Abstract
Background
Observational studies have shown that obesity is closely associated with leukocyte telomere length (LTL). However, the causal relationship between obesity and LTL remains unclear. This study investigated the causal relationship between obesity and LTL through the Mendelian randomization approach.
Materials and Methods
The genome-wide association study (GWAS) summary data of several studies on obesity-related traits with a sample size of more than 600,000 individuals were extracted from the UK Biobank cohort. The summary-level data of LTL-related GWAS (45 6,717 individuals) was obtained from the IEU Open GWAS database. An inverse-variance-weighted (IVW) algorithm was utilized as the primary MR analysis method. Sensitivity analyses were conducted via MR-Egger regression, IVW regression, leave-one-out test, MR-pleiotropy residual sum, and outlier methods.
Results
High body mass index was correlated with a short LTL, and the odds ratio (OR) was 0.957 (95% confidence interval [CI] 0.942–0.973, p = 1.17E−07). The six body fat indexes (whole body fat mass, right leg fat mass, left leg fat mass, right arm fat mass, left arm fat mass, and trunk fat mass) were consistently inversely associated with LTL. Multiple statistical sensitive analysis approaches showed that the adverse effect of obesity on LTL was steady and dependable.
Conclusion
The current study provided robust evidence supporting the causal assumption that genetically caused obesity is negatively associated with LTL. The findings may facilitate the formulation of persistent strategies for maintaining LTL.
Keywords: Body mass index, Telomere length, Body fat mass, GWAS, Single-nucleotide polymorphism
Introduction
Telomeres are DNA-protein complexes located on chromosome ends and play a critical role in guaranteeing the stability and integrality of chromosomes. An abnormal change in telomere length is closely related to human health. Leukocyte telomere length (LTL) is a quickly gauging indicator compared with other tissues. A previous study showed that LTL was closely related to the telomere length of tissues and served as an indicator that reflects telomere length in other tissues (Takubo et al., 2002). Mounting evidence has shown that extreme long LTL is strongly correlated with disease occurrence and development (Antwi & Petersen, 2018; Ayora et al., 2022; D’Mello et al., 2015). The findings of a meta-analysis suggested that short LTL is associated with a high risk of coronary heart disease (Haycock et al., 2014). Likewise, a recent meta-analysis reported that LTL is inversely related to the risk of atrial fibrillation, especially in men (Zheng et al., 2022). Furthermore, aberrant LTL is associated with a variety of diseases, such as major depressive disorder (Pisanu et al., 2020), female fertility (Michaeli et al., 2022), complications of type 2 diabetes mellitus (Testa et al., 2011), Barrett’s esophagus (Risques et al., 2007), and non-obstructive azoospermia (Yang et al., 2018). Hence, screening risk factors leading to the aberrant LTL in the early stage is vital to disease prevention.
Obesity is a severe public health problem worldwide and the main triggering factor for diseases (Adom et al., 2017; Cheng et al., 2020; Wang et al., 2021). Substantial evidence has shown that obesity is associated with abnormal change in LTL (Buxton et al., 2011; Zhang et al., 2021). A negative correlation between obesity and LTL was reported in a recent meta-analysis (Zadeh et al., 2021), and the finding of a recent observational study has shown that obesity is inversely associated with LTL in Asian children (Ooi et al., 2021). Moreover, a meta-analysis including 63 studies and involving 119,439 populations revealed a negative correlation between obesity and LTL (Mundstock et al., 2015). Despite that considerable evidence suggests that obesity is negatively associated with LTL, a large heterogenicity has been found in different studies, and the causal relationship between obesity and LTL remains unclear. Therefore, we propose a hypothesis that obesity may be a potential causal risk factor to cause LTL changes.
Mendelian randomization (MR) study is a powerful epidemiological approach for investigating the causality between exposure and outcome by using genetic variants as instrumental variables (Burgess, Butterworth & Thompson, 2013). Given that the allocation of genetic variants is a randomization process and is not affected by extraneous postnatal factors (Emdin, Khera & Kathiresan, 2017), MR controlling residual confounding factors is the same as a randomized controlled trial (RCT) (Davies, Holmes & Davey Smith, 2018; Ference, Holmes & Smith, 2021). In the present work, a two-sample MR study was conducted to investigate the causal relationship between obesity and LTL. The inverse-variance weighted (IVW) method was used as the primary analysis algorithm to assess the potential causation. We are seeing the perniciousness of obesity in human health. Clarifying obesity’s potential causal influence on LTL is beneficial in drawing up strategies for preventing diseases. As far as we know, this is the first MR study investigating the causality between obesity-related traits and LTL. Nevertheless, more molecular experiments are necessary to investigate further the potential mechanism for the effect of obesity on LTL.
Materials & Methods
Study design
The summary-level data of obesity-related traits, including body mass index (BMI), whole body fat mass, leg fat mass (right), leg fat mass (left), arm fat mass (right), arm fat mass (left), and trunk fat mass were extracted from the IEU Open GWAS database (https://gwas.mrcieu.ac.uk/). The summary statistical data of GWAS associated with LTL were also obtained from the IEU Open GWAS database. The data were from large sample studies and were used in conducting the two-sample MR investigation and exploring the causal relationship between obesity-related traits and LTL.
