Abstract
Transmasculine individuals are often prescribed testosterone (T) for masculinizing hormone therapy. Mouse models mimicking transmasculine T therapy require reliable long-term T administration. The objectives of this study were three-fold, namely, to compare: 1) the release dynamics of three different subcutaneous delivery systems of T enanthate administration (subcutaneous injections, commercially available pellets, and silastic implants) over a 6-week period in postpubertal C57BL/6N mice, 2) to compare the timing for T levels in plasma to return to baseline and cyclicity to resume after cessation of T between injections and pellets, 3) to utilize silastic implants to achieve sustainable increase in T levels utilizing T enanthate and crystalline T. All three modes of T administration resulted in an increase in T levels in plasma. Pharmacokinetic analyses showed a similar overall exposure to T enanthate over 6 weeks (integrated area) for, subcutaneous injection (0.45 mg two times per week and 0.90 mg one time per week), pellet (5mg 60-day release), and silastic implant (5mg 21 week) groups. Crystalline T had lower solubility and a decreased integrated area compared to T enanthate, even when implanted at a higher dosage, indicating different pharmacokinetic profiles based on type of T formulation when utilizing the same silastic delivery method. Surgical removal of pellets and silastic tubing led to a quick drop in T levels and resumption of estrous cyclicity, while cessation of injections required a long washout period for T levels to drop and estrous cycles to resume. Sustained elevation in T levels was achieved for at least 21 weeks with silastic implants. As all three delivery methods are able to elevate T levels in female mice for at least 6 weeks, choice of T administration method should be based on outcomes of interest and study design.
Keywords: Testosterone, administration, estrous cycle, pharmacokinetics, reversibility, subcutaneous implants
1. Introduction
Individuals may identify as transgender or non-binary if their gender identity does not align with their sex assigned at birth. There are an estimated 1.4 million transgender adults in the United States (Flores et al. 2016), although this is most likely an underestimate due to the sensitive nature of disclosing one’s gender identity. Transmasculine individuals assigned female at birth are often prescribed testosterone (T) or T esters for masculinizing hormone therapy (Deutsch 2017 & T’Sjoen et al. 2019).
Routes of T administration for masculinizing hormone therapy include intramuscular and subcutaneous injections or transdermal application. Possible T esters that can be prescribed globally (Deutsch 2017) include T cypionate, T enanthate, and T undecanoate (Figure 1). Native T has a half-life of 10 minutes, while esters have a longer clearance due to a greater solubility in oil of the ester form. T cypionate is commercially dissolved in cottonseed oil, while T enanthate dissolved in sesame oil and T undecanoate is dissolved in refined castor oil (Figueiredo et al. 2021, Shoshkes et al. 2016, Matsumoto & Bremner 2016). Until recently, T undecanoate was unavailable in the United States and now can only be accessed through a restricted program (Deutsch 2017). Therefore, T enanthate and T cypionate are prescribed more readily and can be found more commonly, especially at United States Pharmacopeia (USP) grade (Deutsch 2017).
Figure 1.
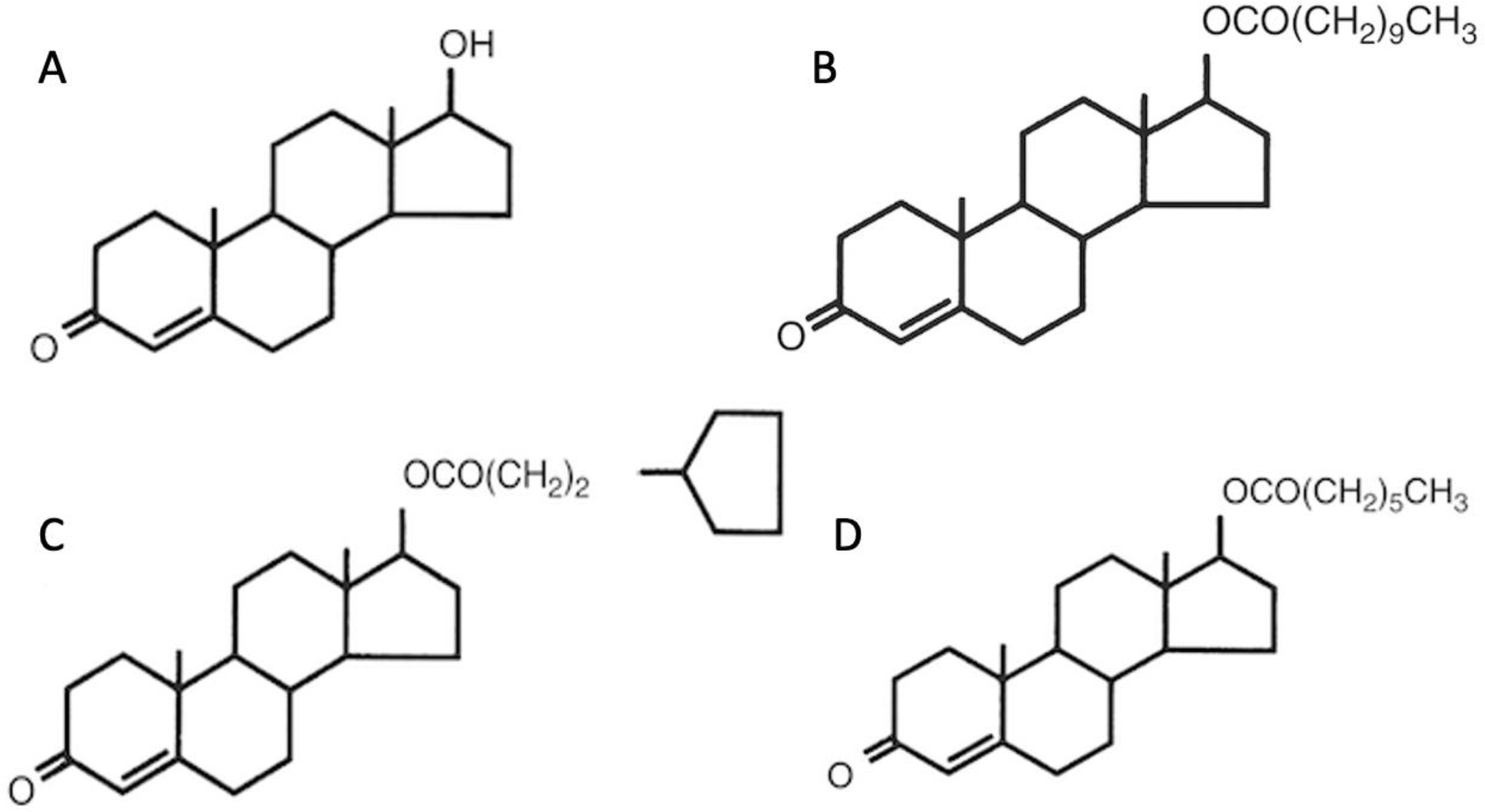
Chemical structure of Testosterone and Testosterone esters A) Testosterone B) Testosterone undecanoate C) Testosterone cypionate D) Testosterone enanthate.
