Abstract
Background
The historic standard of care for adult medulloblastoma has been considered surgery and radiation, while chemotherapy is increasingly being prescribed. This study reviewed 20-year chemotherapy trends at a high-volume center, as well as overall and progression free-survival.
Methods
Adults with medulloblastoma treated at an academic center from January 1, 1999 to –December 31, 2020 were reviewed. Patient baseline data were summarized and Kaplan–Meier estimators were used for survival.
Results
Forty-nine patients were included; median age was 30 years and male: female ratio was 2:1. Desmoplastic and classical histologies were most common. Of all patients, 23 (47%) were high risk and 7 (14%) metastatic at diagnosis. Only 10 (20%) received initial chemotherapy, of which 70% were high risk and 30% metastatic, with most treated from 2010 to 2020. Forty percent of initial chemotherapy patients received salvage chemotherapy for recurrence or metastases (of all patients, 49% required salvage). Initial chemotherapy regimens were mainly cisplatin/lomustine/vincristine, and at recurrence cisplatin/etoposide. Median overall survival was 8.6 years (95% CI 7.5–∞), with 1-, 5-, and 10-year survival at 95.8%, 72%, and 46.7%. Median overall survival for those who did not receive initial chemotherapy was 12.4 years and 7.4 years for those who did (P-value .2).
Conclusions
Twenty years of adult medulloblastoma treatment was reviewed. Initial chemotherapy patients, most of whom were high risk, trended towards worse survival, but this was nonsignificant. The ideal timing and choice of chemotherapy for adult medulloblastoma is unknown—challenges of administering chemotherapy following photon craniospinal irradiation may have prevented it from becoming routine.
Keywords: adult, chemotherapy, medulloblastoma, radiation, retrospective cohort study
Medulloblastoma is the most common pediatric brain tumor but is rare in adults, with an incidence of only 0.58 per million cases in patients over 19, comprising less than 1% of adult brain tumors.1,2 They are classified as WHO grade 4 tumors and are associated with a 5-year overall survival (OS) from 40% to 82%2–7 depending on the stage group studied and severity of disease. The traditional standard of care (SOC) treatment consists of maximal safe resection and adjuvant craniospinal (CSI) radiation with boost to the tumor bed and residual gross disease.8,9 Evidence supporting chemotherapy as part of SOC initial treatment is growing:8 a large meta-analysis showed improved median OS in patients who received neoadjuvant chemotherapy compared to patients with first chemotherapy at recurrence,10 Kann et al., demonstrated a 14.5% absolute 5 year OS benefit of adjuvant chemotherapy in patients without M4 disease,5 and a subsequent review of the same national database review showed OS benefit of either concurrent or adjuvant chemotherapy.11 There are no randomized trials comparing SOC treatment with or without chemotherapy or different drug regimens, mainly due to the low disease incidence.
Classification and treatment of medulloblastoma have evolved over time. Data on molecular subgroups has only recently become available in the clinic. This retrospective study reviewed 20 years of chemotherapy prescribing practices for adult medulloblastoma patients seen at a busy academic center, Princess Margaret Hospital (PMH) in Toronto, Canada. The primary objective of this study was to compare the use of adjuvant chemotherapy across treatment eras. Secondary objectives included overall and progression free-survival analysis, based on risk status and treatment approach, as well as between those who received upfront chemotherapy and those who did not.
Materials and Methods
The Princess Margaret Cancer Registry (PMCR) was used to identify potential patients. The PMCR was established in 1958, with data from all University Health Network hospitals. Eligibility criteria included patients 18 or older, seen at PMH from January 1, 1999 to December 31, 2020, for a diagnosis of medulloblastoma. Initial registry data searched for patients classified as having medulloblastoma, as well as primitive neuroectodermal tumors (PNETs), as this was a historical term for medulloblastoma. We then excluded patients with supratentorial PNETs, PNETs of only the spinal column, patients with insufficient data for analysis or patients who received no treatment at PMH (neither at diagnosis nor recurrence). This resulted in a final sample size of 49 patients. This retrospective study received research ethics board approval with a waiver of patient consent. Chart review and radiation records were used to complete missing data. Archival pathology was reviewed for cases where histologic classification was missing from the original pathology report.
Data collected included baseline patient demographics, surgical, pathology, and diagnostic imaging reports and patient electronic chart notes. Risk group was divided into average risk (residual tumor less than 1.5 cm3, M0–M1, classic or desmoplastic/nodular histology, wingless/WNT or Sonic hedgehog/SHH molecular subtype) and high risk (residual tumor greater than 1.5 cm3, >M1 disease, large cell/anaplastic histology, non-WNT/non-SHH molecular subtype, or SHH-p53mut).12,13 Descriptive statistics were used to present the data and Kaplan–Meier estimators were used for survival analysis. P-values were calculated with Chi-squared test. The R project for Statistical Computing14 was used for calculations and plots.