Assumptions of MR study
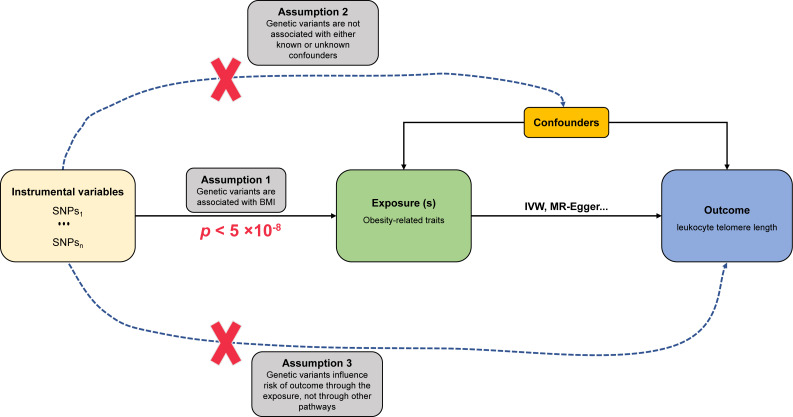
The fundamental assumptions and design of the MR investigation are shown in Fig. 1. (1) Relevance assumption: The genetic variants (instrumental variables) must be strongly correlated with obesity-related traits (exposure). (2) Independence assumption: no unpredictable confounders of the correlations between genetic variants and LTL (outcome) are present. (3) Exclusion restriction assumption: the genetic variants influence LTL only via obesity-related traits.
Figure 1. Schematic diagram of MR investigating the causal relationship between obesity and LTL.
The instrumental variable (IV) assumptions: (1) the IVs must be strongly associated with obesity-related traits (p < 5 × 10−8); (2) the IVs must not be correlated with any unmeasured confounders of obesity-related traits vs. LTL relationship; (3) the IVs should only affect the risk of LTL via obesity-related traits. SNPs, single-nucleotide polymorphisms; LTL, leukocyte telomere length; IVW, inverse-variance-weighted.
Data sources
The GWAS summary statistical data of obesity-related traits were obtained from European populations. The information included whole body fat mass with 330,762 participants, leg fat mass (right) with 331,293 populations, leg fat mass (left) with 331,275 populations, arm fat mass (right) with 331,226 individuals, arm fat mass (left) with 331,164 people, and trunk fat mass with 331,093 volunteers; all above obesity-related traits were obtained from UK Biobank cohort of the Neale lab. Single-nucleotide polymorphisms (SNPs) associated with obesity-related traits were identified as instrumental variables based on these parameters: p < 5 × 10−8 as a genome-wide statistical significance, independence among SNPs in linkage disequilibrium (r2 < 0.001; clump window, 10,000 kb). The F statistic was used in assessing the instrumental variables’ power in the MR and the computational method described by a previous study (Pierce, Ahsan & Vanderweele, 2011). F statistic > 10 was defined as the minimum required threshold. The GWAS summary data associated with LTL from European ancestry (472,174 individuals) were downloaded from the IEU Open GWAS database. All participants in the obesity-related trait research projects were not screened for the LTL cohort.
Statistical analysis
The IVW was used as a primary analysis algorithm in estimating the causal effect size of obesity-related traits on LTL (Burgess, Butterworth & Thompson, 2013). The MR-Egger (Bowden, Davey Smith & Burgess, 2015), maximum likelihood (Xue, Shen & Pan, 2021), MR-pleiotropy residual sum outlier (MR-PRESSO) (Verbanck et al., 2018), and robust adjusted profile score (MR-RAPS) (Zhao et al., 2020) algorithms were utilized in validating the reliability and robustness for the causal relationship between obesity traits and LTL. An available online tool (https://shiny.cnsgenomics.com/mRnd/) (Brion, Shakhbazov & Visscher, 2013) was used to calculate the statistical power of MR analysis, and a power greater than 80% was deemed as a good value. The directionality that obesity-related traits cause LTL was verified via the MR Steiger test (Hemani, Tilling & Davey Smith, 2017). p < 0.05 indicated statistical significance.
Sensitivity analysis
The heterogeneity of SNPs was inspected via the IVW method and MR-Egger regression. MR-PRESSO, MR-Egger, and IVW approaches were used in identifying and removing outliers. The MR-Egger algorithms were utilized to detect potential pleiotropy. A single SNP’s influence on the total effect of IVW was evaluated through leave-one-out permutation analysis.
All MR analyses were conducted using TwoSampleMR (version 0.5.6) and MRPRESSO packages in R software (version 4.1.2; R Core Team, 2021).
