ABSTRACT
Recently, immune checkpoint inhibitors (ICIs) present promising application prospects in treating non-small cell lung cancer (NSCLC). This study aimed to investigate optimal treatment strategy by comparing the first-line treatment strategies with ICIs in NSCLC. We retrieved relevant studies on first-line therapy of NSCLC with ICIs. Primary outcomes were overall survival (OS) and progression-free survival (PFS). Secondary outcomes were treatment-related serious adverse events (tr-SAEs) with grade 3 or higher and objective response rate (ORR). We also conducted a Bayesian network meta-analysis. We included 14 studies involving 7,823 patients and compared seven different interventions. In PD-L1 nonselective NSCLC, nivolumab+ipilimumab had good PFS and ORR, pembrolizumab significantly prolonged OS, and nivolumab had the fewest adverse events (AEs). For PD-L1-positive patients, nivolumab remarkably prolonged OS. For those with negative PD-L1, nivolumab+ipilimumab also showed an advantage. In addition, nivolumab+ipilimumab significantly prolonged the PFS in both PD-L1-negative and -positive patients. For patients with PD-L1 tumor proportion score (TPS) within 1–49%, atezolizumab+chemotherapy remarkably prolonged PFS and OS. For those with PD-L1 TPS ≥50%, pembrolizumab prolonged OS and atezolizumab+chemotherapy significantly prolonged PFS. Nivolumab combined with ipilimumab showed advantages in OS, PFS and ORR in most patients. Nivolumab+ipilimumab may be the optimal first-line therapy for NSCLC.
KEYWORDS: NSCLC, immune checkpoint inhibitor, chemotherapy, first-line therapy, network meta-analysis
Introduction
Non-small cell lung cancer (NSCLC) constitutes 80–85% of lung cancer, which is a predominant cause of deaths,1 As most patients are too advanced for surgical treatment when diagnosed, chemotherapy remains the preferred therapy for NSCLC. But patients receiving chemotherapy had relatively low response rate (<50%)2 and 5-y overall survival rate (about 5%).3 Nowadays, with the advent of targeted drugs, 30–40% of the NSCLC patients with sensitive mutations have benefited. Nevertheless, acquired resistance has limited their availability of targeted drugs.4 Therefore, finding new treatment methods for NSCLC is an issue demands prompt solution.
Recently, accumulating evidence has shown that immune evasion is a central marker for lung cancer.5 Activation of immune checkpoint antibodies programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) pathways were vital mechanisms to evade immune elimination in tumor cells.6 Immune checkpoint inhibitors (ICIs) promote autoimmune function of cancer cells via blocking expression of immune checkpoint antibodies mentioned above.7 The U.S. Food and Drug Administration (FDA) has approved four ICIs for NSCLC patients: pembrolizumab, nivolumab, durvalumab and atezolizumab. The former two target PD-1 receptor, and the latter two target anti-PD-L1.8 Several phase III randomized controlled trials (RCTs) presented that pembrolizumab monotherapy substantially improves survival in advanced NSCLC patients compared with platinum-based chemotherapy, with a lower probability of grade 3 or higher adverse events (AEs).9–11 Recent studies reported that the standard first-line therapy for NSCLC patients with PD-L1 tumor proportional score (TPS) ≥1% is pembrolizumab monotherapy or atezolizumab monotherapy.12–14 PACIFIC trial unveiled that durvalumab monotherapy is suitable for patients with unresectable stage III NSCLC.15
CTLA4 binds to CD80/CD86 to activate inhibitory downstream signaling in lymphocytes.7 Ipilimumab and tremelimumab act as immunosuppressive agents by antagonizing CTLA4 to prevent it from binding to ligands.7 Additionally, the antitumor mechanism of CTLA-4 and repression of PD-1/PD-L1 pathway are complementary.12 Compared with ICI monotherapy, the combination regimen of ipilimumab plus nivolumab can be first-line therapy of advanced NSCLC patients and is independent of PD-L1 levels.16,17 Durvalumab can improve survival in patients with metastatic NSCLC with PD-L1 ≥ 25% whatever being a single agent or in combination with tremelimumab,18
Clinical trials in recent years have also revealed that ICIs combined with chemotherapy as first-line therapy for advanced NSCLC patients have favorable survival and fewer side effects.12 But immune-related AEs caused by ICIs are even more than chemotherapy.19 The main cause of AEs is the activation of autoreactive T cells caused by the blockade of immune checkpoint receptors PD-1/PD-L1 and CTLA-4, mainly involving the endocrine glands, gastrointestinal tract, skin, lung, liver, cardiovascular, nervous system, and blood.20–23 Hence, the treatment and management of AEs need to be considered with therapeutic regimens in clinical practice.
However, the best PD-1/L1 treatment is still unclear. Now it is controversial whether PD-L1 expression affects the immunotherapy effect on patients. Therefore, a network meta-analysis comparing the first-line treatment methods was conducted in our study to determine the best treatment option.
Methods
Literature retrieval
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the PRISMA extension statement for network meta-analysis were applied to conduct this systematic evaluation and meta-analysis.
By 20 August 2020, two investigators did a comprehensive literature search in PubMed, Embase, Web of Science and The Cochrane Library databases independently. Retrieval strategies were as follows: ((((((((((((((((((“Carcinoma, Non-Small-Cell Lung”[MeSH Terms]) OR (“Non-Small Cell Lung Cancer”[Title/Abstract])) OR (“Carcinoma, Non-Small Cell Lung”[Title/Abstract])) OR (“Non Small Cell Lung Carcinoma”[Title/Abstract])) OR (“Non-Small-Cell Lung Carcinoma”[Title/Abstract])) OR (“Nonsmall Cell Lung Cancer”[Title/Abstract])) OR (“Non-Small-Cell Lung Carcinomas”[Title/Abstract])) OR (“Lung Carcinomas, Non-Small-Cell”[Title/Abstract])) OR (“Lung Carcinoma, Non-Small-Cell”[Title/Abstract])) OR (“Carcinomas, Non-Small-Cell Lung”[Title/Abstract])) OR (“Carcinoma, Non Small Cell Lung”[Title/Abstract])) OR (“Lung Cancers”[Title/Abstract])) OR (“Lung Cancer”[Title/Abstract])) OR (“Lung Neoplasms”[Title/Abstract])) OR (“Lung Neoplasm”[Title/Abstract])) OR (“NSCLC”[Title/Abstract])) AND (((((((((((((((((((Ipilimumab[Title/Abstract]) OR (Yervoy[Title/Abstract])) OR (Pembrolizumab[Title/Abstract])) OR (Keytruda[Title/Abstract])) OR (lambrolizumab[Title/Abstract])) OR (Nivolumab[Title/Abstract])) OR (Opdivo[Title/Abstract])) OR (Cemiplimab[Title/Abstract])) OR (Libtayo[Title/Abstract])) OR (Sintilimab[Title/Abstract])) OR (“Da Boshu”[Title/Abstract])) OR (Atezolizumab[Title/Abstract])) OR (Tecentriq[Title/Abstract])) OR (Durvalumab[Title/Abstract])) OR (Imfinzi[Title/Abstract])) OR (Avelumab[Title/Abstract])) OR (Bavencio[Title/Abstract])) OR (Toripalimab[Title/Abstract])) OR (“Tuo Yi”[Title/Abstract]))) AND (first-line[Title/Abstract])) OR (front-line[Title/Abstract])
Literature screening
The following were inclusion criteria: (1) Studies involved patients who were confirmed advanced NSCLC histologically or cytologically; (2) Studies taking ICIs as the first-line therapy; (3) Studies comparing ICI monotherapy and combination therapies involving chemotherapy or targeted therapy; (4) Studies containing ≥1 of outcome indicators including overall survival (OS), progression-free survival (PFS), treatment-related serious AE (tr-SAE) and objective response rate (ORR). Exclusion criteria were as follows: (1) Studies involving patients who had received treatment other than immunotherapy or chemotherapy in first-line treatment; (2) Studies involving patients with sensitive mutations of epidermal growth factor receptor, anaplastic lymphoma kinase, or other genes; (3) Duplicate literature, systematic review, case report, meta-analysis, letter or non-English literature.
