Abstract
Background and Objectives:
Kidney Disease Education (KDE) has been shown to improve informed dialysis selection and home dialysis use, two long-held but underachieved goals of US nephrology community. In 2010, the Center for Medicare and Medicaid Services (CMS) launched a policy of KDE reimbursements for all Medicare beneficiaries with advanced chronic kidney disease. However, incorporation of KDE service in real-world practice, and its associations with the home dialysis utilization has not been examined.
Design, Settings, Participants, and Measurements:
Using the 2016 United States Renal Data System (USRDS) linked to end stage renal disease (ESRD) and pre-ESRD Medicare claims data, we identified all adult incident ESRD patients with active Medicare benefits at their first-ever dialysis during the study period (January 1, 2010 to December 31, 2014). From these, we identified those who had at least one KDE service code before their dialysis initiation (KDE cohort) and compared them to a parsimoniously matched non-KDE control cohort in 1:4 proportions for age, gender, ESRD network and the year of dialysis initiation. The primary outcome was home dialysis use at dialysis initiation, and secondary outcomes were home dialysis use at day 90 and anytime through the course of ESRD.
Results:
Of the 369,968 qualifying incident ESRD Medicare beneficiaries with their first-ever dialysis during the study period, 3,469(0.9%) received KDE services before dialysis initiation. African American race, Hispanic ethnicity, and presence of congestive heart failure and hypoalbuminemia were associated with significantly lower odds of receiving KDE services. Multivariate analyses showed that KDE recipients had twice the odds of initiating dialysis with home modalities [15.0% vs. 6.9%; adjusted odds ratio (aOR):95% confidence interval (CI): 2.0:1.7–2.4] and had significantly higher odds using home dialysis throughout the course of ESRD [home dialysis use at day 90 (17.6% vs. 9.9%, aOR:CI: 1.7:1.4–1.9) and cumulatively (24.7% vs. 15.1%, aOR:CI: 1.7:1.5–1.9)].
Conclusions:
Utilization of pre-ESRD KDE services is associated with significantly greater home dialysis utilizations in the incident ESRD Medicare beneficiaries. The very low rates of utilizations of these services suggest for the need for focused systemic evaluations to identify and address the barriers and facilitators of this important patient-centered endeavor.
BACKGROUND
Despite having equivalent medical outcomes, and trends for better patient-centered and health services outcomes,(1–6) home dialysis therapies—comprising of home peritoneal dialysis and home hemodialysis, are utilized only in about 10% of the US end stage renal disease (ESRD) population. (7) Over the last two decades, major ESRD stakeholders have consistently advocated, and the Center for Medicare and Medicaid Services (CMS) has targeted policies to facilitate greater home dialysis use. (8) Unfortunately, these efforts have resulted in marginal increase in home dialysis rates and 90.0% of the US ESRD patients continue to receive in-center hemodialysis. (9) In July 2019, the US president issued an Executive Order emphasizing the goals for increasing the home dialysis utilization for the management of ESRD in the US.(10)
Major ESRD stakeholders in the US, including National Kidney Foundation and American Society of Nephrology recommend that all patients transitioning to ESRD should be empowered to make informed dialysis modality choice best suited for their lifestyle, values, and preferences. (11–14) Informed choice requires comprehension of complex medical, social, and lifestyle implications of different dialysis modalities.(15) However, awareness of chronic kidney disease (CKD) and its management options is suboptimal among the US advanced CKD patients, (4, 9, 16, 17) and this is an important factor limiting greater home dialysis use. (18, 19)
Several studies, primarily from outside the US, have shown that Kidney Disease Education (KDE) can improve the patients’ awareness of CKD,(20–22) facilitate patient-centered informed dialysis choice, and increase home dialysis utilization.(16, 23–29) In light of these factors, the CMS implemented the policy for KDE reimbursement policy for Medicare beneficiaries beginning January 1, 2010.(30, 31) We conducted this retrospective analysis with the goals of examining the utilizations of pre-ESRD KDE services among the US incident ESRD Medicare beneficiaries and assessing its association with the home dialysis use for the management of ESRD.
METHODS
Study Design:
This retrospective study was approved by the University of Florida Institutional Review Board. In this study, we obtained study data from multiple national databases. Using the standard files from the 2016 United States Renal Data System (USRDS) as a primary source, we identified all adult (≥18 years old) incident ESRD patients who initiated their first-ever dialysis on or after 1/1/2010 (CMS-KDE policy implementation date) to 12/31/2014 (the last date for available data in USRDS-2016 report). To minimize the variations in pre-ESRD Medicare coverage, among those we then selected patients who had an active Medicare recipient status at the time of initiation of dialysis therapy as identified by the Medicare/Medicaid status on the CMS Medical Evidence Report form (CMS-2728). We then used the ESRD and the pre-ESRD Institutional and Physician/Supplier claims data to identify among those who had the Healthcare Common Procedure Coding System (HCPCS) codes of G0420 or G0421 for KDE. We excluded patients whose KDE codes occurred after the first date of dialysis to avoid confounding by factors other than KDE, i.e., prior dialysis or renal transplant. We then created a control non-KDE cohort for comparison from the same cohort selecting the patients that did not have either HCPCS codes for the KDE. The control cohort was parsimoniously matched to the KDE cohort in 1:4 proportions for age ±2 years, gender, year of dialysis initiation, and the ESRD network. The parsimonious matching was used to ensure that we are able to examine the variations in the factors associated with the utilization of the KDE service. Finally, using the CMS-2728 form and the core USRDS dataset, we retrieved information on patient demographics, primary disease causing ESRD, date of the first dialysis, first dialysis modality type, ESRD network, comorbidities [i.e., diabetes mellitus (DM), hypertension, congestive heart failure (CHF), chronic obstructive pulmonary disease, peripheral vascular disease, stroke, coronary heart disease], need for assistance with daily activities, and laboratory data including estimated glomerular filtration rate (eGFR) and serum albumin.
Outcome Measures:
Primary outcome of home dialysis use at dialysis initiation was determined by the first dialysis modality type of PD or HHD. The secondary outcomes of home dialysis use at 90 days after the date of the first dialysis and anytime throughout the course of ESRD were determined according to detailed treatment history data (i.e., Modality Sequence File).