Results
Patient Population
A total of 49 patients were included. Patient age ranged from 18 to 60 years, with median age of 30. Male patients were the majority at approximately 2:1. A minority of patients were metastatic at diagnosis with Chang12 M2 disease or worse (14%; 7/49); these patients had metastatic disease confirmed with MRI brain and MRI spine at least 2 weeks postoperatively. The most common sites of metastases were the spine and leptomeninges. Twenty-three were high risk (47%) and 26 (53%) were average risk, classified by metastases, amount of postoperative residual, histology, and molecular subgroup. Twenty-eight patients had gross total resection and 20 subtotal resections, with one patient missing surgical residual information. Most initial tumors were lateral (58%; 28/49, 18 were bilateral or midline, 37%; 18/49 and 3 patients had unknown initial tumor location). The most common histology was desmoplastic/nodular. Molecular subgroup was available for the 6 most recent patients, with SHH most common (4/6). Four patients received initial treatment at other centers and were treated at PMH upon subsequent recurrence. Complete baseline patient characteristics are described in Table 1.
Table 1.
Baseline characteristics of the entire study population (49 adult patients with medulloblastoma), as well as based on initial chemotherapy treatment decision, treated at Princess Margaret Cancer Centre (PM) from January 1, 1999 to December 30, 2020
| Baseline characteristic | All patients | No initial chemotherapy (n = 39) | Given initial chemotherapy (n = 10) |
|---|---|---|---|
| Age at diagnosis (years) | |||
| Median (IQR) | 30 | 32 (21, 38.5) | 22 (21, 24.3) |
| Range (years) | 18–60 | 18–60 | 18–43 |
| Sex | |||
| Female | 16 (33%) | 13 | 3 |
| Male | 33 (67%) | 26 | 7 |
| Year of diagnosis | |||
| Median (IQR) | 2008 (2004, 2014) | 2008 (2003.5, 2014) | 2013.50 (2006.5, 2016) |
| Range | 1999–2018 | 2000–2018 | 1999–2017 |
| Time from symptoms to diagnosis (days) | |||
| Minimum | 6 | 6 | 12 |
| Median (IQR) | 46.5 (29,95.3) | 46 (30.75, 96.25) | 54 (29.50, 85.75) |
| Mean (sd) | 88 +/− 112.3 | 96.82 ± 125.27 | 58.30 ± 37.5 |
| Maximum | 538 | 538 | 120 |
| Unknown/missing | 5 | 5 | 0 |
| Histology, No. (%) | |||
| Classical | 17 | 14 | 3 |
| Desmoplastic/nodular | 17 | 15 | 1 |
| Large cell/anaplastic | 8 | 5 | 4 |
| Unknown/Missing | 7 | 5 | 2 |
| Molecular subgroup, No. (%) | n = 6 | ||
| Group 3 or 4 | 1 | 0 | 1 |
| Group 4 | 1 | 1 | 0 |
| SHH | 4 | 3 | 1 |
| Unknown/Missing | 43 | 35 | 8 |
| Metastatic at diagnosis, No. (%) | |||
| Yes | 7 (14%) | 4 | 3 |
| No | 42 (86%) | 35 | 7 |
| Sites of metastasis at diagnosis, No. (%) | n = 7 | ||
| Spine | 4 | 1 | 0 |
| Leptomeningeal | 1 | 3 | 1 |
| Spine/Leptomeningeal | 2 | 0 | 2 |
| Unknown/missing | 0 | 35 | 7 |
| Resection extent, No. (%) | |||
| Gross total | 28 (57%) | 23 (59%) | 5 (50%) |
| Subtotal | 20 (41%) | 15 (38%) | 5 (50%) |
| Unknown | 1 (2%) | 1 (3%) | 0 |
| Tumor location, No, (%) | |||
| Lateralized | 28 (57%) | 23 (59%) | 5 (50%) |
| Nonlateral | 18 (37%) | 14 (36%) | 4 (40%) |
| Unknown | 3 (6%) | 2 (3%) | 1 (10%) |
| Residual disease (area cm 2 ) | |||
| Minimum | 0 | 0 | 0 |
| Median (IQR) | 0 (0, 1.41) | 0 (0, 1.73) | 0 (0, 0.30) |
| Mean (sd) | 1.78 | 1.92 ± 4.25 | 1.11 ± 2.69 |
| Maximum | 18.74 | 18.74 | 7.20 |
| Unknown/missing | 9 | 6 | 3 |
| Risk category, No. (%) | |||
| Intermediate | 26 (53%) | 23 | 3 |
| High | 23 (47%) | 16 | 7 |
Chemotherapy
Initial chemotherapy in this study was defined as chemotherapy occurring concurrent with or adjuvant to primary radiation treatment, as opposed to chemotherapy at disease progression or recurrence. Ten patients (20%) received initial chemotherapy; 12 patients were offered initial chemotherapy but 2 did not receive treatment due to patient preference or deterioration in health. One initial chemotherapy regimen was concurrent treatment only, 4 were adjuvant, and 5 were concurrent followed by adjuvant treatment. Of the 10 initial chemotherapy patients, 7 were classified as high risk and 3 were metastatic at diagnosis. Patients who received initial chemotherapy were on average younger and treated in the most recent treatment decade (see Table 1).
