EDITORIAL
Public Health England (PHE) released a report in 2015 that stated: “E-cigarettes around 95% less harmful than tobacco” 1. But based on the evidence provided in two companion papers reported in this issue of ATVB (#: pgs) and other published works, we can disregard the notion that electronic cigarettes (E-cigs) are “less harmful” to the cardiovascular system. In fact, we are likely now to rephrase the PHE statement to read “E-cigs are equally toxic as combustible cigarettes in regard to vascular injury.” No doubt, tobacco smoke is harmful, and tobacco smoke has been characterized as the single greatest modifiable risk factor in cardiovascular disease (CVD) development 2, 3. Extensive evidence demonstrates that exposure to either mainstream (MCS) 4, 5 or secondhand cigarette smoke (SHS) 4, 6 increases CVD risk. Smoking induces endothelial dysfunction (ED) – a common element in atherosclerosis and other CVDs including hypertension, peripheral artery disease and stroke 7.
A shared strength of these two complementary papers is the use of flow-mediated dilation (FMD) for measurement of ED in rodent models and humans. FMD is measured non-invasively by ultrasound of a conduit artery (e.g. brachial or femoral) that dilates robustly following a brief bout (i.e.. 5 min) of ischemia (proximal or distal). As cited in both studies, FMD was established as a key measure of ED during the 1990s in a series of human panel studies of vascular injury from inhaled MCS/SHS 8, 9. However, the constituents in tobacco smoke and the mechanisms by which they impair FMD - and promote CVD - remain, surprisingly, understudied. These two unknowns similarly exist for E-cig-derived aerosols, and appropriately, the two new studies provide novel insights into these 30-year old questions.
Constituents
In the paper by Nabavizadeh et al. 2, FMD is impaired following acute exposures of anesthetized rats to each aerosol derived of an E-cig or a constituent gas alone (i.e., acrolein or acetaldehyde) as well as Marlboro Red smoke when compared with the filtered air control. This is not the first time that Dr. Springer’s group at University of California San Francisco has shown that acute exposure of rats to E-cig aerosols impairs FMD and, remarkably, to a comparable degree as that induced by combustible cigarette smoke 10, 11. Although nicotine has typically been a component of and certainly is a likely suspect of aerosol induced-ED, this present study (and a previous study of marijuana smoke without nicotine 12) exposed rats to the individual gases, acrolein (3 ppm) and acetaldehyde (10-11.5 ppm), that alone also induce ED. Although these gases, which are abundant in E-cig-derived aerosols, impair FMD in the absence of nicotine, it may not be inferred that nicotine or particles, which are also abundant in E-cig aerosols, are without effect 13. To the authors’ credit, their analysis of the varying effect of levels of the constituents indicates that nicotine level proportionally worsens FMD impairment. These data would be quite useful to the Food and Drug Administration and Center for Tobacco Products (FDA/CTP) in light of their regulatory authority over the levels of all constituents in E-cigs. In these acute exposure studies, there appears to be no “safe level” given that each exposure induced significant vascular harm. This contrasts with prior research on inhaled acrolein supports that levels less than 3 ppm are acutely and chronically harmful to the vasculature 14, 15.
Mechanisms
Of note, both studies are complementary in their efforts to better understand mechanisms by which E-cig aerosols impair FMD. However, both papers detail quite different approaches to this issue, which leads to intriguing results and different, yet not mutually exclusive, inferences (Scheme).
