Abstract
Purpose:
To review the current literature on the use of intravitreal methotrexate (IVT MTX) for the treatment and prevention of proliferative vitreoretinopathy (PVR).
Methods:
All reports of IVT MTX to treat and prevent PVR published in PubMed, Google Scholar, and EBSCOhost were reviewed. The relevant current studies are included in this report.
Results:
The literature search yielded 32 articles describing the use of MTX in PVR. These included preclinical studies, 1 case report, and several case series. Early studies found that IVT MTX is a promising medication for the treatment and prevention of PVR. MTX works as a potent anti-inflammatory agent through a new mechanism of action different from that of other medications for use in PVR. Few side effects have been reported and were mostly limited to mild reversible corneal keratopathy. There are 2 current ongoing randomized controlled clinical trials to further evaluate the efficacy of MTX for PVR.
Conclusions:
MTX is a safe and potentially efficacious medication for the treatment and prevention of PVR. Additional clinical trials are needed to further establish this effect.
Keywords: methotrexate, intravitreal methotrexate, IVT methotrexate, IVT MTX, proliferative vitreoretinopathy, PVR, intraocular methotrexate, intra-silicone methotrexate
Introduction
Proliferative vitreoretinopathy (PVR) is a sight-threatening condition characterized by the proliferation of retinal fibrovascular membranes after retinal detachment (RD) surgery. It is a multifactorial scarring process that results in the growth and contraction of fibroglial material within the vitreous, on both surfaces of the retina, and within the retina itself. 1
The hallmarks of the pathophysiology of PVR include (1) migration of proinflammatory, cytokine-producing, and retinal pigment epithelial (RPE) cells, (2) cytokine-induced metaplastic RPE changes resulting in enhanced RPE contractile properties and proliferation of glial cells, and (3) fibrocellular proliferation of contractile membranes. The result is vitreoretinal traction and RD.2,3 The clinical presentation ranges from formation of cell debris within the vitreous to full-thickness retinal folds and tractional RD (TRD). 4
PVR is elicited by various intraocular stimuli but most notably occurs 30 to 90 days after rhegmatogenous RD (RRD), with an incidence of 5% to 10% in such cases.5,6 Despite improvements in retina surgical technology and technique, PVR remains the most common cause of failure in repair of RRD, accounting for 75% of retinal redetachment surgeries. 5 There are no current established medications for the treatment or prevention of PVR; surgical removal of the membranes remains the mainstay of treatment. Given the common occurrence of this devastating complication, there is particular research interest in pharmaceutical agents that might aid in the treatment and prevention of PVR.
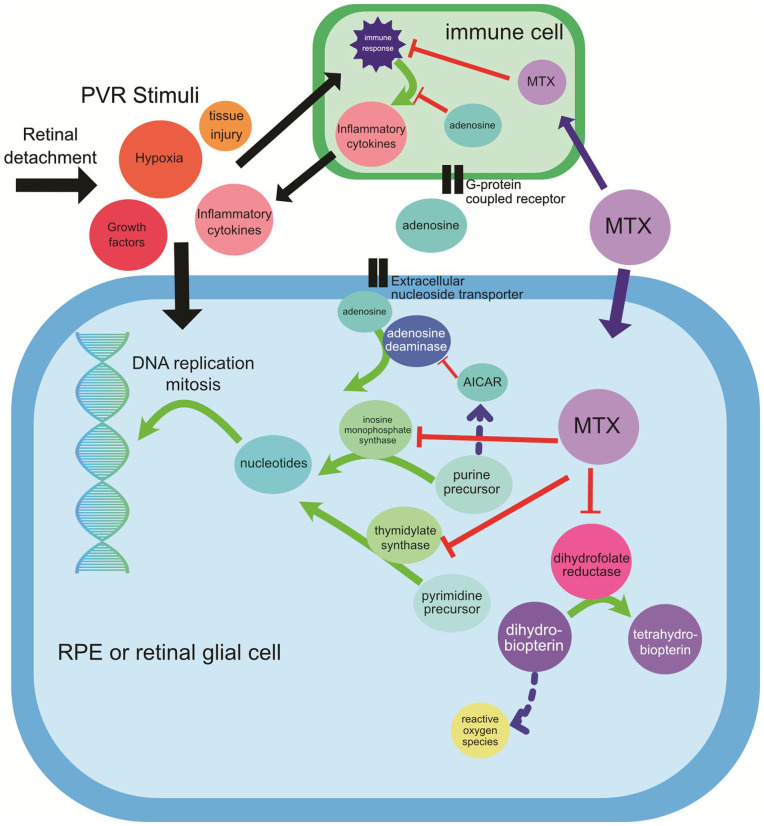
Methotrexate (MTX), a folate antagonist, is one of several treatment modalities that have been studied for the treatment of PVR. It first received US Food and Drug Administration approval in 1953 as a chemotherapeutic agent and later to treat autoimmune disorders. Through the inhibition of the enzymes dihydrofolate reductase and aminoimidazole carboxamide ribonucleotide transformylase, MTX decreases DNA replication and cell proliferation and stimulates adenosine release, a potent anti-inflammatory agent7–10 (Figure 1).
Figure 1.
