Abstract
In this study, we use advanced growth modeling techniques and the rich biospecimen and data repositories of the NICU Hospital Exposures and Long-Term Health (NICU-HEALTH) study to assess the impact of NICU-based phthalate exposure on extrauterine growth trajectories between birth and NICU discharge. Repeated holdout weighed quantile sum (WQS) regression was used to assess the effect of phthalate mixtures on the latency to first growth spurt and on the rate of first growth spurt. Further, we assessed sex as an effect modifier of the relationship between a phthalate mixture and both outcomes. Nine phthalate metabolites, mono-ethyl phthalate (MEP), mono-benzyl phthalate (MBzP), mono-n-butyl phthalate (MBP), mono-isobutyl phthalate (MiBP), mono-(3-carboxypropyl) phthalate (MCPP), mono-2-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) were measured in weekly urine specimens from 101 NICU-HEALTH participants between birth and the first growth spurt. Phthalate levels varied by species but not by infant sex, and decreased over the course of the NICU hospitalization as presented in detail in Stroustrup et al., 2018. There was evidence of nonlinearity when assessing the effect of phthalates on latency to first growth spurt. Above a threshold level, a higher phthalate mixture with dominant contributorsMCPP, MBz, and ME predicted a shorter latency to the first inflection point, or an earlier growth spurt. A higher phthalate mixture with dominant contributors MECPP, MEHHP, and MEOHP was associated with an increased rate of growth. Results of both models were clearly different for boys and girls, consistent with other studies showing the sexually dimorphic impact of early life phthalate exposure. These results suggest that growth curve modeling facilitates evaluation of discrete periods of rapid growth during the NICU hospitalization and exposure to specific phthalates during the NICU hospitalization may both alter the timing of the first growth spurt and result in more rapid growth in a sexually dimorphic manner.
Keywords: Prematurity, Neonatology, Children’s Environmental Health
1. INTRODUCTION
Over the past twenty years, advancements in neonatology have greatly improved preterm infant extrauterine growth in the neonatal intensive care unit (NICU). Poor growth between birth and term equivalent is linked to both adverse neurodevelopmental outcome and metabolic syndrome in survivors of prematurity (1–11). Studies of children born at term have shown that chemical exposures early in development can impact early weight gain and are associated with later life growth trajectory. Specifically, prenatal exposure to ubiquitous organic chemical plasticizers known to disrupt endocrine function, phthalates, has been linked both to intrauterine growth restriction(12), decrements in birth weight (13), poor weight gain in infancy(14), and development of overweight or obesity later in life (15–17). Childhood phthalate exposure is also associated with an increased body mass index (BMI), larger waist circumference, and increased risk of being overweight or obese(18, 19), mimicking associations also seen between phthalate biomarkers and BMI and waist circumference in adults(20–22). Moreover, changes in the timing and rate of early-life growth spurts have been recognized as both susceptible to chemical exposures early in development (5) and predictive of later life obesity (1, 3, 5).
Studies of preterm infant growth between birth and discharge from the neonatal intensive care unit (NICU) have to date focused on maximizing nutrition and minimizing medical morbidities (23–25). The study of preterm infant growth has been hindered by lack of consistency of outcome metrics used to quantify preterm infant growth. To our knowledge, timing and rate of early life growth spurts, metrics found to be useful in studies of term-born children (24), have not been applied to study of preterm infant growth.
Phthalate exposure in the NICU has been well-documented (26–31). Exposure has persisted over time, and has been linked to disposable plastic components of medical equipment, specifically central lines and respiratory support devices (27, 29, 32). Despite the recognized impact of early-life phthalate exposure on somatic growth of term-born children (5, 12, 14–16, 19–22, 33–35), phthalate exposure has not been considered as a potential contributor to atypical preterm infant growth previously.
In this study, we used the rich biospecimen and data repositories of the NICU Hospital Exposures and Long-Term Health (NICU-HEALTH) study to assess the impact of NICU-based phthalate exposure on extrauterine growth trajectories between birth and NICU discharge. Specifically, we hypothesized that phthalate exposure early in life would impact the timing and rate of accelerated weight gain (the “growth spurt”) during the expected period of rapid postnatal growth in the NICU. The objective of this study was to apply a comprehensive growth modeling technique to assess the impact of NICU-based phthalate exposure preterm infant growth between birth and NICU discharge.
2. MATERIALS AND METHODS
2.1. Participant population:
The NICU-HEALTH cohort (ClinicalTrials.gov NCT01420029, NCT01963065(30)), the first prospective environmental health cohort focused on preterm infants from its inception, enrolled a racially, ethnically, and socioeconomically diverse population of very preterm infants born less than 33 weeks gestation at a single urban academic medical center in New York City from September 2011 to February 2020. Infants with congenital anomalies or signs of hypoxic injury at birth were not eligible for enrollment. All families provide written informed consent prior to participation in NICU-HEALTH. The study was approved by the institutional review board of the Icahn School of Medicine at Mount Sinai.
2.2. Exposure assessment:
Serial urine specimens were collected by trained study staff between the week of birth and NICU discharge using methods described previously(28). Diapers are changed every three hours in the NICU, so there is no distinction between“first morning” and “random” void in this population. Specimens were collected during the day, most commonly between 10am and 4pm. Briefly, cotton balls were placed in the infant diaper and retrieved three hours later. Urine was squeezed from cotton not contaminated with stool. Specimens were refrigerated after collection, then frozen within 6 hours of collection at −80°C pending batch analysis. As we measure phthalate metabolites in urine rather than the parent phthalate diester, contamination from collection materials was not anticipated. Nonetheless, collection materials were screened for contamination. Field blanks collected alongside biospecimens were also analyzed for phthalate metabolites.
2.3. Outcome assessment:
Clinical daily weight measurements and once weekly length and head circumference measurements from birth through hospital discharge were abstracted by trained study staff. Study outcomes assess neurodevelopment, growth, and pulmonary outcomes longitudinally through early childhood as part of the Developmental Impact of NICU Exposures (DINE) study, which is in turn part of the National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) program(36, 37). Since it is common for infants to initially lose weight in the first few days of life(9, 38, 39) weight measurements obtained after the first 7 days of life were included in analyses. Additionally, as we were primarily interested in growth during the ex utero prenatal period, we limited curves to growth between 7 days of life and NICU discharge or the due date, postmenstrual age (PMA) of 40 weeks, whichever came first.
2.4. Phthalate biomarker analysis:
Concentrations of nine metabolites of six phthalate diesters were measured at the Senator Frank R. Lautenberg Environmental Health Sciences Laboratory at the Icahn School of Medicine at Mount Sinai from 200 μL urine specimens as described previously(28) with a minor modification. Measured metabolites were mono-ethyl phthalate (MEP), mono-benzyl phthalate (MBzP), mono-n-butyl phthalate (MBP), mono-isobutyl phthalate (MiBP), mono-(3-carboxypropyl) phthalate (MCPP), mono-2-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP). Quantification was based on isotope-dilution liquid chromatography with tandem mass spectrometry. 13C4 or D4 stable isotope labeled internal standards were added to each sample. Metabolites were treated with β-glucuronidase from Escherichia coli-K12 (product # 3707601001, Roche Diagnostics through Sigma Aldrich) followed by solid-phase extraction with an Oasis HLB hydrophilic-lipophilic balanced reversed-phase 96-well plate (30 mg sorbent per well, 30 μm particle size; Waters Corporation, Milford, MA). Low-volume sample aliquoting and cleanup procedure was automated using a liquid handler (epMotion 5075vtc; Eppendorf, Hauppauge, NY). The LC-MS/MS (Agilent 1290 Infinity II UHPLC coupled with 6470A triple quadrupole MS, Agilent Technologies, Wilmington, DE) was operated in electrospray negative mode for ionization and multiple reaction monitoring (MRM) for quantification. Chromatographic separation was achieved on a Zorbax RRHD Eclipse Plus C18, 1.8 μm, 100 × 2.1 mm analytical column with 5 × 2.1 mm guard cartridge (Agilent Technologies, Wilmington, DE). Mobile phase A was 0.1% formic acid in LC-MS grade water, and mobile phase B was 0.1% formic acid in LC-MS grade acetonitrile. Quality assurance measures included the insertion of National Institute of Standards and Technology standard reference materials NIST SRM 3672 Organic Contaminants in Smokers’ Urine and NIST SRM 3673 Organic Contaminants in Non-Smokers’ Urine (two each per batch), and bi-annual participation in the German External Quality Assessment Scheme (G-EQUAS) (http://www.g-equas.de/)(40). Laboratory staff were blinded to field blanks and established standards analyzed alongside participant specimens. Urine specific gravity was measured with a refractometer, and small volume method (10μL) (Rudolph Research Analytical, Hackettstown, NJ, USA), detection limit 1.0000 kg m−3(41).