The most common initial chemotherapy regimen was cisplatin/lomustine/vincristine, followed by etoposide/cisplatin/lomustine/vincristine and etoposide/cisplatin/cyclophosphamide/vincristine. Common regimens at first recurrence were cisplatin/etoposide, etoposide, and temozolomide. This was similar at second recurrence, with the addition of autologous stem cell transplant, which was administered to 2 patients. Toxicity during chemotherapy was most commonly neutropenia, fatigue, anemia, and neuropathy. Complete chemotherapy regimen details are described in Tables 2 and 3.
Table 2.
Initial chemotherapy details for 10 adult medulloblastoma patients (including treatment timing, drug regimen and number of cycles received), treated at Princess Margaret Cancer Centre (PM) from January 1, 1999 to December 30, 2020.
| Chemotherapy patients | Treatment | Concurrent regimen | Adjuvant regimen | Toxicity | CSI dose | Boost dose |
|---|---|---|---|---|---|---|
| 1 | Concurrent, adjuvant | Weekly Vincristine | Cisplatin/Lomustine/Vincristine (3 cycles) | Vincristine related neuropathy, prolonged neutropenia, fatigue | 36/20 | 18/10 |
| 2 | Concurrent, adjuvant | Daily Etoposide | Etoposide + Cisplatin (3 cycles), then cyclophosphamide + Vincristine | Anemia, neutropenia | 36/20 | 18/10 |
| 3 | Concurrent, adjuvant | Daily Etoposide | Etoposide + Cisplatin (3 cycles), then Cyclophosphamide + vincristine (2 cycles) | Pancytopenia | 36/20 | 18/10 |
| 4 | Concurrent, adjuvant | Weekly Vincristine | Cisplatin/Lomustine/Vincristine (0 cycles) | NA | RT at outside center | RT at outside center |
| 5 | Concurrent | Etoposide (1 dose) | NA | PCP, herpetic encephalopathy | 45/25 | No boost |
| 6 | Adjuvant | NA | Cisplatin/Lomustine/Vincristine (2 cycles) | Vincristine related neuropathy, fatigue | 36/20 | 18/10, 5.4/3 |
| 7 | Adjuvant | NA | Cisplatin/Lomustine/Vincristine (4 cycles) | Vincristine related neuropathy, ototoxicity | 36/20 | 18/10, 5.4/3 |
| 8 | Concurrent, adjuvant | Etoposide | Cisplatin/Lomustine/Vincristine (4 cycles) | Neutropenia, severe fatigue | 39.6/22 | 5.4/3, 9/5, 3.6/2 |
| 9 | Adjuvant | NA | Cisplatin/Lomustine/Vincristine (3 cycles) | Anemia, pneumonia, severe fatigue | RT at outside center | RT at outside center |
| 10 | Adjuvant | NA | Cisplatin/Lomustine/Vincristine (5 cycles) | Pancytopenia, fatigue, neuropathy | 36/20 | 18/10, 5.4/3 |
Table 3.