MTX and adenosine signaling. Extracellular signals from retinal detachment stimulate an immune cascade and a proliferation signal of RPE cells and RGCs important in PVR pathogenesis. 10 MTX blocks the enzyme inosine monophosphate synthase (also known as aminoimidazole carboxamide ribonucleotide transformylase), resulting in elevated intracellular AICAR levels, leading to increased intracellular adenosine. 8 Adenosine is transported out of the cell by the extracellular nucleoside transporter, where it might act on G-protein coupled receptors and/or be transported intracellularly to inhibit proinflammatory cytokine release in immune cells. MTX also blocks thymidylate synthase and reduces proliferation of RPE cells and RGCs in the formation of PVR. Finally, MTX inhibits dihydrofolate reductase.
Abbreviations: AICAR, amidoimidazole carboxamide ribonucleotide; MTX, methotrexate; PVR, proliferative vitreoretinopathy; RGCs, retinal glial cells; RPE, retinal pigment epithelium.
Previous studies found that an intraocular dose of less than 400 µg/0.1 mL MTX acts on 2 hallmarks of the pathophysiology of PVR by neutralizing both retinal glial elements and the induction of RPE and decreasing the proliferation of cells with myocontractile properties without a significant effect on cell migration. 2 Rheumatoid arthritis models showed that the specific downstream effects of the adenosine pathway include decreased neutrophil adhesion and macrophage giant cell formation, inhibition of reactive oxygen species (ROS) production, and decreased proinflammatory cytokine production. The effects on lymphocytes include inhibition of T-cell activation and T-cell–induced apoptosis, inhibition of Fas ligand–mediated cell death in CD4 cells, decreased leukocyte recruitment, and increased T-regulatory cells that decrease T-cell activation.8,9 Notably, this mechanism of action is not known to appreciably occur with other studied medications used to treat PVR (eg, corticosteroids).
The reported side effects of intravitreal MTX (IVT MTX) are few. This is in contrast to other medications used to prevent PVR (ie, corticosteroids and 5-fluorouracil [5-FU]), which are associated with significant intraocular toxicity.
IVT injection of MTX has been used to treat several intraocular disorders, including intraocular lymphoma, noninfectious uveitis, complex RD, age-related macular degeneration, diabetic macular edema and TRD, epithelial downgrowth, recalcitrant epiretinal membranes, anterior segment fibrosis, and retinoblastoma.5,7,11,12 Here, we review the current literature on IVT MTX to treat and prevent PVR.
Methods
A PubMed, Google Scholar, and EBSCOhost search was performed in May 2022 using the keywords “intravitreal methotrexate,” “intraocular methotrexate,” “intravitreal MTX,” “intraocular MTX,” “intravitreal methotrexate and proliferative vitreoretinopathy,” and “IVT MTX and PVR” in various combinations. Case reports, case series, original articles, reviews, and clinical trials discussing the use of MTX in the treatment of PVR were included. Articles were excluded if they were abstract-only articles or if they were in non-English languages for which no English translation was readily available. There were no publication date limitations. The authors thoroughly reviewed all articles included in the study.
Results
Using the aforementioned keywords, 430 articles were identified, 31 of which described the use of IVT MTX for PVR. The articles ranged from preclinical studies to case reports and case series to clinical trials. Table 1 shows an overview of the major studies discussed in this review.2,5,13–20
Table 1.
Major Studies of IVT MTX for PVR.
| Study a | Study Type | Groups | Outcome Measures | Results/Conclusions |
|---|---|---|---|---|
| Sunalp et al
13
PMID 6245019 |
Preclinical; rabbit dermal fibroblast injection in rabbit eyes | • BSS • Triamcinolone • Adriamycin (doxorubicin) • 5-FU • MTX • Trifluridine |
• Percentage of animals with RD after treatment | • Significantly fewer RDs in adriamycin, triamcinolone, and 5-FU
treated eyes • No benefit to MTX or trifluridine treatment |
| Eliott and Stryjewski
14
US Patent 10,272,089 |
Preclinical; in vitro assays of cells derived from PVR extracted during human retina surgery | • Control (growth medium) • 400 µg MTX • 200 µg MTX • 100 µg MTX |
• Growth and cellular morphology after 72 h, 1 wk, and 2 wk | • 72 h: Similar growth of PVR cells in all groups with typical
epithelioid morphology • 1 week: All cells exposed to MTX showed growth inhibition, lack of confluency, and less epithelioid-appearing; continued growth in control plate • 2 wk: Similar findings to week 1 with continued growth in the control plates |
| Amarnani et al
15
PMID 28777835 |
Preclinical; in vitro assays of 6 PVR specimens extracted during human retina surgery | • Vehicle control vs MTX (100 µM, 200 µM, or 400 µM) treatment of PVR-derived cell cultures in vitro | • Cell density • Ki-67 immunofluorescence (proliferation marker) • Caspase-3/7 immunofluorescence (apoptosis marker) |
• Reduced cell density 6 wk after MTX treatment (not dose
dependent) • Reduced cell proliferation 24 h after MTX treatment (dose dependent) • Increased apoptosis 6 wk after MTX treatment (dose dependent) |
| Eliott and Stryjewski
14
US Patent 10,272,089 |
Prospective nonrandomized single-arm pilot study | • 10 patients requiring PPV for RD repair with serial postop IVT MTX injections | • Development of PVR • Rate of redetachment • Final visual acuity |