2.5. Statistical Approach:
Growth curves were modeled for all infants with available growth data using proc nlin in SAS v.9.4. For each participant, a double logistic curve was fit to weight measures(42). Curves were examined visually, and when appropriate a triple logistic curve was fit. Model fit was assessed using the mean squared error (MSE). Curves with an MSE greater than the 95th percentile were removed from subsequent analyses. Each inflection point on the growth curve represents a distinct period of rapid growth or a “growth spurt”. Analyses were focused on the first growth spurt, regardless of whether the curve was modeled by double or triple logistic regression. Parameters estimating time to first growth spurt and rate of first growth spurt were pulled from the logistic curve fit per participant. Further details on how parameters were calculated can be found in Tanner et al 2020.
Since phthalate metabolite data were right skewed, values were log2-transformed to assess effects for every doubling of the chemical concentration. Single chemical multivariable regression models were used to assess the linear effect of each log2-transformed phthalate on the latency to first growth spurt relative to PMA (D1) and on the rate of first growth spurt (B1) (Figure 5) using the geometric mean of specific gravity-corrected urinary phthalate metabolite concentrations measured prior to the first inflection point to increase generalizability (Table S2). Adjustment for concentration was calculated for each urinary phthalate concentration using the formula [(Biomarker in μmol)*(median batch specific gravity - 1) / (specific gravity - 1)](28). The typical time to the first growth spurt was 1–3 weeks from birth, meaning that phthalate exposure was estimated by the geometric mean of a small number of individually measured biomarker levels (Table 1). Phthalate biomarker concentrations have previously been shown to fall appreciably over the course of the NICU hospitalization in our cohort (30). Differences in phthalate concentration are related most directly to degree of medical intervention rather than age, however (27–29). Models were adjusted for relevant factors selected by a directed acyclic graph (DAG; Appendix Figure A1).
Figure 5:
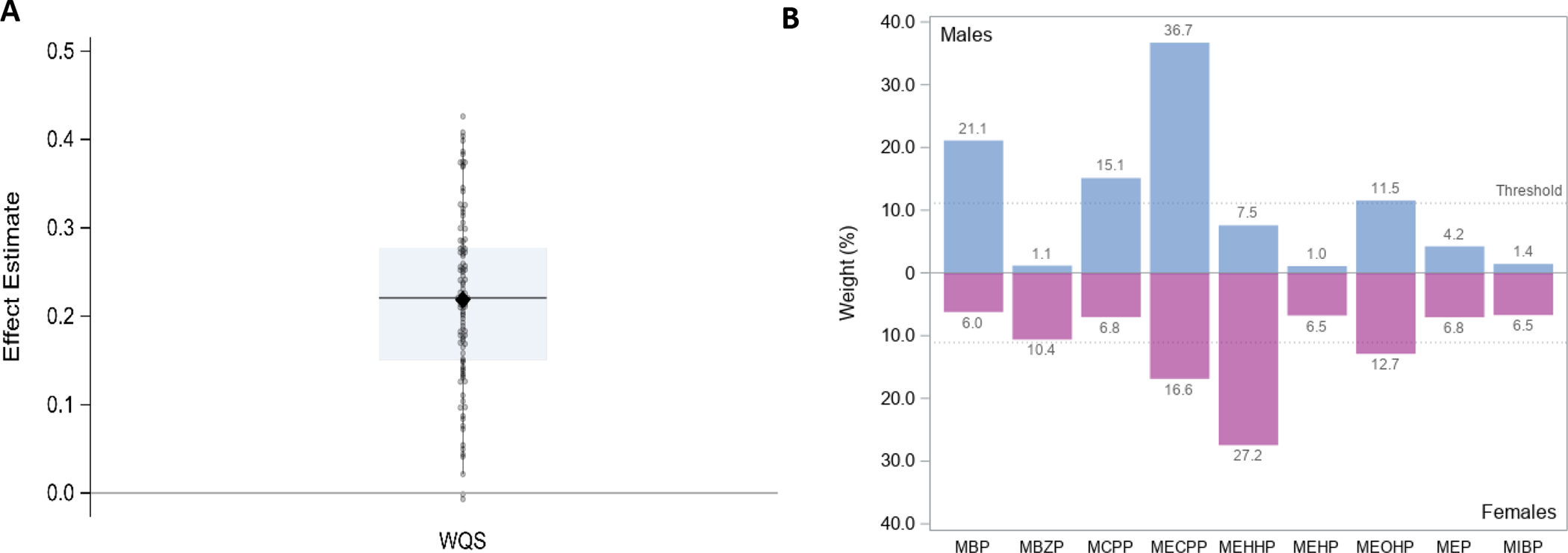
Stratified model for growth spurt rate. Results from 100 repeated holdouts testing the association between the phthalate index and rate of first growth spurt, controlling for sex, SGA, NICU morbidity score, and year of birth, and including stratified weights only (N=96; training n~38, validation n~58). A. shows the distribution of WQS estimates where data points represent the estimate for each holdout and the closed diamond shows the mean effect estimate and the boxplot shows the 25th, 50th, and 75th percentiles and the whiskers show the 2.5th and 97.5th percentiles. B. shows the relative weight of each phthalate metabolite, using the average weight from the 100 holdouts divided by the sum of the averages, stratified by sex.
Table 1:
Characteristics of study cohort, overall and stratified by sex (N=101)
| Overall (N=101) | Males (N=51) | Females (N=50) | |
|---|---|---|---|
|
| |||
| Insurance, N (%) | |||
| Medicaid | 24 (23.8) | 16 (31.4) | 8 (16.0) |
| Private/Self pay | 77 (76.2) | 35 (68.6) | 42 (84.0) |
|
| |||
| Race, N (%) | |||
| White (non-Hispanic) | 48 (49.0) | 19 (39.6) | 29 (58.0) |
| Black (non-Hispanic) | 25 (25.5) | 10 (20.8) | 15 (30.0) |
| Asian (non-Hispanic) | 3 (3.0) | 3 (6.3) | 0 (0.0) |
| Hispanic | 9 (9.2) | 8 (16.7) | 1 (2.0) |
| Mixed Race | 4 (4.1) | 2 (4.2) | 2 (4.0) |
| Other | 9 (9.2) | 6 (12.5) | 3 (6.0) |
| Missing | 3 | 3 | 0 |
|
| |||
| Small for gestational age (SGA), N (%) | 11 (10.9) | 9 (17.6) | 2 (4.0) |
|
| |||
| NEC > stage I, N (%) | 2 (2.0) | 2 (3.9) | 0 (0.0) |
|
| |||
| ROP stage, N (%) | |||
| 0 | 89 (89.0) | 42 (84.0) | 47 (94.0) |
| 1 | 2 (2.0) | 1 (2.0) | 1 (2.0) |
| 2 | 8 (8.0) | 6 (12.0) | 2 (4.0) |
| 3 | 1 (1.0) | 1 (2.0) | 0 (0.0) |
| Missing | 1 (1.0) | 1 (2.0) | 0 (0.0) |
|
| |||
| IVH grade, N (%) | |||
| 0 | 79 (78.2) | 40 (78.4) | 39 (78.0) |
| 1 | 19 (18.8) | 9 (17.3) | 10 (20.0) |
| 2 | 1 (1.0) | 1 (2.0) | 0 (0.0) |
| 3 | 0 (0.0 | 0 (0.0) | 0 (0.0) |
| 4 | 2 (2.0) | 1 (2.0) | 1 (2.0) |
|
| |||
| Sepsis, N (%) | 11 (10.9) | 9 (17.7) | 2 (4.0) |
|
| |||
| BPD, N (%) | 17 (17.0) | 10 (10.0) | 7 (14.0) |
|
| |||
| Gestational age (weeks), Mean (SD) | 30.0 (2.2) | 29.8 (2.4) | 30.3 (1.9) |
|
| |||
| Birth weight (grams), Mean (SD) | 1326 (364) | 1261 (380) | 1393 (338) |
|
| |||
| Apgar score at 1 min, Median (IQR) | 8 (2) | 8 (3) | 8 (2) |
|
| |||
| Apgar score at 5 min, Median (IQR) | 9 (0) | 9 (1) | 9 (0) |
|
| |||
| Maternal age at delivery (years), Mean (SD) | 32.7 (6.4) | 31.3 (6.5) | 34.2 (6.1) |
|
| |||
| Time to first growth spurt (weeks), Mean (SD) | 33.5 (1.71) | 33.5 (1.78) | 33.4 (1.66) |
|
| |||
| Slope of first growth spurt (kg/week), Mean, (SD) | 11.5 (36.9) | 14.1 (46.1) | 8.77 (24.5) |
|
| |||
| # of samples between birth and first growth spurt, Mean (SD) | 2 (1.4) | 2 (1.6) | 2 (1.3) |
Due to the complex correlation pattern of phthalate metabolites, as shown in Figure 1, repeated holdout weighed quantile sum regression(42) was used to assess the effect of the phthalate mixture on the same outcomes. Chemical component weights were estimated on a randomly selected 40% of subjects. The instability in estimation of weights, due to the complex correlations between components, can be improved by focusing the inference in a specified direction with a powerful 1 degree of freedom test. Since this is the first study to assess phthalates on growth of pre-term infants in the NICU, we could not specify a direction a priori and thus tested mixture effects in both the positive and negative directions. Thus, two-sided confidence intervals were reported. The final weighted index was calculated as , where WQS is the mixture term, Wi is the mean weight per variable averaged across 100 bootstraps, and is the deciled measurement per subject/variable. The effect of this index on each outcome was assessed with the remaining 60% of subjects. Typically, WQS assumes linearity of the effect of the mixture on outcome; however, we also tested for nonlinearity with a quadratic term to determine if a more flexible model was needed. The full WQS analysis was repeated for 100 holdouts of randomly selected 40/60 splits of the data. Models were adjusted for the same factors used in single chemical modeling.