Statistical Analysis:
Demographic, clinical and socioeconomic characteristics were compared between the study groups using the chi-square tests for categorical variables, and the one-way analysis of variance and the Kruskal-Wallis tests for normally and non-normally distributed continuous variables respectively. We used conditional logistic regression to determine the association between KDE and home dialysis use at dialysis initiation using univariate and multivariate models. In the multivariate regression model, we adjusted for race, ethnicity, employment status, obesity categorically by BMI score, eGFR, albumin by quartile, DM, CHF, pre-existing cardiovascular disease (CVD, defined as having coronary heart disease, stroke or peripheral vascular disease), cystic kidney disease as primary cause of ESRD, need for assistance with daily activities, and pre-ESRD nephrology care and duration. We also used multivariate conditional logistic regression models for stratified analysis and assessment of the interactions between KDE and variables of interest in the matched cohort. The analytic model for the secondary outcomes of home dialysis use on day-90 of ESRD and at any time during the course of ESRD was similar to the original analysis model. All significance tests were two-sided, with a p-value <0.05 considered statistically significant. Statistical analyses were performed using Statistical Analysis Software, version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Figure 1 shows the selection of the patients for the study. We identified a total of 369,968 patients with active Medicare at the time of their first-ever dialysis initiation during the study period. Of these, 3,681 (~1.0%) patients were identified to have received KDE service by the presence of the KDE HCPCS codes. After excluding 212 patients who received their KDE service after their first date of dialysis, the resultant KDE cohort of 3,469 (0.9% of incident ESRD) was matched in 1:4 ratios to obtain matched control cohort.
Figure 1.
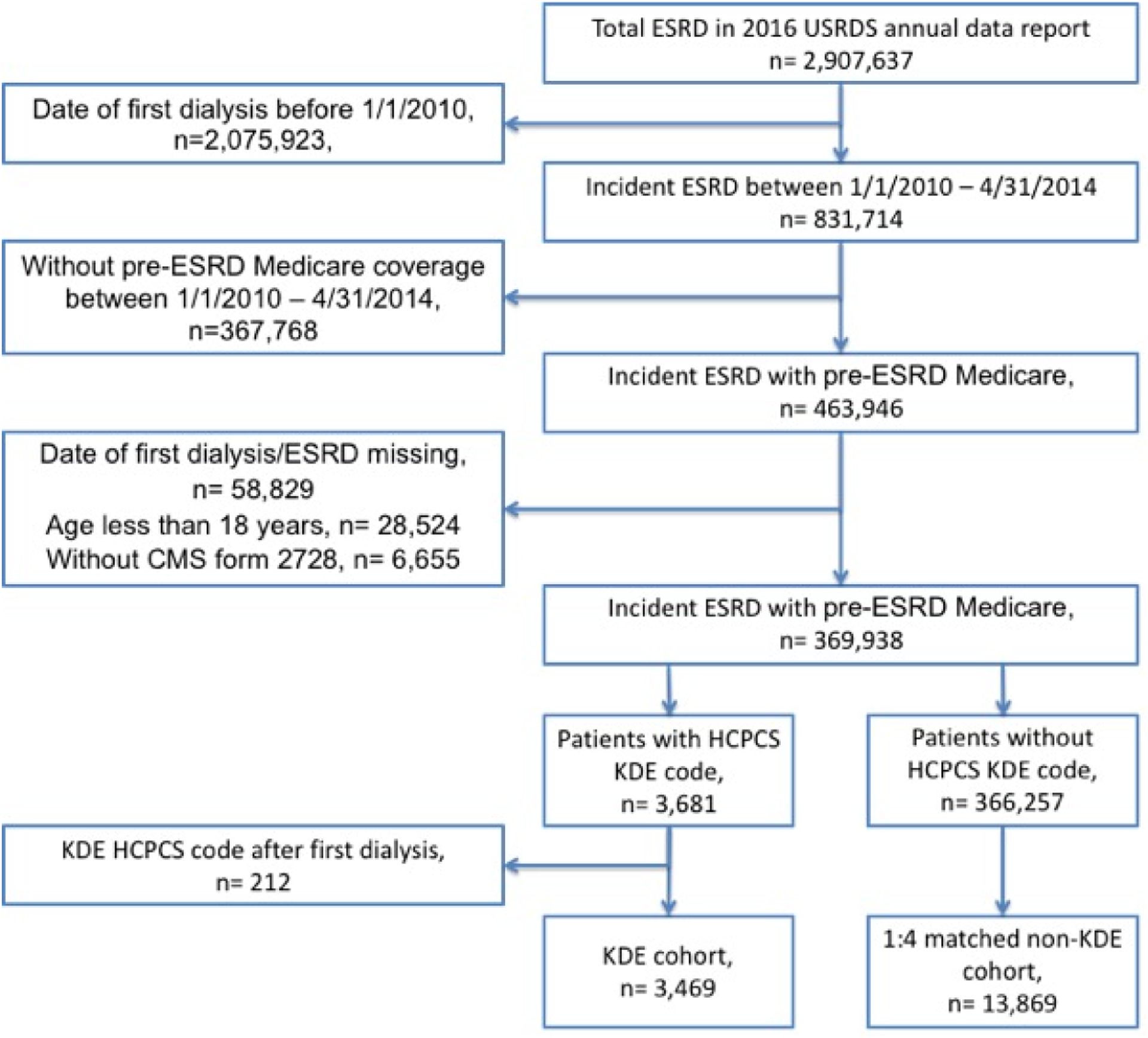
Study Cohort Flow Chart
USRDS: United States Renal Data System, CMS: Center for Medicare and Medicaid Services, HCPCS: Healthcare Common Procedure Coding System, KDE: Kidney Disease Education.
Table 1 shows the baseline characteristics of the KDE cohort, the matched non-KDE cohort, and the wider-USRDS cohort at the initiation of the dialysis. The adjusted odds for receiving KDE were 11% lower among blacks (95%CI:0.89–0.98) and 22% lower among Hispanics (95%CI:0.63–0.95). Employed individuals had lower likelihoods (aOR:0.41, 95%CI:0.3–0.56) and retired individuals had higher likelihoods (aOR:1.29, 95%CI:1.1–1.52) of receiving KDE compared to unemployed individuals. Patients with hypertensive renal disease (OR:1.4, 95%CI:1.24–1.59), DM (OR:1.24, 95%CI:1.14–1.33) and pre-existing CVD (OR:1.22, 95%CI:1.12–1.34) had greater likelihood of receiving KDE, whereas the patients with CHF (aOR:0.85, 95%CI:0.75–0.95) and hypoalbuminemia had a significantly lower likelihood of receiving KDE. Hypoalbuminemia especially, had a strong relation with receiving KDE as each quartile decline in albumin was associated with progressively declining odds of receiving KDE [aOR:95%CI for KDE being 0.78:0.68–0.89 (albumin 3.2–3.6 gm/dl), 0.61:0.52–0.71 (albumin 2.7–3.2 gm/dl), and 0.47:0.4–0.56 (albumin <2.7 gm/dl) compared to those with albumin levels above 3.6gm/dl (p <0.0001)]. Finally, any nephrology care was three times as likely to receive KDE, with a progressively higher correlation between the duration of nephrology care and the likelihood of receiving KDE (aOR for receiving KDE: 2.4, 3.0, and 4.02 for nephrology care duration of less than 6 months, between 6–12 months and greater than 12 months compared to no nephrology care, p<0.0001).
Table 1.