The effects of T enanthate through injections (Kinnear et al. 2019) or silastic T implants (Aflatounian et al. 2020) on reproductive and metabolic changes, the resumption of cyclicity after cessation of short-term T enanthate pellet administration (Kinnear et al. 2021), and the impact of T cypionate injections on egg fertilization (Bartels et al. 2020) have been analyzed in animal models, including our previously published transgender mouse model (Kinnear et al. 2019). Our mouse model utilized subcutaneous T enanthate injections at an optimized dose of 0.45 mg two times per week over a 6-week period (5.4 mg total) to reproduce many of the key reproductive changes observed in transgender men on T including acyclicity, a reduction in luteinizing hormone levels, and polycystic ovary morphology without corpora lutea (Kinnear et al. 2019). Subsequent related studies have found no reduction in egg fertilization rate after 6 weeks of T cypionate injections at 0.4 mg weekly (2.4 mg total) (Bartels et al. 2020), and similar reproductive changes but reduced metabolic changes after 12 weeks of silastic implants (10 mg total) with T as compared to dihydrotestosterone (Aflatounian et al. 2021). Reversibility of T-induced changes has been shown using commercially available T enanthate pellets (5mg 60-day release) (Kinnear et al. 2021). Mice promptly resumed estrous cycles within a week of T pellet removal and were then sacrificed in diestrus after four estrous cycles. Histologic analyses of the ovaries showed comparable numbers of follicles and corpora lutea in control and T-treated mice post-T. There were also no significant differences in the terminal diestrus levels of luteinizing hormone, follicle-stimulating hormone, progesterone, or estradiol (Kinnear et al. 2021).
The differences in T administration regimens across these studies encouraged us to investigate more closely the pharmacokinetics of different T delivery systems. A study conducted in 2015 looked at the release dynamics of three different types of T implants in female Japanese quail (Quispe et al. 2015). Comparing time-release pellets, silastic tubing implants, and beeswax implants that contained T, researchers found that T levels were quite similar, however the temporal aspect of release dynamics and longevity of these implants varied based on implant type (Quispe et al. 2015). While previous studies have looked at the release dynamics of T administration in different animal models, there are currently no studies that compare the pharmacokinetics of multiple T administration methods in a mouse model relevant to a transgender population.
Understanding the temporal aspects and release dynamics of different methods of T administration will be useful for aligning method and study design. Subcutaneous injections utilize T, or a T ester dissolved in oil, and require regular administration. Commercially available pellets are inserted under the skin and can be tailored for overall dosage and duration. Silastic tubing allows for steroids to pass through at a constant rate proportional to the surface area of the implant after an initial period of high release (Frick et al. 1974; Kumar et al. 1981; Christensen et al. 1984; Fusani et al. 2008). Therefore, the goals of this study were to compare: 1) the release dynamics of three different modes of administration of T enanthate (subcutaneous injections, commercially available pellets, and silastic implants) over a 6-week period in postpubertal C57BL/6N mice, 2) to compare the timing for T levels to return to baseline and cyclicity to resume after cessation of T between injections and pellets, 3) to compare the release of T enanthate and crystalline T in silastic implants and see whether silastic implants can sustain elevated T levels for longer durations.
2. Material and Methods:
2.1. Ethical Approval
This study was conducted under Institutional Animal Care and Use Committee of the University of Michigan approved protocols (PRO00007618 & PRO00009635) and was in line with the requirements of National Research Council’s Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act.
2.2. Animal Housing
Female C57BL/6NHsd & Hsd:ICR mice (Envigo, Indianapolis, IN, USA) were housed in ventilated cages within a non-barrier access facility in groups of five at the University of Michigan, Ann Arbor. The animal facility had regular 12 hour light/dark cycles and animals had access to food and water ad libitum.
2.3. Experimental Design
Female mice were administered T enanthate either through subcutaneous injections, silastic implants, or commercially available time-release pellets. T levels were monitored weekly to compare different administration methods in terms of the rise and return to baseline of serum T levels. Commercially available pellets and injections were compared to determine the differences in reversibility of T-induced reproductive changes as previously found (Kinnear et al. 2021). Vaginal cytology was collected daily to track the disruption and resumption of estrous cyclicity, and the release dynamics were compared across cohorts over 6 weeks of T administration. Lastly, silastic implants were utilized to determine whether reliable, long-term T administration is possible. This is summarized in Table 1.
Table 1.