Figure 1:
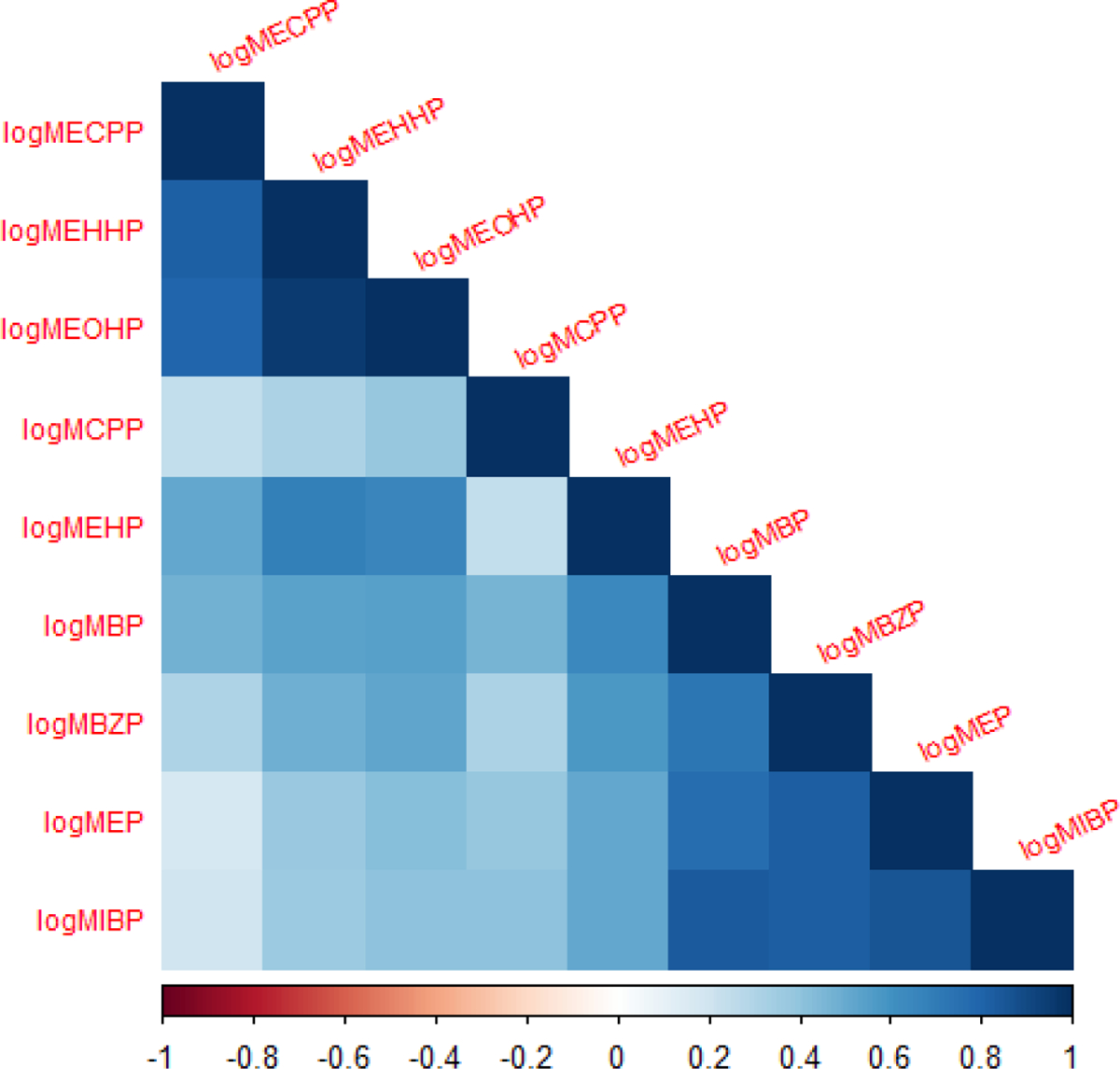
Correlation matrix of phthalate metabolites among the study population.
As a sensitivity analysis, a bootstrapped lasso regression, with 500 bootstraps and 10 nfolds, was run to confirm that metabolites highly weighted in the WQS regression were also identified in the lasso. Specifically, lasso regression is a shrinkage method that identifies a parsimonious model among predictors that may be highly correlated, such that one variable may be retained while the beta coefficient of other variables that are highly correlated are forced to zero. All WQS regression analyses were run with the gWQS package in RStudio (Version 1.2.5001) and all figures were created using SAS (v 9.4).
Distribution of each final chemical weight component and the beta coefficient of the effect on growth measures were examined using the 100 holdouts. We defined significance when at least 97.5% of effect estimates were above or below the null effect using a two-sided test. When at least 90% of the chemical component weight distributions were greater than the 1/c threshold, we defined the component to be a probable contributor to the overall mixture effect. When at least 50% of holdouts were above the threshold, we defined the component to be a possible contributor to the mixture effect.
Further, as phthalate effects have been shown to be sexually dimorphic in other studies(14, 33), we assessed sex as an effect modifier of the relationship between a phthalate mixture and both outcomes. The gWQS package has the capability to include interactions between covariates as well as specify stratification of the model’s weight estimation. Throughout this report, we refer to a model run with an interaction term between WQS and sex in addition to the main effects and other covariates as an “interaction model”. We refer to a WQS model in which weight components are estimated for each strata as a “stratified weight model”. This model had twice as many parameters estimated in the training dataset, one set for males and one set for females but assessed only one overall effect. We refer to a model which includes both components as a “stratified/interaction model”. This model combined both components such that twice as many parameters were estimated in the presence of an interaction term between WQS and sex in addition to the main effect and other covariates.
We began by testing the most flexible model – the stratified/interaction WQS. If the same components were identified as important for both sexes, based on criteria presented previously, then we would test the interaction model which assumes the same weights per sex, but tests if the effect between sexes differ. If the same components were not identified as important between the sexes and the effect did not differ between sexes, then we would test the stratification model which assumes different chemical mixtures per sex resulting in the same effect sizes. When an analysis estimated common weights, meaning one weight was estimated per chemical component for the entire population, the distribution of weight estimates across the 100 holdouts is shown. When an analysis estimated stratified weights, meaning one weight is estimated per chemical component per stratum, the relative contributions were calculated as where is the average weight from the 100 holdouts per chemical/stratum and is the sum of the stratum-specific average weights. The relative weights sum to 100% per stratum.
3. RESULTS
One hundred thirty-eight NICU-HEALTH participants had urinary phthalate biomarkers measured during the NICU hospitalization and 101 had appropriate data for analysis. Appendix Figure A2 is a CONSORT-style diagram that describes progression from initial to final analytic cohort. Table 1 demonstrates clinical and demographic characteristics of the diverse analytic cohort, overall and stratified by sex.
Four hundred ninted-six urine specimens were analyzed for phthalate biomarkers in 14 batches. The mean concentrations of phthalate metabolites were similar across batches (Supplementary Table 1). The coefficients of variance were low and within the acceptable range, indicating good intra-batch consistency. Since NIST provides reference values, mean percent recovery was calculated for each phthalate. All recoveries were within an acceptable tolerance and thus no batch correction was needed. Phthalate levels varied by species but not by infant sex, and decreased over the course of the NICU hospitalization as presented in detail in Stroustrup et al., 2018(28). Phthalate levels by species and sex are presented in Supplementary Table 2.