Results
Causality between BMI and LTL
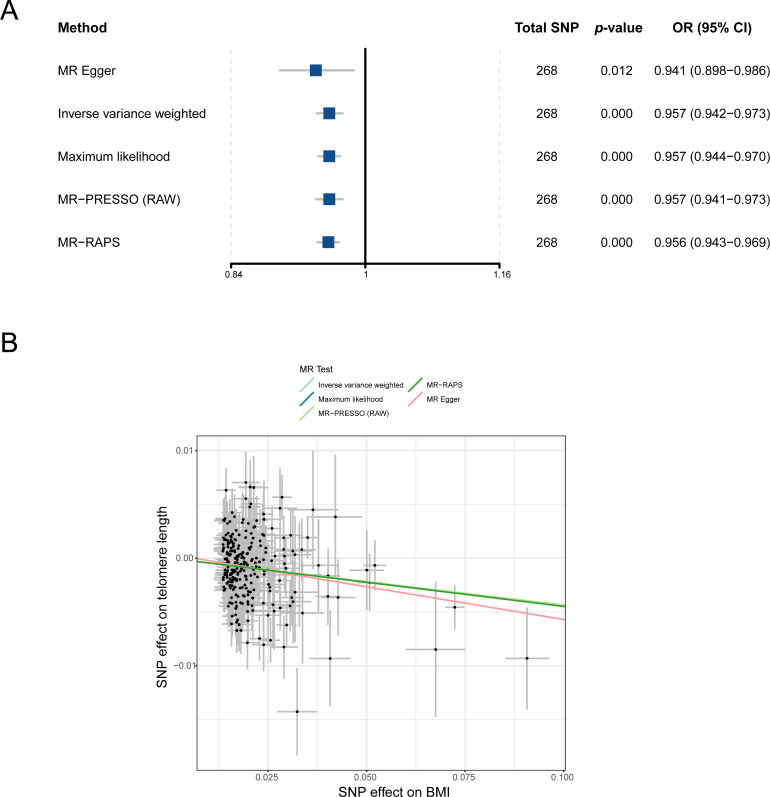
To understand the causal relationship between obesity and LTL preliminarily, we analyzed the effect of BMI on LTL by using the IVW method in the two-sample MR study. After outliers were removed, all 268 independent SNPs associated with BMI were included in the MR for the computation of the pool effect size of BMI on LTL (File S1). The result of the IVW approach showed that a genetically determined 1-SD increase in BMI was correlated with decreased LTL, and the odds ratio (OR) was 0.957 (95% confidence interval [CI] = 0.942–0.973, p = 1.17E−07; Table 1 and Fig. 2A). In the analysis, the F statistic of the SNPs was 61.0, and the statistical power was 100%, indicating the absence of weak-instrument bias and high credibility, respectively (Table 1).
Table 1. MR Results of obesity-related traits’ influence on LTL.
| Exposure | Method | No. of SNPs | p | OR (95% CI) | p -het | p -intercept | F statistic | Power |
|---|---|---|---|---|---|---|---|---|
| BMI | MR Egger | 268 | 0.012 | 0.941 (0.898–0.986) | 4.48E−07 | 0.448 | ||
| Inverse-variance-weighted | 268 | 1.17E−07 | 0.957 (0.942–0.973) | 61.00 | 100% | |||
| Maximum likelihood | 268 | 2.07E−10 | 0.957 (0.944–0.970) | |||||
| MR-PRESSO (RAW) | 268 | 2.45E−07 | 0.957 (0.941–0.973) | |||||
| MR-RAPS | 268 | 6.70E−11 | 0.956 (0.943–0.969) | |||||
| Whole body fat mass | MR Egger | 247 | 0.040 | 0.942 (0.890–0.997) | 3.44E−12 | 0.782 | ||
| Inverse-variance-weighted | 247 | 3.69E−08 | 0.949 (0.932–0.967) | 60.00 | 100% | |||
| Maximum likelihood | 247 | 1.03E−12 | 0.949 (0.936–0.963) | |||||
| MR-PRESSO (RAW) | 247 | 9.26E−08 | 0.949 (0.931–0.968) | |||||
| MR-RAPS | 247 | 1.34E−13 | 0.948 (0.934–0.961) | |||||
| Leg fat mass (right) | MR Egger | 242 | 0.057249543 | 0.931 (0.865–1.002) | 2.36E−15 | 0.976 | ||
| Inverse-variance-weighted | 242 | 6.98E−09 | 0.932 (0.910–0.954) | 58.90 | 100% | |||
| Maximum likelihood | 242 | 2.38E−15 | 0.931 (0.914–0.947) | |||||
| MR-PRESSO (RAW) | 242 | 2.17E−08 | 0.932 (0.909–0.955) | |||||
| MR-RAPS | 242 | 4.44E−16 | 0.930 (0.913–0.946) | |||||
| Leg fat mass (left) | MR Egger | 245 | 0.013970688 | 0.918 (0.858–0.982) | 8.34E−10 | 0.683 | ||
| Inverse-variance-weighted | 245 | 3.79E−10 | 0.931 (0.910–0.952) | 59.20 | 100% | |||
| Maximum likelihood | 245 | 2.85E−15 | 0.931 (0.914–0.947) | |||||
| MR-PRESSO (RAW) | 245 | 1.70E−09 | 0.931 (0.909–0.953) | |||||
| MR-RAPS | 245 | 4.44E−16 | 0.929 (0.912–0.945) | |||||
| Arm fat mass (right) | MR Egger | 240 | 0.114083631 | 0.959 (0.910–1.010) | 7.85E−08 | 0.889 | ||
| Inverse-variance-weighted | 240 | 3.67E−07 | 0.955 (0.939–0.972) | 60.72 | 100% | |||
| Maximum likelihood | 240 | 4.59E−10 | 0.955 (0.942–0.969) | |||||
| MR-PRESSO (RAW) | 240 | 7.41E−07 | 0.955 (0.938–0.973) | |||||
| MR-RAPS | 240 | 1.40E−10 | 0.954 (0.941–0.968) | |||||
| Arm fat mass (left) | MR Egger | 236 | 0.018611648 | 0.939 (0.892–0.989) | 2.44E−07 | 0.689 | ||
| Inverse-variance-weighted | 236 | 4.63E−09 | 0.949 (0.932–0.965) | 61.07 | 100% | |||
| Maximum likelihood | 236 | 9.11E−13 | 0.949 (0.935–0.962) | |||||
| MR-PRESSO (RAW) | 236 | 1.56E−08 | 0.949 (0.931–0.966) | |||||
| MR-RAPS | 236 | 2.18E−13 | 0.947 (0.934–0.961) | |||||
| Trunk fat mass | MR Egger | 247 | 0.034146424 | 0.942 (0.891–0.995) | 3.55E−10 | 0.376 | 58.78 | 100% |
| Inverse-variance-weighted | 247 | 5.53E−05 | 0.964 (0.947–0.981) | |||||
| Maximum likelihood | 247 | 3.43E−07 | 0.964 (0.951–0.978) | |||||
| MR-PRESSO (RAW) | 247 | 7.37E−05 | 0.964 (0.947–0.982) | |||||
| MR-RAPS | 247 | 1.26E−07 | 0.963 (0.950–0.977) |
Notes.