Scheme:
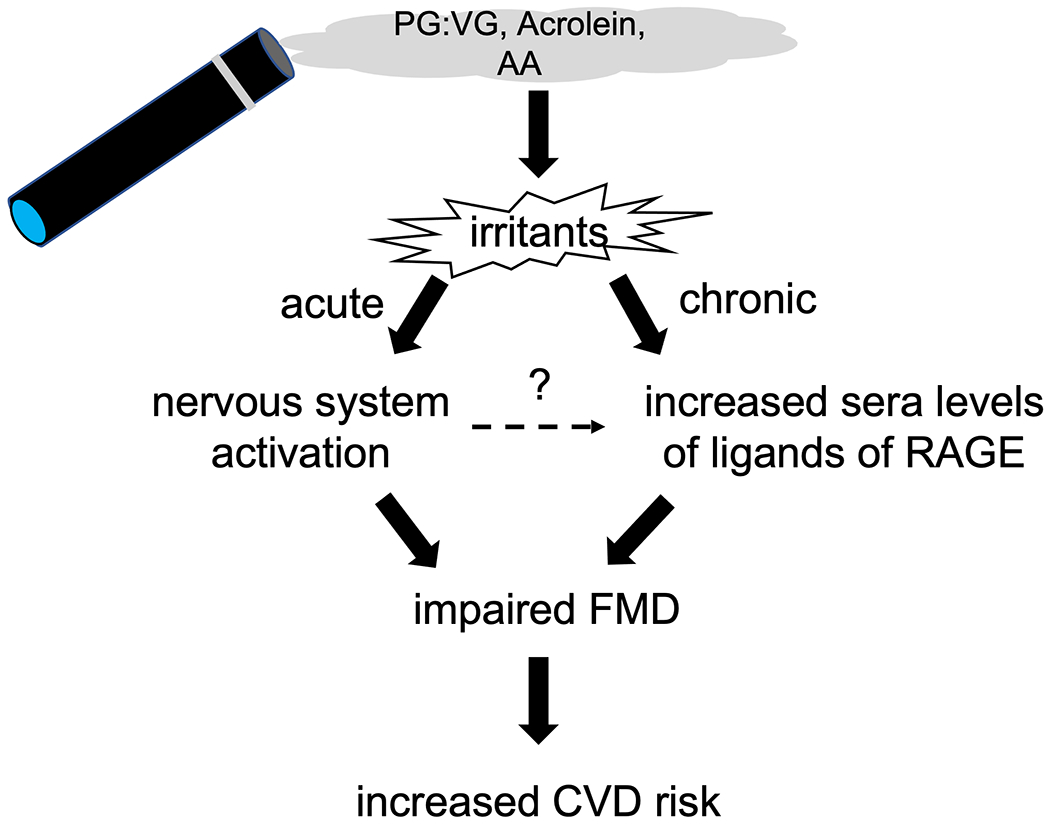
Novel insights into mechanisms of endothelial dysfunction (impaired flow-mediated dilation, FMD) induced by electronic cigarette aerosol exposure (acute pathway: rats) and chronic use (chronic pathway: human E-cig users) as revealed in the complementary studies in this issue of ATVB (#, pgs). Abbreviations: AA, acetaldehyde, PG:G, propylene glycol:glycerin; RAGE, receptor of advanced glycation endproducts.
Acute Exposure and the Role of the Nervous System
In the acute study, the primary interventions include gases alone and bilateral vagotomy. As mentioned above, results of gas alone exposures rule out a necessary role for nicotine (or solvents or flavorings) yet support a sufficient role of irritants in injury. The results of bilateral vagotomy intervention confirm a necessary role of the nervous system (e.g., reflex) in mediating ED. This latter finding dovetails well with findings that showed short-term exposure of female mice to the irritant formaldehyde (5 ppm) gas alone recapitulates both ED and ”respiratory braking” responses seen in exposure of female mice to E-cig solvent-derived aerosols 16. It is worth noting, however, that exposure of female mice to acetaldehyde at 5 ppm did not lead to either ED or a ”respiratory braking” response 13, which differs from the findings of the Nabavizadeh et al. study2. However, they used acetaldehyde at 10-11.5 ppm (2 times greater) perhaps reflecting a species difference in sensitivity to this irritant (e.g., rats vs mice). Overall, a nervous system-dependent (e.g., possibly sympathetic autonomic) reflex that impairs ED is well-founded. For example, a decade ago, the sympathetic nervous system was indicted in arteriolar ED in rats exposed by inhalation to nano-TiO2 17. As a wide variety of irritants are well known to activate airway sensory neural pathways that trigger autonomic reflexes 18, it will be important to investigate the specific steps by which aerosol or gas induce ED via nervous system activation.