Data selection and quality assessment
Two investigators completed data extraction severally, and a third investigator joined negotiation when there was any disagreement. Data included author, publication year, phase of trial, intervention, sample size, histological type, gender and age, and primary outcome measures including OS, PFS, ORR, and tr-SAE.
With the Cochrane bias risk tool, quality assessment was conducted from seven perspectives: (1) random sequence production; (2) allocation hiding; (3) blinding the subjects and investigators; (4) blinding outcome assessors; (5) incomplete data; (6) selective result reporting; and (7) other biases. Items were scored as unclear risk (yellow), low risk (green), and high risk (red).
Statistical analysis
All data were included for comparison of efficacy of different treatments. PFS and OS were taken as the primary outcomes, and incidence of ORR and tr-SAE were taken as the secondary outcomes. The hazard ratio (HR) of PFS and OS were extracted from literature. Odds ratio (OR) of ORR and incidence of tr-SAE were obtained by calculation.
We used Stata 14.0 software to map the network diagram of different interventions with different outcomes to visually reflect the direct or indirect comparisons of the treatment methods in included studies. The gemtc package of R software was applied to summarize data of comparisons directly and indirectly. The R-based ggplot2 package generated a cumulative ranking curve, and surface under cumulative ranking curve showed the probability of different treatments ranking best, second best and third best under different outcome indicators. Besides, we also performed subgroup analysis based on PD-L1 expression. Heterogeneity of studies was measured using Q test and I2 statistics. If p < .1 or I2 > 50%, the heterogeneity was regarded as high and then the random-effects model was adopted. Instead, fixed-effects model was adopted. Statistical significance was considered when p < .05.
Results
Literature retrieval
A total of 789 studies were retrieved through online databases, 103 duplicates were excluded, 648 articles were removed after browsing titles and abstracts, 38 articles were evaluated in full-text, and 14 studies were finally included (Figure 1).
Figure 1.
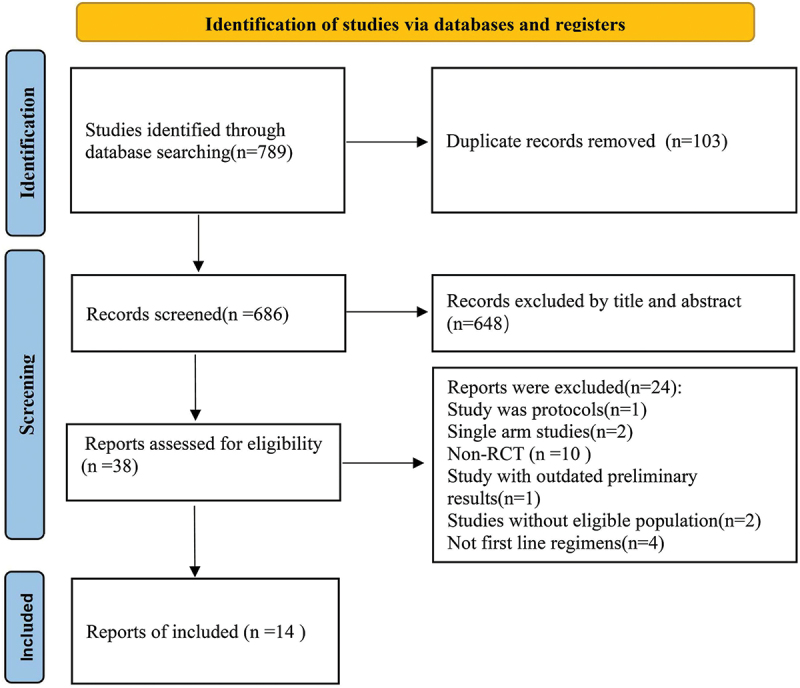
Flow chart of literature screening.
Basic characteristics and quality assessment
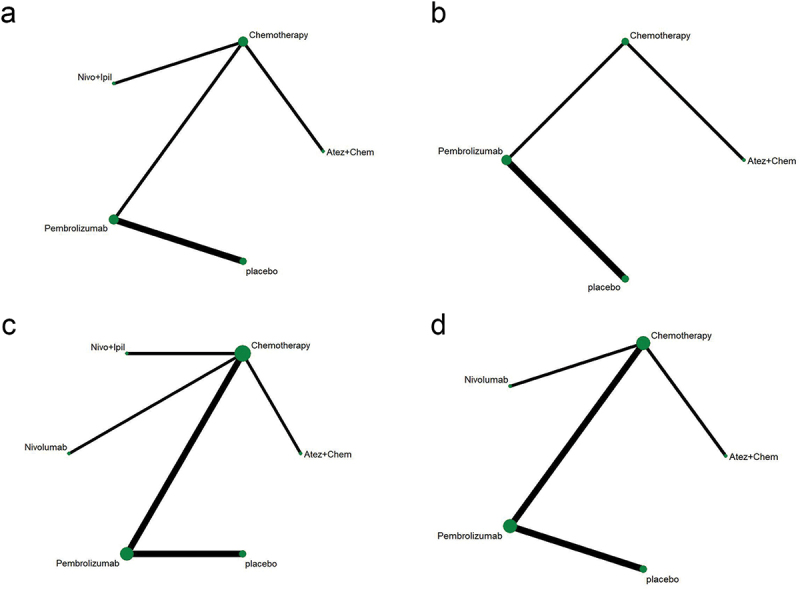
Table 1 shows the basic characteristics of 14 references.9,11,13,17,24–33 A total of 7,823 patients participated in 7 different treatment strategies: Comparison between pembrolizumab, nivolumab, atezolizumab, docetaxel, atezolizumab+chemotherapy, and nivolumab+ipilimumab, and chemotherapy. The included literature included multicenter Phase II or Phase III RCTs. Figure 2 shows detailed results of bias assessment. A comparative network plot for all outcomes is presented in Figure 3. Among PD-L1 nonselective NSCLC patients, PFS was reported in five treatment strategies, OS and AEs in six treatment strategies, and ORR in four treatment strategies.
Table 1.
Basic characteristics of the included literature.