Chemotherapy decision and regimens at initial treatment and recurrence of adult medulloblastoma patients treated at Princess Margaret Cancer Centre (PM) from January 1, 1999 to December 30, 2020.
| Treatment details | |
|---|---|
| Initial chemotherapy (concurrent and/or adjuvant) | Number of patients |
| Yes | 10 |
| No | 39 |
| Received salvage chemotherapy at recurrence | |
| Yes | 35 |
| No | 11 |
| Initial chemotherapy regimen | n = 10 |
| Cisplatin/Lomustine/Vincristine | 5 |
| Etoposide/Cisplatin/Lomustine/Vincristine | 1 |
| Etoposide/Cisplatin/Cyclophosphamide/Vincristine | 3 |
| Carboplatin/Etoposide | 1 |
| Recurrent patients | n = 22 (3 with no initial treatment, 1 never disease free) |
| First recurrence n = 19 | n = 19 (4 initial chemotherapy patients) |
| Cisplatin/Etoposide | 6 |
| Etoposide | 4 |
| Temozolomide | 4 |
| Carboplatin/Etoposide | 1 |
| Carboplatin/Etoposide/Autologous stem cell transplant | 1 |
| Etoposide/Cisplatin/Lomustine/Vincristine | 1 |
| Sonidegib Procarbazine/Lomustine/Vincristine/Etoposide |
1 1 |
| Second recurrence | n = 12 (2 initial chemotherapy patients) |
| Temozolomide | 3 |
| Etoposide | 2 |
| Autologous stem cell transplant | 2 |
| Cyclophosphamide/Autologous stem cell transplant | 1 |
| Etoposide/Cisplatin | 1 |
| Lomustine | 1 |
| Etoposide/Temozolomide | 1 |
| Third recurrence | n = 4 |
| Cyclophosphamide | 2 |
| Lomustine | 1 |
| Imatinib | 1 |
| Fourth recurrence | n = 1 |
| Temozolomide | 1 |
Radiation Therapy
Primary radiation (CSI + boost) was delivered to 47 patients. One patient did not receive radiation and radiation data were missing for two others. One patient received CSI radiation alone without boost. The average time from initial surgery to radiation completion was 78 days (range 16–117). The most common CSI dose was 36 Gy in 20 fractions and the most common boost dose was 18 Gy in 10 fractions. Eleven (11/49) patients received additional boost to gross disease in the brain or spine, with the most common boost dose being 5.4 Gy in 3 fractions (to a total of 59.4 Gy). One patient was unable to complete radiation due to meningitis and stroke, receiving only 900 cGy to the spine and no radiation to the brain.
Outcomes
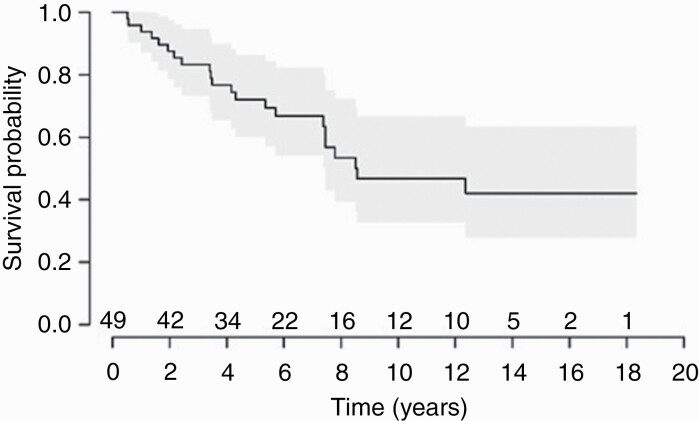
As of December 31, 2020, at most recent follow-up 55% (22/49) of patients were alive. For the entire study population, median OS was 8.6 years (Figure 1). The estimated 1-, 5- and 10-year OS were 96%, 72%, and 47% respectively. There were 23 patients out of the entire cohort who developed recurrence; median progression free survival was 5.1 years. Median time to first progression was 2.8 years for the entire cohort. There were 5 patients out of the initial chemotherapy patients who developed at least 1 recurrence and 4 went on to receive salvage chemotherapy (Table 3). Of the entire population, 49% received chemotherapy as salvage at recurrence. The median follow-up time (calculated using the reverse Kaplan–Meier method) was 10.6 years (interquartile range 5.9–13.6).
Figure 1.
Kaplan–Meier plot of overall survival for 49 adult patients with medulloblastoma treated at the Princess Margaret Cancer Centre (PM) between January 1, 1999 and December 30, 2020.