Summary of Administration Method, Dose, and Number of Mice.
| Administration Method | Dose | Number of Treated Mice | Number of Control Mice |
|---|---|---|---|
|
| |||
| Injections | 0.45 mg 1x/wk | 5 | 5 |
| Injections | 0.45 mg 2x/wk | 5 | 5 |
| Injections | 0.90 mg 1x/wk | 5 | 5 |
| Pellet | 5 mg, 60 day release | 5 | 5 |
| Pellet | 5 mg, 90 day release | 10 | 10 |
| Silastic Implant | 5 mg dissolved in sesame oil | 5 | 5 |
| Silastic Implant | 10 mg crystalline T 6 week | 10 | 10 |
| Silastic Implant | 10 mg crystalline T 12 week | 10 | 10 |
2.3.1. Subcutaneous Injections
Female C57BL/6NHsd mice 9–10-weeks old were subcutaneously injected mid-back with 0.45 mg once per week (n=5), 0.90 mg once per week (n=5), or 0.45 mg twice per week (n=5) T enanthate (Hikma Pharmaceuticals, Portugal) dissolved in sesame oil. Controls received equal volume of vehicle (sterile-filtered USP grade sesame oil, USP/NF grade, Welch, Holme & Clark Co., Inc., Newark, NJ, USA) (n=10 once per week and n=5 twice per week). Control mice were injected prior to treated mice to avoid accidental T exposure in controls. Daily vaginal cytology and weekly blood collection was performed throughout the experimental period. After 6 weeks, injections were discontinued.
2.3.2. Time-Release Pellets
Female C57BL/6NHsd mice 9–10-weeks old were surgically implanted subcutaneously on the upper back with slow-release pellets that either contained T enanthate (5mg/pellet, 60-day release, n=5 or 90-day release, n=10) or a placebo (n=5 for 60-day release; n=10 for 90-day release; Innovative Research of America, Sarasota, FL, USA). Implantation occurred under isoflurane anesthesia. After 6 weeks, pellets of control and experimental groups were surgically removed under isoflurane anesthesia. Daily vaginal cytology and weekly blood collection was performed throughout the experimental period and after implant removal to track T levels and follow resumption of estrous cyclicity. All control mice were implanted prior to experimental mice to avoid accidental T exposure in controls. This approach using a slow-release pellet that either contained T enanthate (5mg/pellet, 60-day release, n=5) or a placebo (n=5) has been previously published (Kinnear et al. 2021) and focused on correlating T cessation to the resumption of estrous cyclicity.
2.3.3. Silastic Tubing
Female C57BL/6NHsd mice 36–37 (n=10) weeks old underwent a surgical procedure under isoflurane anesthesia where a subcutaneous 16 mm silastic implant was placed on the back (Dow Corning, USA; Inner Diameter: 1.98 mm, Outer Diameter: 3.17 mm). Control implants were loaded with 25 μl (8 mm) sterile-filtered USP grade sesame oil (USP/NF grade, Welch, Holme & Clark Co., Inc., Newark, NJ, USA) and sealed with 4 mm of adhesive (Factor II, Incorporated, Lakeside, AZ, USA) at both ends. T implants were loaded with 5mg T enanthate dissolved in sesame oil (25 μl) and similarly sealed. Just before surgical implantation, implants were soaked in 200 proof ethanol (Decon Laboratories, King of Prussia, PA, USA) for three minutes and allowed to air dry prior to being placed in animals. Female mice received either a control implant (n=5) or a T implant (n=5). Implants were surgically inserted and replaced under isoflurane every seven weeks in mice 36–37 weeks old in order to maintain elevated T levels to study the effects of longer T administration with as little fluctuation in T levels as possible. T levels were collected for 21 weeks following the initial implantation.
In a separate set of experiments, female Hsd:ICR mice at 9–10 weeks old underwent a surgical procedure under isoflurane anesthesia where a subcutaneous 16 mm silastic implant was placed on the back (Dow Corning, USA; Inner Diameter: 1.98 mm, Outer Diameter: 3.17 mm). Control implants were loaded with 200 proof ethanol (Decon Laboratories, King of Prussia, PA, USA) and sealed with 4 mm of adhesive (Factor II, Incorporated, Lakeside, AZ, USA) at both ends. Ethanol was allowed to evaporate prior to implantation. T implants were loaded with 10 mg crystalline T dissolved in ethanol and similarly sealed. The ethanol was allowed to evaporate prior to implantation. Just before surgical implantation, implants were soaked in 200 proof ethanol (Decon Laboratories, King of Prussia, PA, USA) for three minutes and allowed to air dry prior to being placed in animals. Female mice were treated with T for either 6.5 weeks (n=10 control; n=10 T) or 13 weeks (n=10 control; n=10 T).
2.4. Protocol Violation
Two mice were sacrificed early due to vaginal prolapse and excluded from subsequent analysis: 0.45 mg 2x/wk (n=1); 5mg60d pellet (n=1).
2.5. Vaginal Cytology
Vaginal cytology was performed daily through lavage of sterile saline in the vaginal canal. Estrous cycles were tracked for at least two weeks prior to treatment, throughout the treatment period, and 4 cycles following resumption of cyclicity after T cessation. Cycle stage was determined based on the presence or absence of leukocytes, nucleated epithelial cells, and cornified epithelial cells (Cora et al. 2015).
2.6. Blood Collection/Hormone Analysis
The lateral tail vein was used to collect blood on a weekly basis. Weekly blood collection did not exceed 0.5% of animal body weight. Blood samples were stored overnight at 4°C and centrifuged the next day at 8100 G for 10 minutes. Serum was separated and kept at −20° C before sending samples out for analysis. Analysis of T levels was performed through the Ligand Assay and Analysis Core at the University of Virginia Center for Research in Reproduction. The reportable range with a coefficient of variation of <20% for the Testosterone Mouse and Rat enzyme-linked immunosorbent assay (Immuno-Biological Laboratories, Inc.; Minneapolis, MN, USA) is 0.10–16 ng/mL (or 0.20–32 ng/mL, with a 2x dilution). The intra-assay coefficient of variability is 6.4% and the inter-assay coefficient of variability is 9.7%.
2.7. Integrated Area
The integrated area was calculated for each experimental group over the six-week T administration period using Origin 2021. The maximum, minimum, and average serum T concentrations were calculated. Serum T concentrations were calculated during the first 6 weeks of T administration and included in subsequent analysis.