| Author | Year | Study name | Phase | Blind | Stage | Histology | Intervening measure | Sample | Median age(range) | Male (%) | PFS HR(95% CI) | OS HR(95% CI) | Grade AE ≥3(%) | Objective response(%) | PD-L1 expression | PD-L1 test | PD-L1 TPS<1% | PD-L1 TPS≥1% | PD-L1 TPS 1–49% | PD-L1 TPS≥50% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Julie Brahmer(1) | 2015 | CheckMate 017 | III | Open-label | IIIB, IV | NSCLC | Nivolumab | 135 | 62(39–85) | 111(82) | 0.62(0.47–0.81) | 0.59(0.44–0.79) | 9(7) | 27(20) | Any | IHC 28–8 pharmDx assay | OS 0.58(0.37–0.92) | OS 0.69(0.45–1.05) | — | — |
| Docetaxel | 137 | 64(42–84) | 97(71) | 71(55) | 12(9) | PFS 0.66(0.43–1.00) | PFS 0.67(0.44–1.01) | — | — | |||||||||||
| H. Borghaei(2)+Dickran Kazandjian(3) | 2015 | CheckMate 057 | III | Open-label | IIIB, IV | Non-squamous | Nivolumab | 292 | 61(37–84) | 151(52) | 0.92(0.77–1.11) | 0.73(0.59–0.89) | 30(10) | 56(19) | — | IHC 28–8 pharmDx assay | OS 0.90(0.66–1.24) | OS 0.59(0.43–0.82) | — | — |
| Docetaxel | 290 | 64(21–85) | 168(58) | 144(54) | 36(12) | PFS 1.19(0.88–1.61) | PFS 0.70(0.53–0.94) | — | — | |||||||||||
| Roy S Herbst(4) | 2016 | KEYNOTE-010 | II/III | Open-label | — | NSCLC | Pembrolizumab | 339 | 63(56–69) | 212(62) | 0.88(0.74–1.05) | 0.71(0.58–0.88) | 43(13) | 62(18) | Any | IHC 22C3 pharmDx assay | — | — | OS 0.76(0.60–0.96) | OS 0.54(0.38–0.77) |
| Docetaxel | 309 | 62(56–69) | 209(61) | 109(35) | 31(9) | — | — | PFS 1.04(0.85–1.27) | PFS 0.59(0.44–0.78) | |||||||||||
| Louis Fehrenbacher(5) | 2016 | POPLAR | II | Open-label | — | NSCLC | Atezolizumab | 144 | 62(42–82) | 93(65) | — | 0.73(0.53–0.99) | 17(12) | 63(45) | Any | — | OS 1.04(0.63–1.75) | OS 0.59(0.40–0.85) | — | OS 0.49(0.22–1.07) |
| Docetaxel | 143 | 62(36–84) | 76(53) | 55(41) | 43(27) | — | — | — | — | |||||||||||
| Martin Reck(6) | 2016 | KEYNOTE-024 | III | Open-label | IV | NSCLC | Pembrolizumab | 154 | 64.5(33–90) | 92(59.7) | 0.50(0.37–0.68) | 0.60(0.41–0.89) | 41(26.6) | 69(44.8) | — | IHC 22C3 pharmDx assay | — | — | — | — |
| Chemotherapy | 151 | 66.0(38–85) | 95(62.9) | 80(53.3) | 42(27.8) | — | — | — | — | |||||||||||
| Achim Rittmeyer(7) | 2016 | OAK | III | Open-label | IIIB, IV | NSCLC | Atezolizumab | 425 | 63(33–82) | 261(61) | — | 0.73(0.62–0.87) | 90(15) | 58(14) | Any | — | OS 0.75(0.59–0.96) | OS 0.74(0.58–0.93) | — | — |
| Docetaxel | 425 | 64(34–85) | 259(61) | 247(43) | 57(13) | — | — | — | — | |||||||||||
| D.P. Carbone(8) | 2017 | CheckMate026 | III | Open-label | IV | NSCLC | Nivolumab | 271 | 63(32–89) | 184(68) | 1.15(0.91–1.45) | 1.02(0.80–1.30) | 47(18) | 55(26) | > = 50 | IHC 28–8 pharmDx assay | — | — | — | OS 0.90(0.64–1.29) |
| Chemotherapy | 270 | 65(29–87) | 148(55) | 133(51) | 71(33) | — | — | — | PFS 1.07(0.77–1.49) | |||||||||||
| M.D. Hellmann(9, 10) | 2018/2019 | CheckMate 227 | I/III | Open-label | IV | NSCLC | Nivolumab plus Ipilimumab | 583 | 64(26–87) | 98(70.5) | 0.58(0.41–0.81) | 0.73(0.64–0.84) | 180(31.2) | 63(45) | Any | IHC 28–8 pharmDx assay | OS 0.62(0.49–0.79) | OS 0.79(0.65–0.96) | OS 0.94(0.75–1.18) | OS 0.70(0.55–0.90) |
| Chemotherapy | 583 | 64(29–87) | 106(66.2) | 206(36.1) | 43(27) | PFS 0.48(0.27–0.85) | PFS 0.62(0.44–0.88) | — | — | |||||||||||
| L. Gandhi(11) | 2018 | KEYNOTE-189 | III | Double-blind | — | NSCLC | Pembrolizumab | 410 | 65.0(34–84) | 254(62.0) | 0.52(0.43–0.64) | 0.49(0.38–0.64) | 272(67.2) | — | Any | IHC 22C3 pharmDx assay | OS 0.59(0.38–0.92) | — | OS 0.55(0.34–0.90) | OS 0.42(0.26–0.68) |
| Placebo | 206 | 63.5(34–84) | 109(52.9) | 133(65.8) | — | PFS 0.75(0.53–1.05) | PFS 0.44(0.34–0.57) | PFS 0.55(0.37–0.81) | PFS 0.36(0.25–0.52) | |||||||||||
| Tony S.K. Mok(12) | 2019 | KEYNOTE-042 | III | Open-label | IV | NSCLC | Pembrolizumab | 637 | 63.0(57–69) | 450(71) | — | — | 113(18) | — | > = 1 | IHC 22C3 pharmDx assay | — | OS 0.81(0.71–0.93) | OS 0.92(0.77–1.11) | OS 0.69(0.56–0.85) |
| Chemotherapy | 637 | 63.0(57–69) | 452(71) | 252(41) | — | — | PFS 1.07(0.94–1.21) | — | PFS 0.81(0.67–0.99) | |||||||||||
| Howard West(13) | 2019 | Impower130 | III | Open-label | IV | NSCLC | Atezolizumab plus chemotherapy | 483 | 64(18–86) | 227(57) | 0.65(0.54–0.77) | 0.80(0.65–0.99) | 354(75) | — | Any | — | OS 0.81(0.61–1.08) | — | OS 0.70(0.45–1.08) | OS 0.84(0.51–1.39) |
| Chemotherapy | 240 | 65(38–85) | 138(58) | 141(61) | — | PFS 0.72(0.56–0.91) | — | PFS 0.61(0.43–0.77) | PFS 0.51(0.34–0.77) | |||||||||||
| L. Paz-Ares(14) | 2020 | KEYNOTE-407 | III | Double-blind | — | Squamous | Pembrolizumab | 278 | 65(19–87) | 220(79.1) | 0.56(0.45–0.7) | 0.64(0.49–0.85) | 194(69.8) | — | Any | IHC 22C3 pharmDx assay | OS 0.61(0.38–0.98) | OS 0.65(0.45–0.92) | OS 0.57(0.36–0.90) | OS 0.64(0.37–1.10) |
| Placebo | 281 | 65(36–88) | 235(83.6) | 191(68.2) | — | PFS 0.68(0.47–0.98) | PFS 0.49(0.38–0.65) | PFS 0.56(0.39–0.80) | PFS 0.37(0.24–0.58) |
Note: NSCLC: non-small cell lung cancer; PFS: progression-free survival; OS: overall survival; IHC: immunohistochemical.
Figure 2.
