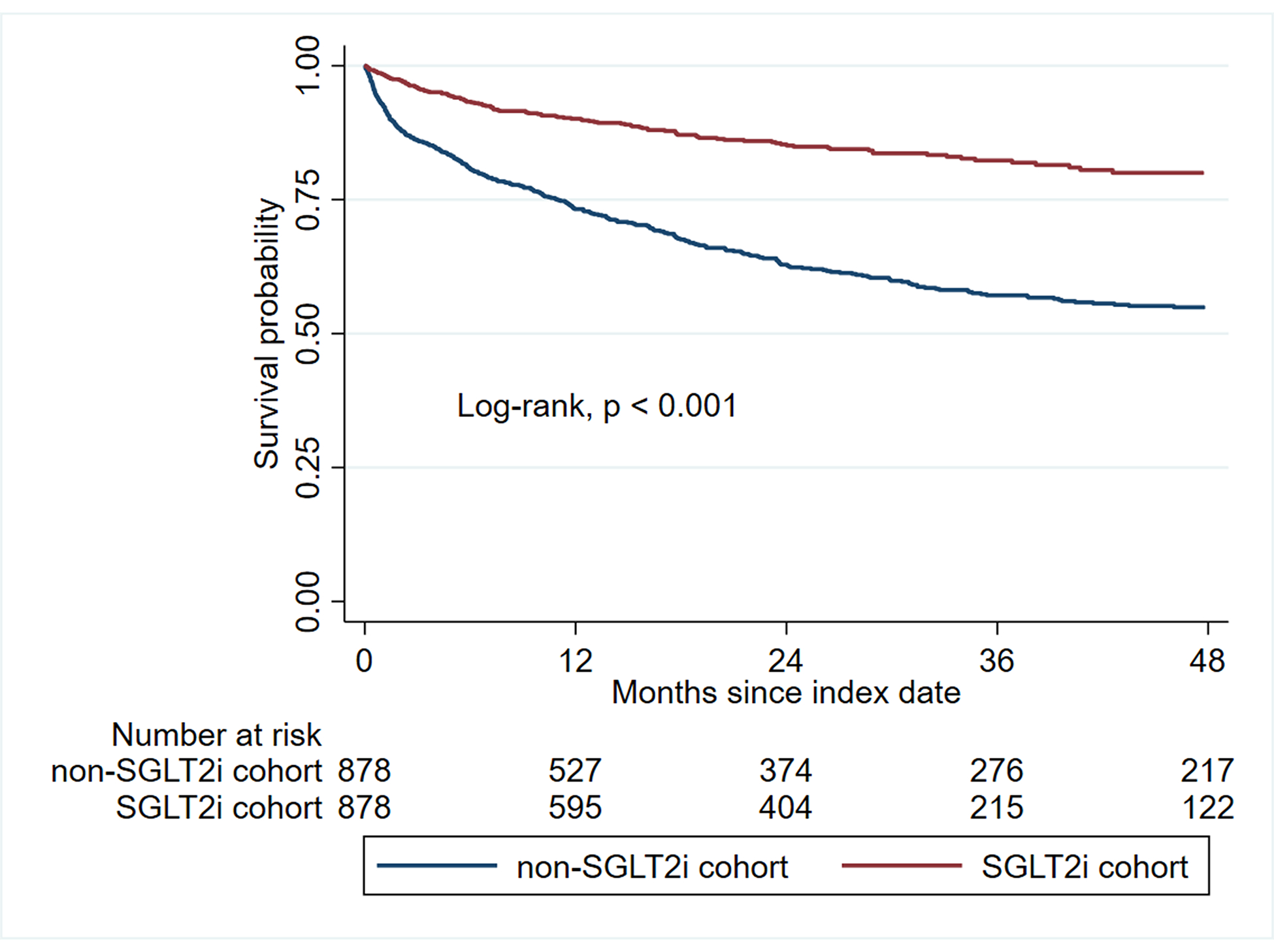
Kaplan-Meier analysis showing the overall survival between SGLT2i and non-SGLT2i cohorts.
Abbreviations: SGLT2i, Sodium-glucose Cotransporter 2 Inhibitors
Other adverse events
The use of SGLT2i was not associated with an increased risk of diabetic ketoacidosis (HR, 1.18 [95% CI: 0.29–4.78, p = 0.82) or acute kidney injury (HR, 0.78 [95% CI: 0.52–1.18, p = 0.24) (Table 3). One patient developed Fournier’s gangrene in the SGLT2i group (0.12%), while no patient had Fournier’s gangrene in the non-SGLT2i group. The use of SGLT2i was associated with a decreased risk of urosepsis (HR, 0.27 [95% CI: 0.13–0.56]), sepsis (HR, 0.31 [95% CI: 0.22–0.44], and hypoglycemia (HR, 0.36 [95% CI: 0.16–0.81]).