2.8. Statistical Analysis
GraphPad Prism 9 was used for statistical analysis, using a single mouse as the unit of analysis. The Shapiro-Wilk normality test was used to determine whether parametric (Paired t test) or non-parametric (Mann-Whitney test) testing was needed. P<0.05 was viewed as statistically significant. Hormone levels below and above the level of detection were assigned the lower and upper limits of quantification. Each experiment was conducted once using 5–10 biological replicates, as we calculated that we would need at least 5 mice to detect expected differences in T levels between T-treated mice and controls at 90% power.
3. Results
3.1. Pharmacokinetic Data Over a 6 Week Period
Pharmacokinetic data is summarized in Table 2. The cumulative T dosage over a 6 week period ranged from 2.7–5.4 ng/mL for all T enanthate cohorts and 10.0 mg for the two crystalline T cohorts. The integrated area (recorded in ng/mL) that denotes the body’s total exposure to T was comparable between most cohorts (Table 2) that received 5–5.4 mg of T enanthate (41.505–56.576). The one exception with this dosage was the cohort that received the 5 mg, 90-day release pellet, which spiked early and did not keep T levels elevated for 6 weeks. The cohort that received 2.7 mg had an integrated area of 21.869 ng/mL. The integrated area for the cohorts that received 10 mg of crystalline T ranged between 16.12–18.98 for the first 6 weeks of treatment. The mean T levels (ng/mL) of T-treated mice in each cohort over the first 6 weeks of T administration were as follows: 0.45 mg 1x/wk, 3.89; 0.45 mg 2x/wk, 9.05; 0.90 mg 1x/wk, 7.38; 5mg60dpellet, 9.83; 5mg90d pellet, 16.04; 5mg21wk silastic, 9.11, 10mg6.5wk silastic, 2.877; 10mg12wk silastic, 3.384.
Table 2.
Pharmacokinetics Comparison. Integrated Area, Cmean, Cmax, and Cmin (all in ng/mL) were calculated from average weekly T levels per group over the first 6 weeks of T administration.
| Dosage | Cumulative Dosage | Integrated Area (ng/mL) | Cmean | Cmax | Cmin | Number of Mice |
|---|---|---|---|---|---|---|
|
| ||||||
| 0.45 mg 1x/wk | 2.7 mg | 21.869 | 3.887 | 6.985 | 1.297 | 5 |
| 0.45 mg 2x/wk | 5.4 mg | 41.505 | 9.047 | 10.644 | 6.866 | 5 |
| 0.90 mg 1x/wk | 5.4 mg | 40.889 | 7.375 | 9.448 | 6.282 | 5 |
| 5mg60d pellet | 5.0 mg | 56.576 | 9.834 | 19.979 | 5.172 | 5 |
| 5mg90d pellet | 5.0 mg | 99.357 | 16.041 | 29.641 | 0.492 | 10 |
| 5mg21wk sesame oil silastic | 5.0 mg | 53.245 | 9.105 | 19.790 | 3.087 | 5 |
| 10 mg -short crystalline T | 10.0 mg | 16.118 | 2.877 | 3.622 | 2.647 | 10 |
| 10 mg - long crystalline T | 10.0 mg | 18.976 | 3.384 | 4.351 | 3.014 | 10 |
Blue shaded box refers to T enanthate administration groups and green shaded box indicates crystalline T administration. Cumulative dosage ranges between 2.7–5.4 mg for T enanthate groups and 10.0 mg for crystalline T groups. Integrated areas, indicating the body’s exposure to T, of T enanthate cohorts typically correspond to the cumulative dosage, except for 5mg-90 day release pellet that demonstrated high initial release and then fell to levels comparable to control mice during the 6 weeks of T treatment. Cmean indicates the average T levels over 6 weeks of treatment. Cmin refers to the lowest average T level during 6 weeks of T administration while Cmax refers to the highest average T level in each group. The last column indicates how many T-treated mice were used in each cohort.
T Levels were measured weekly one week before starting T therapy, throughout T therapy, and up to the time of sacrifice following the end of T therapy. All three modes of administration (injections, pellets, and silastic implants) increased average T levels over 6 weeks (mean ± SD; ng/mL) as compared to controls; injections: 0.45 mg 1x/wk (3.4 ± 2.1 vs. 0.37 ± 0.06, p=0.0006), 0.45 mg 2x/wk (6.9 ± 4.5 vs. 0.6 ± 0.05, p=0.03), 0.90 mg 1x/wk (6.4 ± 2.9 vs. 0.37 ± 0.06, p=0.002); pellets: 5mg60day (8.6 ± 6.1 vs. 0.2 ± 0.04, p=0.009), 5mg90day (13.8 ± 12.9 vs. 0.4 ± 0.3, p=0.03); silastic tubing: 5 mg (7.9 ± 7.1 vs. 0.4 ± 0.2; p=0.004), 10mg6.5wk (2.5 ± 1.0 vs. 0.31 ± 0.09; p=0.007), 10mg12wk (3.0 ± 1.2 vs. 0.3 ± 0.07; p=0.0006). For T enanthate administration, pellets and silastic implants had a higher sustained initial spike in T levels compared to injections (Figure 2, Table 2). As seen in Table 2 (denoted as Cmin and Cmax), T levels for all injection mice ranged from 1.30 ng/mL to 9.05 ng/mL. In comparison, pellet mice had T levels ranging from 5.17 ng/mL to 19.98 ng/mL. Silastic tubing mice had T levels from 3.09 ng/mL to 19.87 ng/mL. For mice that received crystalline T through silastic tubing, the T levels ranged from 2.65 ng/mL to 4.351 ng/mL. The mean serum concentration over the 6-week period is summarized in Table 2, denoted as Cmean.
Figure 2.