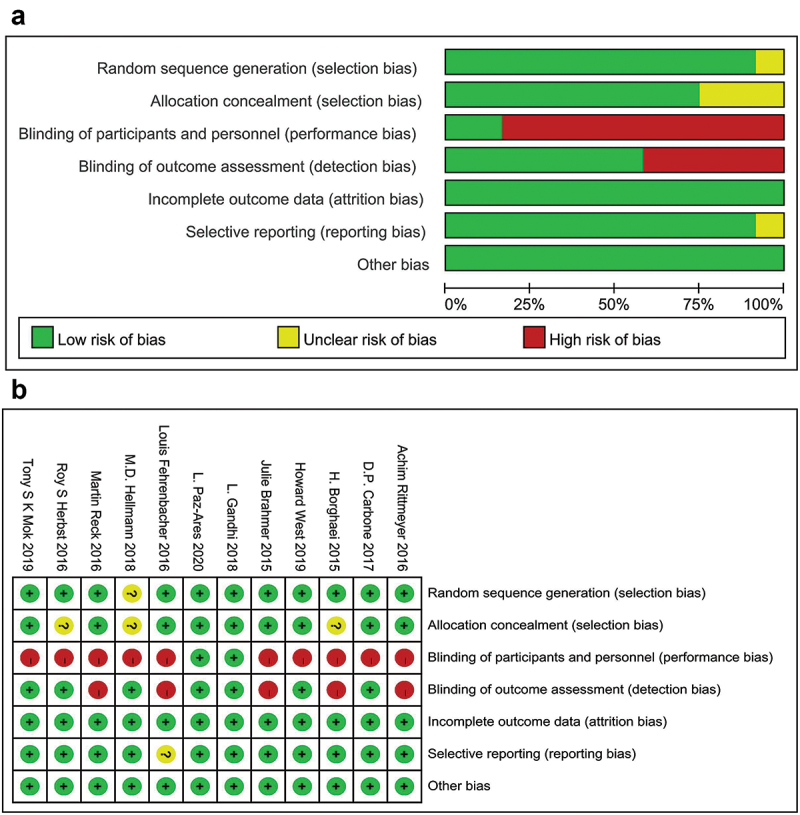
Quality assessment of included studies. Yellow (?): unclear risk; Green (+): low risk; Red (-): high risk.
Figure 3.
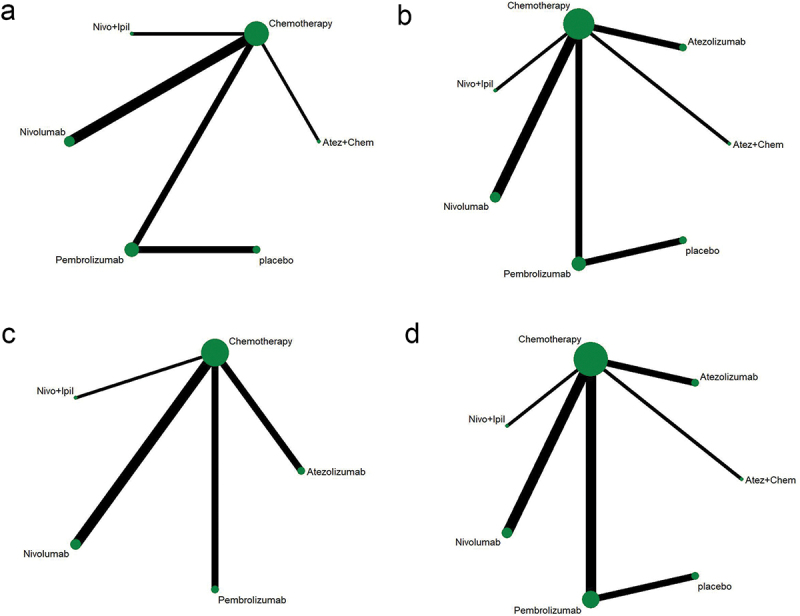
Network diagram. (a) PFS; (b) OS; (c) ORR; (d) AEs ≥3. The dots in the figure represent different treatment methods; the size of the dots represents the sample size using that treatment; the line between the dots represents a direct comparison between the two treatments; the thickness of the line represents the number of studies. PFS: progression-free survival; OS: overall survival; ORR: objective response rate; AEs: adverse events.
Therapeutic effect
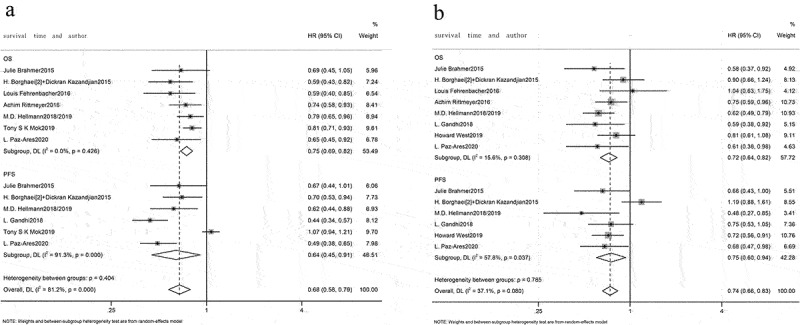
A meta-analysis of overall efficacy across all studies was conducted. As illustrated by forest plot, when compared with chemotherapy, immunotherapy markedly prolonged OS (HR = 0.72, 95% CI: 0.69–0.76, Figure 4a) and PFS (HR = 0.71, 95% CI: 0.66–0.77, Figure 4b) in advanced NSCLC patients, and noticeably reduced the incidence of grade 3 and worse AEs (RR: 0.62, 95% CI: 0.48–0.80, Figure 4c).
Figure 4.
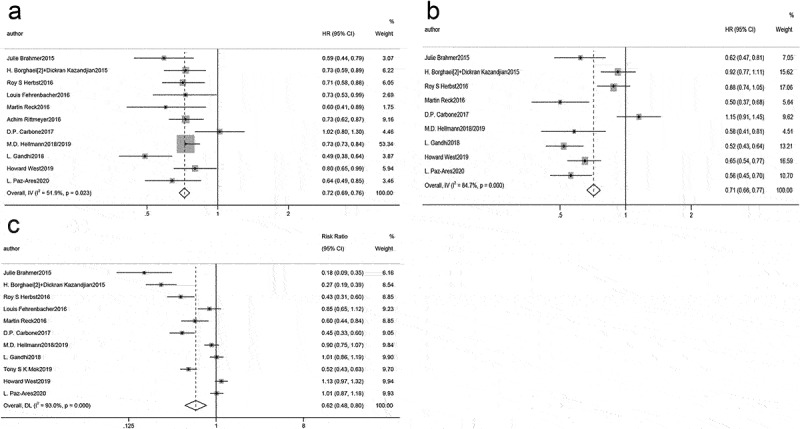
Forest plot of comparison of (a) OS, (b) PFS, and (c) adverse events of grade 3 or higher between immunotherapy and chemotherapy.
Afterward, the efficacy of immunotherapy and chemotherapy on advanced NSCLC patient’s survival was compared through league charts. In terms of OS, immunotherapy was evidently superior to chemotherapy, and no prominent differences were seen in these immunotherapies. In the context of PFS, immunotherapy was notably better than chemotherapy except for nivolumab (HR = 0.90, 95% CI: 0.79–1.02; Figure 5a). Besides, the PFS of nivolumab+ipilimumab was substantially higher than nivolumab monotherapy (HR = 0.64, 95% CI: 0.45–0.93; Figure 5a).
Figure 5.