• 0/10 eyes developed PVR during injection period • 2/10 eyes had redetachment • Median postop visual acuity 20/200 • Mild corneal toxicity in 1/10 eyes |
| Benner et al
5
PMID 31179399 |
Retrospective chart review of series of patients with PVR | • Serial low-dose MTX IVT injection after RD surgery (5 patients) | • 6-month visual acuity • Re-detachment rate |
• Final VA range from 20/60 to HM • Redetachment in 0/5 patients • Mild corneal toxicity in 1/5 patients |
| Nourinia et al
16
PMID 28777835 [15] |
Prospective single-arm interventional pilot study | • 11 patients with RD and PVR, with 250 µg MTX injected into silicone oil after RD surgery, then repeat injections @ 3 wk + 6 wk | • Postop redetachment rate • Postop VA |
• 0/11 redetachment rate posteriorly; 2/11 peripheral retina
re-detachment rate • Improved mean postop logMAR VA: 1.02 ± 0.51 (postop) vs 2.62 ± 0.04 (preop) (P = .003) |
| Ghasemi Falavarjani et al
17
PMID 31136255 |
Prospective randomized comparative interventional | • Intra-silicone-oil injection of 250 µg MTX after RD surgery
(n, 22) • No MTX after RD surgery (n, 22) |
• Postop redetachment rate • Final postop VA |
• 1/22 (MTX) vs 5/22 (control) eyes had redetachment
(P = .18) • Final mean logMAR VA: 1.51 ± 0.63 (MTX) vs 1.57 ± 0.44 (control) (P = .77) |
| Roca et al
18
PMID 33900444 |
Retrospective case-control study of eyes with RD and PVR | • Eyes with weekly MTX injections • 9 control eyes |
• Postop reattachment rate • Final postop VA |
• 86% of MTX eyes completely reattached vs. 22% of control eyes
completely reattached (P = .0406) • Final VA (logMAR mean) 1.92 (MTX) vs 2.05 (control, P, NS) |
| Sadaka et al
19
PMID 27698550 |
Retrospective chart review of patients with RD having PPV; judged to be at risk for PVR | • 29 patients requiring PPV for RD repair with 40 mg MTX added to 500 mL BSS during PPV | • Improved or stable VA at 6 months • VA ≥ 20/200 • Recurrent PVR without RD • Redetachment requiring reoperation |
• 24/29 eyes with improved or stable VA • 19/29 eyes with VA ≥ 20/200 • 3/29 eyes with recurrent PVR without RD • 3/29 eyes with redetachment requiring reoperation |
| Jahangir et al
20
PMID 34462712 |
Prospective nonrandomized single-arm study | • 30 patients with or at risk for PVR requiring PPV for RD repair with MTX added to BSS bottle intraop | • Reattachment rate • Final VA |
• Retina remained reattached at 4 mo in 24/30 eyes • Postop logMAR BCVA 1.01 vs. 1.35 preoperative (P < .05) |
| El Baha et al
2
PMID 34336257 |
Prospective nonrandomized case series of MTX added to intraop BSS vs retrospective chart review of controls | • I: RD with PVR +MTX (n = 42) • II: Recent RD, high risk for PVR, +MTX (n = 35) • III: Recent RD, no risk for PVR, +MTX (n = 24) • Ia: Retrospective with established PVR (n = 30) • IIa: Retrospective with high risk for PVR (n = 30) • IIIa: Retrospective with no risk for PVR (n = 29) |
• Anatomic outcome of retinal reattachment • Final VA |
• Group I (31/42 reattachment rate) vs. Group Ia (26/30
reattachment rate) (P = .2) • Group II (27/35 reattachment rate) vs. Group IIa (28/30 reattachment rate) (P = .07) • Group III (23/24 reattachment rate) vs. Group IIIa (23/29 reattachment rate) (P = .07) • Group I (0.11 ± 0.15 BCVA) vs. Group Ia (0.04 ± 0.04) (P = .07) • Group II (0.15 ± 0.12 BCVA) vs. Group IIa (0.08 ± 0.06) (P = .03) • Group III (0.16 ± 0.14 BCVA) vs. Group IIIa (0.08 ± 0.05) (P = .03) |
Abbreviations: 5-FU, 5-fluorouracil; BCVA, best-corrected visual acuity; BSS, balanced salt solution; HM, hand motion vision; IVT, intravitreal; MTX, methotrexate; NS, not significant; PPV, pars plana vitrectomy; PVR, proliferative vitreoretinopathy; RD, retinal detachment; VA, visual acuity.
First author.
Preclinical Studies
In a 1984 study, Sunalp et al 13 reported the in vivo effects of cytotoxic drugs on PVR. They created an experimental model of PVR using dermal fibroblasts and rabbit eyes. The models were subjected to balanced salt solution (control), MTX, 5-FU, doxorubicin, triamcinolone, or trifluridine. MTX reduced the rate of RD (50% vs 63% in the control). However, the authors concluded that all studied drugs, except MTX, significantly inhibited fibroblast cell proliferation in vitro.
Eliott and Stryjewski 14 and Amarnani et al 15 isolated PVR membranes from human subjects and subjected them to MTX in vitro. Together, these studies showed that MTX inhibited growth, diminished cell confluency, altered cell appearance, reduced band formation, and promoted apoptosis in PVR. However, Amarnani et al showed that MTX did not have a significant effect on cell migration. Schulz et al 21 later performed an in vitro comparison between MTX and 5-FU using human RPE cells, fibroblasts, and photoreceptors. This study showed that MTX was capable of inhibiting RPE cells but, unlike 5-FU, was not associated with photoreceptor toxicity.
Clinical Studies
Intravitreal/Intra-silicone Oil Injections
Several studies have evaluated the role of multiple postoperative MTX injections for PVR. The half-life of intraocular MTX (5.9 to 7.6 hours in rabbit models)22,23 is relatively short in relation to the 30- to 90-day window of cell proliferation after RD.5,24 Multiple injections are therefore necessary to provide adequate coverage over the entire timespan that active proliferation is present in PVR.