- BMI
- body mass index
- OR
- odds ratio
- CI
- confidence interval
- p-het
- p-value for heterogeneity using Cochran Q test
- p-intercept
- p-value for MR-Egger intercept
- MR-PRESSO
- Mendelian randomization-pleiotropy residual sum outlier
- MR-RAPS
- robust adjusted profile score
- SNP
- single-nucleotide polymorphism
Figure 2. MR analysis of the causal relationship between BMI and LTL. (A) Forest plot for MR analysis visualization; (B) scatter plots of the correlation between BMI and LTL.
MR, Mendelian randomization; SNP, single-nucleotide polymorphism; BMI, body mass index; MR-PRESSO, MR-pleiotropy residual sum outlier; MR-RAPS, robust adjusted profile score; OR, odds ratio; CI, confidence interval.
A series of sensitivity analysis approaches was used in verifying the robustness and reliability of the above result. First, as shown in Fig. 2B, four methods (MR-Egger, maximum likelihood, MR-PRESSO, and MR-RAPS) consistently exhibited causal direction from BMI to LTL. The result suggested that the causality from BMI to LTL was stable. Second, the results from Cochran’s Q test in the IVW model (p-het = 4.76E−07) and MR-Egger model (p-het = 4.48E−07) indicated heterogeneity among the SNPs, which may have been caused by the random allocation of alleles. Third, the statistical result of the MR-Egger intercepts indicated no directional pleiotropy in the MR analysis (p-intercept = 0.448). Fourth, leave-one-out analysis showed that no single SNP significantly influenced the causal association between BMI and LTL (p < 0.05; File S2). Finally, statistical evidence from the MR Steiger test suggested that BMI influencing LTL was a correct causal direction (p < 0.001; Table 1).
Causality between six body fat indexes and LTL
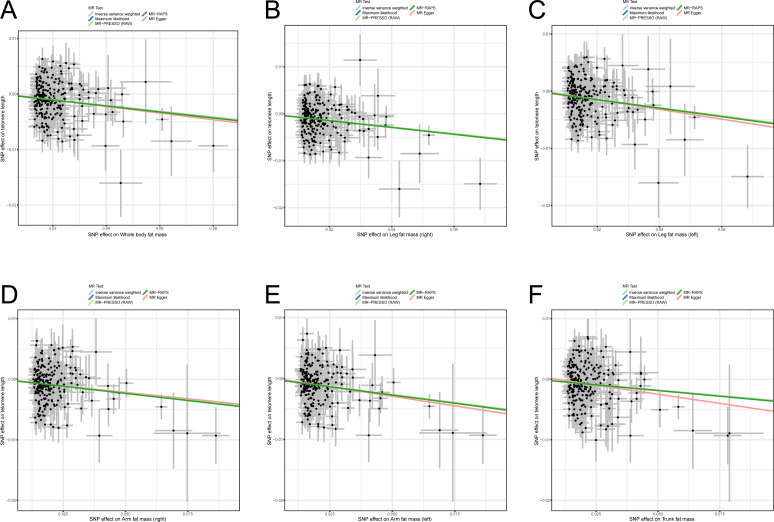
Previous studies reported some bias when only BMI was used as an indicator for measuring obesity and estimating the correlation between obesity and diseases (Nimptsch, Konigorski & Pischon, 2019; Piche, Tchernof & Despres, 2020; Vecchie et al., 2018) because BMI cannot reflect body fat distribution. Therefore, to avoid the bias of causality, six body fat indexes (whole body fat mass, right leg fat mass, left leg fat mass, right arm fat mass, left arm fat mass, and trunk fat mass) were used to confirm the casual assumption of obesity impact on LTL. After outliers were deleted, 247, 242, 245, 240, 236, and 247 independently available SNPs were associated with whole body fat mass, right leg fat mass, left leg fat mass, right arm fat mass, left arm fat mass, and trunk fat mass, respectively (File S1). The SNPs were then utilized in proving the genetically predicted causality between obesity and LTL. The result of the IVW algorithm indicated that enhanced body fat indexes are causally associated with a decrease in LTL. The effect size of six body fat indexes’ influences on LTL were as follows: whole body fat mass: OR = 0.949 (95% CI [0.932–0.967]; p = 3.69E−08), right leg fat mass: OR = 0.932 (95% CI [0.910–0.954]; p = 6.98E−09), left leg fat mass: OR = 0.931 (95% CI [0.910–0.952]; p = 3.79E−10), right arm fat mass: OR = 0.955 (95% CI [0.939–0.972]; p = 3.67E−07), left arm fat mass: OR = 0.949 (95% CI [0.932–0.965]; p = 4.63E−09), and trunk fat mass: OR = 0.964 (95% CI [0.947–0.981]; p = 5.53E−05; Table 1). In the analyses, the F statistics of the SNPs were larger than 10.0 (range: 58.8–61.1), indicating the absence of potential weak instrument bias, and all statistical power rates were approximately 100%, indicating high credibility (Table 1).