Patient characteristics at baseline or the initiation of dialysis.
| KDE group (n = 3,469) | Matched Controls, non-KDE Group (n=13,869) | USRDS cohort (n=366,257) | p value* | p value** | |
|---|---|---|---|---|---|
| Age (years) | 72.9 ± 10.9 | 72.9 ± 10.9 | 64.9 ± 14.9 | N/A | <0.0001 |
| 18–44 years, n (%) | 68 (1.96) | 274 (1.98) | 38,232 (10.4) | N/A | |
| 45–64 years, n (%) | 567 (16.3) | 2295 (16.5) | 132,852 (46.7) | ||
| >65 years, n (%) | 2834 (81.7) | 11300 (81.5) | 195,173 (53.3) | ||
| Gender, Male, n (%) | 1,955 (56.4) | 7,816 (56.4) | 212,294 (57.9) | N/A | 0.056 |
| BMI (kg/m2), n(%) | 28.9 ± 7.7 | 28.7 ± 7.6 | 29.5 ± 8 | 0.098 | 0.0001 |
| BMI ≥ 30 | 1,247 (36.3) | 4,931 (35.9) | 143,402 (39.5) | 0.6040 | 0.0001 |
| Race, n(%) | |||||
| White | 2,584 (74.5) | 10,152 (73.2) | 252,528 (68.9) | 0.022 | <0.0001 |
| Black | 739 (21.3) | 3,207 (23.1) | 94,696 (25.8) | ||
| Other | 146 (3.7) | 510 (3.7) | 19,043 ((5.2) | ||
| Hispanic ethnicity, n(%) | 283 (8.1%) | 1,388 (10) | 52,643 (14.3) | 0.0016 | <0.0001 |
| Employment status, n(%) | |||||
| Employed | 93 (2.7) | 865 (6.3) | 47,236 (12.9) | <0.0001 | <0.0001 |
| Unemployed | 468 (13.5) | 2,214 (16) | 232,434 (63.5) | ||
| Retired | 2,908 (83.8) | 10,790 (77.8) | 86,587 (23.6) | ||
| Primary Disease, n(%) | |||||
| Diabetes Mellitus | 6042 (43.6) | 1687 (48.6) | 171,040 (46.7) | <0.0001 | <0.0001 |
| Hypertension | 4920 (35.5) | 1279 (36.9) | 110,657 (30.2) | ||
| Glomerulonephritis | 661 (4.8) | 163 (4.7) | 25,829 (7.05) | ||
| Polycystic kidney disease | 156 (1.1) | 39 (1.12) | 6,278 (1.71) | ||
| Comorbidity, n (%) | |||||
| Diabetes Mellitus§ | 2,279 (64.3) | 8,375 (60.4) | 224,403 (61.3) | <0.0001 | 0.0003 |
| Congestive heart failure | 1,237 (35.7) | 5,327 (38.4) | 122,932 (33.6) | 0.002 | 0.0092 |
| Hypertension | 3,143 (90.6) | 12,108 (87.3) | 318,958 (87.1) | <0.0001 | <0.0001 |
| Obstructive pulmonary disease | 441 (12.7) | 1,867 (13.5) | 39,608 (10.8) | 0.247 | 0.0003 |
| Coronary heart disease | 905 (26.1) | 3,123 (22.5) | 71,978 (19.7) | <0.0001 | <0.0001 |
| Peripheral vascular disease | 555 (16) | 2,060 (14.7) | 49,342 (13.5) | 0.091 | <0.0001 |
| Stroke | 372 (10.7) | 1,498 (10.8) | 34,977 (9.6) | 0.8902 | 0.0192 |
| Pre-existing CVD§§ | 1,365 (39.4) | 4,972 (35.9) | 116,849 (31.9) | <0.0001 | <0.0001 |
| Cancer | 328 (9.5) | 1,387 (10) | 29,079 (7.94) | 0.333 | 0.001 |
| Albumin (g/dl), n(%) | 3.3 ± 0.6 | 3.1 ± 0.7 | 3.2 ± 4.4 | <0.0001 | <0.0001 |
| Below 2.7 | 367 (14.6) | 2350 (24.1) | 64,435 (24.47) | <0.0001 | <0.0001 |
| 2.7 to 3.2 | 552 (21.9) | 2570 (26.3) | 66,627 (25.3) | ||
| 3.2 to 3.6 | 793 (31.4) | 2707 (27.7) | 71,014 (27) | ||
| Above 3.6 | 809 (32.1) | 2134 (21.9) | 61,249 (23.2) | ||
| Need for assistance, n(%) | 489 (14.1) | 2,461 (17.7) | 52996 (14.5) | <0.0001 | 0.534 |
| Under the care of Nephrologist, n(%) | |||||
| None | 573 (16.5) | 5472 (39.5) | 150,523(41.1) | <0.0001 | <0.0001 |
| Less than 6 months | 487 (14) | 1885 (13.6) | 51,303 (14) | ||
| 6–12 months | 770 (22.2) | 2492 (18) | 66,244 (18.1) | ||
| More than 12 months | 1639 (47.3) | 4020 (29) | 98,187 (26.8) | ||
| MDRD eGFR at dialysis initiation (mL/min/1.73 m2) | 13.1 ± 5 | 12.7 ± 5.4 | 11.95 ± 5.4 | <0.0001 | <0.0001 |
Data is reported as mean±SD unless otherwise indicated.
Diabetes as a comorbidity was decided based on patients either having diabetes as a cause of ESRD or diabetes listed in other medical problems.
pre-existing CVD was calculated by patient having an existing diagnosis of any of the coronary heart disease, stroke or peripheral vascular disease.
p values for comparison between the KDE and matched non-KDE control cohort, by conditional logistic regression.
p values for comparison between the KDE the cotemporaneous USRDS cohort, by one-way analysis of variance or Kruskal-Wallis tests (continuous variables) and chi-square test (categorical variables).
Abbreviations. KDE: Kidney Disease education, BMI: body mass index, CVD: cardiovascular disease, SD, standard deviation.
Home dialysis utilizations:
Figure 2 shows the dialysis modality utilizations through the three study time points: initiation of dialysis, at day-90 into dialysis care, and cumulatively at any time during the course of ESRD in the study cohorts. KDE cohort patients used home dialysis at more than twice the rates (15.0% vs. 6.9%) at the initiation of dialysis. Cumulatively, over the lifespan the KDE service recepients used home dialysis at 66% greater rates, compared to their matched controls; a quarter of all patients who received KDE (24.7%) used home dialysis eventually through their course of ESRD vs. 15.1% in matched control (Table 2). Subgroup analysis of the KDE cohort suggested the overall timing of KDE services (317 ± 317 days before ESRD), or frequency of KDE service (one session, n=2420, 70% of the KDE cohort; two sessions: n=499, or three sessions: n=538, 14.6% of KDE cohort) was not associated with significant difference in the use of home dialysis (p>0.81).
