Abstract
Synovial iron deposition associated with rheumatoid disease may result in the production of highly reactive oxygen free radicals, leading to tissue damage. This chain of events can be interrupted by iron chelation. Families of strong iron (III) chelators have been tested for their iron scavenging properties in vitro and their effects assessed in vivo using a rat model of inflammation. All the chelators competed successfully for iron with apotransferrin, and some removed up to 34% of iron from ferritin. The best anti-inflammatory effects were achieved with the most hydrophilic chelators and those which chelated iron most avidly. Activity was dependent on dose. The route of administration was also an important factor with lower affinity chelators. This work introduces a range of simple bidentate iron chelators, which under certain conditions exceed desferrioxamine in their iron scavenging abilities, and some of which, in this simple animal model, approach indomethacin in their anti-inflammatory capabilities.
Full text
PDF
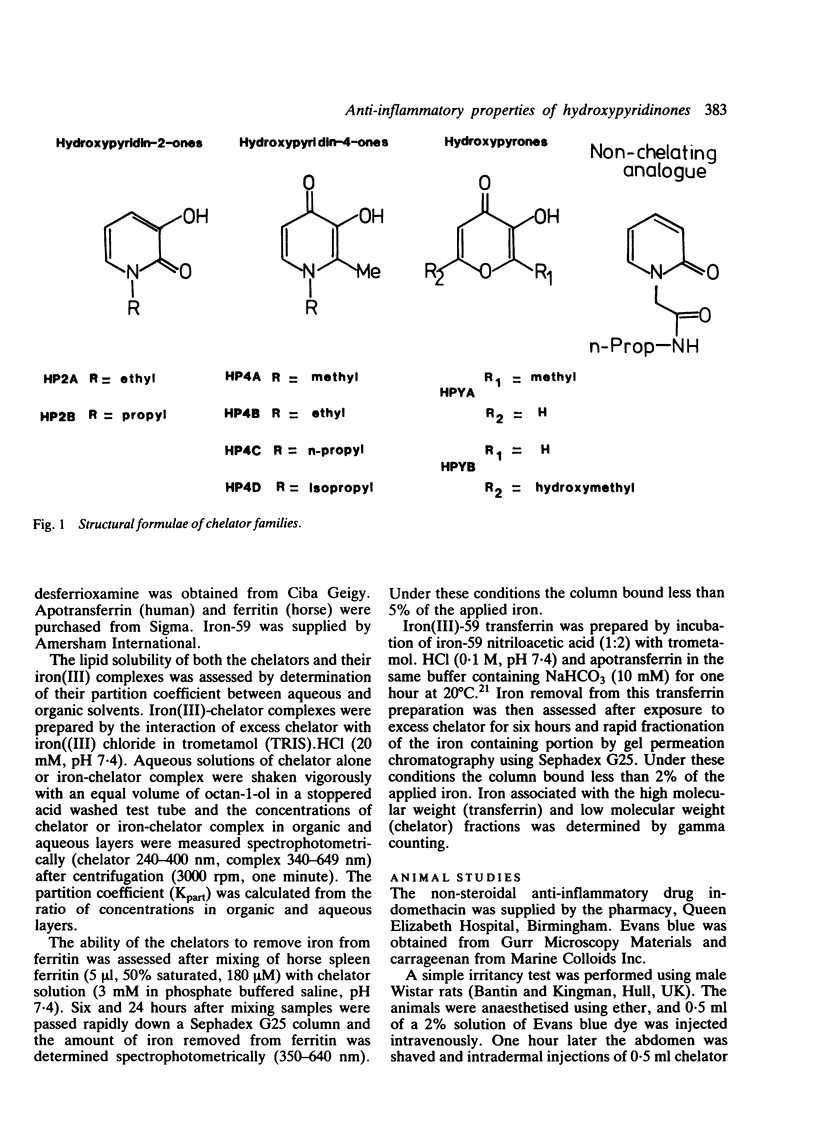

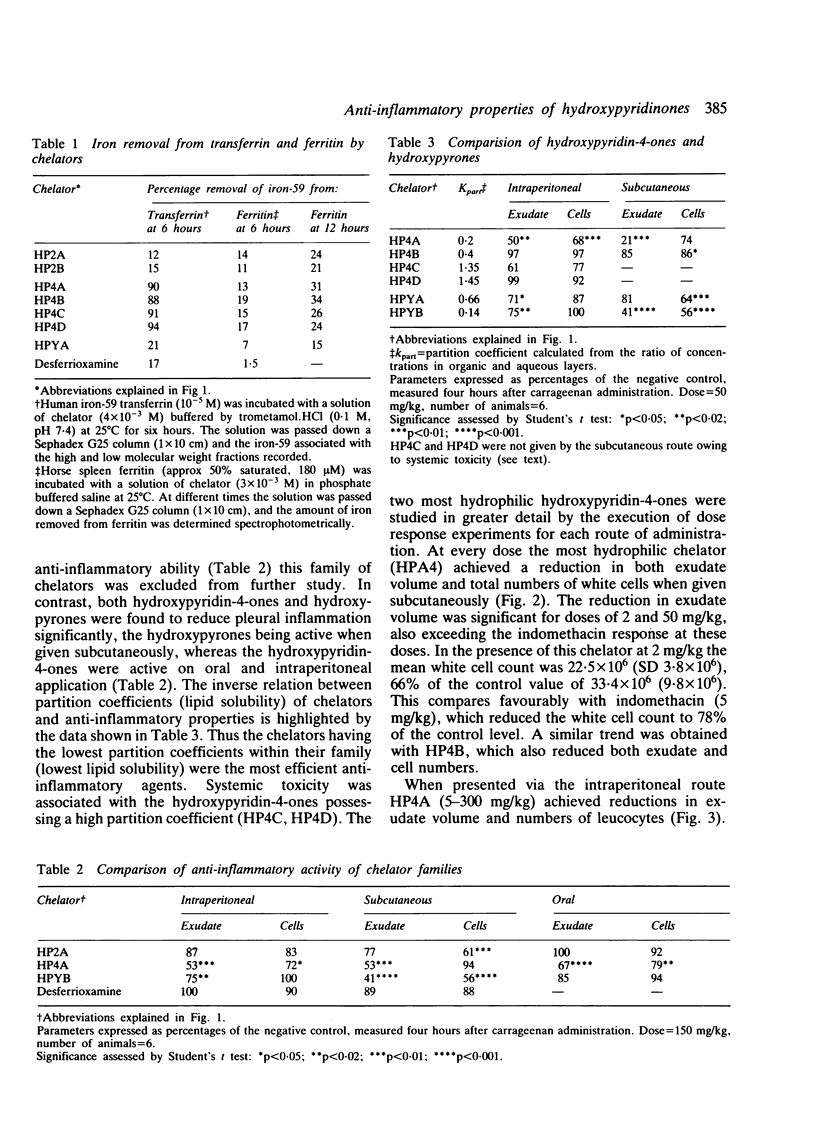
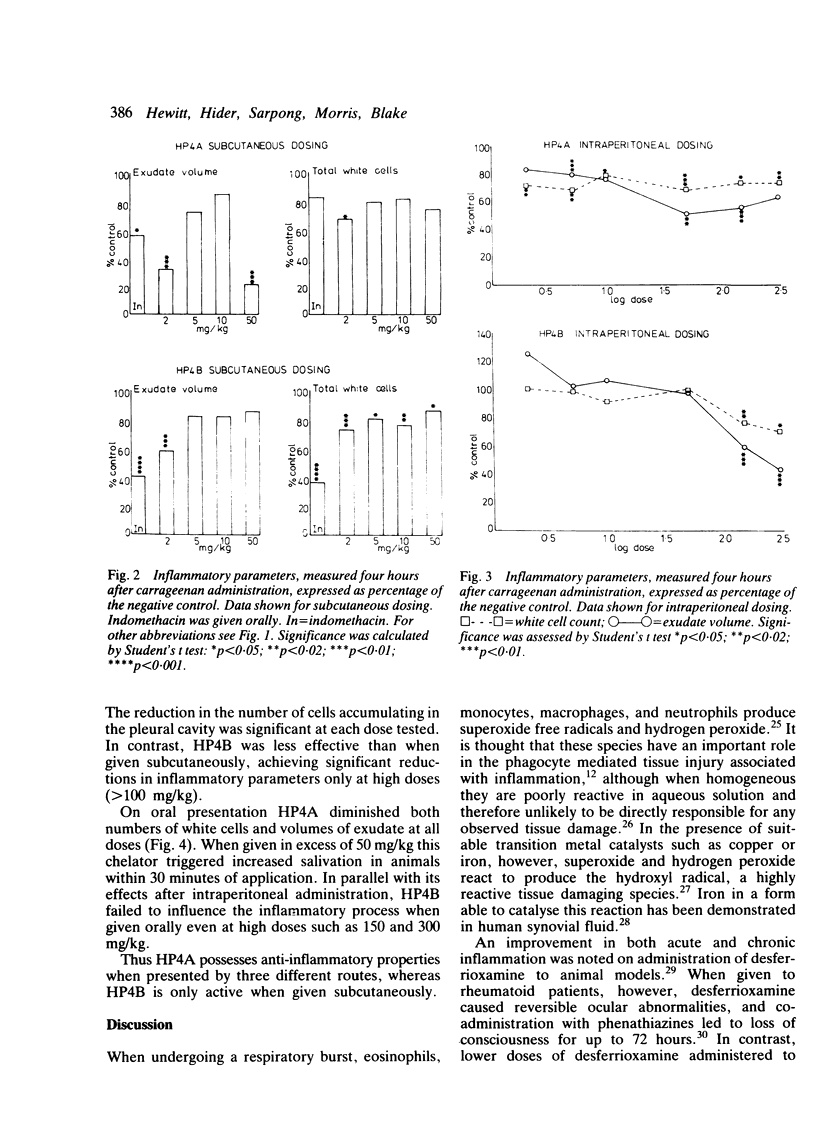

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews F. J., Morris C. J., Kondratowicz G., Blake D. R. Effect of iron chelation on inflammatory joint disease. Ann Rheum Dis. 1987 Apr;46(4):327–333. doi: 10.1136/ard.46.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Wernicke J. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J Biol Chem. 1971 Jun 10;246(11):3679–3685. [PubMed] [Google Scholar]