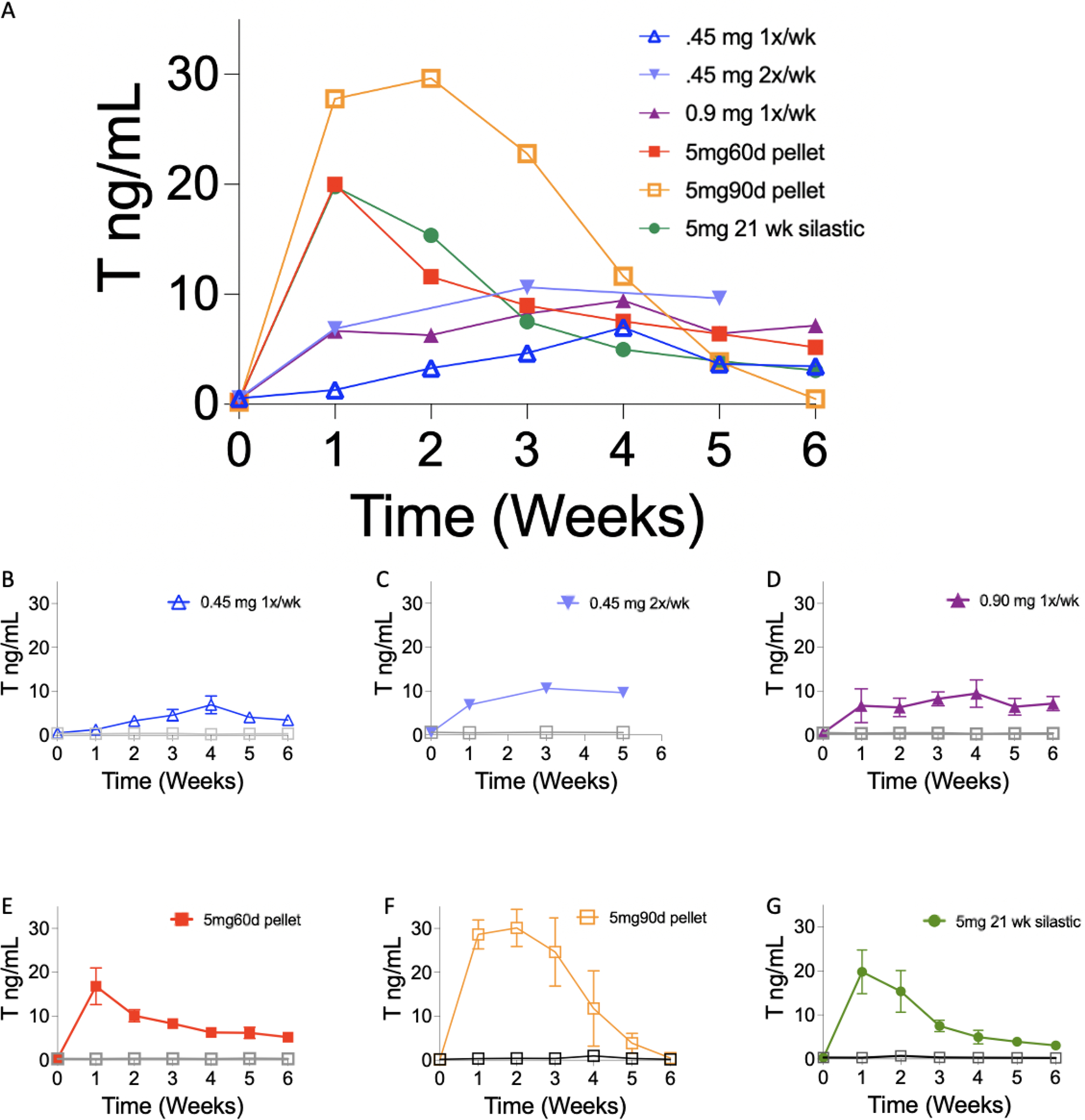
Longitudinal weekly T enanthate levels (A) Average T levels (ng/mL; mean ± SD) in serum after subcutaneous injections of 0.45 mg once per week (blue; B), 0.45 mg twice per week (light blue; C) 0.90 mg once per week (purple, D), of T enanthate dissolved in sesame oil, subcutaneous implantations of pellets with T enanthate (5mg/pellet, 60-day release, red; E, or 90-day release, orange; F) and silastic tubing loaded with 5mg T enanthate dissolved in sesame oil(green; G) in all cohorts from Week 0 (prior to T treatment) and through 6 weeks of T treatment. (B-G) Individual cohorts of mice. Controls are depicted as gray squares (mean ± SD, error bars that are shorter than symbol are not denoted). T levels for 0.45 mg twice per week (light blue) were measured at week 0 and 1 and subsequently every other week as this study had a long total duration after T cessation as seen in Figure 3.
3.2. T Level Reversal and Resumption of Estrous Cyclicity after T Cessation
Oil injections led to a longer washout period following the last injection as compared to pellet removal (Kinnear et al. 2021). T levels of 5mg60d pellet mice were comparable to controls within one week after implant removal (average T level of treated = 0.51 ng/mL; average T level of controls = 0.47 ng/mL; p=0.511). The T levels of the 0.45 mg 2x/wk injection mice were still significantly different as compared to controls at 21 weeks, a total of 15 weeks after the last injection (average T levels of treated = 1.01 ng/mL; average T level of controls = 0.33 ng/mL; p=0.001) (Figure 3).
Figure 3.