Table 3.
Incidence rate ratio and cox proportional analysis of adverse outcomes between SGLT2i and non-SGLT2i cohorts.
| Outcomes | Exposure | Cases | Incidence per 1000 patient-years | Incidence rate ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | SHR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Total adverse events | Non-SGLT2i | 215 | 109.0 | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 |
| SGLT2i | 97 | 57.7 | 0.53 (0.41–0.68) | 0.45 (0.35–0.57) | 0.50 (0.39–0.63) | ||||
| Diabetic ketoacidosis | Non-SGLT2i | 5 | 2.25 | Reference | 0.97 | Reference | 0.82 | Reference | 0.74 |
| SGLT2i | 4 | 2.29 | 1.02 (0.20–4.72) | 1.18 (0.29–4.78) | 1.25 (0.34–4.51) | ||||
| Urosepsis | Non-SGLT2i | 34 | 15.7 | Reference | 0.001 | Reference | <0.001 | Reference | 0.002 |
| SGLT2i | 9 | 5.15 | 0.33 (0.14–0.70) | 0.27 (0.13–0.56) | 0.31 (0.15–0.65) | ||||
| Sepsis | Non-SGLT2i | 131 | 64.9 | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 |
| SGLT2i | 40 | 24.4 | 0.38 (0.26–0.54) | 0.31 (0.22–0.44) | 0.34 (0.24–0.49) | ||||
| Hypoglycemia | Non-SGLT2i | 24 | 11.1 | Reference | 0.025 | Reference | 0.014 | Reference | 0.037 |
| SGLT2i | 8 | 4.64 | 0.41 (0.16–0.95) | 0.36 (0.16–0.81) | 0.42 (0.19–0.95) | ||||
| Acute kidney injury | Non-SGLT2i | 61 | 28.9 | Reference | 0.38 | Reference | 0.24 | Reference | 0.54 |
| SGLT2i | 41 | 24.2 | 0.84 (0.55–1.26) | 0.78 (0.52–1.18) | 0.88 (0.58–1.33) | ||||
| Fournier’s gangrene | Non-SGLT2i | 0 | 0.0 | Reference | - | Reference | - | Reference | - |
| SGLT2i | 1 | 0.57 | - | - | - |
Abbreviations: CI, confidence interval; SGLT2i, Sodium-glucose Cotransporter-2 Inhibitors; SHR, sub-distribution hazard ratio
Sensitivity analysis
Patients who received SGLT2i showed a trend of lower risk of hospitalization for incident HF and all-cause mortality compared to those who did not receive SGLT2i regardless of the time (before or after 2015) during which the non-SGLT2i control was selected (Supplemental Figure 6 and 7). Additionally, different classes of SGLT2i showed similar efficacy in reducing the risk of HF and mortality. Patients who received SGLT2i for at least 6 months experienced a greater risk reduction in HF and mortality compared to patients who received SGLT2i for a shorter period. Compared with patients who received different classes of non-SGLT2i medications, patients who received SGLT2i had a lower risk of HF and mortality.
Discussion
In this retrospective study, we found that the use of SGLT2i was associated with a reduction in hospitalization for HF and improved overall survival in patients with cancer. Furthermore, SGLT2i use was not associated with an increased risk of adverse events among cancer patients. Our results suggest that SGLT2i may provide cardiovascular and survival benefits for patients with diabetes mellitus and cancer.
Our findings that SGLT2i reduce hospitalization for incident HF among patients with cancer are novel, and they are supported by findings in non-cancer populations. The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial was the first to report a 35% relative reduction in hospitalization with HF associated with the use of empagliflozin in type 2 diabetes mellitus (14). Subsequent trials generalized these findings to other classes of SGLT2i and the non-diabetic population (15–17). In our study, the use of SGLT2i was associated with a 70% relative reduction in hospitalization for HF. It is likely that some of the same beneficial effects of SGLT2i in countering heart failure progression that are seen in patients without cancer, also occur in those with cancer. Whether SGLT2i also confers additional heart failure benefits specific to patients with cancer is still unclear. In our study, only 136 (8%) were treated with an anthracycline, and among the patients who were hospitalized with HF, only 1 was treated with anthracyclines. Thus, we were unable to examine the risk of HF hospitalization in an anthracycline-specific population.