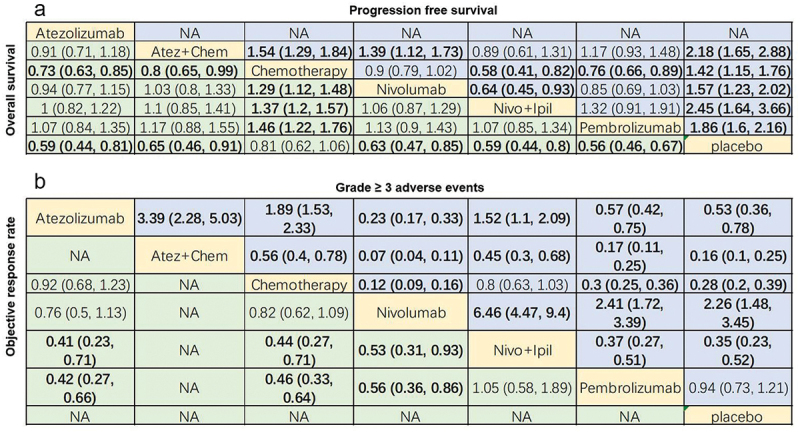
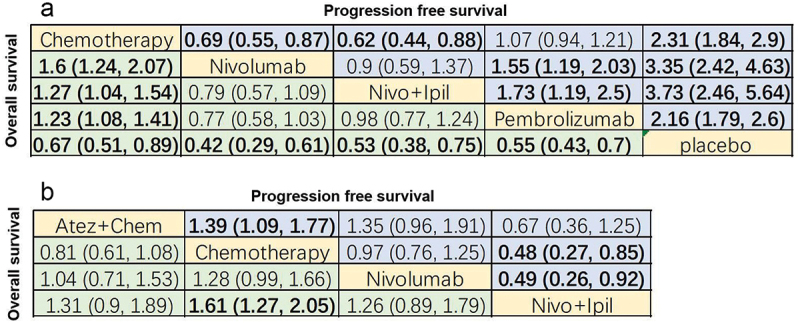
League chart. (a) Combined HR (95%CI) of PFS (upper triangle) and OS (lower triangle); (b) Combined or (95% CI) of grade 3 or higher AEs (upper triangle) and ORR (lower triangle); the data in each cell is HR or OR (95% CI) comparing row definition processing and column definition processing. HR <1 or OR >1 indicates better results. The results in bold are significant. PFS: progression-free survival; OS: overall survival; HR: hazard ratio; OR: odds ratio; ORR: objective response rate; AEs: adverse events; Atez: atezolizumab; Chem: chemotherapy; Nivo: nivolumab; Ipil: ipilimumab.
Regarding ORR, ORR in atezolizumab (OR = 0.41, 95% CI: 0.23–0.71), chemotherapy (OR = 0.44, 95% CI: 0.27–0.71) and nivolumab (OR = 0.53, 95% CI: 0.31–0.93) groups were significantly lower than that in the nivolumab+ipilimumab group (Figure 5b). Additionally, nivolumab+ipilimumab had a similar ORR as pembrolizumab (OR = 1.05, 95% CI: 0.58–1.89; Figure 5b).
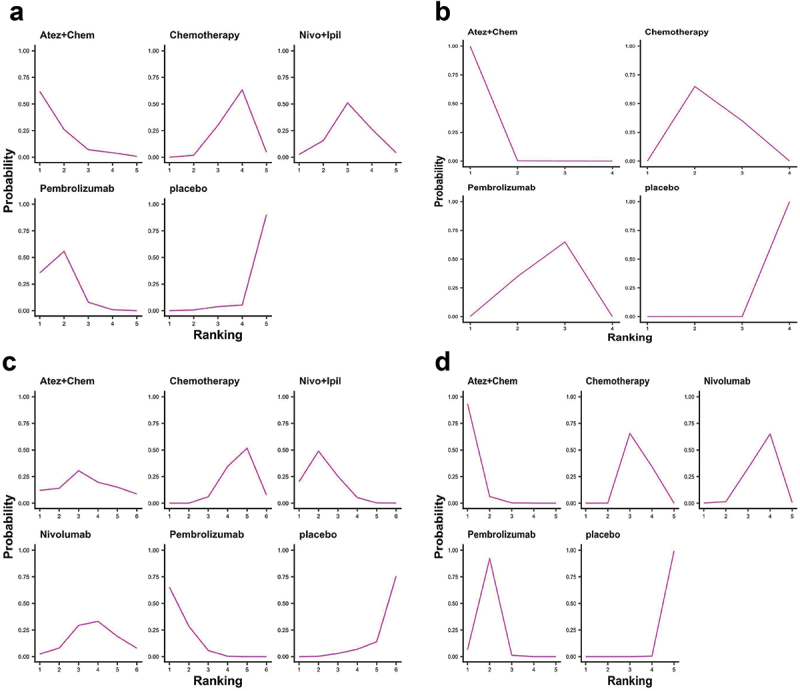
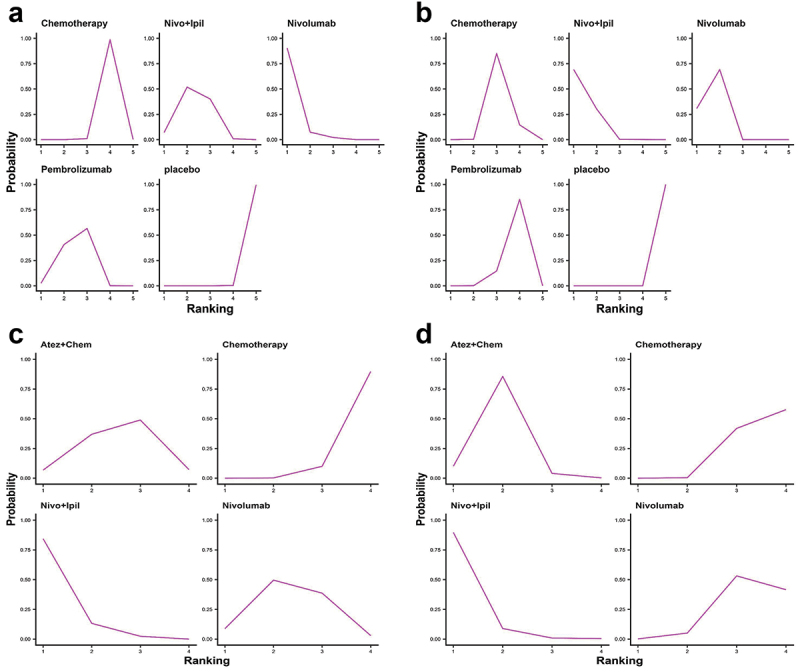
We ranked OS and PFS of treatment strategies by SUCRA value. The results showed that pembrolizumab ranked first in OS among patients with PD-L1 nonselective NSCLC (53.9% probability; Figure 6a). Nivolumab+ipilimumab was most likely to rank first in PFS (71.5% probability; Figure 6b) and ORR (55.8% probability; Figure 6c). Nivolumab ranked first in AE (99.9% probability; Figure 6d). Overall, nivolumab had the fewest AEs compared to other treatments.
Figure 6.
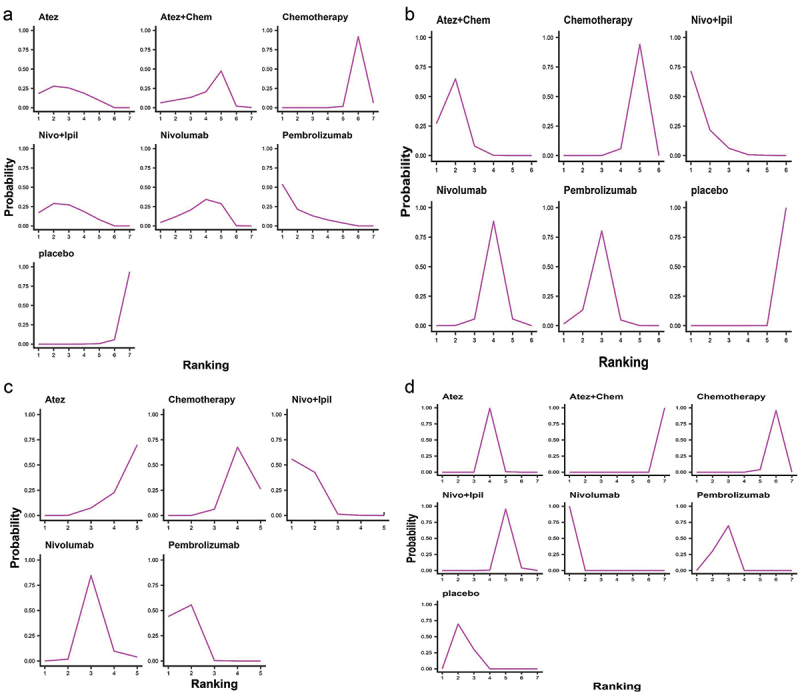
Ranking diagram. (a) Ranking diagram of OS of PD-L1 nonselective NSCLC patients; (b) Ranking diagram of PFS of PD-L1 nonselective NSCLC patients; (c) 7 Ranking diagram of ORR of PD-L1 nonselective NSCLC patients; (d) Ranking diagram of tr-SAE of PD-L1 nonselective NSCLC patients. Atez: atezolizumab; Chem: chemotherapy; Nivo: nivolumab; Ipil: ipilimumab.