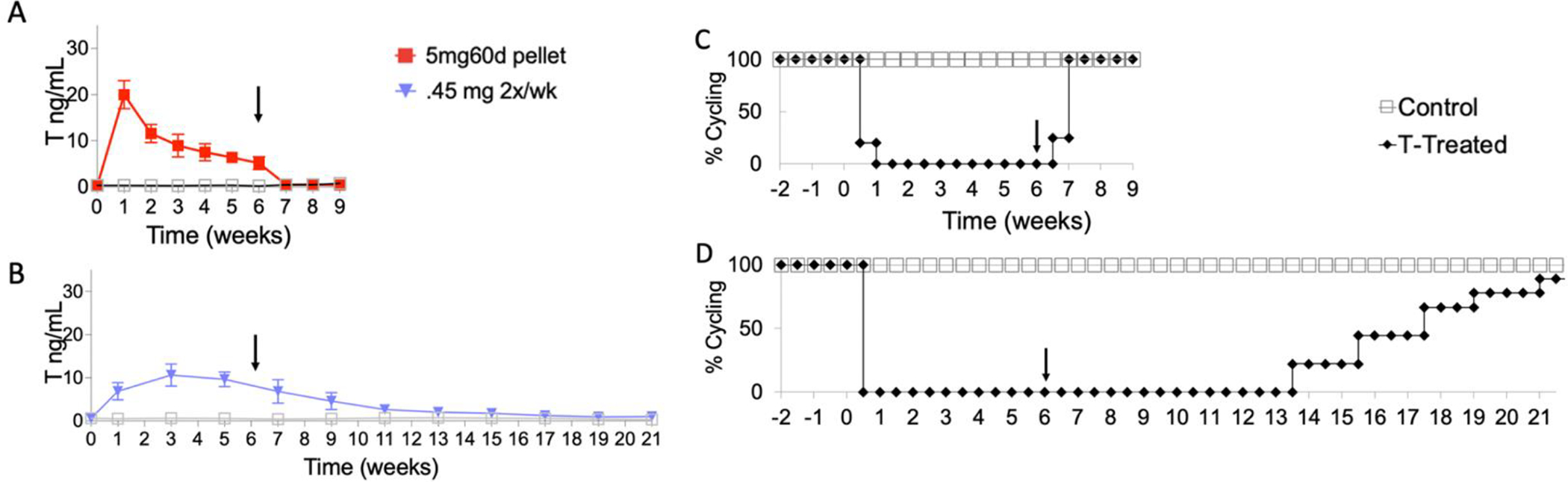
T-Induced changes and reversibility of estrous cycling vary based on administration method. Black arrow points to the end of T administration. T levels are shown are depicted as mean ± SD. Controls are depicted as gray squares. (A) T levels (ng/mL) in female mice treated with a 5mg 60 day release pellet. At pretreatment (Week 0), all mice were comparable to controls. Within one week, T levels rose and were in a physiologically male range until the end of treatment (Week 6). After explantation (Week 6), T levels dropped and were comparable to controls. (B) T levels in female mice receiving 0.45 mg T enanthate, twice per week for 6 weeks. At pretreatment (Week 0), all mice were comparable to controls. Within one week, T levels rose and were in a physiologically male range until the end of treatment (Week 6). T levels subsequently remained elevated until week 21. (C-D) All T treated mice stopped cycling within one week after beginning T treatment and remained in persistent diestrus. (C) 100% of mice treated with the pellet resumed cycling within one week of ending treatment (Week 6). (D) Mice receiving twice weekly injections took 7–16 weeks after ending treatment to resume cycling.
Vaginal cytology was performed daily to track estrous cyclicity. Mice treated with T stopped cycling and remained in persistent diestrus throughout the treatment period. Age-matched controls regularly cycled throughout the study. Mice resumed cycling after the cessation of T therapy, although the time needed for resumption of cyclicity varied based upon T administration method (Figure 3). Mice who received 5mg, 60-day release pellets showed a quick reversal of T levels as well as resumption of estrous cyclicity within one week following surgical removal of pellets, while mice that received 0.45 mg injections twice per week had a longer washout period lasting 7–15 weeks after the last injection before regular estrous cycle resumed. T levels and cyclicity data for the 5mg, 60-day release pellet cohort was previously published in a study that focused on correlating T cessation to the resumption of estrous cyclicity (Kinnear et al. 2021).
3.3. Long-Term T Administration
Silastic tubing implants loaded with 5 mg T enanthate dissolved in sesame oil were replaced every 7 weeks to investigate their suitability for long-term administration. Analyses of the weekly T levels (Figure 4A) demonstrated that the T levels were still significantly elevated after 7 weeks (mean ± SD) as compared to controls (Week 7: treated: 2.15 ± 0.4 ng/mL versus controls: 0.21 ± 0.03 ng/mL, p=.000015; Week 13: treated: 4.74 ± 1.5 ng/mL versus control: 0.34 ng/mL ± 0.2, p=.000028; Week 21: treated: 1.87 ± 0.5 ng/mL versus control: 0.34 ± 0.1 ng/mL, p= 0.00022).
Figure 4.