Early clinical studies showed promise for MTX in treating PVR. In 2016, Eliott and Stryjewski 14 reported a case series of 10 IVT MTX injections (400 µg/0.1 mL) over a 3-month period. The series comprised 10 eyes with grade C PVR (or higher) or were at high risk for PVR. All eyes had retinal reattachment surgery. IVT MTX was administered intraoperatively and then weekly in the immediate postoperative period at weeks 1 through 8 and postoperatively at month 3 for a total of 10 IVT injections per eye. There was remarkable follow-up in this study in that all except 1 patient received all scheduled injections; the 1 patient missed only a single injection. After oil removal at 3 months, 80% of the retinas remained attached over a median follow-up of 25 months (range, 4-39 months).
In the Eliott and Stryjewski 14 study, the median preoperative visual acuity (VA) was hand motion (HM) at 2 ft (0.61 m). The final VA ranged from 20/70 to HM (median 20/200). The only reported adverse effects were mild superficial punctate keratopathy (SPK) and ocular hypertension in the same eye. The elevated intraocular pressure was postulated to be a result of steroid responsiveness. Before this study, Hardwig et al 25 described a single intracameral injection of MTX for PVR, which improved VA. These studies led to further investigations of MTX for PVR.
Benner et al 5 published a retrospective review of 5 patients with complex, recurrent RD complicated by severe PVR. All patients were treated with a relaxing retinectomy with perfluorocarbon (PFCL) and multiple MTX injections. Postoperatively, patients received 100 to 200 µg/0.05 mL MTX every 2 weeks for 5 injections and 1 patient received 12 injections (because of an ocular history of Vogt-Koyanagi-Harada [VKH] and retinopathy of prematurity [ROP]). All 5 retinas remained attached over a mean follow-up of 13.4 months (range, 7-23 months). The VA improved or remained stable (range 20/60 to 20/400). The MTX was tolerated well without significant complications. The VA in the patient with VKH and ROP remained at HM. This patient had prolonged hypotony after surgery, which the authors said was not likely caused by the MTX. One patient developed mild SPK that resolved in 2 weeks without treatment. The authors noted that no patient developed the significant postoperative inflammation often associated with PFCL.
Nourinia et al 16 also found the efficacy of multiple MTX injections for high-grade PVR. They reported a prospective case series of intra-silicone oil MTX injections for PVR. All 11 eyes had total RD with PVR grade C before surgery. Each eye had pars plana vitrectomy (PPV) and silicone oil placement followed by 250 µg MTX injections immediately postoperatively and at 3 weeks and 6 weeks. The retina in all eyes remained attached posteriorly over a mean follow-up of 9 months (range, 6-15 months). The retina detached again peripherally in 2 eyes but remained stable without posterior involvement. The mean VA logMAR improved to 1.02 ± 0.51 (~20/200 Snellen) from 2.62 ± 0.04 (~20/8300 Snellen). Again, no significant adverse effects were reported.
One of the first prospective studies comparing IVT MTX against a control group was performed by Falavarjani et al. 24 They recruited patients with RRD and grade C PVR who had vitrectomy with silicone oil. After the procedure, all patients received sub-Tenon triamcinolone; the experimental group also had a single intra-silicone oil injection of 250 µg MTX. There was a trend toward fewer retinal redetachments from PVR in the MTX group (1 of 22 patients) than in the control group (5 of 22 patients); however, the difference was not statistically significant. The time to redetachment after surgery was 8 months in the MTX group and 3 to 12 months in the control group. The difference did not achieve statistical significance (P = .18), partially because of the small sample.
Roca et al 18 performed a retrospective case-control study to evaluate serial postoperative IVT MTX injections vs controls. They evaluated 7 study eyes and 9 control eyes with grade C PVR who had PPV with silicone oil or perfluoropropane. The study group received 400 µg MTX at the end of surgery followed by repeat postoperative injections on a weekly basis for a mean of 6 total injections (range, 4-9 injections). At the final follow-up, 86% of study eyes and 22.2% of control eyes had complete retinal reattachment (P = .04). The change in best-corrected VA (BCVA) was not statistically significant. No adverse toxic effects occurred.
Denstedt et al 26 published a report of a single patient with a RD secondary to a full-thickness globe perforation from an acupuncture needle. The female patient had 2 vitrectomies with silicone oil instillation and developed recurrent PVR, which did not respond to IVT triamcinolone. She was started on 200 µg IVT injections of MTX every 2 to 3 weeks for 9 total treatments. Six months after initial presentation, there was no progression of PVR or tractional detachment. She later had a third vitrectomy for PVR removal with repeat silicone oil placement. At 15 months, the retina remained flat with stable residual fibrosis.
The use of MTX has also been studied in TRD. Ghasemi Falavarjani et al 17 found no risk reduction for retinal redetachment in cases of diabetic TRD with a single postoperative 250 µg MTX injection. In 1 study, a series of patients received MTX 200 to 400 µg for diabetic TRD or cystoid macular edema. They had mixed results in terms of improvement in VA.7,25
Intraoperative MTX Infusions
Three studies used continuous MTX infusions during PPV for the treatment and prevention of PVR after RD. The continuous infusion of MTX during surgery is postulated to load the intraocular tissues. This results in a depot of medication with a slow release during the postoperative period, obviating the need for repeat postoperative injections. 5
Sadaka et al 19 were the first to describe the intraoperative infusion of MTX. They infused the equivalent of 400 µg of MTX during vitrectomy in eyes that had or were at high risk for grade C PVR. Of the 29 eyes at the 6-month follow-up, 20% had recurrent PVR, 10% had recurrent RDs, and 83% had improved VA, with 66% improving to 20/200 or better. No adverse effects from MTX were noted. There was no reported issue of the MTX infusion disrupting the silicone oil tamponade for the retinal breaks/detachments.