Growth curves were fit for 135 participants. Goodness of fit was assessed using MSE, such that curves with the top 5% of MSE were removed due to poor fit. Appendix Figure A3 shows the distribution of MSE and the 95% percentile. In addition, example curves are shown above and below this threshold to demonstrate what was considered a “good fit” and what was considered a “bad fit”.
Single chemical species were modeled as a preliminary step to mixture analyses (Table S2). Regression diagnostics revealed that models assessing slope did not meet assumptions necessary for linear regression (residuals were not normally distributed); thus, the natural log of growth spurt speed was used for all subsequent analyses. Initially, several phthalate metabolites were identified as being significantly related to latency to first growth spurt and natural log transformed speed of growth spurt. Only MEHHP and MEOHP remained significantly and negatively associated with latency after false discovery rate correction to account for multiple comparisons.
Mixture models were assessed with both a positive and negative constraint. There were no positive estimates found when assessing the phthalate mixture on latency to growth spurt in the positive direction. There was evidence of nonlinearity when assessing the mixture on latency in the negative direction (Figure S4). To account for this nonlinearity, an additional WQS regression model, with a quadratic term for the WQS, was repeated 100 times using different sets of participants for training and validation. Table 2 provides a summary of the distribution of effect estimates for both the linear WQS term and the quadratic WQS2 term across the 100 holdouts. Figure 2 provides the distribution of the 100 regression models.. Seventy of the holdouts resulted in a positive linear effect of the index and 86 holdouts resulted in a negative nonlinear effect (i.e. WQS2; Figure 2A). Though not considered significant, based on criteria described previously, there is some evidence that at lower levels, the phthalate mixture has no effect or a slight delay in latency to first growth spurt (as indicated by positive/negligible WQS term) but at higher phthalate mixture levels, the growth spurt occurs earlier (as indicated by a highly negative WQS2 term). Figure 2B provides the distribution of each phthalates weight contributions across the 100 holdouts. MCPP, MBzP, and MEP are possibly important contributors to the phthalate mixture effect.
Table 2:
Results from 100 repeated holdouts testing the association between the phthalate WQS index and PMA at first growth spurt (latency) and slope of the first growth spurt (growth rate), controlling for sex, SGA, NICU morbidity score, and year of birth.
| Outcome | Parameter | Mean | Median | 2.5th percentile | 97.5th percentile |
|---|---|---|---|---|---|
| Latencya | WQS | 0.17 | 0.21 | −0.39 | 0.64 |
| WQS2 | −0.045 | −0.049 | −0.103 | 0.030 | |
| Growth rateb | WQS | 0.13 | 0.13 | 0.05 | 0.21 |
N=101 (training n~40, validation n~61); a nonlinear effect was assessed by including a WQS*WQS term
N=96 (training n~38, validation ~58); βs were constrained in the positive direction; log transformed growth rate to meet assumptions of linear regression
Figure 2:
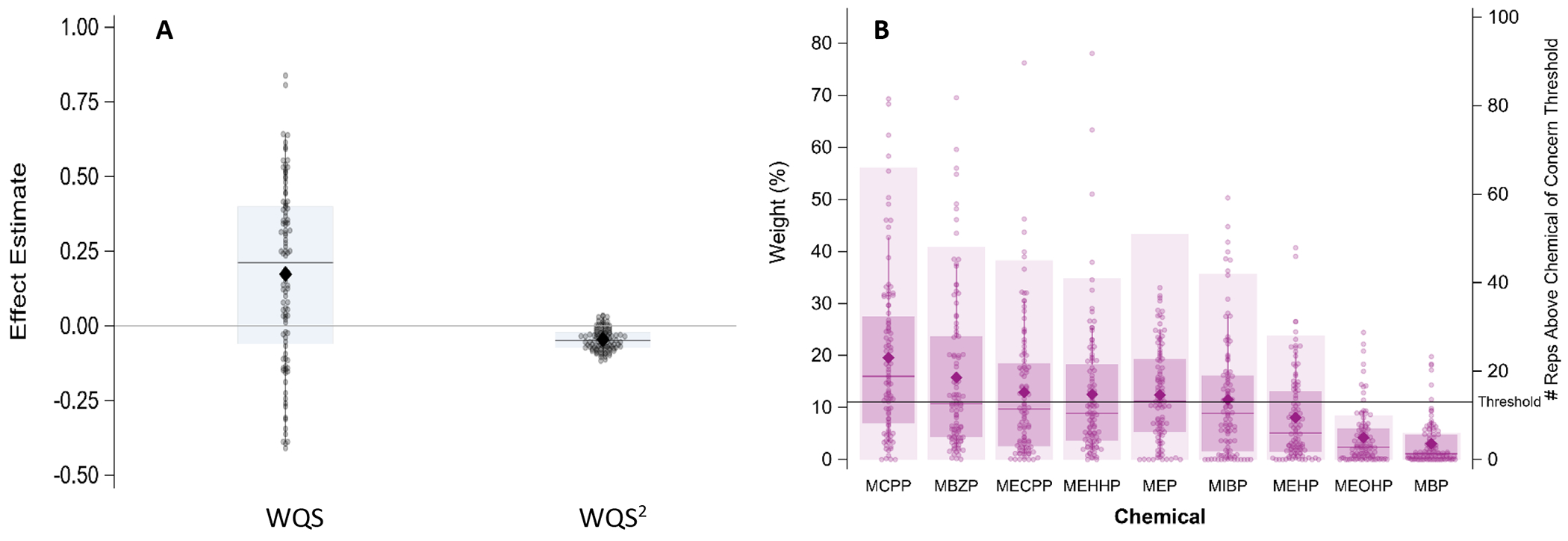
Results from 100 repeated holdouts testing the association between the phthalate index and latency to the first growth spurt, adjusting for sex, SGA, NICU morbidity score, and year of birth (N=101; training n~40, validation n~61). A. shows the distribution of WQS and WQS2 estimates and B. shows the distribution of weights for each phthalate metabolite. For both A. and B., data points represent the estimate for each holdout and the closed diamond shows the mean effect estimate. For A., the boxplot shows the 25th, 50th, and 75th percentiles and the whiskers show the 2.5th and 97.5th percentiles and for B., the boxplot shows the 25th, 50th, and 75th percentiles and the whiskers show the 10th and 90th percentiles.
WQS regression modeling, was then repeated 100 times to examine the effect of phthalate exposure on the natural log transformed slope at the first inflection point, or the rate of the first growth spurt. Of the 101 curves, 96 had growth spurt speeds that were within two standard deviations of the mean. Table 2 summarizes the distribution of linear effect estimates across the 100 holdouts and suggests a linear positive association between the phthalate mixture and slope of the first growth spurt without evidence of nonlinearity. Figure 3 provides the distribution of each phthalate metabolite’s weight contribution across the 100 regression models. All holdouts resulted in a positive effect of the mixture on growth rate (Figure 3A). The effect was driven by MECPP with MEHHP and MEOHP as possibly important contributors (Figure 3B).