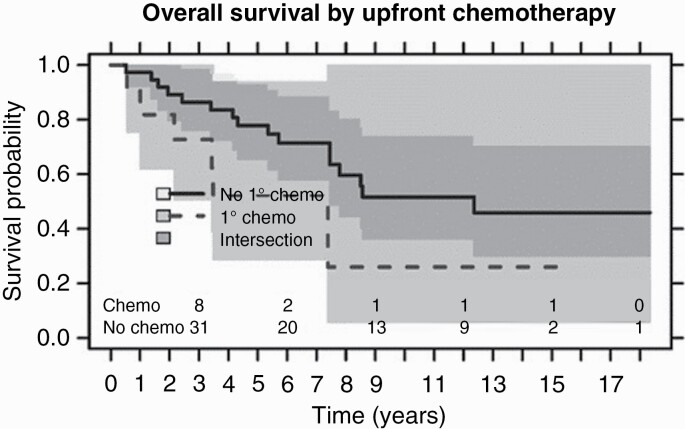
Patients who received initial chemotherapy had a lower median OS of 7.4 years compared to those who did not receive initial chemotherapy with median OS of 12.4 years (P-value .2) (Figure 2). Median progression free survival was 5.1 years in patients without initial chemotherapy and 3.1 years in patients with initial chemotherapy; P-value .8. There were 5 patients out of the initial chemotherapy patients who developed at least one recurrence and 4 went on to receive salvage chemotherapy (Table 3). Of the 10 chemotherapy patients, 3 were average risk, and 7 were high risk; there was no significant OS difference in these patients based on risk group (P-value .7).
Figure 2.
Kaplan–Meier plots of overall survival of adult patients who received (dashed line) and who did not receive chemotherapy (solid line) as part of their initial treatment for medulloblastoma at the Princess Margaret Cancer Centre (PM) between January 1, 1999 and December 30, 2020; P-value .2.
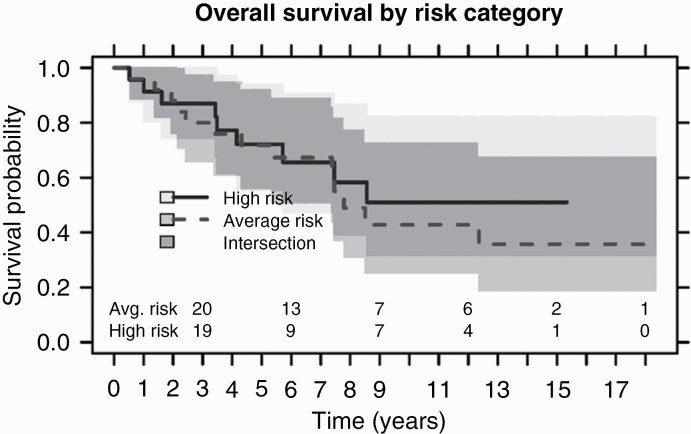
High-risk patients did not reach median survival compared to average risk patients with a medial survival of 7.79 years; P-value .6 (Figure 3). Median progression free survival for high-risk patients was 4.16 years compared to 6.47 years for the average risk group. There was no significant difference in OS between patients with total vs subtotal resection status (P-value .9).
Figure 3.
Kaplan–Meier plots of overall survival of high risk (solid line) and average risk (dashed line) adult patients with medulloblastoma treated at the Princess Margaret Cancer Centre (PM) between January 1, 1999 and December 30, 2020; P-value .6.
When comparing the earlier and most recent treatment decades (1999–2009 vs 2010–2020), 25 patients were treated in the first treatment decade and 24 in the later treatment decade. There was no significant difference between the two treatment groups in terms of median overall and progression free survival (P-value .9 and .7, respectively).
Discussion
This study reviewed 20 years of patients with adult medulloblastoma treated at a busy academic center, with a focus on chemotherapy as part of the initial treatment regimen. Our analysis of 49 patients showed that only 20% of patients received initial chemotherapy and these patients were typically younger and treated in the most recent decade of the study.