- Blake D. R., Bacon P. A. Synovial fluid ferritin in rheumatoid arthritis: an index or cause of inflammation? Br Med J (Clin Res Ed) 1981 Jan 17;282(6259):189–189. doi: 10.1136/bmj.282.6259.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Gallagher P. J., Potter A. R., Bell M. J., Bacon P. A. The effect of synovial iron on the progression of rheumatoid disease. A histologic assessment of patients with early rheumatoid synovitis. Arthritis Rheum. 1984 May;27(5):495–501. doi: 10.1002/art.1780270503. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Bacon P. A., Dieppe P. A., Halliwell B., Gutteridge J. M. Effect of a specific iron chelating agent on animal models of inflammation. Ann Rheum Dis. 1983 Feb;42(1):89–93. doi: 10.1136/ard.42.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Winyard P., Lunec J., Williams A., Good P. A., Crewes S. J., Gutteridge J. M., Rowley D., Halliwell B., Cornish A. Cerebral and ocular toxicity induced by desferrioxamine. Q J Med. 1985 Jul;56(219):345–355. [PubMed] [Google Scholar]
- Giordano N., Sancasciani S., Borghi C., Fioravanti A., Marcolongo R. Antianemic and potential anti-inflammatory activity of desferrioxamine: possible usefulness in rheumatoid arthritis. Clin Exp Rheumatol. 1986 Jan-Mar;4(1):25–29. [PubMed] [Google Scholar]
- Gyparaki M., Porter J. B., Hirani S., Streater M., Hider R. C., Huehns E. R. In vivo evaluation of hydroxypyridone iron chelators in a mouse model. Acta Haematol. 1987;78(2-3):217–221. doi: 10.1159/000205878. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978 Dec 15;96(2):238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Use of desferrioxamine as a 'probe' for iron-dependent formation of hydroxyl radicals. Evidence for a direct reaction between desferal and the superoxide radical. Biochem Pharmacol. 1985 Jan 15;34(2):229–233. doi: 10.1016/0006-2952(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Harvey A. R., Clarke B. J., Chui D. H., Kean W. F., Buchanan W. W. Anemia associated with rheumatoid disease. Inverse correlation between erythropoiesis and both IgM and rheumatoid factor levels. Arthritis Rheum. 