Jahangir et al 20 performed a prospective interventional case series of 30 eyes to assess the intraoperative infusion of MTX for the treatment and prevention of PVR. Patients with open-globe trauma, recurrent RD, and grade C PVR were included. They delivered 400 µg of MTX by intraoperative infusion. The mean BCVA improved from 20/447 preoperatively to 20/204 at the 4-month follow-up. Also, 40% of patients achieved a postoperative BCVA of 20/100. Three patients (10.7%) developed recurrent PVR. Of the 30 eyes, 24 (80%) had an attached retina after a single surgery at 4 months. The success of retinal reattachment was 93.3% after a second surgery. No complications of intraocular MTX were observed.
Finally, El Baha et al 2 evaluated the intraoperative infusion of MTX for PVR in a comparative interventional nonrandomized study. All patients had vitrectomy for RRD, and 91% received silicone oil. Patients were placed in a study group or a control group for comparison based on the presence of established PVR before surgery or the risk for developing PVR postoperatively. Patients in the study group received a similar intraoperative infusion of 400 µg MTX.
In the El Baha et al 2 study, the median rate of retinal reattachment 6 months after a single procedure was 82% and 86% in the study group and control group, respectively, although the difference did not reach statistical significance (P = .08). In addition, there was a statistically significantly greater 6-month survival rate for retinal attachment in the control group in patients at high risk for PVR (P = .003). The BCVA improved by an average of 4 lines at 6 months in the study groups but was only statistically significant in patients at high risk for PVR (P = .03), not in those with established PVR or who were at low risk for PVR. No complications attributable to MTX were reported. The authors concluded that MTX infusions prevent PVR and might be an efficacious alternative to repeat IVT injections.
These 3 infusion studies again showed the safety and possible efficacy of intraocular MTX for PVR and further highlight the need for a prospective randomized clinical trial.
Clinical Trials
Gain Understanding Against Retinal Disease (GUARD) (NCT04136366) is a multicenter randomized controlled phase 3 clinical trial evaluating IVT MTX 0.8% for preventing recurrent RD caused by PVR. 27 In part 1, the trial enrolled 110 patients with at least 3 clock hours of PVR or those with RD secondary to open-globe injury. Study patients receive IVT MTX at the end of vitrectomy with silicone oil every week for 8 weeks and then every other week through 16 weeks after surgery. The primary endpoint is the rate of recurrent RD over a 24-week postoperative period; the secondary endpoint is BCVA. The study is estimated to be completed by December 2022. 27
SIGHT (NCT04830878) is a phase I multicenter uncontrolled open-label study designed to assess the safety and efficacy of systemic and IVT MTX to prevent PVR in pediatric patients with RRD. 28 All 20 patients in the study are 18 years or younger. This study is estimated to be completed by April 2024. 28
Adverse Effects of Intraocular Methotrexate
Safety is a major concern when repurposing a drug for intraocular use. MTX has been used for many years to treat other ocular diseases, such as intraocular lymphoma, with minimal side effects. The most commonly reported ocular side effect of MTX is corneal epithelial keratopathy, which most commonly occurs after the third injection. 29 In a retrospective case series, 30 2 of 13 eyes (15.4%) developed mild corneal epitheliopathy. Keratopathy can occur with shorter intervals between MTX injections; however, its incidence is lower with monthly injections. 31
It is hypothesized that IVT MTX might result in leakage of the medication into the subconjunctival space, resulting in transient limbal stem cell deficiency. 32 In phakic patients having vitrectomy with silicone and IVT MTX, there might be accelerated cataract formation, likely as a result of the surgery itself. Other rare anterior segment side effects include neovascular glaucoma and band keratopathy. Neovascularization of the iris/angle was reported in 2 patients with preexisting diabetes mellitus without evidence of neovascularization before the MTX injections. 29 Choudhury et al 32 reported 2 patients in India who developed endophthalmitis secondary to Ralstonia pickettii after treatment with contaminated MTX. Others described a rare maculopathy (ie, RPE disturbances) that occurred with IVT MTX to treat primary central nervous system (CNS) lymphoma for ocular involvement, although the causal association with this rare effect is unclear. Each of these patients also had blood–brain barrier disrupting chemotherapy, which is also known to cause maculopathy.33,34
A retrospective case series 35 evaluated the safety of cumulative dosing of intra-silicone injections of MTX for nonprimary CNS lymphoma. At the end of the 9-month follow-up, none of the 13 patients had adverse effects; 12 of 13 patients had cumulative doses up to 1200 µg. This study did not necessarily attempt to evaluate the efficacy of MTX for PVR.