The impact of treatment decade on initial chemotherapy decision demonstrated here could reflect increasing tendency to offer initial chemotherapy based on studies showing potential benefit of chemotherapy in adult medulloblastoma patients in recent years.5,8,10,11 However, it is difficult to explore this result of our study further as only 20% of the study population in this series received initial chemotherapy. This is lower than other reported studies, where chemotherapy rates ranged from 33% to 95% either concurrently or adjuvantly.3,4,6,7,10,11,15,16 With the inclusion of patients who were offered chemotherapy but did not proceed with the treatment recommendation, the proportion increased modestly from 20% to 25%. Our review included all M stage patients and the 14% with metastatic disease were all M2 or higher; these patients may have been less well overall and not expected to tolerate systemic treatment if offered. In terms of risk stratification, our population was more evenly split between high and average risk patients (47% and 53%, respectively) compared with other study populations that consisted of fewer high-risk patients,6,7,16 and although higher risk patients could be argued to derive the most benefit from chemotherapy, their clinical fitness to proceed could be influenced by disease factors making them high risk. This may have also impacted their ability to complete chemotherapy, either concurrently or adjuvantly. Other than patient age and treatment decade, there was no clear trend in patient factors for the treatment decision of initial chemotherapy. Our population did include patients who came to our center at time of recurrence, meaning their initial treatment decisions and factors influencing those decisions were not made at our institution—this could influence the low chemotherapy rate in our cohort further. Recent work by Liu et al. demonstrated the role of cfDNA CSF liquid biopsies in detecting measurable residual disease and treatment response in medulloblastoma patients. This marker of disease could be another tool in treatment decision making, both adjuvantly and at suspicion of recurrence.17
OS at 5 years was 67.5% and was slightly worse in patients with metastatic or high-risk disease; the small proportion of patients who received initial chemotherapy had a worse OS than those who did not. In terms of secondary endpoints, our study was in line with previously shown rates of OS,3–7 with disease free survival at 5 years of 67.5%, despite the increased representation of patients with high risk and advanced M stage disease in our study population. With respect to pediatric outcomes, the HIT trial reported 5-year progression free survival of 80.3% and 5 year OS of 85%.18 The significance of patients receiving initial chemotherapy having slightly worse OS is unclear. There could be multiple explanations: the toxicity associated with chemotherapy in adults who received high-dose photon CSI irradiation (compared to less intense chemotherapy regimens such as oral temozolomide in patients with glioblastoma) which could reduce bone marrow reserve, these patients being sicker to begin with or simply the small number (20%) of patients receiving chemotherapy in this study. Toxicity of combination systemic and radiation therapy in older children was studied by Tabori et al.19—grade 3 or 4 hematotoxicity occurred in 95%, along with high rates grade 2 ototoxicity (45%) and neurotoxicity (71%). Other confounding factors could also have influenced the decision to administer chemotherapy, including risk category. Our cohort had a more even proportion of high vs average risk patients as mentioned previously, and although they likely stood to benefit more from chemotherapy, their disease was more severe and this may have influenced their survival, regardless of receiving chemotherapy or not. Beier et al.,20 prospectively explored the feasibility of concurrent (vincristine) and at least 1 cycle adjuvant chemotherapy (cisplatin/lomustine/vincristine) in adult medulloblastoma patients. Four cycles of their adjuvant regimen were feasible in 70% of patients; all required dose reductions and feasibility decreased with increasing patient age past 45 years old, with an increase in severe adverse events past that age.20 As initially discussed, our study had fewer patients receiving initial chemotherapy than most and the difference in survival trends could accordingly be different due to chance. The median time to recurrence in our cohort was slightly over 24 months; other adult studies have quoted longer time periods such as 47 months.7 Our cohort included all M risk category patients and had a large number of high-risk patients, potentially explaining the worse time to recurrence. A pediatric study of recurrence patterns showed a range of median time to recurrence of 12–18.3 months.21
Guidelines by EANO-EURACAN give level IIA recommendations for chemotherapy as part of the primary treatment for patients in any risk category.8 The meta-analysis from Kocakaya et al.,10 demonstrated significant improvement in OS in patients who received chemotherapy at diagnosis vs only at recurrence and Kann et al.,5 demonstrated a 14.5% absolute OS benefit in patients treated with postoperative chemotherapy on their database review. In work published in 2020, Haque et al. reviewed the same database and found again an improvement in survival with chemotherapy, whether delivered concurrently or adjuvantly. This work was limited by multivariate analysis for chemotherapy benefit not reaching significance and the database itself does not include results on residual disease status or molecular classification in both works.5,11 Atalar et al., reviewed 296 adult medulloblastoma patients and found that chemotherapy at initial treatment (neoadjuvant, concurrent, or adjuvant with radiation) was associated with better survival and local control.15 In other studies, including Franceschi et al.,22 that investigated average risk patients, chemotherapy was not a prognostic factor for survival.6,7,23 The concept of preoperative chemotherapy has also been explored but results were disappointing.19
The result of patients with metastatic and high-risk disease at diagnosis trending towards having worse OS is not unexpected; metastatic disease is an aspect of the risk classification of medulloblastoma; therefore, it is logical if one factor had a survival implication, which the other would as well. In our study, patients with metastatic disease were all at least Chang stage M2; while the number of metastatic patients was low (14%), the severity of their metastatic disease likely plays a role in their lower survival shown here. Studies in adult medulloblastoma show prognostic factors are much more heterogeneous compared to pediatric populations—in some studies the presence of metastatic disease is significantly associated with survival and in others it is not.4,5,7,10,11,16,23,24 Other prognostic factors vary as well in their significance between series, including histology, resection extent, performance time between surgery and completion of radiation, and location of initial disease.3–6,10,16,23–25 As previously discussed, our population had more high-risk patients compared to most studies, but this did not bring our overall population survival out of the range of 5-year OS seen in adult medulloblastoma patients.