Inhibition of SGLT2 has been postulated to be a potential candidate for augmenting cancer therapy (25). Preclinical studies showed that SGLT-specific positron emission tomography (PET) tracers accumulate in tumor cells and patient-derived xenografts and were reduced by SGLT2i (25, 26). Our results showed that cancer patients treated with SGLT2i had significantly better survival outcomes than patients who were not treated with SGLT2i. This finding was noted despite the SGLT2i recipients having a higher baseline cardiovascular and mortality risk. In clinical trials of the general diabetes population, patients who received SGLT2i had an estimated mean survival difference of 1 to 5 years compared with placebo (14, 28). The postulated mechanisms involved include natriuresis and osmotic diuresis leading to blood pressure reduction, weight loss, reduced oxidative stress, and improved vascular endothelial function (14, 28).
Although SGLT2i have demonstrated a favorable safety profile in data from clinical trials and post-marketing pharmacovigilance, several serious, though infrequent safety issues have been reported. One commonly reported adverse event from the use of SGLT2i is diabetic ketoacidosis. In our study, there were only 4 cases (0.46%) of diabetic ketoacidosis and its incidence was not higher than that reported in previous studies (29). Another adverse event of concern is urinary tract infections. However, both our data and those from previous studies conducted in the non-cancer populations did not show an increased risk of urinary tract infections associated with SGLT2i (14, 16, 30). This is clinically important because cancer patients have a higher susceptibility to infections and sepsis (20).
The results of this study should be interpreted in the context of the study design. This was a retrospective study, and there may remain residual confounders that were not included in the propensity score matching model. However, we included most of the known covariates that were deemed to influence the use of SGLT2i in our model. There was an expected loss to follow-up as this was a cancer population; this might have affected the risk estimates of the cardiovascular and non-cardiovascular outcomes. We also do not have data on the exact cause of death and response to treatment; therefore, we could not determine if the survival benefits associated with SGLT2i were attributed to its antitumor effects or cardiovascular benefits but suspect the former. The magnitude of the effect on overall mortality was large. The groups appeared to be matched on standard variables associated with mortality in cancer patients, but the presence of residual confounding cannot be excluded and the effect of SGLT2i on overall survival and progression-free survival needs to be tested in a randomized trial. Potential bias might arise from the crossover of SGLT2i-treated patients to the non-SGLT2i group during the study period. However, our sensitivity analysis indicates that patients who discontinued or switched therapy within 6 months of SGLT2i had significantly less cardiovascular and survival benefits than those who were placed on SGLT2i for a longer period. Our data only apply to cancer patients with type 2 diabetes. Whether similar SGLT2i-related benefits can be seen in cancer patients without diabetes is unknown and should be tested. We did not have HbA1c data available for analysis, but this does not influence the decision for which patients are placed on SGLT2i versus other diabetes medications. These results could only be generalized to canagliflozin, dapagliflozin, or empagliflozin, and the effects of other types of SGLT2i on cancer patients remain to be determined. There might be bias resulting from the use of non-SGLT2i controls selected from the time period before SGLT2i were used in clinical practice. Nevertheless, our sensitivity analysis indicates that the cardiovascular and survival benefits associated with SGLT2i were observed regardless of the time period from which the controls were selected. Finally, this study was conducted in an Asian population, which has a different cardiovascular risk profile and cancer demographics than other regions.
Conclusion
In this large retrospective cohort study, the use of SGLT2i was associated with a reduction in hospitalization for HF and improved overall survival among cancer patients with type 2 diabetes mellitus. There were no increased rates of serious adverse events associated with SGLT2i. Prospective randomized trials are needed to further investigate and specify the benefit of SGLT2i in patients with cancer.
Supplementary Material
What is already known on this topic.
SGLT2 inhibitors are associated with a lower risk of hospitalization for heart failure. However, the effects of SGLT2 inhibitors on hospitalization for heart failure and mortality in cancer patients are unclear.
What this study adds.
SGLT2 inhibitors are associated with a lower risk of hospitalization for heart failure and all-cause mortality in patients with cancer and diabetes.
How this study might affect research, practice or policy.
SGLT2 inhibitors need to be tested in randomized trials whether they lower heart failure risk and improve survival among cancer patients.
Acknowledgments:
We thank the staff members of the Da Vinci Minimally Invasive Surgery Center at Chung Shan Medical University Hospital for technical assistance.