Likewise, sensitivity analysis methods were used in testing the robustness and reliability of the above result. First, MR-Egger, maximum likelihood, MR-PRESSO, and MR-RAPS were utilized in validating the stability of the causal hypothesis. The results uniformly showed that the causality between per body fat index and LTL was stable (Figs. 3A–3F). We analyzed the heterogeneity of per body fat index-related SNPs by using Cochran’s Q test in the IVW model and the MR-Egger model. The results suggested heterogeneity among SNPs (all p-het < 0.05; Table 1). Furthermore, uncorrelated horizontal pleiotropy was detected via the MR-Egger method, and the result showed that the MR analyses had no uncorrelated horizontal pleiotropy (all p-intercept < 0.01; Table 1). Moreover, we used the iterative leave-one-out analysis method to determine whether a single SNP significantly modifies the pool effect value of the IVW. The result indicated that no single SNP significantly disrupted the combined effect of IVW (all p < 0.05; File S2). Finally, the causal hypothesis of the six body fat indexes’ influences on LTL was examined using the MR Steiger algorithm. The results suggested that the six body fat indexes affecting LTL was the correct causal direction (all p < 0.001).
Figure 3. Scatter plots of the correlation between six body fat indexes and LTL.
(A) Whole body fat mass; (B) right leg fat mass; (C) left leg fat mass; (D) right arm fat mass; (E) left arm fat mass; (F) Trunk fat mass. MR, Mendelian randomization; SNP, single-nucleotide polymorphism; MR-PRESSO, MR-pleiotropy residual sum outlier; MR-RAPS, robust adjusted profile score.
Discussion
In the present work, we first used the extensive sample GWAS summary data of European populations to investigate the causal relationship between obesity-related features and LTL comprehensively. Results show that genetically predicted higher BMI, whole body fat mass, right leg fat mass, left leg fat mass, right arm fat mass, left arm fat mass, and trunk fat mass were all causally associated with a lower LTL. These findings are the first to prove that obesity was a key causal risk factor that led to a decrease in LTL.
Previous studies have shown that aberrant LTL can increase the risk of many diseases (Codd et al., 2013; Purdue-Smithe et al., 2021; Sanders & Newman, 2013). Thus, protecting LTL may be an essential way of preventing illness. Obesity is a highly prevalent disease and seriously harms people (Conway & Rene, 2004). Although substantial evidence supports that obesity is inversely correlated with LTL, the strength of the correlation is uneven, and the causality between obesity and LTL remains unexplained. This situation might be associated with the use of different indicators in measuring obesity. A meta-analysis included 16 original studies to investigate the correlation between BMI and LTL and found a negative association between BMI and LTL in adults (Muezzinler, Zaineddin & Brenner, 2014). Similarly, a cross-sectional study with 1000 participants found that BMI and LTL has a negative association (Muezzinler et al., 2016). A negative association between BMI and LTL was reported in a cross-sectional study with 35,096 individuals (Williams et al., 2016). A recent large meta-analysis including 87 studies and involving 146,114 individuals investigated the association between BMI and LTL in different age categories, gender, and ethnicity; a negative correlation between BMI and LTL was found, especially in younger participants (Gielen et al., 2018). In the current study, the standardized data of LTL was used to analyze the causal relationship between BMI and LTL and found BMI’s impact on LTL was a correct causal direction (OR = 0.957 [95% CI [0.942–0.973]], p = 1.17E−07). The finding initially illustrated the causal relationship between obesity and LTL. The statistical evidence of several sensitivity analyses confirmed that our result was stable and reliable.
Despite that BMI remains to be a primary indicator for assessing obesity in clinical works, bias occurs when obesity is measured with BMI alone because BMI does not reflect body fat distribution (Antonopoulos et al., 2016). Body fat mass may be a better and more direct indicator for describing obesity (Kesztyus, Lampl & Kesztyus, 2021; Lee & Giovannucci, 2019). A previous observational study with a small sample (45 women) reported body fat mass was negatively correlated with LTL (Shin & Lee, 2016). Similarly, the finding of an observational study including 145 healthy term-born infants indicated that body fat mass was negatively associated with LTL (De Fluiter et al., 2021). Although these observational studies suggested a negative association between fat mass and LTL, the evidence was limited because they only used a single fat mass to estimate the causality between obesity and LTL. Therefore, to accurately assess the causal relationship between obesity and LTL, we used six body fat mass indexes (whole body fat mass, right leg fat mass, left leg fat mass, right arm fat mass, left arm fat mass, and trunk fat mass) to evaluate obesity’s influence on LTL. We found that the indexes were all inversely associated with LTL. These results again proved that the causal direction from obesity to LTL was correct. The present study overcomes the limitations of traditional observational studies that neither well control for unmeasured confounding nor prove causality between exposure and outcome (Davies, Holmes & Davey Smith, 2018).