Figure 3:
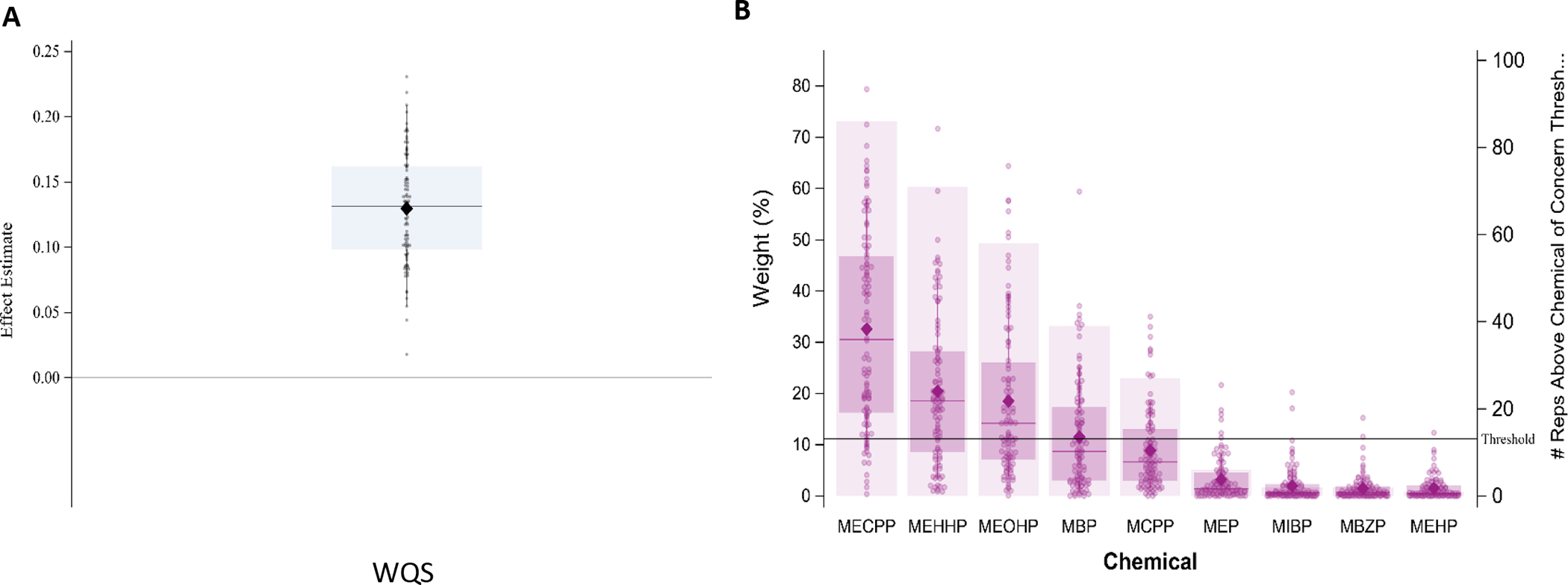
Results from 100 repeated holdouts testing the association between the phthalate index and rate of the first growth spurt, controlling for sex, SGA, NICU morbidity score, and year of birth (N=96; training n~38, validation n~58). A. shows the distribution of WQS estimates and B. shows the distribution of weights for each phthalate metabolite. For both A. and B., data points represent the estimate for each holdout and the closed diamond shows the mean effect estimate. For A., the boxplot shows the 25th, 50th, and 75th percentiles and the whiskers show the 2.5th and 97.5th percentiles and for B., the boxplot shows the 25th, 50th, and 75th percentiles and the whiskers show the 10th and 90th percentiles.
Next, we tested sex as an effect modifier on the relationship between phthalates and latency of first growth spurt. There was no evidence of non-linearity, so models were fit with a linear WQS term. The majority of holdouts for males resulted in a negative association (93 out of 100) and 91 of the holdouts resulted in females having a larger magnitude of effect compared to males (Figure 4A). MECPP, MBzP, and MCPP were most important in the males’ mixture and MECPP, MEHHP, MBzP and MEP were most important in the females’ mixture (Figure 4B).
Figure 4:
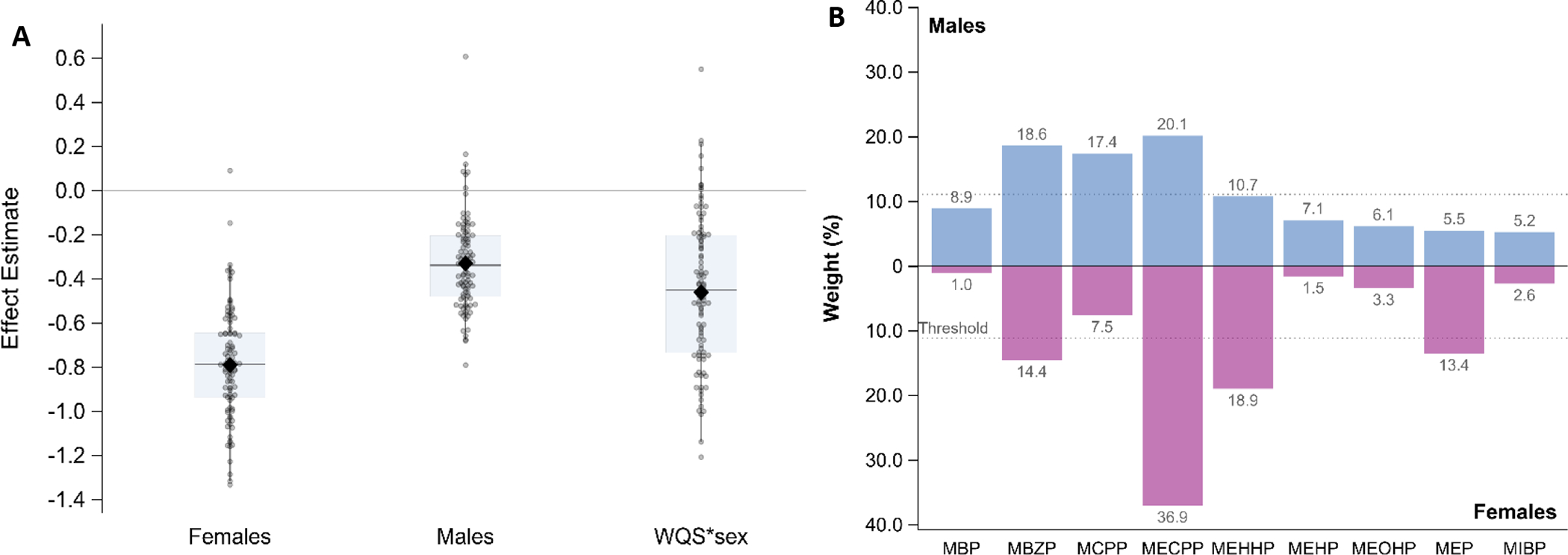
Stratified/interaction model for latency to first growth spurt. Results from 100 repeated holdouts testing the association between the phthalate index and latency to first growth spurt (in PMA weeks), controlling for sex, SGA, NICU morbidity score, and year of birth, and including stratified weights and an interaction between the mixture and sex (N=101; training n~40, validation n~61). A. shows the distribution of WQS and WQS*sex estimates where data points represent the estimate for each holdout and the closed diamond shows the mean effect estimate and the boxplot shows the 25th, 50th, and 75th percentiles and the whiskers show the 2.5th and 97.5th percentiles. B. shows the relative weight of each phthalate metabolite, using the average weight from the 100 holdouts divided by the sum of the averages, stratified by sex.
Finally, we tested sex as an effect modifier on the relationship between phthalates and rate of first growth spurt. The stratified/interaction model, with a negative constraint, resulted in a slightly stronger magnitude of effect estimates for both males and females, with the interaction term being negligible (Appendix Figure A4). However, this model informed us that mixture components differed between males and females, such that the males’ mixture was dominated by MECPP, MBP, MBzP, and MCPP whereas for females MECPP, MEHHP, and MEOHP, were the most important contributors to the mixture. Since this model showed different mixtures for males and females but did not have a significant difference in effect, we tested the stratified weight only model and found that most effect estimates were positive (98 out of 100); Figure 5A). The mixture for males was dominated by MECPP, MBP, MCPP, and MEOHP and while the mixture for females was dominated by MECPP, MEHHP, and MEOHP (Figure 5B).
Results from lasso regression confirm results from WQS analyses, using log transformed growth rate as the outcome (Table S3). As compared to the WQS regression analyses, where MECPP, MEHHP, and MEOHP were all possible contributors to the mixture effect, the lasso regression retained MECPP and MEOHP, but only MEOHP had a significant positive beta coefficient. As described in the methods, lasso uses a shrinkage method where it randomly choses one variable and reduces down or forces the highly correlated variables to zero. In this case, Figure 1 supports high correlations among these three possible contributors. Thus, lasso supports the WQS findings that at least one of these high molecular phthalate metabolites is related to increased growth rate. Those metabolites that were identified as probably not contributors in the WQS analyses were all retained in the lasso but with negative beta coefficients. Only MBZP has a significantly negative beta. We did not run the lasso for latency for simplicity since the WQS regression assessed a nonlinear term.
4. DISCUSSION
In this report, we describe the application of a comprehensive growth modeling technique(42) to the novel question of the impact of chemical exposure on in-hospital preterm infant growth. Our study has four main findings: 1) in single chemical analysis, exposure to MEHHP and MEOHP, two metabolites of the common plasticizer DEHP, is associated with shorter latency of the first growth spurt; 2) in mixture analysis, exposure to MCPP, MBzP and MEP, metabolites of phthalates found in polyvinyl chloride flooring, sealants, and paints as well as vinyl gloves and personal care products (e.g. creams, shampoos), is associated with a shorter latency to the first growth spurt (i.e. an earlier growth spurt); 3) exposure to MECPP, MEHHP, and MEOHP, all DEHP metabolites, is assocated with an increased rate of growth at the first growth spurt in the overall study population; and 4) the impact of phthalate exposure on timing of the first growth spurt was sexually dimorphic, with different mixtures demonstrating stronger impact on girls than boys.