Chronic E-cig Use, AGE-RAGE and ED
As compelling as the acute studies are in reflecting the potent nature of E-cig aerosol to impair FMD in animals, there is the question of whether chronic E-cig use impairs FMD in humans. In the second study, Mohammadi et al.3 adroitly address this question in three groups of humans: non-users, sole E-cig users, and sole combustible cigarette users. Although cross-sectional in design, the cohorts were highly controlled, FMD was measured in all groups, and the results clearly show robust FMD impairment in chronic E-cig and combustible cigarette user groups. The study, however, went a step further and extensively characterized sera toxicity in two types of cultured human endothelial cells with an emphasis on basal and agonist-induced NO production and functionality. Sera of chronic E-cig users contains factors that impair formation of eNOS-derived NO and enhance permeability in a manner separate and distinct from sera of chronic users of combustible cigarettes and from E-cig condensates. Factor in E-cig users sera are S100A8 and HMGB1 -- the pro-permeability effects of which are prevented by Receptor of AGE (RAGE) inhibitor, FPS-ZM1. The presence of elevated levels of RAGE ligands in E-cig users sera does not bode well for vascular health 19.
Limitations and Future Directions
Conduit arteries including the brachial and femoral arteries, used in human and rat FMD studies, respectively, rely, almost exclusively, on NO. Thus, it is unsurprising that impairment of FMD in rats exposed acutely to E-cig-derived aerosols or in humans chronically using E-cig is a consequence of change in NO. Impaired FMD can be a consequence of impaired NO production, enhanced loss/destruction of NO, and/or loss of vascular smooth muscle cell (VSMC) responsiveness to NO. However, as FMD is an integrated physiological measure, one cannot assess the contribution of individual processes - either production, destruction or responsiveness - to the sum total of impairment. To confirm that the latter is not causally part of impaired FMD, typically, an NO donor, e.g., glyceryl trinitrate, or a calcium channel blocker is given to fully dilate the conduit artery 9. Such a ”VSMC control” is not performed in either study, and its absence leaves open a question as to whether VSMC sensitivity to NO is impaired under either acute or chronic conditions.
The authors found a novel pathway in E-cig users, but absent in smokers, wherein elevated S100A8 and HMGB1 –increase EC permeability via RAGE.3 This is a unique observation that surprisingly differentiates the mechanism of ED of chronic E-cig use from use of combustible cigarettes. However, the degree of FMD impairment is not highly correlated with per person sera NO inhibition, and although vexing, it remains to be seen whether circulating factors result from acute stimulation of the nervous system or as a distinct secondary result of chronic use. Perhaps, in a trial where chronic E-cig users receive a RAGE inhibitor or abstain from use would allow for temporal assessment of the reversibility of FMD impairment and sera-induced EC toxicity, and thus, potentially reveal their interdependence. Answers to these mechanistic questions will help in understanding how best to track progression and mitigate deleterious vascular effects of E-cig exposures. These answers may apply to a wide variety of environmental irritant exposures.
Conclusions
CVD is the leading cause of morbidity and mortality worldwide 20, 21, and more than one-fourth of the almost 57 million global deaths are attributed to CVD deaths in 2016. Notably, ischemic heart disease and stroke are the two leading causes of death for the last 20 years 20. Thus, when any determinant induces ED -- especially a key measure such as FMD – it should be taken -- 150% -- seriously!
ACKNOWLEDGEMENTS
The author thanks A-TRAC colleagues for intellectual input and encouragement.
FUNDING
This work was supported by grant funds from the National Institutes of Health (HL122676, HL149351, P42ES023716, P30GM127607, U54HL120106) and the Jewish Heritage Fund for Excellence Research Enhancement Grant Program at the University of Louisville, School of Medicine.
Abbreviations:
- CVD
cardiovascular disease
- E-cig
electronic cigarette
- ED
endothelial dysfunction
- FMD
flow-mediated dilation
- MCS
mainstream cigarette smoke
- NO
nitric oxide
- RAGE
receptor of AGE
- SHS
secondhand smoke
- VSMC
vascular smooth muscle cell
Footnotes
CONFLICTS OF INTEREST
Author declares no conflicts of interest in this paper. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health, the Food and Drug Administration, the U.S. Environmental Protection Agency, or the American Heart Association.