Limitations
The current study possesses some shortcomings. First, obesity was divided into four types: metabolically healthy obesity, metabolically obese normal weight phenotype, normal weight obese syndrome, and sarcopenic obesity (Vecchie et al., 2018). Whether our result applies to all types is unknown. Second, we failed to achieve stratification analysis according to age and gender. Third, genetic variation is only one of the factors causing exposure changes, and many more remain to be further explored, such as environmental and epigenetic factors. Finally, given that the MR study was conducted in European ancestry, whether it can be popularized in non-European ancestry need to be investigated further.
Conclusions
In conclusion, using the extensive GWAS summary data, we implemented two-sample MR investigations to examine the causal relationship between BMI and LTL. We identified potential causal effects of several obesity-related traits on LTL. Our results suggested that genetically predicted obesity is inversely associated with LTL. In addition, given that the lifelong adverse effects of obesity on LTL are due to genetic variants, our findings may be useful in formulating persistent strategies for maintaining LTL and promoting health.
Supplemental Information
Funding Statement
This work was funded by grants from the Hainan Province Clinical Medical Center (QWYH202175), the National Natural Science Foundation of China (82160544), the Research and Cultivation Fund of Hainan Medical University (HYPY2020015), the Natural Science Foundation of Hainan Province (820RC771), and the Key R&D Projects of Hainan Province (ZDYF2022SHFZ074). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Bangbei Wan, Email: 939313612@qq.com.
Cai Lv, Email: 198312170@csu.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Bangbei Wan conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Ning Ma conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Cai Lv conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The STROBE-MR checklist, RAW data, and R code are available in the Supplemental Files.
References
- Adom et al. (2017).Adom T, Puoane T, De Villiers A, Kengne AP. Prevalence of obesity and overweight in African learners: a protocol for systematic review and meta-analysis. BMJ Open. 2017;7:e013538. doi: 10.1136/bmjopen-2016-013538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos et al. (2016).Antonopoulos AS, Oikonomou EK, Antoniades C, Tousoulis D. From the BMI paradox to the obesity paradox: the obesity-mortality association in coronary heart disease. Obesity Reviews. 2016;17:989–1000. doi: 10.1111/obr.12440. [DOI] [PubMed] [Google Scholar]
- Antwi & Petersen (2018).Antwi SO, Petersen GM. Leukocyte telomere length and pancreatic cancer risk: updated epidemiologic review. Pancreas. 2018;47:265–271. doi: 10.1097/MPA.0000000000000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayora et al. (2022).Ayora M, Fraguas D, Abregu-Crespo R, Recio S, Blasco MA, Moises A, Derevyanko A, Arango C, Diaz-Caneja CM. Leukocyte telomere length in patients with schizophrenia and related disorders: a meta-analysis of case-control studies. Molecular Psychiatry. 2022;27:2968–2975. doi: 10.1038/s41380-022-01541-7. [DOI] [PubMed] [Google Scholar]
- Bowden, Davey Smith & Burgess (2015).Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. International Journal of Epidemiology. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion, Shakhbazov & Visscher (2013).Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. International Journal of Epidemiology. 2013;42:1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, Butterworth & Thompson (2013).Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genetic Epidemiology. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton et al. (2011).Buxton JL, Walters RG, Visvikis-Siest S, Meyre D, Froguel P, Blakemore AI. Childhood obesity is associated with shorter leukocyte telomere length. The Journal of Clinical Endocrinology and Metabolism. 2011;96:1500–1505. doi: 10.1210/jc.2010-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng et al. (2020).Cheng F, Luk AO, Tam CHT, Fan B, Wu H, Yang A, Lau ESH, Ng ACW, Lim CKP, Lee HM, Chow E, Kong AP, Keech AC, Joglekar MV, So WY, Jenkins AJ, Chan JCN, Hardikar AA, Ma RCW. Shortened relative leukocyte telomere length is associated with prevalent and incident cardiovascular complications in type 2 diabetes: analysis from the Hong Kong diabetes register. Diabetes Care. 2020;43:2257–2265. doi: 10.2337/dc20-0028. [DOI] [PubMed] [Google Scholar]