The hospital environment is known to be a source of significant exposure to endocrine disrupting organic chemicals including phthalates(43, 44). Since the early 2000s, multiple cohorts have demonstrated clinically meaningful phthalate exposure among hospitalized preterm infants(26–29, 31, 45). Although third trimester in utero phthalate exposure is known to be associated with alterations in growth trajectories in term-born populations (14, 15, 33, 35), the role of NICU-based phthalate exposure on preterm infant growth has not been examined previously. Notably, our preterm infant population demonstrates much higher levels of phthalate exposure than pregnant women participating in NHANES during the same time period of fetal neurodevelopment (Appendix Figure 5). Specifically, studies in term-born populations have shown associations between phthalate exposure and both early growth restriction and higher BMI later in life(12, 17, 35). Preterm infants are known to be at risk for later life adverse neurodevelopmental outcomes, obesity, and metabolic syndrome(6–11). As early life phthalate exposure may impact growth trajectories, including timing and rate of weight gain, and as preterm infants are known both to face significant phthalate exposure and experience abnormal growth trajectories, we were interested in whether hospital-based phthalate exposure could impact specific parameters of preterm infant weight gain.
Pediatric growth is marked by periods of rapid growth, or discrete growth spurts, interspersed with periods of more moderate growth rate(46). On a smaller timescale, this pattern is also seen in ex utero growth during the “preterm period” between preterm infant birth and term-equivalent(9). Preterm infants typically lose weight – largely water weight – in the first week after birth, then establish daily weight gain until discharge(9, 38, 39). Prior studies of preterm infant growth typically consider only absolute and relative weight gain across the NICU hospitalization, rather than growth metrics such as the timing and peak growth velocity of NICU-based growth spurt(s). We know from study of term-born children that early life growth spurts are both (1) sensitive to phthalate exposure and (2) linked to later life overweight and obesity(1, 3), although the mechanism behind these epidemiologic observations remain poorly understood. Based on this limited existing data, we chose the timing and rate of the first ex utero growth spurt as a relevant outcome metric for this study(5) as predictive of later life obesity.
Our statistical approach, first described by Tanner et al. in a study of the impact of prenatal exposure to perfluorooctanoic acid on childhood growth of term-born children in Sweden(42), allows for determination of the impact of chemical exposure on specific aspects of infant weight gain over time. Our growth models demonstrate that despite differences in medical course, nutritional approach, and enteral feeding tolerance between study participants, the vast majority of preterm infants studied had growth that could be modeled similarly, demonstrating at least one discrete period of rapid growth – a growth spurt – during the NICU hospitalization. Our approach allowed identification in differences in growth dynamics not available by more traditional approaches used to evaluate preterm infant growth that compare growth rates or weight parameter z-scores cumulatively across the NICU hospitalization.
It is well documented in the neonatology literature that in-hospital growth is of critical importance to the long-term health of children born preterm. Catch-up growth from the first week of life to term equivalent is associated with improved performance on neurodevelopmental testing later in childhood(47). It has previously been shown that phthalate exposure in the NICU is associated with improved neurobehavioral performance at NICU discharge (28). The findings of this study provide a possible mechanism ripe for further study of how this link may occur. Rather than direct effect on neurological development, phthalate exposure during this sensitive period may improve catch-up growth, which may in-turn contribute to improved neurodevelopment.
Similar to growth restricted fetuses, preterm infants are at known risk of overweight, obesity, and metabolic syndrome later in life(7, 8). Phthalate exposure early in life has also been linked to later overweight and obesity (15–19). Prior studies of ex utero preterm infant growth focus on nutritional practices and/or the impact of medical illness without consideration of environmental exposures both known to be prevalent in the NICU and known to alter growth trajectories in other populations(6, 9–11, 38, 39), making this study a highly novel investigation. As alteration of timing and rate of early-life growth spurts are predictive of later obesity (1, 3, 5), chemical exposure in the NICU may be a previously unrecognized yet modifiable contributor to to later-life obesity among individuals born preterm. Long-term follow-up of our cohort is needed to fully evaluate this hypothesis.
By using double and triple logistic modeling approaches, we identified specific mixtures of phthalate metabolites that are associated with both earlier onset and increased growth rate during the primary NICU-based growth spurt. Consistent with other studies of endocrine disrupting chemicals, our results are sexually dimorphic, with different mixtures and different effects on girls compared to boys. Phthalates share key similarities in chemical structure to endogenous sex hormones. As such, they exert their effet through interaction with estrogen and androgen hormone receptors (48–50). Multiple studies have demonstrated that phthaltes may act in a sexually dimorphic manner through epigenetic mechanisms as well (49, 51, 52). In the NICU population, where overall mortality and certain morbidities are known to have differing rates by baby sex (53–55), our findings may indicate that phthalate exposure could contribute to this observation.
Identification of a NICU-based growth spurt with timing and growth rate associated with phthalate exposure indicates that, despite nutritional practices aimed at consistent weight gain across the NICU hospitalization, environmental chemical exposures may impact ultimate weight gain trajectory for preterm infants.
Our findings are provocative considering what is known about infant growth and long-term outcome. In-hospital growth between birth and NICU discharge is associated both with lifetime risk of metabolic syndrome and with neurodevelopmental outcome(6–8, 11, 47). In terms of obesity risk specifically, this mimics the numerous studies that have documented the association between poor growth in early life followed by excess weight gain leading to metabolic syndrome(56, 57). First described by Barker in relation to fetal undernutrition during the Dutch Famine(58), the pattern of poor early weight gain followed by excess weight gain has been replicated in numerous studies of endocrine disrupting chemicals, including phthalates, over the subsequent decades(12, 17, 18, 35). In parallel, poor growth during the ex utero prenatal period has been associated with later life overweight, obesity, and cardiovascular risk(8, 9, 56, 57). These findings persist despite significant improvements in preterm infant nutrition(39) and widespread understanding of the importance of nutrition and growth during the NICU stay(6, 10, 11, 39). As the first study of the impact of NICU-based chemical exposure on preterm infant growth, we raise the question of whether potentially modifiable hospital-based exposures could impact growth trajectory. Although the exact mechanism of action of early life phthalate exposure on growth remains under investigation, a number of plausible pathways via endocrine receptor activation or blockade, endocrine hormone level alteration, and epigenetic changes have been suggested(34). A conceptual framework for the impact of hospital-based phthalate exposure on preterm infant weight gain is presented in Figure 6.
Figure 6:
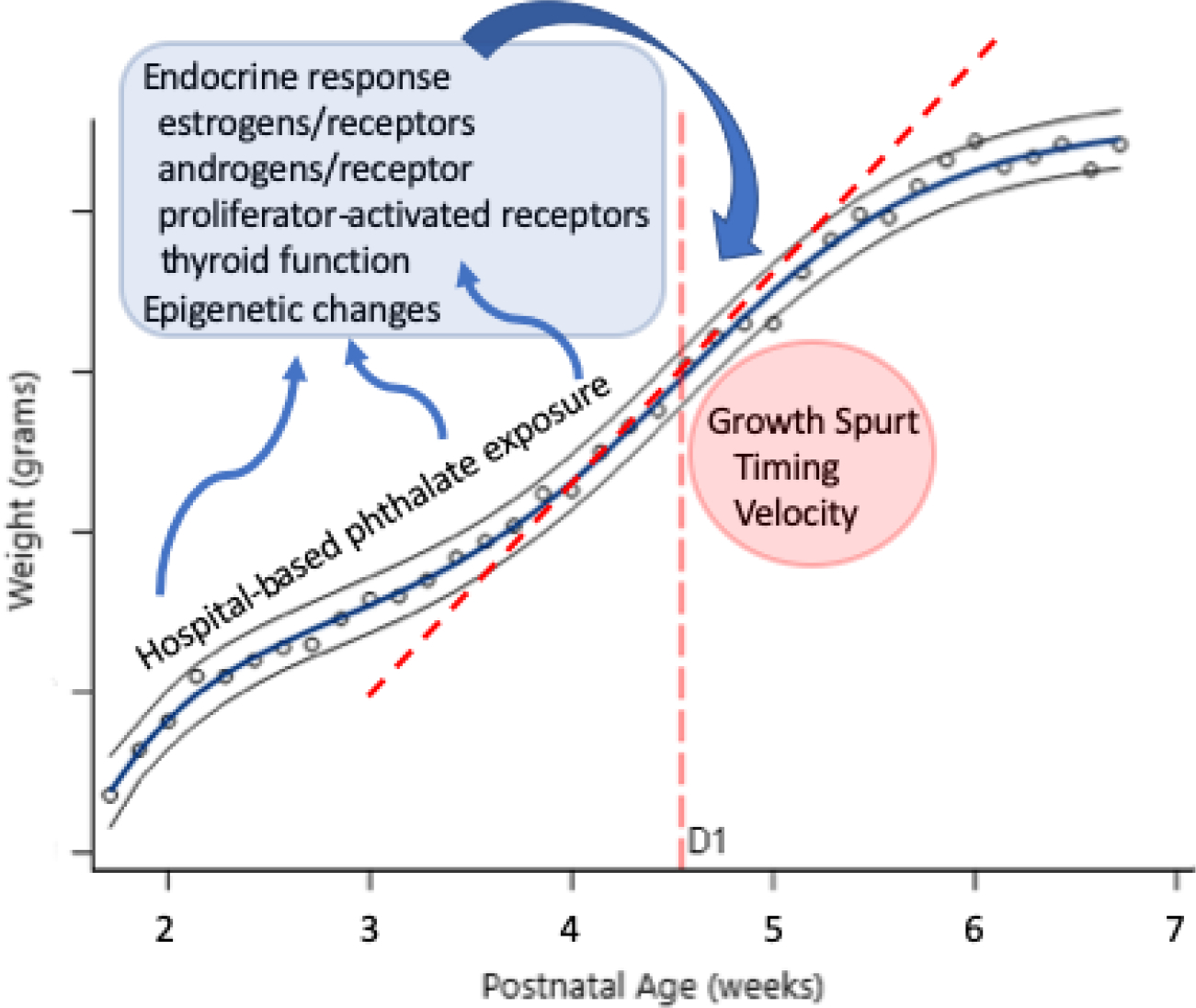
Proposed conceptual framework for NICU-based phthalate exposure and alteration in early growth trajectory during the neonatal period. Phthalate exposure causes endocrine disruption and epigenetic changes in vitro, in animal models, and in non-preterm human studies. These changes could all impact the timing and velocity of the first growth spurt.