Funding/Support:
This work was supported by the National Institutes of Health/National Heart, Lung, Blood Institute (R01HL137562, R01HL130539, K24HL150238 to Dr. Neilan). Dr. T. Neilan holds the Michael and Kathryn Park Chair in Cardiology and was also supported, in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg, Christina and Paul Kazilionis and a Hassenfeld Scholar Award. Dr. Thavendiranathan is supported by a Canada Research Chair in Cardiooncology.
Disclosures:
Dr. Neilan has been a consultant to and received fees from Parexel Imaging, Intrinsic Imaging, BMS, H3-Biomedicine, AbbVie, C4-Therapeutics, Roche, Sanofi, and Genentech, outside of the current work. Dr. Neilan has received grant funding from Astra Zeneca and BMS. Dr. Armand has served as a consultant on the advisory board for Astra Zeneca. Dr. Peterson has stock in Exact Sciences. All other authors have no conflict of interest to disclose.
Footnotes
Data availability:
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Bradley CJ, Yabroff KR, Mariotto AB, Zeruto C, Tran Q, Warren JL. Antineoplastic Treatment of Advanced-Stage Non-Small-Cell Lung Cancer: Treatment, Survival, and Spending (2000 to 2011). J Clin Oncol. 2017;35(5):529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campia U, Moslehi JJ, Amiri-Kordestani L, Barac A, Beckman JA, Chism DD, et al. Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement From the American Heart Association. Circulation. 2019;139(13):e579–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43(4):280–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bright CJ, Hawkins MM, Guha J, Henson KE, Winter DL, Kelly JS, et al. Risk of Cerebrovascular Events in 178 962 Five-Year Survivors of Cancer Diagnosed at 15 to 39 Years of Age. Circulation. 2017;135(13):1194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henson KE, Reulen RC, Winter DL, Bright CJ, Fidler MM, Frobisher C, et al. Cardiac Mortality Among 200 000 Five-Year Survivors of Cancer Diagnosed at 15 to 39 Years of Age. Circulation. 2016;134(20):1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolladille C, Akroun J, Morice PM, Dompmartin A, Ezine E, Sassier M, et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J. 2021;42(48):4964–77. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira de Souza T, Quinaglia ACST, Osorio Costa F, Shah R, Neilan TG, Velloso L, et al. Anthracycline Therapy Is Associated With Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc Imaging. 2018;11(8):1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pun SC, Neilan TG. Cardiovascular side effects of small molecule therapies for cancer. Eur Heart J. 2016;37(36):2742–5. [DOI] [PubMed] [Google Scholar]
- 9.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation. 2020;142(24):2299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang CH, Chiang CH, Ma KS, Hsia YP, Lee YW, Wu HR, et al. The incidence and risk of cardiovascular events associated with immune checkpoint inhibitors in Asian populations. Jpn J Clin Oncol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenlee H, Iribarren C, Rana JS, Cheng R, Nguyen-Huynh M, Rillamas-Sun E, et al. Risk of Cardiovascular Disease in Women With and Without Breast Cancer: The Pathways Heart Study. J Clin Oncol. 2022:Jco2101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Sendón J, Álvarez-Ortega C, Zamora Auñon P, Buño Soto A, Lyon AR, Farmakis D, et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41(18):1720–9. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413–24. [DOI] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–28. [DOI] [PubMed] [Google Scholar]
- 15.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–57. [DOI] [PubMed] [Google Scholar]
- 16.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380(4):347–57. [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 18.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–306. [DOI] [PubMed] [Google Scholar]
- 19.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022. AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 0(0): 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 21.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, et al. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl Sci. 2018;3(5):575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gongora CA, Drobni ZD, Quinaglia Araujo Costa Silva T, Zafar A, Gong J, Zlotoff DA, et al. Sodium-Glucose Co-Transporter-2 Inhibitors and Cardiac Outcomes Among Patients Treated With Anthracyclines. JACC Heart Fail. 2022;10(8):559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scafoglio CR, Villegas B, Abdelhady G, Bailey ST, Liu J, Shirali AS, et al. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci Transl Med. 2018;10(467). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A. 2015;112(30):E4111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claggett B, Lachin JM, Hantel S, Fitchett D, Inzucchi SE, Woerle HJ, et al. Long-Term Benefit of Empagliflozin on Life Expectancy in Patients With Type 2 Diabetes Mellitus and Established Cardiovascular Disease. Circulation. 2018;138(15):1599–601. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Li L, Li S, Wang Y, Qin X, Deng K, et al. Sodium-glucose cotransporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020;22(9):1619–27. [DOI] [PubMed] [Google Scholar]
- 30.Lega IC, Bronskill SE, Campitelli MA, Guan J, Stall NM, Lam K, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: A population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019;21(11):2394–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