Figure 2:
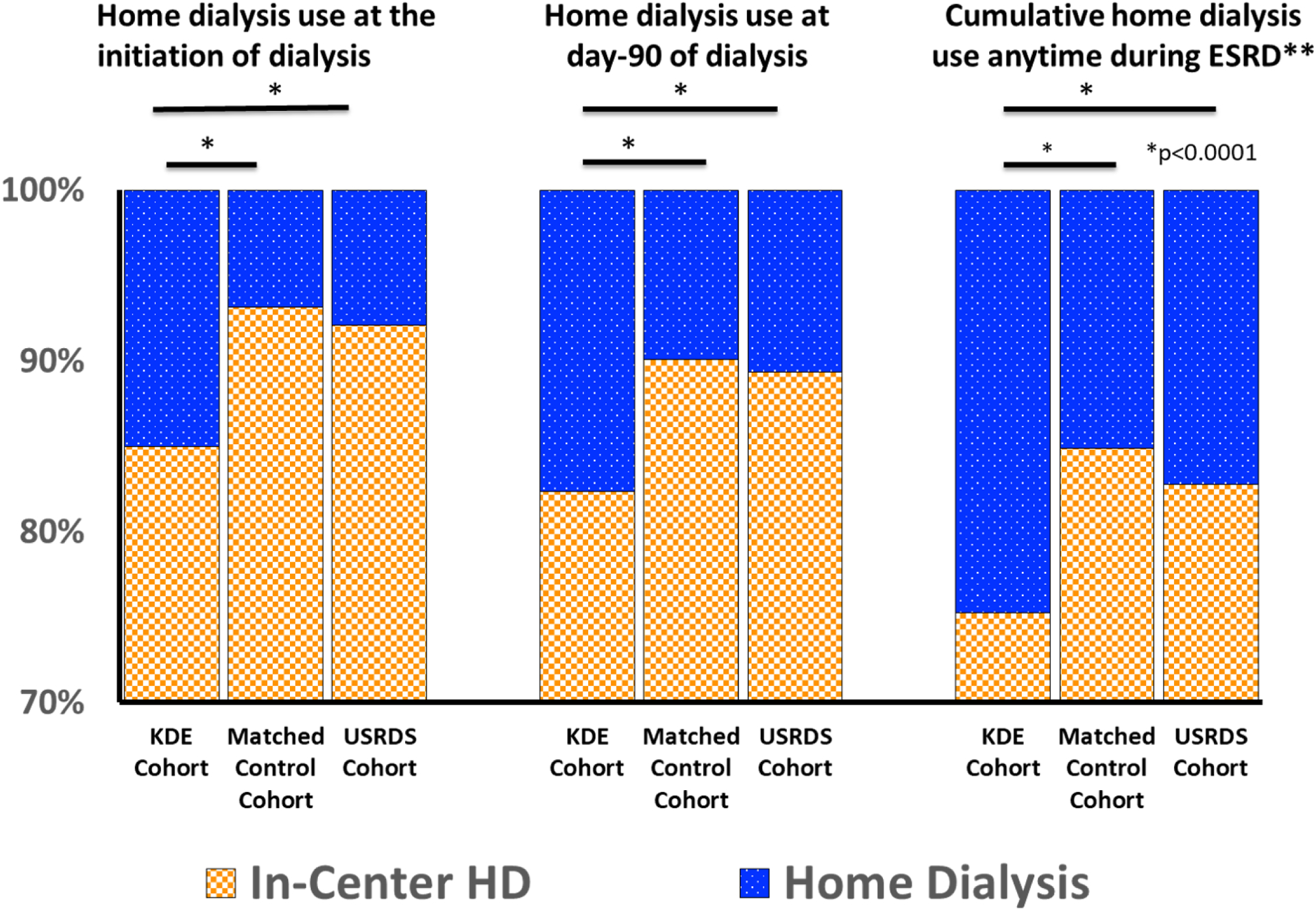
Dialysis Modality Distribution Patterns among the study cohorts
Representation of the In-Center Hemodialysis and home Dialysis use as a fraction of the total dialysis population at different time points through ESRD. The in-center dialysis in the cumulative use, represent proportion of patients who never used home dialysis. The minimum value on Y-axis has been limited at 70%.
Table 2.
Dialysis Modality Utilizations at the initiation of dialysis, at day 90 of dialysis, and Cumulatively throughout the course of ESRD among different study cohorts
| Modality Distribution at the initiation of Dialysis, n(%) | Modality Distribution at the day 90 of ESRD n(%) | Cumulative HoD, and IHD alone use through the course of ESRD n(%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| KDE Cohort | Matched Control Cohort | USRDS Cohort | KDE Cohort | Matched Control Cohort | USRDS Cohort | KDE Cohort | Matched Control Cohort | USRDS Cohort | |
| IHD | 2937 (85.0) | 12875 (93.1) | 335,551 (92.1) | 2859 (82.9) | 11755 (90.1) | 311,310 (89.4) | 2610 (75.2) | 11775 (84.9) | 303216 (82.8) |
| Home dialysis | 520 (15.0) | 947 (6.9) | 28,766 (7.9) | 591 (17.1) | 1295 (9.9) | 37,054 (10.6) | 859 (24.8) | 2094 (15.1) | 63041 (17.2) |
| PD | 503 (14.6) | 888 (6.4) | 27,291 (7.5) | 546 (15.8) | 1,091 (8.4) | 33,429 (9.6) | 739 (21.3) | 1,658 (12.0) | 52,399 (14.3) |
| HHD | 17 (0.4) | 59 (0.4) | 1,475 (0.4) | 45 (1.3) | 204 (1.6) | 3,625 (1.0) | 145 (4.2) | 484 (3.5) | 12,331 (3.4) |
Abbreviations.KDE: Kidney Disease Education, IHD: In-center hemodialysis, PD: Any type of Peritoneal Dialysis, HHD: includes those with true home hemodialysis as well as self-care center hemodialysis
Table 3 shows significant univariate and multivariate analyses examining the associations between the patient variables and home dialysis use at the initiation of dialysis. Both patients who received KDE (vs. matched controls) as well as those who initiated home dialysis (vs. those who initiated IHD), initiated dialysis therapies earlier as defined by their higher eGFR values. Being black was associated with significantly lower odds of home dialysis use, whereas having Hispanic ethnicity did not significantly alter the probability of home dialysis use on multivariate analysis. Comorbidities such as DM or CVD with greater likelihood of receiving KDE were negatively associated with home dialysis use on univariate analysis, an effect that dissipated on multivariate model. Whereas poor functional status as defined by the need for assistance, and comorbidities such as CHF and hypoalbuminemia with lower likelihood of receiving KDE continued to have significantly lower likelihood of using home dialysis. Progressive severity of hypoalbuminemia had strong and progressively declining associations with home dialysis use. Among all variables, prior nephrology care had the strongest positive correlations with home dialysis use with progressively increasing correlations between the duration of nephrology care and home dialysis use (p<0.0001). In both univariate and multivariate analyses receiving KDE showed a strong and independent effect on home dialysis use at the dialysis initiation (aOR: 1.99, 95% CI: 1.67–2.39), at day-90 on dialysis (aOR: 1.7, 95% CI: 1.4–1.9) as well as cumulatively anytime during their course of ESRD (aOR: 1.69, 95% CI: 1.47–1.93). We also performed a subgroup analysis after excluding patients with functional limitations, defined as those with the need for assistance for ADLs, inability to transfer or institutionalized from KDE- and Non-KDE cohort, and found that the analysis was congruent with primary findings; KDE service continued to have strong association with home dialysis, with aOR: 1.76, 95% CI: 1.47–2.11 (Supplemental Table 1). Uni- and multivariate analyses for the secondary outcomes mimicked those in the primary outcome as well, and are available in Supplemental Tables 2 and 3. Similar findings were also evident for a separate analysis examining the associations between KDE and home hemodialysis (data not shown).