Phthalate exposure in the NICU is likely to occur via a variety of sources. The phthalate metabolites highlighted for concern in this study include biomarkers of the low molecular weight phthalates butylbenzyl phthalate, diethyl phthalate, and di-n-butyl phthalate as well as high molecular weight phthalates di-n-octyl phthalate and di(2-ethylhexyl) phthalate. These parent phthalate compounds can leach or off-gas from the built NICU environment (floor tiles), plastic packaging of disposable medical supplies, personal care products used by NICU families or staff, or directly from disposable medical equipment including flexible plastic tubing used for intravenous access or respiratory support (28, 29). As components of the highly controlled NICU environment, these exposures may be modifiable with close attention to NICU construction, medical materials manufacture, and care practices. One unavoidable limitation of our study is that we cannot comment on the impact of chemical species not included in our analytic panel. Nonphthalate plasticisers and other emerging DEHP replacements may also play an important role in preterm infant growth and development.
As a novel approach to preterm infant growth modeling, our study has a number of specific strengths. We provide a more complete characterization of preterm infant growth compared to cumulative weight measures examining only differences between weight at birth and NICU discharge. The ability to identify simple, individual child growth metrics allows for flexibility in regression modeling of the impact of various factors, including environmental exposures, on growth.
Although we applied our technique to weight gain, the most common measure of growth in NICU-based studies, it could also be applied to other important measures including length and head circumference. We focused on weight in this study due to the increased density of data – weight is measured in our NICU daily while length and head circumference are measured weekly – as well as improved precision of electric scale-based weight measurement compared to clinical length and head circumference measures that are obtained manually with a tape measure. For certain long-term outcomes including neurocognitive and pulmonary development, length or head circumference may be the more appropriate surrogate marker. Finally, we modeled the unconditional growth curve of each child individually, after Tanner et al.(42) As noted in that report, there are more complex strategies available to model all study participants simultaneously that could also have been chosen.
5. CONCLUSIONS
Growth curve modeling allows for evaluation of discrete periods of rapid growth during the NICU hospitalization. Exposure to specific phthalates during the NICU hospitalization may both result in an earlier onset of the first growth spurt and result in more rapid growth at the time of the first growth spurt in a sexually dimorphic manner. Phthalate exposure during the NICU hospitalization may impact ex utero growth during the preterm period.
Supplementary Material
ACKNOWLEDGEMENTS
The Senator Frank R. Lautenberg Environmental Health Sciences Laboratory at the Icahn School of Medicine at Mount Sinai acknowledges Anil Meher, PhD, and Shirisha Yelamanchili, MS, who performed the measurements of phthalates metabolites and specific gravity in urine.
Funding Sources:
This work was supported by the National Institutes of Health [K23ES022268, UG3OD023320, UH3OD023320, P30ES023515]. and by the Passport Foundation and the Mount Sinai Children’s Environmental Health Center through pilot grants.
Financial Disclosure:
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations:
- BMI
body mass index
- DAG
directed acyclic graph
- DINE
Developmental Impact of NICU Exposures cohort
- ECHO
Environmental Influences on Child Health Outcomes program
- IQR
interquartile range
- MBP
mono-n-butyl phthalate
- MBzP
mono-benzyl phthalate
- MCPP
mono-(3-carboxypropyl) phthalate
- MECPP
mono-(2-ethyl-5-carboxypentyl) phthalate
- MEHHP
mono-(2-ethyl-5-hydroxyhexyl) phthalate
- MEHP
mono-2-ethylhexyl phthalate
- MEOHP
mono-(2-ethyl-5-oxohexyl) phthalate
- MEP
mono-ethyl phthalate
- MiBP
mono-isobutyl phthalate
- MSE
mean squared error
- NICU
neonatal intensive care unit
- NICU-HEALTH
NICU Hospital Exposures and Long-Term Health study
- NIST SRM
National Institute of Standards and Technology standard reference material
- PMA
postmenstrual age
- QC
quality control
- SGA
small for gestational age
- WQS
weighted quantile sum
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
CRediT AUTHOR STATEMENT
Stefanie A. Busgang: Conceptualization, Methodology, Formal Analysis, Investigation, Writing – Original Draft, Visualization
Emily A. Spear: Conceptualization, Formal Analysis, Investigation, Data Curation, Writing – Review and Editing
Syam S. Andra: Methodology, Validation, Investigation, Resources, Writing – Review and Editing
Srinivasan Narasimhan: Formal Analysis, Validation, Investigation, Writing – Review and Editing
Jennifer B. Bragg: Conceptualization, Investigation, Writing – Review and Editing
Stefano Renzetti: Methodology, Formal Analysis, Investigation, Writing – Review and Editing
Paul Curtin: Methodology, Writing – Review and Editing
Mia Bates: Investigation, Writing – Review and Editing
Manish Arora: Methodology, Resources, Writing – Review and Editing, Supervision
Chris Gennings: Conceptualization, Methodology, Writing – Review and Editing, Supervision, Project administration
Annemarie Stroustrup: Conceptualization, Investigation, Writing – Original Draft, Supervision, Project administration, Funding acquisition
REFERENCES
- 1.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Smith GD, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26(1):19–26. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318(7181):427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gishti O, Gaillard R, Manniesing R, Abrahamse-Berkeveld M, van der Beek EM, Heppe DH, et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. J Clin Endocrinol Metab. 2014;99(7):2557–66. [DOI] [PubMed] [Google Scholar]
- 4.Singhal A Long-Term Adverse Effects of Early Growth Acceleration or Catch-Up Growth. Ann Nutr Metab. 2017;70(3):236–40. [DOI] [PubMed] [Google Scholar]
- 5.Svensson K, Tanner E, Gennings C, Lindh C, Kiviranta H, Wikstrom S, et al. Prenatal exposures to mixtures of endocrine disrupting chemicals and children’s weight trajectory up to age 5.5 in the SELMA study. Sci Rep. 2021;11(1):11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey L, Burnett A, Spittle AJ, Roberts G, Anderson P, Lee K, et al. Extreme prematurity, growth and neurodevelopment at 8 years: a cohort study. Arch Dis Child. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Baldassarre ME, Di Mauro A, Caroli M, Schettini F, Rizzo V, Panza R, et al. Premature Birth is an Independent Risk Factor for Early Adiposity Rebound: Longitudinal Analysis of BMI Data from Birth to 7 Years. Nutrients. 2020;12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni Y, Beckmann J, Hurst JR, Morris JK, Marlow N. Size at birth, growth trajectory in early life, and cardiovascular and metabolic risks in early adulthood: EPICure study. Arch Dis Child Fetal Neonatal Ed. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niklasson A, Engstrom E, Hard AL, Wikland KA, Hellstrom A. Growth in very preterm children: a longitudinal study. Pediatr Res. 2003;54(6):899–905. [DOI] [PubMed] [Google Scholar]
- 10.Sammallahti S, Pyhala R, Lahti M, Lahti J, Pesonen AK, Heinonen K, et al. Infant growth after preterm birth and neurocognitive abilities in young adulthood. J Pediatr. 2014;165(6):1109–15 e3. [DOI] [PubMed] [Google Scholar]
- 11.Tzarouchi LC, Drougia A, Zikou A, Kosta P, Astrakas LG, Andronikou S, et al. Body growth and brain development in premature babies: an MRI study. Pediatr Radiol. 2014;44(3):297–304. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Chen L, Li LX, Xie CM, Li D, Shi HJ, et al. Gender-specific relationship between prenatal exposure to phthalates and intrauterine growth restriction. Pediatr Res. 2014;76(4):401–8. [DOI] [PubMed] [Google Scholar]