REFERENCES
- 1.McNeill AB, L. S; Calder R; Hitchman SC; Hajek P’ McRobbie H. E-cigarettes: An evidence update. A report commissioned by public health england. 2015:109 [Google Scholar]
- 2.Bhatnagar A Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705 [DOI] [PubMed] [Google Scholar]
- 3.American Heart Association. Smoking & cardiovascular disease (heart disease). 2014;2017 [Google Scholar]
- 4.U.S. Department of Health and Human Services. The health consequences of smoking—50 years of progress: A report of the surgeon general, 2014. 2014 [Google Scholar]
- 5.U.S. Food & Drug Administration. Harmful and potentially harmful constituents in tobacco products and tobacco smoke: Established list. 2012;2017 [Google Scholar]
- 6.Centers for Disease Control and Prevention. Health effects of secondhand smoke. 2017;2017 [Google Scholar]
- 7.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 2004;43:1731–1737 [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N. Engl. J. Med 1996;334:150–154 [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155 [DOI] [PubMed] [Google Scholar]
- 10.Nabavizadeh P, Liu J, Havel CM, Ibrahim S, Derakhshandeh R, Jacob Iii P, Springer ML. Vascular endothelial function is impaired by aerosol from a single iqos heatstick to the same extent as by cigarette smoke. Tob Control. 2018;27:s13–s19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao P, Liu J, Springer ML. Juul and combusted cigarettes comparably impair endothelial function. Tob Regul Sci. 2020;6:30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Derakhshandeh R, Liu J, Narayan S, Nabavizadeh P, Le S, Danforth OM, Pinnamaneni K, Rodriguez HJ, Luu E, Sievers RE, Schick SF, Glantz SA, Springer ML. One minute of marijuana secondhand smoke exposure substantially impairs vascular endothelial function. J Am Heart Assoc. 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conklin DJ, Ogunwale MA, Chen Y, Theis WS, Nantz MH, Fu XA, Chen LC, Riggs DW, Lorkiewicz P, Bhatnagar A, Srivastava S. Electronic cigarette-generated aldehydes: The contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol Sci Technol. 2018;52:1219–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheat LA, Haberzettl P, Hellmann J, Baba SP, Bertke M, Lee J, McCracken J, O’Toole TE, Bhatnagar A, Conklin DJ. Acrolein inhalation prevents vascular endothelial growth factor-induced mobilization of flk-1+/sca-1+ cells in mice. Arterioscler Thromb Vasc Biol. 2011;31:1598–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conklin DJ, Malovichko MV, Zeller I, Das TP, Krivokhizhina TV, Lynch BH, Lorkiewicz P, Agarwal A, Wickramasinghe N, Haberzettl P, Sithu SD, Shah J, O’Toole TE, Rai SN, Bhatnagar A, Srivastava S. Biomarkers of chronic acrolein inhalation exposure in mice: Implications for tobacco product-induced toxicity. Toxicol Sci. 2017;158:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L, Lynch J, Richardson A, Lorkiewicz P, Srivastava S, Theis W, Shirk G, Hand A, Bhatnagar A, Srivastava S, Conklin DJ. Electronic cigarette solvents, pulmonary irritation, and endothelial dysfunction: Role of acetaldehyde and formaldehyde. Am J Physiol Heart Circ Physiol. 2021;320:H1510–H1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knuckles TL, Yi J, Frazer DG, Leonard HD, Chen BT, Castranova V, Nurkiewicz TR. Nanoparticle inhalation alters systemic arteriolar vasoreactivity through sympathetic and cyclooxygenase-mediated pathways. Nanotoxicology. 2012;6:724–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessac BF, Jordt SE. Breathtaking trp channels: Trpa1 and trpv1 in airway chemosensation and reflex control. Physiology (Bethesda.). 2008;23:360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramasamy R, Shekhtman A, Schmidt AM. The rage/diaph1 signaling axis & implications for the pathogenesis of diabetic complications. Int J Mol Sci. 2022;23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. The top 10 causes of death. 2018;2018 [Google Scholar]
- 21.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]