All patients on the present study were treated with photon radiotherapy. This treatment irradiates more of the bone marrow in the axial skeleton than proton radiotherapy and can adversely affect tolerance to post-RT chemotherapy. Proton therapy is an emerging standard treatment for medulloblastoma in children and young adults because of its ability to more precisely target the neuraxis.26 The increased precision of proton therapy reduces bone marrow dose and hematologic toxicity and has been shown to be tolerable when combined with chemotherapy.27 This treatment represents an area of future study as proton therapy becomes more broadly available around the world. Another ongoing area of study is the use of targeted agents such as SHH inhibitors; although treatment is not currently driven by molecular status, these agents have potential to address medulloblastoma as a diverse disease entity.28,29
Limitations and Future Perspectives
Our study is limited by overall small sample size and retrospective nature. With only 10 of our patients receiving initial chemotherapy, the power to detect survival differences is limited. Treatment decision details and rationale can be difficult to discern on chart reviews from decades prior and our review may not adequately demonstrate the discussions involved in deciding optional regimens for these patients. There was heterogeneity of those who received initial chemotherapy, some concurrently with radiation and some adjuvantly; we pooled these patients based on the small number who received chemotherapy and survival results may have been resultingly skewed. Medulloblastoma classification was updated in 2016 to include molecular subgrouping in addition to the traditional histologic groupings; as well, further divisions within the molecular subgroups have been identified and may have prognostic implications.30 Our review spanned 2 decades, only the most recent 5 years of the study period was when molecular subgrouping became SOC (currently the SOC at our institution). Accordingly, only 6 of our patients had this data available; with the first occurring in 2011. Not all patients with negative spine imaging had CSF cytology checked, so some patients in this cohort might have been understaged.
Conclusions
This analysis demonstrated that chemotherapy, while infrequently part of the initial treatment regimen for adult medulloblastoma patients treated at our institution, was more commonly prescribed in the most recent treatment decade and to younger patients. Our analysis showed the significant heterogeneity of this rare patient population across a 20-year treatment period. The rarity of this adult malignancy necessitates enrollment in clinical trials to further understand the subtleties of the disease behavior and treatment options.
Acknowledgements
Special thanks to Dr. Julia Keith, Dr. Claire Coire, and Dr. David Munoz for their contribution to the archival pathology review for this study.
Contributor Information
Marissa Sherwood, Department of Radiation Oncology, University of Toronto, Toronto, Ontario M5T 1P5, Canada; Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network (UHN), Toronto, Ontario M5G 2M9, Canada.
Seth Climans, Department of Medicine, Divisions of Neurology and Department of Medical Oncology and Hematology, University of Toronto, Toronto, Ontario M5G 2C1, Canada.
Ronald Ramos, Department of Medicine, Divisions of Neurology and Department of Medical Oncology and Hematology, University of Toronto, Toronto, Ontario M5G 2C1, Canada.
Normand J Laperriere, Department of Radiation Oncology, University of Toronto, Toronto, Ontario M5T 1P5, Canada; Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network (UHN), Toronto, Ontario M5G 2M9, Canada.
Andrew F Gao, Laboratory Medicine Program, University Health Network (UHN), Toronto, Ontario M5G 2C4, Canada.
Barbara-Ann Millar, Department of Radiation Oncology, University of Toronto, Toronto, Ontario M5T 1P5, Canada; Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network (UHN), Toronto, Ontario M5G 2M9, Canada.
David B Shultz, Department of Radiation Oncology, University of Toronto, Toronto, Ontario M5T 1P5, Canada; Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network (UHN), Toronto, Ontario M5G 2M9, Canada.
Derek S Tsang, Department of Radiation Oncology, University of Toronto, Toronto, Ontario M5T 1P5, Canada; Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network (UHN), Toronto, Ontario M5G 2M9, Canada.
Warren P Mason, Department of Medicine, Divisions of Neurology and Department of Medical Oncology and Hematology, University of Toronto, Toronto, Ontario M5G 2C1, Canada.
Funding
This was a non-funded study.
Conflict of interest statement. The authors have no conflicts of interest to declare.