Results of subgroup analysis
PD-L1 TPS within 1–49% and PD-L1 TPS ≥50%
All studies were categorized per different cutoffs for PD-L1 TPS: PD-L1 TPS 1–49% and PD-L1 TPS ≥50%. A pooled analysis of patient’s survival with PD-L1 TPS 1–49% and PD-L1 TPS ≥50% revealed significant heterogeneity (I2 = 65.8%, p = .002, Figure 7a; I2 = 68.4%, p < .001, Figure 7b), and random effects model was utilized for analysis. Compared with chemotherapy, immunotherapy was beneficial to prolong OS (HR = 0.79, 95% CI: 0.67–0.93, Figure 7a; HR = 0.67, 95% CI: 0.57–0.77, Figure 7b) and PFS (HR = 0.68, 95% CI: 0.48–0.97, Figure 7a; HR = 0.58, 95% CI: 0.42–0.81, Figure 7b) in advanced NSCLC patients with PD-L1 TPS 1–49% and PD-L1 TPS ≥50%.
Figure 7.
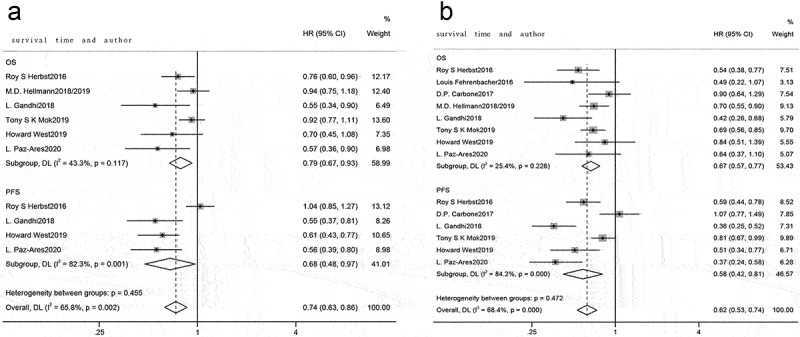
Forest plots for PD-L1 TPS 1–49% and TPS ≥50%. (a) PD-L1 TPS 1–49%, forest plot comparing OS and PFS between immunotherapy and chemotherapy; (b) PD-L1 TPS ≥50%, forest plot comparing OS and PFS between immunotherapy and chemotherapy.
Next, survival analysis of patients with PD-L1 TPS 1–49% and PD-L1 TPS ≥50% receiving different therapies was done. In those with PD-L1 TPS within 1–49%, OS was reported in four treatments (Figure 8a) and PFS was reported in three treatments (Figure 8b). For OS, pembrolizumab notably prolonged OS in comparison with chemotherapy (HR = 1.32, 95% CI: 1.04–1.66; Figure 9a). For PFS, atezolizumab+chemotherapy showed prolonged PFS than chemotherapy (HR = 1.64, 95% CI: 1.23–2.19) and pembrolizumab (HR = 1.71, 95% CI: 1.2–2.43; Figure 9a).
Figure 8.
Network diagram of subgroup analysis. (a-b) OS and PFS of patients with PD-L1 1–49%; (c-d) OS and PFS of patients with PD-L1 ≥ 50%. The dots in the figure represent different treatment methods; the size of the dots represents the sample size using that treatment; the line between the dots represents a direct comparison between the two treatments; the thickness of the line represents the number of studies. Atez: atezolizumab; Chem: chemotherapy; Nivo: nivolumab; Ipil: ipilimumab.
Figure 9.
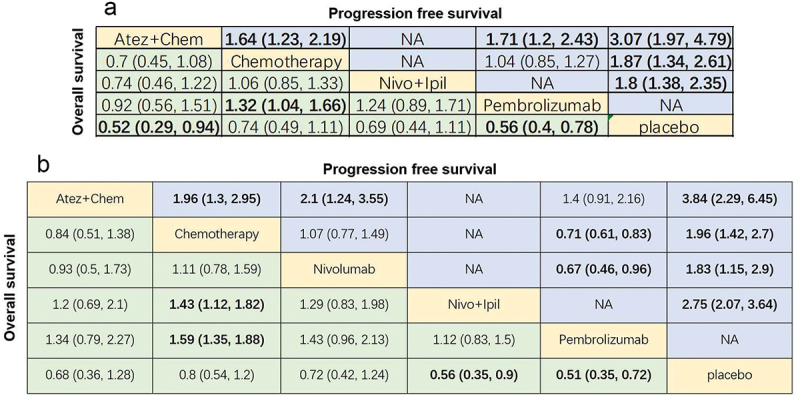
League chart of subgroup analysis. (a) Combined HR (95%CI) for PFS (upper triangle) and OS (lower triangle) of patients with PD-L1 1–49%; (b) Combined HR (95%CI) for PFS (upper triangle) and OS (lower triangle) of patients with PD-L1 ≥ 50%; the data in each cell is HR or OR (95% CI) comparing row definition processing and column definition processing. HR <1 and OR >1 indicate better results. Significant results are shown in bold. PFS: progression-free survival; OS: overall survival; HR: hazard ratio; OR: odds ratio; Atez: atezolizumab; Chem: chemotherapy; Nivo: nivolumab; Ipil: ipilimumab.
Among patients with PD-L1 TPS ≥50%, OS was reported in 5 treatments (Figure 8c) and PFS was reported in 4 treatments (Figure 8d). For OS, nivolumab+ipilimumab (HR = 1.43, 95% CI: 1.12–1.82) and pembrolizumab (HR = 1.59, 95% CI: 1.35–1.88) remarkably prolonged OS compared with chemotherapy (Figure 9b). For PFS, atezolizumab + chemotherapy (HR = 1.96, 95% CI: 1.3–2.95) and pembrolizumab (HR = 0.71, 95% CI: 0.61–0.83; Figure 9b) were significantly superior to chemotherapy.
The ranking results showed that atezolizumab+chemotherapy was the most possible therapy to rank first in PFS (99.8% probability) and OS (61.8% probability) among the PD-L1 1–49% population (Figure 10a,b). In patients with PD-L1 ≥50%, pembrolizumab ranked first in OS (65.2% probability) and atezolizumab+chemotherapy ranked first in PFS (93.4% probability) (Figure 10c,d).
Figure 10.
Ranking diagram of PD-L1 TPS 1–49% and TPS ≥50% subgroups. (a) Ranking diagram of OS of NSCLC patients with PD-L1 1–49%; (b) Ranking diagram of PFS of NSCLC patients with PD-L1 1–49%; (c) Ranking diagram of OS of NSCLC patients with PD-L1 ≥ 50%; (d) Ranking diagram of PFS of NSCLC patients with PD-L1 ≥ 50%. Atez: atezolizumab; Chem: chemotherapy; Nivo: nivolumab; Ipil: ipilimumab.
PD-L1 TPS ≥1% and PD-L1 TPS <1%
According to PD-L1 TPS level, it was divided into PD-L1 TPS <1% and PD-L1 TPS ≥1%. With PD-L1 TPS ≥1%, the pooled analysis revealed large heterogeneity among studies (I2 = 81.2%, p < .001, Figure 11a), and random effects model was utilized for analysis. With PD-L1 TPS <1%, pooled analysis illustrated little heterogeneity among studies (I2 = 37.1%, p = .080, Figure 11b), and fixed effects model was utilized for analysis. Compared with chemotherapy, immunotherapy was beneficial to prolong OS (HR = 0.75, 95% CI: 0.69–0.82, Figure 11a; HR = 0.72, 95% CI: 0.64–0.82, Figure 11b) and PFS (HR = 0.64, 95% CI: 0.45–0.91, Figure 11a; HR = 0.75, 95% CI: 0.60–0.94, Figure 11b) when PD-L1 TPS ≥1% and PD-L1 TPS <1%.