Studies of Alternate Agents
Several other medications (corticosteroids, 5-FU, daunorubicin, aflibercept, bevacizumab, doxorubicin [Adriamycin], and low-molecular-weight heparin) have been studied and published in the literature for use in PVR. These have been shown not to be efficacious in treating or preventing PVR or they are associated with significant ocular toxicity.36–44
A randomized clinical trial evaluating postoperative IVT triamcinolone for PVR with vitrectomy and silicone oil found no significant difference in redetachment rates or PVR recurrence. 36 Another randomized trial assessing slow-release dexamethasone for PVR also found no significant difference in redetachment rates. 39 Although there are studies showing the safety and efficacy of daunorubicin for PVR, they appear to be limited by a small therapeutic window and a very short half-life (131 minutes) in the vitreous.44,45 The role of antivascular endothelial growth factor agents for PVR is still being investigated.41,46 PREVENT-PVR (NCT04580147) is an ongoing phase II randomized clinical trial investigating aflibercept for PVR. 46
Conclusions
PVR is a sight-threatening complication of RD and the most common cause of surgical failure to repair RD. The complex pathophysiology and prolonged duration of fibrosis and proliferation of this disease present significant challenges to discovering efficacious medications and their correct dosing to treat and/or prevent PVR. The mainstay of treatment for PVR remains surgical membrane removal. Nonetheless, there is interest among vitreoretinal surgeons in pharmacological agents to help combat this clinical entity.
Few clinical studies of IVT MTX use for PVR have been published in the literature. They used doses between 100 µg and 400 µg as a single postoperative injection, serial postoperative injections, or intraoperative infusions. All current studies of this topic are limited by relatively small samples, nonrandomized interventions, retrospective study designs, a lack of controls, selection bias, or a combination. Nevertheless, they show that IVT MTX might be efficacious in preventing PVR and associated recurrent RD.
Based on the current literature, we recommend the judicious use of IVT MTX for the treatment and prevention of PVR in select patients. The current practice is to select those with RD who have PVR or are at high-risk for developing it. The most common indications in clinical practice for IVT MTX are patients with (1) RD with PVR, (2) RD after open-globe injuries, (3) a history of PVR in the fellow eye, or (4) diabetic TRD with preretinal fibrosis.
To our knowledge, the most frequently used treatment regimen is similar to that described initially by Eliott and Stryjewski. 14 Our current protocol includes 400 µg/0.1 mL MTX injections intraoperatively followed by weekly injections for 6 weeks and then repeat injections every 2 weeks, for a total of 3 months after surgery. The rationale behind this approach is that the duration of active proliferation in PVR has been shown to be up to 90 days and the half-life of MTX is short (5.9-7.6 hours).5,22–24 Therefore, single treatments are likely to be ineffective. The downside to multiple injections is the theoretical risk for infection and possible corneal toxicity. Nonetheless, the benefits of maintaining retinal reattachment and improved VA postoperatively with serial IVT MTX treatments in these patients outweigh the associated risks in this visually devastating condition.
Other less commonly used regimens include the aforementioned intraoperative MTX infusions and oral MTX. Because of the short IVT half-life of MTX, single intraoperative infusions will not remain in the vitreous cavity long enough to prevent the months-long process of PVR formation. As noted, some vitreoretinal surgeons have hypothesized that infusion of medication might result in a depot-like effect in the intraocular tissues with slow release of MTX, which would negate the need for multiple injections. However, further studies are needed to prove this effect. At present, there are no clinical data in the current literature to substantiate the use of oral MTX for the prevention of postoperative PVR formation. Furthermore, most vitreoretinal surgeons defer oral therapy because of the potential for the known substantial systemic side effects of MTX.
In conclusion, IVT MTX is a safe and potentially efficacious medication for the treatment and prevention of PVR. Further randomized clinical trials are necessary to fully assess the efficacy of its use for this indication; 2 such trials are currently underway. The use of IVT MTX is promising for a potentially devastating, sight-threatening condition.
Footnotes
Ethical Approval: Ethical approval was not applicable in this case due to the nature of literature review in this study. No patient identifiers are included in this study. The authors did not personally perform any chart reviews or clinical studies represented in this review.
Statement Of Informed Consent: This was not applicable in this literature review.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Mark A. McAllister  https://orcid.org/0000-0002-2020-4196
https://orcid.org/0000-0002-2020-4196
Brenna Bullock  https://orcid.org/0000-0002-0240-5963
https://orcid.org/0000-0002-0240-5963
References
- 1. Pastor JC, de la Rua ER, Martin F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002;21:127-144. doi: 10.1016/s1350-9462(01)00023-4 [DOI] [PubMed] [Google Scholar]