1983 Jan;26(1):28–34. doi: 10.1002/art.1780260105. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J., Hoffbrand A. V. Orally active alpha-ketohydroxy pyridine iron chelators intended for clinical use: in vivo studies in rabbits. Br J Haematol. 1986 Apr;62(4):607–613. doi: 10.1111/j.1365-2141.1986.tb04082.x. [DOI] [PubMed] [Google Scholar]
- Lawson A. A., Owen E. T., Mowat A. G. Nature of anaemia in rheumatoid arthritis. VII. Storage of iron in rheumatoid disease. Ann Rheum Dis. 1967 Nov;26(6):552–559. doi: 10.1136/ard.26.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirden K. D. Ferritin in synovial cells in patients with rheumatoid arthritis. Ann Rheum Dis. 1966 Sep;25(5):387–401. doi: 10.1136/ard.25.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick A. D., Blake D. R., Winwood P., Moore A. R., Al-Duaij A., Willoughby D. A. Studies into the effects of the iron chelator desferrioxamine on the inflammatory process. Eur J Rheumatol Inflamm. 1984;7(3):87–94. [PubMed] [Google Scholar]
- Senator G. B., Muirden K. D. Concentration of iron in synovial membrane, synovial fluid, and serum in rheumatoid arthritis and other joint diseases. Ann Rheum Dis. 1968 Jan;27(1):49–54. doi: 10.1136/ard.27.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Hider R. C. Colorimetric detection of the hydroxyl radical: comparison of the hydroxyl-radical-generating ability of various iron complexes. Anal Biochem. 1988 May 15;171(1):47–54. doi: 10.1016/0003-2697(88)90123-6. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S., Blake D. R., Bacon P. A. The effect of desferrioxamine on antigen-induced inflammation in the rat air pouch. J Pharm Pharmacol. 1984 Aug;36(8):543–545. doi: 10.1111/j.2042-7158.1984.tb04448.x. [DOI] [PubMed] [Google Scholar]