Figure 11.
Forest plots for PD-L1 TPS ≥1% and TPS <1%. (a) PD-L1 TPS ≥1%, forest plot comparing OS and PFS between immunotherapy and chemotherapy; (b) PD-L1 TPS <1%, forest plot comparing OS and PFS between immunotherapy and chemotherapy.
Additionally, network diagram depicted that with PD-L1 TPS ≥1%, OS and PFS were reported in 4 treatments (Figure 12a,b). Regarding OS, all immunotherapies had superior efficiency than chemotherapy (Figure 13a). For PFS, both nivolumab+ipilimumab (HR = 0.62, 95% CI: 0.44–0.88) and nivolumab (HR = 0.69, 95% CI: 0.55–0.87) were better than chemotherapy except pembrolizumab (HR = 1.07, 95% CI: 0.94–1.21) (Figure 13a).
Figure 12.
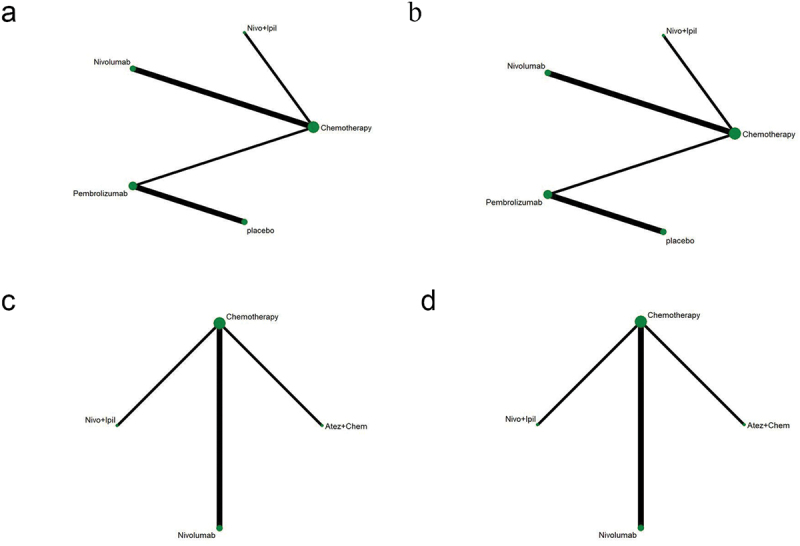
Network diagram of PD-L1-positive and -negative subgroups. (a-b) OS and PFS of patients with PD-L1 ≥ 1%; (c-d) OS and PFS of patients with PD-L1 < 1%. The dots in the figure represent different treatment methods; the size of the dots represents the sample size using that treatment; the line between the dots represents a direct comparison between the two treatments; the thickness of the line represents the number of studies. Atez: atezolizumab; Chem: chemotherapy; Nivo: nivolumab; Ipil: ipilimumab.
Figure 13.
League chart of PD-L1-positive and -negative subgroups. (a) Combined HR (95%CI) for PFS (upper triangle) and OS (lower triangle) of patients with PD-L1 ≥ 1%; (b) Combined HR (95%CI) for PFS (upper triangle) and OS (lower triangle) of patients with PD-L1 < 1%; the data in each cell is HR or OR (95%CI) comparing row definition processing and column definition processing. HR <1 and OR >1 indicate better results. Significant results are shown in bold. PFS: progression-free survival; OS: overall survival; HR: hazard ratio; OR: odds ratio; Atez: atezolizumab; Chem: chemotherapy; Nivo: nivolumab; Ipil: ipilimumab.
With PD-L1 TPS <1%, OS and PFS were reported in three treatments (Figure 12c,d). For OS, nivolumab+ipilimumab (HR = 1.61, 95% CI: 1.27–2.05) had significantly better efficiency than chemotherapy (Figure 13b). For PFS, in addition to nivolumab (HR = 0.97, 95% CI: 0.76–1.25), atezolizumab+chemotherapy (HR = 1.39, 95% CI: 1.09–1.77) and nivolumab+ipilimumab (HR = 0.48, 95% CI: 0.27–0.85) were both prominently better than chemotherapy (Figure 13b).
The ranking results showed that nivolumab ranked first in OS (90.4% probability, Figure 14a) and nivolumab+ipilimumab ranked first in PFS (69.3% probability, Figure 14b) among patients with positive PD-L1. Among PD-L1-negative patients, nivolumab+ipilimumab was likely to rank first in PFS (89.7% probability, Figure 14c) and OS (84.3% probability, Figure 14d).
Figure 14.
Ranking diagram of PD-L1-positive and -negative subgroups. (a) Ranking diagram of OS of PD-L1-positive NSCLC patients; (b) Ranking diagram of PFS of PD-L1-positive NSCLC patients; (c) Ranking diagram of OS of PD-L1-negative NSCLC patients; (d) Ranking diagram of PFS of PD-L1-negative NSCLC patients Atez: atezolizumab; Chem: chemotherapy; Nivo: nivolumab; Ipil: ipilimumab.
Discussion
Immunotherapy takes a vital part in first-line therapy of NSCLC. Herein, network meta-analysis was performed on 14 RCTs. Nivolumab+ipilimumab had better PFS and ORR of PD-L1 nonselective NSCLC patients. Additionally, in those with PD-L1 TPS ≥1% or PD-L1 TPS <1%, nivolumab+ipilimumab were beneficial to improve the survival benefit of advanced NSCLC patients. Nivolumab monotherapy had the fewest AEs, and in PD-L1-positive patients, OS of those who received nivolumab monotherapy was substantially prolonged. A phase III CheckMate 227 trial reported that nivolumab+ipilimumab can be used as the first-line therapy in patients suffering advanced NSCLC with high tumor mutational burden (TMB), with longer OS and PFS and fewer AEs than chemotherapy, and is independent of PD-L1 expression.17,31,34 The CheckMate 9LA trial revealed that nivolumab+ipilimumab in combination with two cycles of chemotherapy have longer OS and favorable risk-benefit profile than four cycles of chemotherapy alone, further supporting its use as first-line therapeutic avenue for advanced NSCLC patients.35 A recent meta-analysis of nivolumab+ipilimumab therapy and existing regimens revealed that compared with existing immunotherapy regimens, the nivolumab+ipilimumab therapy is more likely to be tolerated but has no benefit in PFS in PD-L1-positive patients with advanced NSCLC.36 The reason for the difference from our results may be that only four documents were included, with small sample size. Therefore, more clinical trials are warranted to deeply investigate the optimal efficacy of dual immunotherapy.
We manifested that different PD-L1 TPS scores indicated different therapeutic effects, which made the optimal treatment for patients substantially different. When PD-L1 TPS within 1–49%, atezolizumab+chemotherapy evidently prolonged PFS as well as OS. When PD-L1 TPS ≥50%, pembrolizumab prolonged OS and atezolizumab+chemotherapy significantly prolonged PFS. We analyzed the reasons from the perspective of drug mechanism. For one thing, PD-1 inhibitors have different mechanisms with PD-L1 inhibitors. A recent meta-analysis showed that PD-L1 inhibitors block the PD-L1/PD-1 and PD-L1/B7–1 pathways and therefore have a stronger immune response than PD-1 inhibitors.37 For another thing, PD-1 inhibitors have different binding sites with PD-L1 inhibitors. For PD-1 inhibitors, the binding region of nivolumab is completely different from that of pembrolizumab. These two antibodies bind to PD-1 from two different directions, causing a spatial conflict. The binding region of nivolumab is near the binding region of pembrolizumab on PD-1 without overlap.38 Regarding PD-L1 inhibitors, BMS-963559 AND atezolizumab bind on the upper side near N-end of PD-L1. Different from that, avelumab and durvalumab bind vertically to PD-L1, meaning that specific drugs bind to PD-L1 by specific way.39 However, some articles reported that effect of PD-1/L1 inhibitors is independent of molecular differences between drugs.40 Therefore, whether differences in the mechanism and binding sites affect the efficacy of PD-1/PD-L1 inhibitors awaits to be explored.