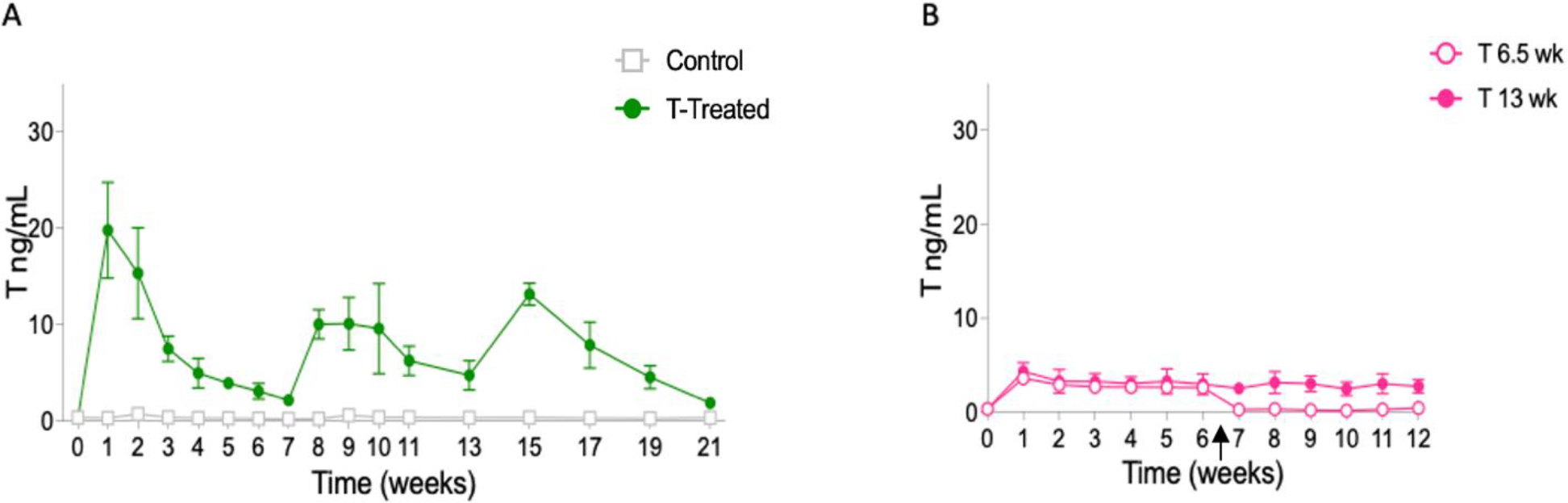
Silastic implants allowed for long-term T administration. (A) Average longitudinal weekly (Weeks 0–11) and biweekly (13–21) T levels (ng/mL; mean ± SD) for female C57BL/6N mice implanted with silastic tubing filled with 5 mg T enanthate or a sesame oil vehicle control. Implants were administered under anesthesia and replaced every 7 weeks (weeks 0, 7, 14). T levels were tracked on a weekly or biweekly basis and remained elevated in T-treated mice through the 21 weeks of treatment. (B) Silastic implants with crystalline T provide stable and sustained release with rapid washout. Average longitudinal weekly T levels (ng/mL; mean ± SD). Female Hsd:ICR mice were implanted with silastic tubing filled with 10 mg crystalline T or a blank control implant. Implants were administered and removed under anesthesia. T levels remained elevated through 12 weeks of treatment (solid fill) and promptly dropped to T levels comparable to controls within one week of removal at 6.5 weeks (outline fill). Arrow indicates when implants were removed for 6.5 weeks on T group.
3.4. Increased Stability with Crystalline T
Previous experiments showed that T enanthate delivered through silastic tubing provides a more sustained release compared to injections and pellets, however the need to replace implants can be detrimental to long-term studies. Silastic implants loaded with 10 mg of crystalline T and implanted in Hsd:ICR mice provided sustained release for extended periods of time, without replacement (Figure 4B). Elevated T levels (mean ± SD) were observed up to for 12 weeks (average treated: 2.8 ± 0.7 ng/mL versus average control: 0.40 ± 0.2 ng/mL, p=0.01). Importantly, after removal of implants, T levels decreased within one week to reach levels comparable to controls.
4. Discussion
This study compared three subcutaneous delivery methods of T: injections, slow-release pellets, and silastic tubing implants. The integrated area indicates that the body’s overall exposure to T enanthate over 6 weeks did not differ across administration methods (Table 2), when similar cumulative dosage was administered (Injections: 0.45 mg 2x/wk, 0.90 mg 1x/wk; Pellet: 5mg60d; Silastic Implants: 5mg21wk). Similarly, when the cumulative dosage was about half compared to what other cohorts received, the integrated area was also about half of what was seen in other cohorts. The exception was the 5mg90d pellet that had a much higher integrated area. This method of administration also failed to keep T levels elevated for the entire 6 weeks (Figure 2).
Pellets and silastic implants with T enanthate in sesame oil led to prolonged spikes in serum levels of T that were not observed with injections. In contrast, oil depots left behind by injections resulted in an extended washout period which was eliminated or significantly shortened when subcutaneous implants or pellets were surgically removed, indicating that different administration methods can be better suited for different experimental designs. The initial spike in serum T levels was also not observed with crystalline T in silastic tubing. Notably, this approach provided a consistent and sustained release pattern, when followed for up to 12 weeks (Figure 4B) of T administration.
Each of the administration methods are advantageous for different applications and desired outcomes (Table 3). Slow-release pellets are expensive; however, they are commercially available. Silastic implants and injections are made or diluted in-house and are more economical. For researchers interested in studying reversibility of T induced changes, such as the study conducted by Kinnear et al. 2021, removable implants are a better choice compared to injections. Injections can be used for smaller animals as they are non-invasive and can be adjusted for smaller volumes and lower doses where quick reversal of T levels and resumption of cyclicity are not required.
Table 3.
Comparing Administration Methods.
| Administration Method | Elevates T Levels for at least 6 weeks | Reversible | Ease of Administration | Cost per Dose | Accessibility | Considerations |
|---|---|---|---|---|---|---|
|
| ||||||
| Injections | Yes | Not reliable | SQ (noninvasive) | $2 | Make in-house | Long washout period |
| Pellets | Yes | Quick reversal | SQ (noninvasive) | $50 | Commercially available | Variability between lots; prolonged initial T spike |
| Silastic Implants | Yes | Quick reversal | SQ (noninvasive) | $0.50 | Make in-house | Prolonged initial T spike for T enanthate; need to replace implants for longer administrations for T enanthate |
All three subcutaneous administration methods elevated T levels in female mice. Reversibility and time for resumption of estrous cyclicity varied. Silastic implants and injections are less expensive than commercially available options. Cost per dose includes total cost over 6-week period: 6 injections (double cost for twice per week injections), 1 pellet, or 1 silastic implant.
Several studies have looked at the reproductive implications of T administration and the reversibility of T induced changes (Kinnear et al. 2021; Bartels et al. 2020; Kinnear et al. 2019). These studies have looked at shorter-term T administration averaging around 6 weeks. Researchers pursuing longer-term administration may be interested in the use of silastic implants. Our study demonstrated that T levels can be elevated for up to 21 weeks by swapping silastic implants containing 5 mg of T enanthate every seven weeks (Figure 4). Although there is a prolonged T spike in the first week following implantation, successive implantations produced slightly lower post-implantation spikes. During successive implantations, implants were inserted in slightly different locations subcutaneously to minimize impact of scar tissue from previous implants. Notably, silastic implants made with 10 mg of crystalline T allowed T levels to remain stably elevated for at least 12 weeks without need for replacement and without an initial T spike.