- Codd et al. (2013).Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I, Broer L, Nyholt DR, Mateo Leach I, Salo P, Hagg S, Matthews MK, Palmen J, Norata GD, O’Reilly PF, Saleheen D, Amin N, Balmforth AJ, Beekman M, De Boer RA, Bohringer S, Braund PS, Burton PR, De Craen AJ, Denniff M, Dong Y, Douroudis K, Dubinina E, Eriksson JG, Garlaschelli K, Guo D, Hartikainen AL, Henders AK, Houwing-Duistermaat JJ, Kananen L, Karssen LC, Kettunen J, Klopp N, Lagou V, Van Leeuwen EM, Madden PA, Magi R, Magnusson PK, Mannisto S, McCarthy MI, Medland SE, Mihailov E, Montgomery GW, Oostra BA, Palotie A, Peters A, Pollard H, Pouta A, Prokopenko I, Ripatti S, Salomaa V, Suchiman HE, Valdes AM, Verweij N, Vinuela A, Wang X, Wichmann HE, Widen E, Willemsen G, Wright MJ, Xia K, Xiao X, Van Veldhuisen DJ, Catapano AL, Tobin MD, Hall AS, Blakemore AI, Van Gilst WH, Zhu H, consortium CA, Erdmann J, Reilly MP, Kathiresan S, Schunkert H, Talmud PJ, Pedersen NL, Perola M, Ouwehand W, Kaprio J, Martin NG, Van Duijn CM, Hovatta I, Gieger C, Metspalu A, Boomsma DI, Jarvelin MR, Slagboom PE, Thompson JR, Spector TD, Van der Harst P, Samani NJ. Identification of seven loci affecting mean telomere length and their association with disease. Nature Genetics. 2013;45:422–427. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway & Rene (2004).Conway B, Rene A. Obesity as a disease: no lightweight matter. Obesity Reviews. 2004;5:145–151. doi: 10.1111/j.1467-789X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- Davies, Holmes & Davey Smith (2018).Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fluiter et al. (2021).De Fluiter KS, Codd V, Denniff M, Kerkhof GF, Van Beijsterveldt I, Breij LM, Samani NJ, Abrahamse-Berkeveld M, Hokken-Koelega ACS. Longitudinal telomere length and body composition in healthy term-born infants during the first two years of life. PLOS ONE. 2021;16:e0246400. doi: 10.1371/journal.pone.0246400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello et al. (2015).D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, Pare G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circulation-Cardiovascular Genetics. 2015;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- Emdin, Khera & Kathiresan (2017).Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318:1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- Ference, Holmes & Smith (2021).Ference BA, Holmes MV, Smith GD. Using mendelian randomization to improve the design of randomized trials. Cold Spring Harbor Perspectives in Medicine. 2021;11(7):a040980. doi: 10.1101/cshperspect.a040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen et al. (2018).Gielen M, Hageman GJ, Antoniou EE, Nordfjall K, Mangino M, Balasubramanyam M, De Meyer T, Hendricks AE, Giltay EJ, Hunt SC, Nettleton JA, Salpea KD, Diaz VA, Farzaneh-Far R, Atzmon G, Harris SE, Hou L, Gilley D, Hovatta I, Kark JD, Nassar H, Kurz DJ, Mather KA, Willeit P, Zheng YL, Pavanello S, Demerath EW, Rode L, Bunout D, Steptoe A, Boardman L, Marti A, Needham B, Zheng W, Ramsey-Goldman R, Pellatt AJ, Kaprio J, Hofmann JN, Gieger C, Paolisso G, Hjelmborg JBH, Mirabello L, Seeman T, Wong J, Van der Harst P, Broer L, Kronenberg F, Kollerits B, Strandberg T, Eisenberg DTA, Duggan C, Verhoeven JE, Schaakxs R, Zannolli R, Reis RMRDos, Charchar FJ, Tomaszewski M, Mons U, Demuth I, Iglesias Molli AE, Cheng G, Krasnienkov D, D’Antono B, Kasielski M, McDonnell BJ, Ebstein RP, Sundquist K, Pare G, Chong M, Zeegers MP, Group T. Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. The American Journal of Clinical Nutrition. 2018;108:453–475. doi: 10.1093/ajcn/nqy107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock et al. (2014).Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani, Tilling & Davey Smith (2017).Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLOS Genetics. 2017;13:e1007081. doi: 10.1371/journal.pgen.1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesztyus, Lampl & Kesztyus (2021).Kesztyus D, Lampl J, Kesztyus T. The weight problem: overview of the most common concepts for body mass and fat distribution and critical consideration of their usefulness for risk assessment and practice. International Journal of Environmental Research and Public Health. 2021;18(21):11070. doi: 10.3390/ijerph182111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee & Giovannucci (2019).Lee DH, Giovannucci EL. The obesity paradox in cancer: epidemiologic insights and perspectives. Current Nutrition Reports. 2019;8:175–181. doi: 10.1007/s13668-019-00280-6. [DOI] [PubMed] [Google Scholar]
- Michaeli et al. (2022).Michaeli J, Smoom R, Serruya N, El Ayoubi H, Rotshenker-Olshinka K, Srebnik N, Michaeli O, Eldar-Geva T, Tzfati Y. Leukocyte telomere length correlates with extended female fertility. Cells. 2022;11(3):513. doi: 10.3390/cells11030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muezzinler et al. (2016).Muezzinler A, Mons U, Dieffenbach AK, Butterbach K, Saum KU, Schick M, Stammer H, Boukamp P, Holleczek B, Stegmaier C, Brenner H. Body mass index and leukocyte telomere length dynamics among older adults: results from the ESTHER cohort. Experimental Gerontology. 2016;74:1–8. doi: 10.1016/j.exger.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Muezzinler, Zaineddin & Brenner (2014).Muezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obesity Reviews. 2014;15:192–201. doi: 10.1111/obr.12126. [DOI] [PubMed] [Google Scholar]
- Mundstock et al. (2015).Mundstock E, Sarria EE, Zatti H, Mattos Louzada F, Kich Grun L, Herbert Jones M, Guma FT, Mazzola In Memoriam J, Epifanio M, Stein RT, Barbe-Tuana FM, Mattiello R. Effect of obesity on telomere length: systematic review and meta-analysis. Obesity. 2015;23:2165–2174. doi: 10.1002/oby.21183. [DOI] [PubMed] [Google Scholar]
- Nimptsch, Konigorski & Pischon (2019).Nimptsch K, Konigorski S, Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019;92:61–70. doi: 10.1016/j.metabol.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Ooi et al. (2021).Ooi DSQ, Dorajoo R, Gurung RL, Dehghan R, Lim YY, Ho CWL, Tay V, Karuppiah V, Loke KY, Lim SC, Liu JJ, Sng AA, Lee YS. Association of leukocyte telomere length with obesity-related traits in Asian children with early-onset obesity. Pediatric Obesity. 2021;16:e12771. doi: 10.1111/ijpo.12771. [DOI] [PubMed] [Google Scholar]