From perspective of clinical trial design, the differences between these immunotherapies can also be considered in the following aspects. First, there is heterogeneity between combination regimens. For example, we should also consider differences in synergies between chemotherapy and immunotherapy. Studies have shown that in addition to cytotoxic effects, conventional chemotherapy can also exert an immunomodulatory function by inducing immunogenic cell death or destroying immunosuppressive tumor microenvironment.41 This reasonably explains the fact that immunotherapy combined with chemotherapy has a survival advantage over chemotherapy alone, especially in the non-immunogenic tumor microenvironment, which may be transformed into immunogenic microenvironment by chemotherapy to enhance the activity of immunotherapy.42 Second, it is also related to the clinical features of the patients enrolled in our study, including smoking history, location, proportion of tumor tissues, and differences in PD-L1 determination methods.
Limitations also exist in our study. First, subgroup analysis was done on PD-L1 level only, and the results were controversial. Prediction based on PD-L1 level upon PD-1/L1 inhibitor efficacy cannot be applied to NSCLC patients.43 Second, the characteristics of patients included in different RCTs may affect the efficacy of immunotherapy, such as patients’ metastatic sites. Results of a meta-analysis demonstrated that patients with brain metastases obtained improved OS by ICI+chemotherapy, while patients with liver metastasis benefit in OS from both ICI monotherapy and ICI+chemotherapy+anti-VEGF therapy.44 Hence, subgroup analysis of baseline characteristics of patients is necessary to confirm our results. Last, most comparisons between interventions were indirect, and our main conclusions were based on relatively few clinical trials. Therefore, more experiments with more complete results are needed to support our conclusions.
In 2020, the FDA approved nivolumab+ipilimumab in combination with two cycles of chemotherapy for metastatic or recurrent NSCLC patients as first-line therapy.45 This study also suggests that nivolumab+ipilimumab can be an optimal first-line therapy for NSCLC.
Funding Statement
This study was supported in part by grants from the Health and Family Planning Commission of Hunan Province Research Project [No.C2019119] and the Natural Science Foundation of Hunan Province [No.2021JJ50097] . The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
Y L contributed to conceptualization and data curation. MY Z contributed to methodology and formal analysis. XY Z contributed to writing. JY N contributed to visualization. All authors have reviewed and approved the final manuscript.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–15. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arulananda S, Mitchell P.. Elderly patients with stage III NSCLC survive longer when chemotherapy is added to radiotherapy-fortune favours the bold. Transl Lung Cancer Res. 2018;7(Suppl 4):S388–92. doi: 10.21037/tlcr.2018.08.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Meador CB, Hata AN. Acquired resistance to targeted therapies in NSCLC: updates and evolving insights. Pharmacol Ther. 2020;210:210107522. doi: 10.1016/j.pharmthera.2020.107522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin A, Coffey DG, Warren EH, Ramnath N. Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Med. 2016;5(9):2567–78. doi: 10.1002/cam4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, Zhou GQ, Li WF, Mao YP, Hsu C, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoint inhibitors in clinical trials. Chin J Cancer. 2014;33(9):434–44. doi: 10.5732/cjc.014.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, Dubinett SM, Ferris A, Gandhi L, Garon EB, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018;6(1):75. doi: 10.1186/s40425-018-0382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr., Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 10.Brahmer JR, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600–09. doi: 10.1016/S1470-2045(17)30690-3. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F, Qiao M, Zhou C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell Mol Immunol. 2021;18(2):279–93. doi: 10.1038/s41423-020-00577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West H, Mccleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, Mccune S, Mekhail T, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 14.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–46. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 15.Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, Spigel DR, Garassino MC, Reck M, Senan S, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC-an update from the PACIFIC trial. J Thorac Oncol. 2021;16(5):860–67. doi: 10.1016/j.jtho.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, De La Mora Jimenez E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, Van Den Heuvel MM, Cobo M, Vicente D, Smolin A, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661–74. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remon J, Passiglia F, Ahn MJ, Barlesi F, Forde PM, Garon EB, Gettinger S, Goldberg SB, Herbst RS, Horn L, et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15(6):914–47. doi: 10.1016/j.jtho.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Suazo-Zepeda E, Bokern M, Vinke PC, Hiltermann TJN, De Bock GH, Sidorenkov G. Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Immunol Immunother. 2021;70(11):3069–80. doi: 10.1007/s00262-021-02996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:4451–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–28. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634–42. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 28.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 29.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, Van Den Heuvel MM, Ciuleanu TE, Badin F, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 33.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 34.Reck M, Schenker M, Lee KH, Provencio M, Nishio M, Lesniewski-Kmak K, Sangha R, Ahmed S, Raimbourg J, Feeney K, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non–small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137–47. doi: 10.1016/j.ejca.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 36.Ando K, Kishino Y, Homma T, Kusumoto S, Yamaoka T, Tanaka A, Ohmori T, Ohnishi T, Sagara H. Nivolumab plus ipilimumab versus existing immunotherapies in patients with PD-L1-positive advanced non-small cell lung cancer: a systematic review and network meta-analysis. Cancers (Basel). 2020;12(7):1905. doi: 10.3390/cancers12071905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You W, Liu M, Miao JD, Liao YQ, Song YB, Cai DK, Gao Y, Peng H. A network meta-analysis comparing the efficacy and safety of anti-PD-1 with anti-PD-L1 in non-small cell lung cancer. J Cancer. 2018;9(7):1200–06. doi: 10.7150/jca.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X, Paterson AM, Watanabe T, Vanguri V, Yagita H, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011;187(3):1113–19. doi: 10.4049/jimmunol.1100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HT, Lee JY, Lim H, Lee SH, Moon YJ, Pyo HJ, Ryu SE, Shin W, Heo YS. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci Rep. 2017;7(1):5532. doi: 10.1038/s41598-017-06002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fessas P, Lee H, Ikemizu S, Janowitz T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol. 2017;44(2):136–40. doi: 10.1053/j.seminoncol.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31(1):3151–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 42.Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–54. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang B, Liu Y, Zhou S, Jiang H, Zhu K, Wang R. Predictive effect of PD-L1 expression for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatment for non-small cell lung cancer: a meta-analysis. Int Immunopharmacol. 2020;80:80106214. doi: 10.1016/j.intimp.2020.106214. [DOI] [PubMed] [Google Scholar]
- 44.Yang K, Li J, Bai C, Sun Z, Zhao L. Efficacy of immune checkpoint inhibitors in non-small-cell lung cancer patients with different metastatic sites: a systematic review and meta-analysis. Front Oncol. 2020;10:101098. doi: 10.3389/fonc.2020.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vellanki PJ, Mulkey F, Jaigirdar AA, Rodriguez L, Wang Y, Xu Y, Zhao H, Liu J, Howe G, Wang J, et al. FDA approval summary: nivolumab with ipilimumab and chemotherapy for metastatic non–small cell lung cancer, a collaborative project orbis review. Clin Cancer Res. 2021;27(13):3522–27. doi: 10.1158/1078-0432.CCR-20-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