Table 3.
Patient demographic, medical and socioeconomical characteristics associated with home dialysis use at the start of ESRD in univariate and multivariate logistic regression model.
| Variable | Referent | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
P value | ||
| KDE | No KDE | 2.42 (2.17–2.74) | <0.0001 | 1.99 (1.67 – 2.39) | <0.0001 |
| Black race | White | 0.49 (0.41–0.58) | <0.0001 | 0.58 (0.45 – 0.76) | <0.0001 |
| Hispanic Ethnicity | Non-Hispanic | 0.81 (0.64–1.02) | 0.07 | 0.90 (0.63 – 1.30) | 0.57 |
| Polycystic kidney disease | No PCKD | 2.93 (1.88–4.56) | <0.0001 | 1.21 (0.59 – 2.5) | 0.60 |
| Employed | Unemployed | 3.3 (2.56–4.26) | <0.0001 | 2.63 (1.78 – 3.88) | <0.0001 |
| Retired | Unemployed | 1.38 (1.14–1.67) | 0.0007 | 1.29 (0.97 – 1.71) | 0.08 |
| CHF | No CHF | 0.62 (0.54–0.71) | <0.0001 | 0.74 (0.60 – 0.92) | 0.007 |
| Diabetes Mellitus | No DM | 0.83 (0.73–0.94) | 0.004 | 0.87 (0.71 – 1.08) | 0.2 |
| Pre-existing CVD | No CVD | 0.72 (0.63–0.83) | <0.001 | 0.90 (0.73 – 1.11) | 0.3 |
| Need for assistance | No need for assistance | 0.45 (0.36–0.55) | <0.0001 | 0.55 (0.40 – 0.77) | 0.0004 |
| BMI 30–≤35 | BMI < 30 | 1.05 (0.90–1.23) | 0.51 | 0.97 (0.76–1.24) | 0.8 |
| BMI 35–40 | BMI < 30 | 1.01 (0.82–1.24) | 0.91 | 0.91 (0.66–1.27) | 0.59 |
| BMI >40 | BMI < 30 | 0.76 (0.60–0.95) | 0.02 | 0.63 (0.44–0.90) | 0.01 |
| eGFR, MDRD | (per 1 mL/min/1.73 m2 increase) | 1.03 (1.01–1.04) | <0.0001 | 1.04 (1.02 – 1.06) | 0.0003 |
| Albumin <2.7 | Albumin >3.6 | 0.11 (0.06–0.15) | <0.0001 | 0.15 (0.11 – 0.21) | <0.0001 |
| Albumin 2.7–3.2 | Albumin > 3.6 | 0.26 (0.21– 0.33) | <0.0001 | 0.37 (0.28 – 0.48) | <0.0001 |
| Albumin 3.2–3.6 | Albumin > 3.6 | 0.50 (0.42– 0.6) | 0.61 (0.49 – 0.76) | <0.0001 | |
| Renal care < 6 months | No renal care | 4.00 (3.17–5.05) | <0.0001 | 2.79 (1.98 – 3.92) | <0.0001 |
| Renal care 6–12 months | No renal care | 4.89 (3.95–6.04) | <0.0001 | 3.5 (2.56 – 4.79) | <0.0001 |
| Renal care > 12 months | No renal care | 5.88 (4.85–7.14) | <0.0001 | 3.84 (2.87 – 5.14) | <0.0001 |
KDE: Kidney Disease education, BMI: body mass index, CHF: congestive heart failure, PCKD: polycystic kidney disease, CVD: cardiovascular disease, DM: diabetes mellitus. Uni- and multivariate analyses were performed using race, ethnicity, BMI, employment status, primary renal diagnosis, CHF, albumin level, need for assistance, prior nephrology care, and KDE status. Variables with a significant p value in univariate and/or multivariate models have been shown in the table. Patients who received KDE were matched (1:4) with patients who did not receive KDE on age ±2 years, gender, year of dialysis initiation, and the ESRD network. Conditional logistic regression models were used for analyzing matched cohort.
Clarifying the effects of KDE on home dialysis use:
In order to account for the colinearity between the KDE service use and the home dialysis use, we examined the interaction effects between KDE and each individual variable, and performed stratified analyses to evaluate the impact of KDE on home dialysis use across the different strata of each individual variable, while controlling for other variables. Among the examined variables, KDE had significant interactions only with BMI (p=0.018) and serum albumin (p= 0.032). However despite these, the stratified analysis showed that KDE use continued to have positive associations with home dialysis across most strata albumin and BMI distributions, except for those with BMI between 35–40. Furthermore, KDE continued to show significant positive associations with home dialysis use across all other examined variables. Noteworthy, the duration of prior nephrology care did not have significant interactions with KDE’s associations with home dialysis use (p=0.707), and KDE use had strong positive associations with home dialysis use across all strata of patients with established nephrology care in the pre-ESRD period (Figure 3) including those who had long-term ongoing nephrology care (>12 months duration). However, this association was found not significant among those without any nephrology care at the time of initiation of dialysis. Overall, when examined across the variables and their strata, patients receiving KDE had greater use of home dialysis therapies.
Figure 3.