- Piche, Tchernof & Despres (2020).Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circulation Research. 2020;126:1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- Pierce, Ahsan & Vanderweele (2011).Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. International Journal of Epidemiology. 2011;40:740–752. doi: 10.1093/ije/dyq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanu et al. (2020).Pisanu C, Tsermpini EE, Skokou M, Kordou Z, Gourzis P, Assimakopoulos K, Congiu D, Meloni A, Balasopoulos D, Patrinos GP, Squassina A. Leukocyte telomere length is reduced in patients with major depressive disorder. Drug Development Research. 2020;81:268–273. doi: 10.1002/ddr.21612. [DOI] [PubMed] [Google Scholar]
- Purdue-Smithe et al. (2021).Purdue-Smithe AC, Kim K, Andriessen VC, Pollack AZ, Sjaarda LA, Silver RM, Schisterman EF, Mumford SL. Preconception leukocyte telomere length and pregnancy outcomes among women with demonstrated fecundity. Human Reproduction. 2021;36:3122–3130. doi: 10.1093/humrep/deab201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2021).R Core Team . Vienna: R Foundation for Statistical Computing; 2021. [Google Scholar]
- Risques et al. (2007).Risques RA, Vaughan TL, Li X, Odze RD, Blount PL, Ayub K, Gallaher JL, Reid BJ, Rabinovitch PS. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:2649–2655. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- Sanders & Newman (2013).Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiologic Reviews. 2013;35:112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin & Lee (2016).Shin YA, Lee KY. Low estrogen levels and obesity are associated with shorter telomere lengths in pre- and postmenopausal women. Journal of Exercise Rehabilitation. 2016;12:238–246. doi: 10.12965/jer.1632584.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo et al. (2002).Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, Oshimura M, Nakamura K. Telomere lengths are characteristic in each human individual. Experimental Gerontology. 2002;37:523–531. doi: 10.1016/s0531-5565(01)00218-2. [DOI] [PubMed] [Google Scholar]
- Testa et al. (2011).Testa R, Olivieri F, Sirolla C, Spazzafumo L, Rippo MR, Marra M, Bonfigli AR, Ceriello A, Antonicelli R, Franceschi C, Castellucci C, Testa I, Procopio AD. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabetic Medicine. 2011;28:1388–1394. doi: 10.1111/j.1464-5491.2011.03370.x. [DOI] [PubMed] [Google Scholar]
- Vecchie et al. (2018).Vecchie A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, Fruhbeck G, Montecucco F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. European Journal of Internal Medicine. 2018;48:6–17. doi: 10.1016/j.ejim.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Verbanck et al. (2018).Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2021).Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. The Lancet Diabetes & Endocrinology. 2021;9:446–461. doi: 10.1016/S2213-8587(21)00118-2. [DOI] [PubMed] [Google Scholar]
- Williams et al. (2016).Williams DM, Palaniswamy S, Sebert S, Buxton JL, Blakemore AI, Hypponen E, Jarvelin MR. 25-Hydroxyvitamin D concentration and leukocyte telomere length in young adults: findings from the Northern Finland birth cohort 1966. American Journal of Epidemiology. 2016;183:191–198. doi: 10.1093/aje/kwv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Shen & Pan (2021).Xue H, Shen X, Pan W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects. American Journal of Human Genetics. 2021;108:1251–1269. doi: 10.1016/j.ajhg.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2018).Yang Q, Luo X, Bai R, Zhao F, Dai S, Li F, Zhu J, Liu J, Niu W, Sun Y. Shorter leukocyte telomere length is associated with risk of nonobstructive azoospermia. Fertility and Sterility. 2018;110:648–654 e641. doi: 10.1016/j.fertnstert.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Zadeh et al. (2021).Zadeh FA, Bokov DO, Yasin G, Vahdat S, Abbasalizad-Farhangi M. Central obesity accelerates leukocyte telomere length (LTL) shortening in apparently healthy adults: a systematic review and meta-analysis. Critical Reviews in Food Science and Nutrition. 2021:1–10. doi: 10.1080/10408398.2021.1971155. Epub ahead of print Sep 1 2021. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2021).Zhang Y, Xu Z, Yang Y, Cao S, Lyu S, Duan W. Association between weight change and leukocyte telomere length in US adults. Frontiers in Endocrinology. 2021;12:650988. doi: 10.3389/fendo.2021.650988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2020).Zhao Q, Wang J, Hemani G, Bowden J, Small DSJTAoS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Annals of Statistics. 2020;48:1742–1769. doi: 10.1214/19-AOS1866. [DOI] [Google Scholar]
- Zheng et al. (2022).Zheng Y, Zhang N, Wang Y, Wang F, Li G, Tse G, Liu T. Association between leucocyte telomere length and the risk of atrial fibrillation: an updated systematic review and meta-analysis. Ageing Research Reviews. 2022;81:101707. doi: 10.1016/j.arr.2022.101707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The STROBE-MR checklist, RAW data, and R code are available in the Supplemental Files.