- 13.Goodrich JM, Ingle ME, Domino SE, Treadwell MC, Dolinoy DC, Burant C, et al. First trimester maternal exposures to endocrine disrupting chemicals and metals and fetal size in the Michigan Mother-Infant Pairs study. J Dev Orig Health Dis. 2019;10(4):447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015;123(10):1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley JP, Engel SM, Braun JM, Whyatt RM, Daniels JL, Mendez MA, et al. Prenatal Phthalate Exposures and Body Mass Index Among 4- to 7-Year-old Children: A Pooled Analysis. Epidemiology. 2016;27(3):449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13(3):161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang TC, Peterson KE, Meeker JD, Sanchez BN, Zhang Z, Cantoral A, et al. Exposure to Bisphenol A and phthalates metabolites in the third trimester of pregnancy and BMI trajectories. Pediatr Obes. 2018;13(9):550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teitelbaum SL, Mervish N, Moshier EL, Vangeepuram N, Galvez MP, Calafat AM, et al. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ Res. 2012;112:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ Health Perspect. 2013;121(4):501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115(6):876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang-Peronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev. 2011;12(8):622–36. [DOI] [PubMed] [Google Scholar]
- 23.Fabrizio V, Shabanova V, Taylor SN. Factors in Early Feeding Practices That May Influence Growth and the Challenges that Arise in Growth Outcomes Research. Nutrients. 2020;12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenton TR, Chan HT, Madhu A, Griffin IJ, Hoyos A, Ziegler EE, et al. Preterm Infant Growth Velocity Calculations: A Systematic Review. Pediatrics. 2017;139(3). [DOI] [PubMed] [Google Scholar]
- 25.Fabrizio V, Trzaski JM, Brownell EA, Esposito P, Lainwala S, Lussier MM, et al. Individualized versus standard diet fortification for growth and development in preterm infants receiving human milk. Cochrane Database Syst Rev. 2020;11:CD013465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113(5):e429–34. [DOI] [PubMed] [Google Scholar]
- 27.Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, et al. Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environ Health Perspect. 2005;113(9):1222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroustrup A, Bragg JB, Andra SS, Curtin PC, Spear EA, Sison DB, et al. Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLoS One. 2018;13(3):e0193835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroustrup A, Bragg JB, Busgang SA, Andra SS, Curtin P, Spear EA, et al. Sources of clinically significant neonatal intensive care unit phthalate exposure. J Expo Sci Environ Epidemiol. 2020;30(1):137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroustrup A, Bragg JB, Spear EA, Aguiar A, Zimmerman E, Isler JR, et al. Cohort profile: the Neonatal Intensive Care Unit Hospital Exposures and Long-Term Health (NICU-HEALTH) cohort, a prospective preterm birth cohort in New York City. BMJ Open. 2019;9(11):e032758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weuve J, Sanchez BN, Calafat AM, Schettler T, Green RA, Hu H, et al. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ Health Perspect. 2006;114(9):1424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallow EB, Fox MA. Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks. J Perinatol. 2014;34(12):892–7. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YW, Gao H, Mao LJ, Tao XY, Ge X, Huang K, et al. Effects of the phthalate exposure during three gestation periods on birth weight and their gender differences: A birth cohort study in China. Sci Total Environ. 2018;613–614:1573–8. [DOI] [PubMed] [Google Scholar]
- 34.Muscogiuri G, Barrea L, Laudisio D, Savastano S, Colao A. Obesogenic endocrine disruptors and obesity: myths and truths. Arch Toxicol. 2017;91(11):3469–75. [DOI] [PubMed] [Google Scholar]
- 35.de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. First year growth in relation to prenatal exposure to endocrine disruptors - a Dutch prospective cohort study. Int J Environ Res Public Health. 2014;11(7):7001–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schantz SL, Eskenazi B, Buckley JP, Braun JM, Sprowles JN, Bennett DH, et al. A framework for assessing the impact of chemical exposures on neurodevelopment in ECHO: Opportunities and challenges. Environ Res. 2020;188:109709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Services USDoHaH. National Institutes of Health. Environmental Influences on Child Health Outcomes (ECHO) Program 2020. [Available from: https://www.nih.gov/research-training/environmental-influences-child-health-outcomes-echo-program.
- 38.Saenz de Pipaon M, Martinez-Biarge M, Dorronsoro I, Salas S, Madero R, Martos GA, et al. Growth in preterm infants until 36 weeks’ postmenstrual age is close to target recommendations. Neonatology. 2014;106(1):30–6. [DOI] [PubMed] [Google Scholar]
- 39.Scharf RJ, Stroustrup A, Conaway MR, DeBoer MD. Growth and development in children born very low birthweight. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goen T, Schaller KH, Drexler H. External quality assessment of human biomonitoring in the range of environmental exposure levels. Int J Hyg Environ Health. 2012;215(2):229–32. [DOI] [PubMed] [Google Scholar]
- 41.Cone EJ, Caplan YH, Moser F, Robert T, Shelby MK, Black DL. Normalization of urinary drug concentrations with specific gravity and creatinine. J Anal Toxicol. 2009;33(1):1–7. [DOI] [PubMed] [Google Scholar]
- 42.Tanner EM, Bornehag CG, Gennings C. Dynamic growth metrics for examining prenatal exposure impacts on child growth trajectories: Application to perfluorooctanoic acid (PFOA) and postnatal weight gain. Environ Res. 2020;182:109044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos J, Pearce SE, Stroustrup A. Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr. 2015;27(2):254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stroustrup A, Teitelbaum SL, Aschner JL. The Value of Preterm Infant Environmental Health Cohorts: The Canary in the Coal Mine. JAMA Pediatr. 2017;171(12):1139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117(4):639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regnault N, Gillman MW. Importance of characterizing growth trajectories. Ann Nutr Metab. 2014;65(2–3):110–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123(1):e101–9. [DOI] [PubMed] [Google Scholar]
- 48.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lite C, Raja GL, Juliet M, Sridhar VV, Subhashree KD, Kumar P, et al. In utero exposure to endocrine-disrupting chemicals, maternal factors and alterations in the epigenetic landscape underlying later-life health effects. Environ Toxicol Pharmacol. 2022;89:103779. [DOI] [PubMed] [Google Scholar]
- 50.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24(1):6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8(1):e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Y, Wu N, Wang S, Gao M, Song P, Lou J, et al. Transgenerational impaired male fertility with an Igf2 epigenetic defect in the rat are induced by the endocrine disruptor p,p’-DDE. Hum Reprod. 2014;29(11):2512–21. [DOI] [PubMed] [Google Scholar]
- 53.Van Nostrand SM, Bennett LN, Coraglio VJ, Guo R, Muraskas JK. Factors influencing independent oral feeding in preterm infants. J Neonatal Perinatal Med. 2015. [DOI] [PubMed] [Google Scholar]
- 54.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71(3):305–10. [DOI] [PubMed] [Google Scholar]
- 55.Zisk JL, Genen LH, Kirkby S, Webb D, Greenspan J, Dysart K. Do premature female infants really do better than their male counterparts? Am J Perinatol. 2011;28(3):241–6. [DOI] [PubMed] [Google Scholar]
- 56.Briana DD, Malamitsi-Puchner A. Intrauterine growth restriction: the controversial role of perinatal adipocytokines in the prediction of metabolic adult disease. J Matern Fetal Neonatal Med. 2019:1–6. [DOI] [PubMed] [Google Scholar]
- 57.Kesavan K, Devaskar SU. Intrauterine Growth Restriction: Postnatal Monitoring and Outcomes. Pediatr Clin North Am. 2019;66(2):403–23. [DOI] [PubMed] [Google Scholar]
- 58.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