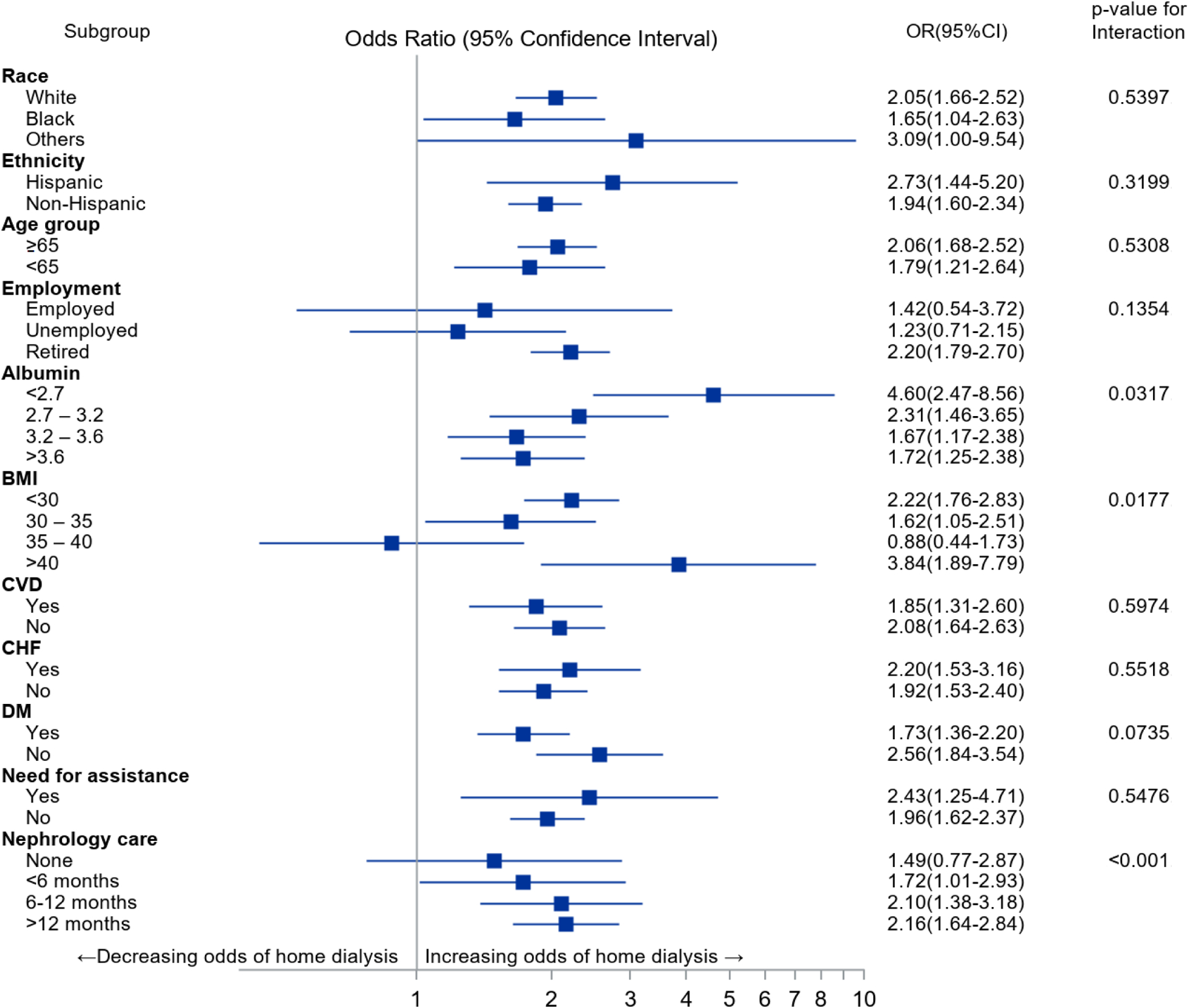
Stratified analysis clarifying the impact of KDE on home dialysis use at the start of ESRD across different strata of the variables of interest
KDE: Kidney Disease education, BMI: body mass index, CHF: congestive heart failure, CVD: cardiovascular disease, DM: diabetes mellitus. Conditional logistic regression model was used for analyzing matched cohort and adjusted for age, race, ethnicity, BMI, employment status, primary renal diagnosis, CHF, DM, combined cardiovascular disease, eGFR, albumin level, need for assistance, prior nephrology care, and KDE status.
DISCUSSION
Our study provides a number of important insights of public health significance. First, our analysis is consistent with the evidence provided by researchers in the international community and supports the broad intent of the CMS-KDE policy. It shows a strong positive association between the utilization of pre-ESRD KDE service and home dialysis use, with a near doubling of home dialysis use at the time of dialysis initiation, and an eventual home dialysis use among a quarter of all recipients. This is among the strongest association any intervention has shown on home dialysis rates on a system-wide scale. Prior studies have estimated that mere doubling of the current home dialysis rates can result in the annual Medicare savings of ~0.6–1 billion dollars.(32–34) Of the multiple CMS policies targeting the unintended unfavorability of home dialysis in the US healthcare system,(31) the Prospective Payment System (PPS) for ESRD, better known as ESRD bundling, is most noticeable and has been credited with the much-awaited reversal of the declining home dialysis trends of more than two decades. Examining four years around the introduction of PPS, Zhang et al showed that PPS introduction was associated with a rise in home dialysis use by about 2% over the evaluation period.(35) Compared to these, near doubling in the home dialysis use among KDE recipients is remarkable. Though differential evaluation of these policies is not the target of current analysis, the strength of data points towards the importance of KDE as a measure to improve informed home dialysis use.
Our study further shows that the positive associations between the KDE service and home dialysis utilization are independent of the socio-demographic characteristics, comorbidity burden, and pre-ESRD nephrology care. These independent associations are evident not only in the multivariate analysis but in a more detailed stratified analyses, which evaluated the associations between the KDE service across the different strata of each individual variable. These analyses showed that the positive associations between the KDE service and home dialysis use are constant and strong across the measured variables – whether the individual variables themselves had a positive or a negative association with home dialysis use. For example, the employment status and pre-ESRD nephrology care had among the strongest associations with home dialysis use on multivariate analysis. However, these associations were further strengthened by the presence of KDE service across all strata of these variables. Similarly, progressive hypoalbuminemia had declining odds of home dialysis use. However, provision of KDE services was associated with strong and positive odds for home dialysis use across all strata of hypoalbuminemia (Figure 3).
At the same time, this is among the lowest effect size assessed for KDE services on home dialysis rates in the reported studies. Multiple single-center studies have shown that nearly 50% of those receiving kidney disease education use home dialysis therapies, with reported range between 35 to 85%.(23–26, 28, 29, 36) In the only available large organization-based initiative, Lacson et al. found that nearly quarter of all educated chose home dialysis therapies.(27) However, assessment of such low effect size is not surprising in our cohort based analysis since our analysis does not aim to evaluate the association between the KDE and home dialysis use but, merely the delivery/coding of the KDE service with the home dialysis use. It is reasonable to assume that some form of KDE must have occurred without the appropriate KDE coding, even among those in non-KDE cohort for these patients to be able to choose conventional rates of home dialysis utilizations. Furthermore, CMS-KDE policy only loosely defines KDE and provides for significant latitude in the format (group vs. individual KDE), educator expertise, and above all the content of KDE.(30) Heterogeneity in KDE protocols, varying educator expertise, and physician comfort and proficiency with home dialysis therapies likely explains the impressive yet comparatively low rates of home dialysis in the pragmatic community-wide KDE service cohort of the analysis, and establishes the need for future research in this area.