References
- 1. Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012;19(11):1541–1544. [DOI] [PubMed] [Google Scholar]
- 2. Walker EV, Davis FG. Malignant primary brain and other central nervous system tumors diagnosed in Canada from 2009 to 2013. Neuro Oncol. 2019;21(3):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abacioglu U, Uzel O, Sengoz M, Turkan S, Ober A. Medulloblastoma in adults: treatment results and prognostic factors. Int J Radiat Oncol Biol Phys. 2002;54(3):855–860. [DOI] [PubMed] [Google Scholar]
- 4. Brandes AA, Franceschi E, Tosoni A, Blatt V, Ermani M. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer. 2007;110(9):2035–2041. [DOI] [PubMed] [Google Scholar]
- 5. Kann BH, Lester-Coll NH, Park HS, et al. Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro Oncol. 2017;19(2):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Padovani L, Sunyach MP, Perol D, et al. Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys. 2007;68(2):433–440. [DOI] [PubMed] [Google Scholar]
- 7. Zhang N, Ouyang T, Kang H, et al. Adult medulloblastoma: clinical characters, prognostic factors, outcomes and patterns of relapse. J Neurooncol. 2015;124(2):255–264. [DOI] [PubMed] [Google Scholar]
- 8. Franceschi E, Hofer S, Brandes AA, et al. EANO-EURACAN clinical practice guideline for diagnosis, treatment, and follow-up of post-pubertal and adult patients with medulloblastoma. Lancet Oncol. 2019;20(12):e715–e728. [DOI] [PubMed] [Google Scholar]
- 9. NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) - Central Nervous System Cancers, Version 5. 2020; https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed April 24, 2022. [DOI] [PubMed] [Google Scholar]
- 10. Kocakaya S, Beier CP, Beier D. Chemotherapy increases long-term survival in patients with adult medulloblastoma--a literature-based meta-analysis. Neuro Oncol. 2016;18(3):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haque W, Verma V, Brian Butler E, Teh BS. Prognostic role of chemotherapy, radiotherapy dose, and extent of surgical resection in adult medulloblastoma. J Clin Neurosci. 2020;76:154–160. [DOI] [PubMed] [Google Scholar]
- 12. Chang CH, Housepian EM, HerbertC, Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 13. Franceschi E, Seidel C, Sahm F, Pajtler KW, Hau P. How we treat medulloblastoma in adults. ESMO Open. 2021;6(4):100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 15. Atalar B, Ozsahin M, Call J, et al. Treatment outcome and prognostic factors for adult patients with medulloblastoma: the Rare Cancer Network (RCN) experience. Radiother Oncol. 2018;127(1):96–102. [DOI] [PubMed] [Google Scholar]
- 16. Hadi I, Roengvoraphoj O, Niyazi M, et al. Medulloblastoma in adults: a retrospective single institution analysis. Strahlenther Onkol. 2018;194(3):225–234. [DOI] [PubMed] [Google Scholar]
- 17. Liu APY, Smith KS, Kumar R, et al. Serial assessment of measurable residual disease in medulloblastoma liquid biopsies. Cancer Cell. 2021;39(11):1519–1530.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dietzsch S, Placzek F, Pietschmann K, et al. Evaluation of prognostic factors and role of participation in a randomized trial or a prospective registry in pediatric and adolescent Nnnmetastatic medulloblastoma - a report from the HIT 2000 trial. Adv Radiat Oncol. 2020;5(6):1158–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tabori U, Sung L, Hukin J, et al. Medulloblastoma in the second decade of life: a specific group with respect to toxicity and management: a Canadian Pediatric Brain Tumor Consortium Study. Cancer. 2005;103(9):1874–1880. [DOI] [PubMed] [Google Scholar]
- 20. Beier D, Proescholdt M, Reinert C, et al. Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro Oncol. 2018;20(3):400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franceschi E, Bartolotti M, Paccapelo A, et al. Adjuvant chemotherapy in adult medulloblastoma: is it an option for average-risk patients? J Neurooncol. 2016;128(2):235–240. [DOI] [PubMed] [Google Scholar]
- 23. Friedrich C, von Bueren AO, von Hoff K, et al. Treatment of adult nonmetastatic medulloblastoma patients according to the paediatric HIT 2000 protocol: a prospective observational multicentre study. Eur J Cancer. 2013;49(4):893–903. [DOI] [PubMed] [Google Scholar]
- 24. Moots PL, O’Neill A, Londer H, et al. Preradiation chemotherapy for adult high-risk medulloblastoma: a trial of the ECOG-ACRIN cancer research group (E4397). Am J Clin Oncol. 2018;41(6):588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan H, Zabih V, Bartels U, et al. Prognostic factors related to overall survival in adolescent and young adults with medulloblastoma: a systematic review. Neuro-Oncology Advances. 2022;4(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsang DS, Patel S. Proton beam therapy for cancer. CMAJ. 2019;191(24):E664–E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86(2):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samkari A, White J, Packer R. SHH inhibitors for the treatment of medulloblastoma. Expert Rev Neurother. 2015;15(7):763–770. [DOI] [PubMed] [Google Scholar]
- 30. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]