- 2. El Baha S, Leila M, Amr A, Lolah MMA. Anatomical and functional outcomes of vitrectomy with/without intravitreal methotrexate infusion for management of proliferative vitreoretinopathy secondary to rhegmatogenous retinal detachment. J Ophthalmol. 2021;2021:3648134. doi: 10.1155/2021/3648134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garweg JG, Tappeiner C, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv Ophthalmol. 2013;58:321-329. doi: 10.1016/j.survophthal.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 4. Leiderman YI, Miller JW. Proliferative vitreoretinopathy: pathobiology and therapeutic targets. Semin Ophthalmol. 2009;24:62-69. doi: 10.1016/j.survophthal.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 5. Benner JD, Dao D, Butler JW, Hamill KI. Intravitreal methotrexate for the treatment of proliferative vitreoretinopathy. BMJ Open Ophthalmol. 2019;4:e000293. doi: 10.1136/bmjophth-2019-000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowley M, Conway BP, Campochiaro PA, Kaiser D, Gaskin H. Clinical risk factors for proliferative vitreoretinopathy. Arch Ophthalmol. 1989;107:1147-1151. doi: 10.1001/archopht.1989.01070020213027 [DOI] [PubMed] [Google Scholar]
- 7. Abdi F, Mohammadi SS, Falavarjani KG. Intravitreal methotrexate. J Ophthalmic Vis Res. 2021;16:657-669. doi: 10.1001/archopht.1989.01070020213027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol. 2020;16:145-154. doi: 10.1038/s41584-020-0373-9 [DOI] [PubMed] [Google Scholar]
- 9. Friedman B, Cronstein B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine. 2019;86(3):301-307. doi: 10.1016/j.jbspin.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Idrees S, Sridhar J, Kuriyan AE. Proliferative vitreoretinopathy: a review. Int Ophthalmol Clin. 2019;59:221-240. doi: 10.1097/IIO.0000000000000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ralph A, Lemire CA, Seto B, Arroyo JG. Intravitreal methotrexate for recalcitrant epiretinal membrane reproliferation. Ophthalmic Surg Lasers Imaging Retina. 2022;53:49-51. doi: 10.3928/23258160-20211209-02 [DOI] [PubMed] [Google Scholar]
- 12. Ahmad TR, Han Y, Stewart JM. Successful treatment of anterior segment fibrosis with intravitreal methotrexate. Am J Ophthalmol Case Rep. 2021;25:101247. doi: 10.1016/j.ajoc.2021.101247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sunalp M, Wiedemann P, Sorgente N, Ryan SJ. Effects of cytotoxic drugs on proliferative vitreoretinopathy in the rabbit cell injection model. Curr Eye Res. 1984;3:619-623. doi: 10.3109/02713688409003063 [DOI] [PubMed] [Google Scholar]
- 14. Eliott D, Stryjewski TP. Methotrexate for Proliferative Vitreoretinopathy. Office USPaT. Massachusetts Eye and Ear Infirmary; 2016. [Google Scholar]
- 15. Amarnani D, Machuca-Parra AI, Wong LL, et al. Effect of methotrexate on an in vitro patient-derived model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2017;58:3940-3949. doi: 10.1167/iovs.16-20912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nourinia R, Borna F, Rahimi A, et al. Repeated injection of methotrexate into silicone oil-filled eyes for grade C proliferative vitreoretinopathy: a pilot study. Ophthalmologica. 2019;242:113-117. doi: 10.1159/000500271 [DOI] [PubMed] [Google Scholar]
- 17. Ghasemi Falavarjani K, Modarres M, Hadavandkhani A, Karimi Moghaddam A. Intra-silicone oil injection of methotrexate at the end of vitrectomy for advanced proliferative diabetic retinopathy. Eye (Lond). 2015;29:1199-1203. https://doi-org.ezproxy1.library.arizona.edu/10.1038/eye.2015.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roca JA, Yon-Mendoza A, Huaman N, Wu L. Adjunctive serial post-operative intravitreal methotrexate injections in the management of advanced proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 2021;259:2913-2917. doi: 10.1007/s00417-021-05206-z [DOI] [PubMed] [Google Scholar]
- 19. Sadaka A, Sisk RA, Osher JM, Toygar O, Duncan MK, Riemann CD. Intravitreal methotrexate infusion for proliferative vitreoretinopathy. Clin Ophthalmol. 2016;10:1811-1817. doi: 10.2147/OPTH.S111893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jahangir S, Jahangir T, Ali MH, Lateef Q, Hamza U, Tayyab H. Use of intravitreal methotrexate infusion in complicated retinal detachment for prevention of proliferative vitreoretinopathy in a pilot study. Cureus. 2021;13:e17439. doi: 10.7759/cureus.17439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulz A, Rickmann A, Julich-Haertel H, et al. Comparative cytotoxic and antiproliferative profile of methotrexate and fluorouracil on different ocular cells. Acta Ophthalmol. 2021;99:e1070-e1076. doi: 10.1111/aos.14735 [DOI] [PubMed] [Google Scholar]
- 22. Ozkan EB, Ozcan AA, Alparslan N. Intravitreal injection of methotrexate in an experimental rabbit model: determination of pharmacokinetics. Indian J Ophthalmol. 2011;59(3):197-200. doi: 10.4103/0301-4738.81026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Velez G, Yuan P, Sung C, et al. Pharmacokinetics and toxicity of intravitreal chemotherapy for primary intraocular lymphoma. Arch Ophthalmol. 2001;119(10):1518-1524. doi: 10.1001/archopht.119.10.1518 [DOI] [PubMed] [Google Scholar]
- 24. Falavarjani KG, Hadavandkhani A, Parvaresh MM, Modarres M, Naseripour M, Alemzadeh SA. Intra-silicone oil injection of methotrexate in retinal reattachment surgery for proliferative vitreoretinopathy. Ocul Immunol Inflamm. 2020;28:513-516. doi: 10.1080/09273948.2019.1597894 [DOI] [PubMed] [Google Scholar]