Finally our analysis shows that despite the recommendations by the American Society of Nephrology and CMS that ‘all’ patients transitioning to ESRD be provided with the KDE and opportunities for informed dialysis choice,(11, 30) incorporation of KDE services in the routine clinical practice is uncommon to rare for US Medicare beneficiaries. Less than 1% of the beneficiaries between 2010–2014 were coded to have received KDE while nearly 85% of non-recipients had some ongoing nephrology care at the time of ESRD with more than 2/3rd having nephrology care for more than six months. Such gross underutilizations of a universally recommended practice is alarming. Lack of the granular practice level data makes it difficult to comment on the reasons though; based on available literature, few assumptions can be made. First, the imbalances between the reimbursements and resource-utilizations may have reduced the provision KDE or the use of KDE service codes. CMS-KDE policy requires a high-level practitioner to provider KDE but, allows for only a small time-based billing for KDE (~$125/hour for an individual session and $26/hour for a group session) with the applicable 20% copays and deductibles for patients.(30, 31) Studies have shown that KDE is a resource-consuming multidisciplinary process.(28, 37) These imbalances may deter nephrology practices from establishing infrastructure and/or billing for KDE in favor of more lucrative clinical visit codes resulting in underreporting of the existent KDE within the community. Alternatively, many practices may have provided KDE through the affiliated dialysis organizations.(27, 35, 38) Such KDE could not be coded using the same HCPCS codes, and thus may have caused underreporting in control group. Evidence-based alterations in the KDE reimbursements may increase its utilizations. Second, the pre-existing bias among the providers may be limiting a wider, more universal provision of KDE services. Prior studies from the US have shown that persons with greater socio-economic affluence or favorable comorbidity status are more likely to receive KDE as well as use home dialysis.(27, 36) Our analysis corroborates these findings as multiple socio-demographic characteristics (i.e., African American race, Hispanic ethnicity etc.) and medical comorbidities (i.e., presence of CHF and hypoalbuminemia, etc.) conventionally considered unfavorable for peritoneal dialysis, had strong negative correlations with KDE and eventual home dialysis. Finally, the paucity of data demonstrating consistent and significant benefits in clinical outcomes from within the US may have limited the adoption of KDE services. Validated, evidence-based KDE protocols that are easily reproducible may facilitate wider adoption of these services.(39, 40) Overall, while these concerns may explain marginal errors in the KDE reporting, the gross underutilization as seen in our data needs further evaluations.
Considering the nature of clinical and claims database analysis, our study has a few limitations. Although we can report strong associations between the provision of KDE service and home dialysis use, the study does not allow for ascribing causality or analysis of additional patient-related factors such as patients’ level of education, language barriers, fears, choice, or motivation, as such data are unavailable in the USRDS dataset. (41) Improved quality of pre-ESRD care or provider bias may have led to greater use of KDE among those with greater likelihood to use home dialysis. To address these, we performed stratified analyses evaluating the impact of KDE services utilization on home dialysis use across the different strata of each sociodemographic, comorbidity and prior nephrology care parameters (Figure 3), and found that the positive impacts of KDE is maintained across most strata of all studied variables. Second, we acknowledge that the extremely low rates of KDE service utilization may be concerning for the applicability of these results in the wider-USRDS population. To ensure that our results are applicable to the broader cohort, we compared the KDE cohort with the larger cotemporaneous USRDS cohort and evaluated the factors impacting home dialysis use in the wider USRDS cohort (see Table 1 and Supplemental Table 3). The congruity of findings between our matched analysis and this alternate analysis further highlights the robustness of our methodology. Finally, we may be underestimating the true prevalence of KDE within the community either due to lack of administrative codes or suboptimal reimbursements. However, such a scenario would only further strengthen our conclusions since such provisions of KDE would likely have a greater impact on the home dialysis rates among the non-KDE cohort.
In conclusion, the professional nephrology bodies as well as the agencies of the US government, including the CMS and the Veteran Health Administration, have emphasized the need for greater utilization of home dialysis. Outside an unlikely mandate for home dialysis-first model of healthcare, any viable method to increase the home dialysis to achieve the aspirational goals of home dialysis utilization set forth by the recent Executive Order will require advanced CKD patients to embrace home dialysis as the preferred dialysis modality. Trends over the last three decades suggest that in the current model of advanced CKD care, few (less than 10%) patients are able to select and use home dialysis. The strength of association seen between the provision of the KDE service and the utilization of home dialysis in our report validates the intent of CMS-KDE policy for universal KDE as a mean to improve home dialysis utilizations within the US. Unfortunately, despite such strong associations, it appears that the incorporation of KDE services into routine clinical practice is sparse among the US incident ESRD Medicare beneficiaries, and when incorporated its application is disparate. Thus, these results provide a strong rationale for targeted efforts to better understand and remedy the reasons for gross underutilization of KDE in the US Medicare beneficiaries such that more comprehensive implementation of KDE for ‘all’ patients with advanced CKD can be accomplished.
Supplementary Material
Acknowledgements:
Ashutosh M Shukla was supported in part by the U.S. Department of Veterans Affairs Merit Review Awards I01CX001661 from Clinical Sciences Research and Development and I01HX002639 from Health Services Research and Development Program.
Funding:
Shukla AM reports the following VA Merit Grant supports from the Department of Veterans Affairs (I01HX002639: A system-wide strategy for KDE to improve the health and health services outcomes among Veterans and I01CX001661: Management of cardiovascular disease in advanced CKD)
Footnotes
Disclosures: Authors have nothing to disclose.