This study found similar integrated areas over 6 weeks when comparable dosages were used for three different delivery systems of T (injections, commercially available pellets, and silastic implants). T levels promptly returned to baseline with resumption of regular estrous cyclicity after surgical explant of pellets and silastic tubing in contrast to oil injections, likely due to the subcutaneous oil pocket buildup seen with injections. Silastic implants with T enanthate in oil were able to elevate T levels for up to 21 weeks when replaced every 7 weeks, indicating that long-term T administration is possible in a mouse model. Notably, long-term administration for up to 12 weeks was improved using silastic implants made with crystalline T. Future studies will look at utilizing silastic implants with crystalline T for durations greater than 12 weeks as well as repeating studies with crystalline T in C57BL/6N mice in addition to Hsd:ICR mice. Multiple doses of crystalline T in silastic tubing can be further evaluated to see whether their pharmacokinetic profile is impacted.
Acknowledgements
The authors thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for performing hormone analyses.
Funding
Supported by National Institutes of Health (R01-HD098233 to M.B.M., F30-HD100163 and T32-HD079342 to H.M.K.), American Society for Reproductive Medicine/Society for Reproductive Endocrinology and Infertility grant to M.B.M., University of Michigan Office of Research funding (U058227) to A.S., and Michigan Institute for Clinical & Health Research (KL2 TR 002241 and UL1 TR 002240) to C.D.C. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) grant (P50-HD28934 & R24-HD102061).
Footnotes
Competing Interests
The authors do not have any competing interests to disclose.
References
- 1.Flores A, Herman J, Gates G, Brown T 2016. How many adults identify as transgender in the United States? Available from: https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- 2.Deutsch M 2016. Overview of masculinizing hormone therapy. UCSF Transgender Care. Available from: https://transcare.ucsf.edu/guidelines/masculinizing-therapy [Google Scholar]
- 3.T’Sjoen G, Arcelus J, Gooren L, Klink DT, Tangpricha V, 2019. Endocrinology of Transgender Medicine. Endocr. Rev. 40, 97–117. 10.1210/er.2018-00011 [DOI] [PubMed] [Google Scholar]
- 4.Figueiredo MG, Gagliano-Juca T, Basaria S 2021. Testosterone Therapy With Subcutaneous Injections: A Safe, Practical, and Reasonable Option. JCEM. 107(3): 614–626. 10.1210/clinem/dgab772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoskes JJ, Wilson MK, Spinner ML, 2016. Pharmacology of testosterone replacement therapy preparations. Transl. Androl. Urol. 5, 834–843. 10.21037/tau.2016.07.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto AM & Bremner WJ 2016. Chapter 19 - Testicular Disorders. Williams Textbook of Endocrinology. Thirteenth Edition, 694–784. 10.1016/B978-0-323-29738-7.00019-8 [DOI] [Google Scholar]
- 7.Kinnear HM, Constance ES, David A, Marsh EE, Padmanabhan V, Shikanov A, Moravek MB, 2019. A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Hum. Reprod. 34, 2009–2017. 10.1093/humrep/dez177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels CB, Uliasz TF, Lestz L, Mehlmann LM, 2020. Short-term testosterone use in female mice does not impair fertilizability of eggs: implications for the fertility care of transgender males. Hum. Reprod. 1–10. 10.1093/humrep/deaa282 [DOI] [PubMed] [Google Scholar]
- 9.Quispe R, Trappschuh M, Gahr M, Goymann W, 2015. Towards more physiological manipulations of hormones in field studies: Comparing the release dynamics of three kinds of testosterone implants, silastic tubing, time-release pellets, and beeswax. Gen. Comp. Endocrinol. 212, 100–105. 10.1016/j.ygcen.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 10.Frick J, Marberger M, Marberger H, 1974. Hormonal therapy with steroid filled silastic rubber implants. Urol. Int. 29, 81–92. 10.1159/000279896 [DOI] [PubMed] [Google Scholar]
- 11.Kumar D, Farooq A, Laumas KR, 1981. Fluid-filled silastic capsules: A new approach to a more constant steroidal drug delivery system. Contraception 23, 261–268. 10.1016/0010-7824(81)90048-2 [DOI] [PubMed] [Google Scholar]
- 12.Christensen DA, Kesler DJ, 1984. Passage of testosterone, testosterone propionate and testosterone enanthate from silastic implants and the retention of testosterone once it enters the blood of ewes. Anim. Reprod. Sci. 7, 531–536. 10.1016/0378-4320(84)90058-7 [DOI] [Google Scholar]
- 13.Fusani L, 2008. Endocrinology in field studies: Problems and solutions for the experimental design. Gen. Comp. Endocrinol. 157, 249–253. 10.1016/j.ygcen.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 14.Kinnear HM, Hashim PH, Dela Cruz C, Rubenstein G, Chang FL, Nimmagadda L, Brunette MA, Padmanabhan V, Shikanov A, Moravek MB, 2021. Reversibility of testosterone-induced acyclicity after testosterone cessation in a transgender mouse model. F&S Sci. 2, 116–123. 10.1016/j.xfss.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aflatounian A, Edwards MC, Rodriguez Paris V, Bertoldo MJ, Desai R, Gilchrist RB, Ledger WL, Handelsman DJ, Walters KA, 2020. Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. J. Endocrinol. 245, 381–395. 10.1530/JOE-19-0530 [DOI] [PubMed] [Google Scholar]
- 16.Cora MC, Kooistra L, Travlos G, 2015. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 43, 776–793. 10.1177/0192623315570339 [DOI] [PMC free article] [PubMed] [Google Scholar]