- 25. Hardwig PW, Pulido JS, Erie JC, Baratz H, Buettner H. Intraocular methotrexate in ocular diseases other than primary central nervous system lymphoma. Am J Ophthalmol. 2006;142(5):883-885. doi: 10.1016/j.ajo.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 26. Denstedt J, Schulz DC, Diaconita V, Sheidow TG. Acupuncture resulting in eye penetration and proliferative vitreoretinopathy – Surgical and medical management with intraocular methotrexate. Am J Ophthalmol Case Rep. 2020;18:100605. doi: 10.1016/j.ajoc.2020.100605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Medicine UNLo. The GUARD Trial - Part 1: A Phase 3 Clinical Trial for Prevention of Proliferative Vitreoretinopathy. National Institutes for Health; 2022. [Google Scholar]
- 28. Medicine UNLo. Methotrexate for The Prevention and Treatment of Proliferative Vitreoretinopathy in Pediatric Patients (SIGHT). National Institutes for Health; 2021. [Google Scholar]
- 29. Frenkel S, Hendler K, Siegal T, Shalom E, Pe’er J. Intravitreal methotrexate for treating vitreoretinal lymphoma: 10 years of experience. Br J Ophthalmol. 2008;92:383-388. doi: 10.1136/bjo.2007.127928 [DOI] [PubMed] [Google Scholar]
- 30. Zhou X, Zhou X, Shi H, et al. Reduced frequency of intravitreal methotrexate injection lowers the risk of keratopathy in vitreoretinal lymphoma patients. BMC Ophthalmol. 2020;20:189. doi: 10.1186/s12886-020-01464-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gorovoy I, Prechanond T, Abia M, Afshar AR, Stewart JM. Toxic corneal epitheliopathy after intravitreal methotrexate and its treatment with oral folic acid. Cornea. 2013;32:1171-1173. doi: 10.1097/ICO.0b013e3182910106 [DOI] [PubMed] [Google Scholar]
- 32. Choudhury H, Jindal A, Pathengay A, Flynn HW., Jr. An outbreak of Ralstonia pickettii endophthalmitis following intravitreal methotrexate injection. Clin Ophthalmol. 2015;9:1117-1120. doi: 10.2147/OPTH.S81218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109:1709-1716. doi: 10.1016/s0161-6420(02)01125-9 [DOI] [PubMed] [Google Scholar]
- 34. Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy—developments in adjunctive treatment and retinal pathology. Eye. 2002;16:369-374. doi: 10.1038/sj.eye.6700194 [DOI] [PubMed] [Google Scholar]
- 35. Hardwig PW, Pulido JS, Bakri SJ. The safety of intraocular methotrexate in silicone-filled eyes. Retina. 2008;28:1082-1086. doi: 10.1097/IAE.0b013e3181754231 [DOI] [PubMed] [Google Scholar]
- 36. Anvari P, Falavarjani KG. Adjunctive pharmacological therapies in the management of proliferative vitreoretinopathy. In: Spandau U, Tomic Z, Ruiz-Casas D, eds. Retinal Detachment Surgery and Proliferative Vitreoretinopathy. Springer Cham; 2018:25-33. doi: 10.1007/978-3-319-78446-5_3 [DOI] [Google Scholar]
- 37. Chen C, Chen P, Li H. Combined 5-fluorouracil and low molecular weight heparin for the prevention of postoperative proliferative vitreoretinopathy in patients with retinal detachment: a meta-analysis. Font Med. 2021;8:790460. doi: 10.3389/fmed.2021.790460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williamson TH. Proliferative vitreoretinopathy. In: Williamson TH, ed. Vitreoretinal Surgery. Springer; 2013:189-208. doi: 10.1007/978-3-642-31872-6_8 [DOI] [Google Scholar]
- 39. Banerjee PJ, Quartilho A, Bunce C, et al. Slow-release dexamethasone in proliferative vitreoretinopathy. Ophthalmology. 2017;124(6):757-767. doi: 10.1016/j.ophtha.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 40. Wickham L, Bunce C, Wong D, McGurn D, Charteris DG. Randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in the management of unselected rhegmatogenous retinal detachments undergoing primary vitrectomy. Ophthalmology. 2007;114(4):698-704. doi: 10.1016/j.ophtha.2006.08.042 [DOI] [PubMed] [Google Scholar]
- 41. Zhao XY, Xia S, Wang EQ, Chen YX. Efficacy of intravitreal injection of bevacizumab in vitrectomy for patients with proliferative vitreoretinopathy retinal detachment: a meta-analysis of prospective studies. Retina. 2018;38(3):462-470. doi: 10.1097/IAE.0000000000001584 [DOI] [PubMed] [Google Scholar]
- 42. Sunalp MA, Wiedemann P, Sorgente N, Ryan SJ. Effect of adriamycin on experimental proliferative vitreoretinopathy in the rabbit. Exp Eye Res. 1985;41(1):105-115. doi: 10.1016/0014-4835(85)9099-5 [DOI] [PubMed] [Google Scholar]
- 43. Ahmadieh H, Feghhi M, Tabatabaei H, Shoeibi N, Ramezani A, Mohebbi MR. Triamcinolone acetonide in silicone-filled eyes as adjunctive treatment for proliferative vitreoretinopathy: a randomized clinical trial. Ophthalmology. 2008;115(11):1938-1943. doi: 10.1016/j.ophtha.2008.05.016 [DOI] [PubMed] [Google Scholar]
- 44. Hou H, Huffman K, Rios S, Freeman WR, Sailor MJ, Cheng L. A novel approach of daunorubicin application on formation of proliferative retinopathy using a porous silicon controlled delivery system: pharmacodynamics. Invest Ophthalmol Vis Sci. 2015;56:2755-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiedemann P, Hilgers RD, Bauer P, Heimann K. Adjunctive daunorubicin in the treatment of proliferative vitreoretinopathy: results of a multicenter clinical trial. Daunomycin Study Group. Am J Ophthalmol. 1998;126(4):550-559. doi: 10.1016/s0002-9394(98)00115-9 [DOI] [PubMed] [Google Scholar]
- 46. Medicine UNLo. The PREVENT-PVR Trial – A Multi-Center, Randomized, Sham-Controlled, Phase II Clinical Trial Evaluating Intravitreal Aflibercept for The Prevention of Proliferative Vitreoretinopathy Following Macula Off Rhegmatogenous Retinal Detachment Repair. National Institutes for Health; 2022. [Google Scholar]