Financial Disclosure: Authors have no financial conflict of interest to disclose
References:
- 1.R BFC, Michael W, Scott WK, Garth M, Narine S-D, Robert RQ, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA. 2017; 298:1291–1299. [DOI] [PubMed] [Google Scholar]
- 2.Zazzeroni L, Pasquinelli G, Nanni E, Cremonini V, Rubbi I. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: A systematic review and meta-analysis. Kidney Blood Press Res. 2017; 42:717–727. [DOI] [PubMed] [Google Scholar]
- 3.Lee MB, Bargman JM. Survival by dialysis modality—who cares? Clinical Journal of the American Society of Nephrology. 2016; 11:1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadem SZ, Walker DR, Abbott G, Friedman AL, Goldman R, Sexton S, et al. Satisfaction with renal replacement therapy and education: The american association of kidney patients survey. Clinical Journal of the American Society of Nephrology. 2011; 6:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juergensen E, Wuerth D, Finkelstein SH, Juergensen PH, Bekui A, Finkelstein FO. Hemodialysis and peritoneal dialysis: Patients’ assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clinical Journal of the American Society of Nephrology. 2006; 1:1191–1196. [DOI] [PubMed] [Google Scholar]
- 6.Liu FX, Walton SM, Leipold R, Isbell D, Golper TA. Financial implications to medicare from changing the dialysis modality mix under the bundled prospective payment system. Peritoneal Dialysis International. 2014; 34:749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehrotra R, Chiu Y, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Archives of Internal Medicine. 2011; 171:110–118. [DOI] [PubMed] [Google Scholar]
- 8.Golper TA. A view of the bundle from a home dialysis perspective. Clinical Journal of the American Society of Nephrology. 2018; 13:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, et al. Us renal data system 2017 annual data report: Epidemiology of kidney disease in the united states. Am J Kidney Dis. 2018; 71:A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.House TW. Executive order on advancing american kidney health. Executive orders. https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/2019, July 10. [Google Scholar]
- 11.GALLA JH. Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. Journal of the American Society of Nephrology. 2000; 11:1340–1342. [DOI] [PubMed] [Google Scholar]
- 12.Barry MJ, Edgman-Levitan S. Shared decision making — the pinnacle of patient-centered care. New England Journal of Medicine. 2012; 366:780–781. [DOI] [PubMed] [Google Scholar]
- 13.Asn releases benchmarks for home dialysis training. Kidney news online. 2017. [Google Scholar]
- 14.Covic A, Bammens B, Lobbedez T, Segall L, Heimbürger O, van Biesen W, et al. Educating end-stage renal disease patients on dialysis modality selection. NDT Plus. 2010; 3:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berns JS. Honoring patient preferences: The 2016 national kidney foundation presidential address. American Journal of Kidney Diseases. 2016; 68:661–664. [DOI] [PubMed] [Google Scholar]
- 16.Kutner NG, Zhang R, Huang Y, Wasse H. Patient awareness and initiation of peritoneal dialysis. Archives of Internal Medicine. 2011; 171:119–124. [DOI] [PubMed] [Google Scholar]
- 17.Whaley-Connell A, Shlipak MG, Inker LA, Tamura MK, Bomback AS, Saab G, et al. Awareness of kidney disease and relationship to end-stage renal disease and mortality. The American Journal of Medicine. 2012; 125:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehrotra R, Marsh D, Vonesh E, Peters V, Nissenson A. Patient education and access of esrd patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int. 2005; 68:378–390. [DOI] [PubMed] [Google Scholar]
- 19.Golper TA, Saxena AB, Piraino B, Teitelbaum I, Burkart J, Finkelstein FO, et al. Systematic barriers to the effective delivery of home dialysis in the united states: A report from the public policy/advocacy committee of the north american chapter of the international society for peritoneal dialysis. American Journal of Kidney Diseases. 2011; 58:879–885. [DOI] [PubMed] [Google Scholar]
- 20.Ghafari A, Sepehrvand N, Hatami S, Ahmadnejad E, Ayubian B, Maghsudi R, et al. Effect of an educational program on awareness about peritoneal dialysis among patients on hemodialysis. Saudi J Kidney Dis Transpl. 2010; 21:636–40. [PubMed] [Google Scholar]
- 21.Yen M, Huang JJ, Teng HL. Education for patients with chronic kidney disease in taiwan: A prospective repeated measures study. J Clin Nurs. 2008; 17:2927–34. [DOI] [PubMed] [Google Scholar]
- 22.Mason J, Khunti K, Stone M, Farooqi A, Carr S. Educational interventions in kidney disease care: A systematic review of randomized trials. American Journal of Kidney Diseases. 51:933–951. [DOI] [PubMed] [Google Scholar]
- 23.Goovaerts T, Jadoul M, Goffin E. Influence of a pre-dialysis education programme (pdep) on the mode of renal replacement therapy. Nephrology Dialysis Transplantation. 2005; 20:1842–1847. [DOI] [PubMed] [Google Scholar]
- 24.Wu IW, Wang SY, Hsu KH, Lee CC, Sun CY, Tsai CJ, et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality--a controlled cohort study based on the nkf/doqi guidelines. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009; 24:3426–3433. [DOI] [PubMed] [Google Scholar]
- 25.Prieto-Velasco M, Quiros P, Remon C, Spanish Group for the Implementation of a Shared Decision Making Process for RRTCwPDAT. The concordance between patients’ renal replacement therapy choice and definitive modality: Is it a utopia? PLOS ONE. 2015; 10:e0138811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribitsch W, Haditsch B, Otto R, Schilcher G, Quehenberger F, Roob JM, et al. Effects of a pre-dialysis patient education program on the relative frequencies of dialysis modalities. Peritoneal Dialysis International : Journal of the International Society for Peritoneal Dialysis. 2013; 33:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacson E Jr, Wang W, DeVries C, Leste K, Hakim RM, Lazarus M, et al. Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. American Journal of Kidney Diseases. 58:235–242. [DOI] [PubMed] [Google Scholar]
- 28.Shukla AM, Easom A, Singh M, Pandey R, Rotaru D, Wen X, et al. Effects of a comprehensive predialysis education program on the home dialysis therapies: A retrospective cohort study. Perit Dial Int. 2017; 37:542–547. [DOI] [PubMed] [Google Scholar]
- 29.Shukla AM, Hinkamp C, Segal E, Ozrazgat Baslanti T, Martinez T, Thomas M, et al. What do the us advanced kidney disease patients want? Comprehensive pre-esrd patient education (cpe) and choice of dialysis modality. PLOS ONE. 2019; 14:e0215091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Services CfMaM. Kidney disease education coverage. In: Services HaH, ed. https://www.medicare.gov/coverage/kidney-disease-education: Department of Health and Human Services; 2010. [Google Scholar]
- 31.Lin E, Cheng XS, Chin K-K, Zubair T, Chertow GM, Bendavid E, et al. Home dialysis in the prospective payment system era. Journal of the American Society of Nephrology. 2017; 28:2993–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkelstein FO, Story K, Firanek C, Barre P, Takano T, Soroka S, et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int. 2008; 74:1178–1184. [DOI] [PubMed] [Google Scholar]
- 33.Liu FX, Treharne C, Culleton B, Crowe L, Arici M. The financial impact of increasing home-based high dose haemodialysis and peritoneal dialysis. BMC Nephrology. 2014; 15:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neil N, Guest S, Wong L, Inglese G, Bhattacharyya SK, Gehr T, et al. The financial implications for medicare of greater use of peritoneal dialysis. Clinical Therapeutics. 2009; 31:880–888. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Thamer M, Kshirsagar O, Zhang Y. Impact of the end stage renal disease prospective payment system on the use of peritoneal dialysis. Kidney international reports. 2016; 2:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liebman SE, Bushinsky DA, Dolan JG, Veazie P. Differences between dialysis modality selection and initiation. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012; 59:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin A, Lewis M, Mortiboy P, Faber S, Hare I, Porter EC, et al. Multidisciplinary predialysis programs: Quantification and limitations of their impact on patient outcomes in two canadian settings. American Journal of Kidney Diseases. 1997; 29:533–540. [DOI] [PubMed] [Google Scholar]
- 38.Inc. DKC. Kidney smart classes. Vol. 2019. https://www.davita.com/education/kidney-smart-classes: Davita Kidney Care Inc.; 2019. [Google Scholar]
- 39.Mehrotra R Bridging the care gap around dialysis initiation: Is ckd education part of the solution? American Journal of Kidney Diseases. 2011; 58:160–161. [DOI] [PubMed] [Google Scholar]
- 40.Van den Bosch J, Warren DS, Rutherford PA. Review of predialysis education programs: A need for standardization. Patient preference and adherence. 2015; 9:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Bulck L, Claes K, Dierickx K, Hellemans A, Jamar S, Smets S, et al. Patient and treatment characteristics associated with patient activation in patients undergoing hemodialysis: A cross-sectional study. BMC Nephrology. 2018; 19:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


